Abstract
RNA interference (RNAi) has rapidly become a powerful tool for target discovery and therapeutics. Small interfering RNAs (siRNAs) are highly effective in mediating sequence-specific gene silencing. However, the major obstacle for using siRNAs as cancer therapeutics is their systemic delivery from the administration site to target cells in vivo. This chapter describes approaches to deliver siRNA effectively for cancer treatment and discuss in detail the current methods to assess pharmacokinetics and biodistribution of siRNAs in vivo.
Keywords: siRNA, Ovarian Cancer, Delivery, Cancer Therapy, stem-loop RT-PCR
1. Introduction
Classical analyses of gene function are performed by generating knockout (KO) mouse models and observing a phenotype (1). Even though KO of genes offers powerful means to discover disease related-genes such as oncogenes in vivo, development of drugs such as small molecule compounds or antibodies are required for clinically relevant therapeutic strategies. However, these approaches do have limitations (2). Small molecule inhibitors are frequently associated with undesirable toxicities and antibodies are only useful for targets accessible in the circulation or located on the surface of target cells. Since the discovery of RNA interference (RNAi) (3) and the application of small interfering RNA (siRNA) to silence desired target genes (4), siRNA has become an alternative technology to analyze gene function and discover drug targets. Since siRNAs can inhibit the expression of any gene of interest, we can utilize this technology for targeting previously undruggable genes. Hence, the use of siRNA is attractive for cancer therapy.
Despite the promise, several hurdles must be overcome for successful use of siRNA in the clinic. SiRNA is easily degraded in the bloodstream by ribonucleases (RNase), eliminated by renal excretion, and cannot pass through a cellular membrane readily because of its large molecular weight, high hydrophilicity and negative charge (5). Thus, effective siRNA delivery systems are needed for this approach to be successful. Many groups are developing siRNA delivery systems for cancer using a variety of formulations, such as liposomes, polymers, or micelles (5-7). The physical properties of delivery systems such as size, shape and surface charge are critical factors for delivery of nanoparticles to tumors after systemic administration. It is well established that long-circulating nanoparticles with an average diameter of ≤100 nm accumulate efficiently in tumor tissues via the enhanced permeability and retention (EPR) effect based on the fact that tumor vessels are irregularly shaped, defective, leaky and have varying widths compared with normal capillaries (8,9). Intratumoral mobility of nanoparticles may be affected by higher interstitial fluid pressure and soluble factors in solid tumors, population of stromal cells and density of extracellular matrix in tumor. For example, polymeric micelles of 30 nm in diameter showed penetration in stromal-rich pancreatic tumors but that of 70 nm showed no penetration (10). After being taken up by target cells through endocytosis, siRNAs need to be released from endosomes into cytosol. These sequential steps from administration site to cytosol in target cells should be considered for development of siRNA delivery systems for cancer treatment (2,5,7). Importantly, siRNAs need to be effectively delivered to tumors to exert therapeutic effect. Therefore, determination of pharmacokinetic profiles of administrated siRNA in the body is an important issue for the clinical development of siRNA medicine. Here, we describe in vivo siRNA delivery in orthotopic ovarian cancer (OvCa) models using chitosan/siRNA nanoparticles (11,12), and quantification of siRNAs by stem-loop quantitative reverse transcribed (qRT)-PCR and fluorescence-based assays (13).
2. Materials
2.1. Commercial Reagents
Chitosan (CH), low molecular weight (Sigma-Aldrich)
Sodium tripolyphosphate (TPP; Sigma-Aldrich)
Glacial acetic acid (Thermo Scientific)
siRNAs (Sigma-Aldrich)
Human ovarian cancer cell lines, SKOV3, HeyA8 and A2780 (ATCC).
RPMI1640 (Thermo Scientific)
Hank's Balanced Salt Solution (HBSS) (Mediatech, Inc)
0.5 M EDTA (Invitrogen)
Trizol (Life Technologies)
Direct-zol RNA Kit (Zymo Research)
Verso cDNA Synthesis Kit (Thermo Scientific)
TaqMan miRNA assays (Applied Biosystems)
2× Fast SYBR Green Master Mix (Applied Biosystems)
2.2. Equipment
Centrifuges, 5417R and 5810R (Eppendorf)
A homogenizer, TISSUE MASTER 125 homogenizer (OMNI International, Kennesaw GA, USA) for homogenization of tissue samples
A spectrophotometer, NanoDrop 2000c (Thermo Scientific) for RNA quantification
A thermal cycler, Mastercycler pro (Eppendorf) for RT-PCR
A real-time thermal cycler, 7500 Fast Real-Time PCR System (Applied Biosystmes) for real-time PCR
In vivo imaging system, IVIS 200 system (Xenogen) for ex vivo imaging for siRNAs
3. Methods
3.1. Preparation of siRNA/Chitosan (siRNA/CH) nanoparticle
Chitosan (CH) is a linear polysaccharide composed of randomly distributed β-linked D-glucosamine and N-acetyl-D-glucosamine. CH is biodegradable, biocompatible, low immunogenic and low toxic, which makes it as a very attractive tool for clinical and biological applications (14–16). Due to the presence of protonated amino groups, negatively charged nucleic acids can be loaded in CH, and siRNA/CH nanoparticles can effectively interact with cell membranes. Therefore, we developed CH nanoparticles to deliver siRNA into tumors (11,12). siRNA/CH nanoparticles are prepared based on ionic gelation of anionic TPP and siRNA with cationic CH.
0.25% acetic acid is prepared by dissolving 0.25 ml glacial acetic acid in 99.75 ml of water.
CH solution is obtained by dissolving CH (2 mg/ml) in 0.25% acetic acid.
TPP is prepared by dissolving 0.25 g of TPP in 100 ml of water.
Nanoparticles are spontaneously generated by the addition of TPP (0.25% w/v) and siRNA (1 μg/μl) to CH solution under constant stirring at room temperature.
After incubating at 4°C for 40 min, siRNA/CH nanoparticles are collected by centrifugation at 14,000 rpm for 40 min at 4°C.
The pellet is washed 3 times to remove unbound chemicals or siRNA and siRNA/CH nanoparticles are stored at 4°C until use.
3.2. Development of orthotopic in vivo models of ovarian cancer
Female athymic nude mice (8-12 weeks old) are obtained from the National Cancer Institute.
Human ovarian cancer cells such as SKOV3ip1, HeyA8, or A2780 are cultured in RPMI1640 supplemented with 15% fetal bovine serum in 10 or less passage prior to injection into mice.
Cells are detached with Trypsin and complete media is added. Cells are then pelleted at 1,200 rpm for 5 min at 4°C
Cells are then washed twice with PBS.
Resuspend cells using HBSS at a concentration of 5 × 106 cells per ml.
Cells (1 × 106 cells per 200 μl per mouse) are injected into the peritoneal cavity using 30G needles.
After tumors have been established, siRNA/CH-nanoparticles are injected intraperitoneally into tumor-bearing mice at a dose of 1.25~5.0 μg siRNA per mouse.
3.3. RNA isolation from blood, plasma and tissue
- Sample preparation from blood:
-
1.1At different time points, mice are put under anesthesia using isoflurane and blood is collected from abdominal vena cava or by cardiac puncture into RNase-free cryotubes using 25-G needles.
-
1.2Blood (typically 200 μl) is mixed with three times volume of Trizol (600 μL). Vortex the mixture thoroughly (see Note 1).
-
1.3The mixture is centrifuged at 12,000 × g for 10 min at 4°C to obtain supernatants. The resulting supernatant is processed for total RNA isolation.
-
1.1
- Sample preparation from plasma:
-
2.1Blood is to be stored in RNase-free tubes with each tube containing 84.3 μL K2EDTA per mL of blood.
-
2.2Mix blood and anticoagulant thoroughly by inverting tube immediately 10 times (see Note 2).
-
2.3Centrifuge the mixture at 12,000 × g for 10 min at 4°C. This will give three layers: (from top to bottom) plasma, leucocytes (buffy coat), erythrocytes. Carefully aspirate the supernatant (plasma) to a tube. Prior to use, the plasma can be stored at −80°C.
-
2.4Plasma (typically 200 μl) is mixed with three times volume of Trizol (600 μL).
-
2.5The mixture is centrifuged at 12,000 × g for 10 min at 4°C to obtain the supernatants. The resulting supernatant is processed for total RNA isolation.
-
2.1
- Sample preparation from tissues:
-
3.1Tissues, such as tumor, brain, lung, heart, liver, spleen and kidney are collected in RNase-free cryotubes using sharp scissors and forceps, and snap freeze them in liquid nitrogen. The organs can be stored at −80°C until use.
-
3.2Just prior to use, thaw the tissues on ice. Weighed tissue (typically 50~100 mg) is transferred into 5 ml polystyrene round-bottom tubes with 750 μL of Trizol (see Note 3).
-
3.3A tissue is homogenized with a homogenizer (see Note 4).
-
3.4The resulting tissue homogenate is centrifuged at 12,000 × g for 10 min at 4°C to obtain the supernatants. The resulting supernatant is processed for total RNA isolation.
-
3.1
Total RNA is isolated from the supernatant of blood, plasma or tissue using Direct-zol RNA Kit according to the manufacturer’s protocol.
RNA concentration is quantified using a spectrophotometer.
3.4. siRNA quantification in blood and tissue by stem-loop qRT-PCR
Stem-loop qRT-PCR has been utilized to quantify small RNA fragments (e.g., miRNA) (17). First, a miRNA-specific stem-loop RT primer is hybridized to the miRNA and then reverse transcribed. Next, the RT product, cDNA is amplified by regular real-time PCR using a miRNA-specific forward primer and the universal reverse primer. Stem-loop qRT-PCR method also can be applied for quantification of siRNA, which gives high sensitivity, selectivity and wide dynamic range of detection of siRNA as compared with other means such as enzyme-linked immunosorbent assay (ELISA) or high performance liquid chromatography (HPLC) (18). Therefore, stem-loop qRT-PCR technique can be used for the quantification of administered siRNA in blood and tissues. The primer for siRNA in stem-loop PCR is designed for each sequence. The forward primer in PCR amplification is designed based on the siRNA sequence and a universal reverse primer (5′-GACCTGTCCGATCACGACGAG-3′) is used.
Standard siRNA is prepared by serial 2-fold dilutions of siRNA with RNase free water.
Standard blood sample is prepared by directly adding 5 ng siRNA to 200 μl of naïve blood or plasma obtained from non-treated mouse, and the siRNA/blood or plasma mixture is subjected to RNA isolation as mentioned before.
Isolated total RNA (1~10 μg) from blood and tissues, and standard siRNAs (~2.5 pg) is subjected to stem-loop PCR using TaqMan miRNA assays according to the manufacturer’s protocol. 10 μl of total RNA/siRNA is combined with 5 μl of master mix of stem-loop PCR and 2 pmol stem-loop PCR primer, then stem-loop PCR is carried using Mastercycler pro. Condition for the stem-loop PCR reaction is as follows: 16°C for 30 min, 42°C for 30 min, 85°C for 5 min, then hold at 4°C.
1.3 μl of cDNA is added into the PCR amplification reaction mix (10 μl 2× Fast SYBR Green Master Mix and 20 pmol of forward and reverse primer sets at a volume of 20 μl).
PCR amplification is carried out using the 7500 Fast Real-Time PCR System. Condition for the RT reaction is as follows: 95 °C for 15min enzyme activation, then 40 cycles of 95°C for 15 sec denaturation and 60°C for 1 min annealing/extension.
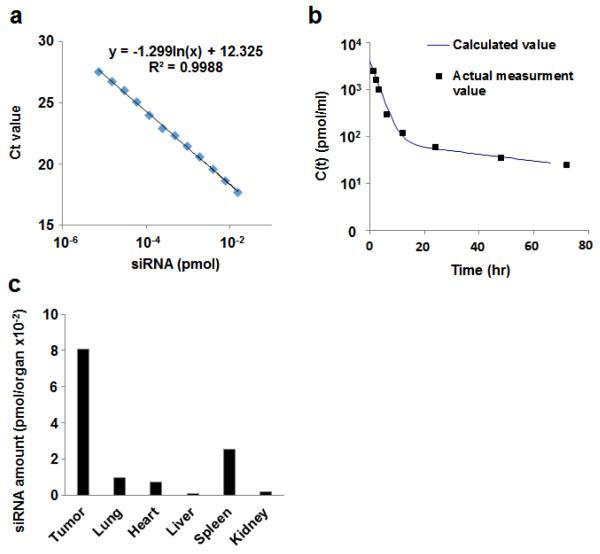
siRNA amount in blood sample is calculated using the standard curve (Fig.1a).
Blood concentrations of siRNA at collected time points, C(t) is expressed as amount of siRNA per ml of blood.
-
Pharmacokinetic analysis is performed as described below:
C(t) is fitted by an appropriate equation for one or two-compartment models using software such as Graphpad prism, MULTI, or other appropriate programs (Fig. 1b) (see Note 5).
One compartment: C(t) = Ae−αt, Tow compartment: C(t) = Ae−αt + Be−βt
Area under the curve (AUC0-t) of blood concentration is calculated by integration of C(t) up to a given time point. Levels of siRNAs in various oragans are also calculated using standard siRNA curve (Fig. 1c).
Fig.1.
Stem-loop PCR for siRNA quantification. (a) Standard curve showing a plot of log amount of siRNA vs. Ct values quantified using qRT-PCR method. The equation derived from this plot is used for calculating the absolute amount of siRNA. (b) Time profile of plasma siRNA concentration. Closed squares and line represent actual concentration of siRNA in blood and calculated curve of siRNA concentration by fitting the data using two-compartment model using MULTI program, respectively. (c) siRNA amount in organs is calculated using the siRNA standard curve shown in (a).
3.5. Fluorescence-based biodistribution study
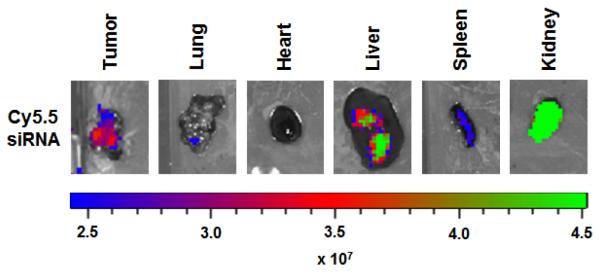
In vivo imaging has become an important tool for the development of drug delivery systems. The near–infrared (NIR) fluorescence provides simultaneous acquisition of full-color white light imaging with NIR images, deeper penetration of NIR signal and decreased tissue autofluorescence compared to visible light (19). Therefore, nanoparticle labeled with NIR fluorophore such as Cy5.5 or DiR allows determination of amount in organs ex vivo and non-invasive evaluation of biodistribution in vivo after their administration.
Cy5.5-labeled siRNAs loaded nanoparticle is administered i.p. into tumor bearing mice at a dose of 2.5 μg siRNA.
At time points (typically 24 or 48 hours), tumor and organs are excised.
Excised organs are washed with cold PBS and put them on 6-well plate.
Fluorescence image in excised organs are captured using the Xenogen IVIS 200 system with Cy5.5 fluorophore excitation (678 nm) and Emission (703 nm) filter.
Fluorescent images are analyzed using Living image 2.5 software. Regions of interest are drawn for each organ and total radiant efficiency ps−1 μW−1 cm2 are measured (Fig. 2).
Fig. 2.
Fluorescence-based images of Cy5.5 labeled siRNA in organs. The levels of Cy5.5-siRNA were measured in various organs at 48 hr post administration of nanoparticles in tumor-bearing mice. The scale bar represents the fluorescence intensity in ps−1 mW−1 cm2.
3.6. Quantifying levels of gene knockdown using quantitative reverse transcription PCR (qRT-PCR)
Isolated RNA (500-1000 ng) from tumor tissue is reverse transcribed using a Verso cDNA Synthesis Kit according as per the manufacturer’s instructions using a Mastercycler pro.
2 μl diluted cDNA (typically 2-10 folds dilution) is then subjected to PCR amplification with 10 μl 2× Fast SYBR Green Master Mix and 20 pmol of forward and reverse primer sets at a volume of 20 μl.
PCR is performed using the 7500 Fast Real-Time PCR System. Each cycle consists of 15 sec of denaturation at 95 °C and 1 min of annealing and extension at 60 °C (40 cycles).
Relative levels of gene expression are quantified using the ΔΔCt method.
Acknowledgments
H. H. is supported by JSPS Postdoctoral Fellowships for Research Abroad. S.Y.W. is supported by Ovarian Cancer Research Fund, Inc., Foundation for Women’s Cancer, and Cancer Prevention Research Institute of Texas training grants (RP101502 and RP101489). Portions of this work were supported by NIH grants (P50CA083639, CA109298, P50CA098258, U54CA151668, UH2TR000943, CA016672, U54CA96300, and U54CA96297), CPRIT (RP110595 and RP120214), an Ovarian Cancer Research Fund Program Project Development Grant, the Betty Ann Asche Murray Distinguished Professorship, the RGK Foundation, the Gilder Foundation, the Judi A. Rees Ovarian Cancer Research Fund, the Chapman Foundation, and the Meyer and Ida Gordon Foundation. This research was also supported, in part, by the Blanton-Davis Ovarian Cancer Research Program.
4. Notes
Blood samples should be vortexed immediately after mixing with Trizol, or blood solidifies in Trizol. If storage is necessary prior to use, blood need to be collected in a tube with anticoagulant agent and stored at −80 °C.
Process samples as soon as possible. If storage is necessary prior to use, store the blood at room temperature, shielded from light.
Hard tissues should be cut into small pieces with scissors before adding Trizol, which results in efficient homogenization.
Output power of homogenizer should be adjusted depending on softness of organ. Soft organs such as brain and liver are homogenized at low power to avoid bubble; hard organs such as spleen and heart are homogenized at high power. Homogenization should be done with tubes immersed in ice cold water to avoid generating heat.
Five or more time points are required for curve fitting of the time profile of siRNA concentration in blood or plasma.
Reference
- 1.Gondo Y. Trends in large-scale mouse mutagenesis: from genetics to functional genomics. Nat Rev Genet. 2008;9:803–810. doi: 10.1038/nrg2431. [DOI] [PubMed] [Google Scholar]
- 2.Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 4.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 5.Hatakeyama H, Akita H, Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv Drug Deliv Rev. 2011;63:152–160. doi: 10.1016/j.addr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Materials. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 7.Wu SY, Lopez-Berestein G, Calin GA, Sood AK. RNAi therapies: drugging the undruggable. Sci Transl Med. 2014;6:240ps7. doi: 10.1126/scitranslmed.3008362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 9.McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 10.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 11.Kim HS, Han HD, Armaiz-Pena GN, Stone RL, Nam EJ, Lee JW, et al. Functional roles of Src and Fgr in ovarian carcinoma. Clin Cancer Res. 2011;17:1713–1721. doi: 10.1158/1078-0432.CCR-10-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gharpure KM, Chu KS, Bowerman CJ, Miyake T, Pradeep S, Mangala SL, et al. Metronomic docetaxel in PRINT nanoparticles and EZH2 silencing have synergistic antitumor effect in ovarian cancer. Mol Cancer Ther. 2014;13:1750–1757. doi: 10.1158/1535-7163.MCT-13-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu SY, Yang X, Gharpure KM, Hatakeyama H, Egli M, McGuire MH, et al. 2′-OMe-phosphorodithioate-modified siRNAs show increased loading into the RISC complex and enhanced anti-tumour activity. Nat Commun. 2014;5:3459. doi: 10.1038/ncomms4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han HD, Song CK, Park YS, Noh KH, Kim JH, Hwang T, et al. A chitosan hydrogel-based cancer drug delivery system exhibits synergistic antitumor effects by combining with a vaccinia viral vaccine. Int J Pharm. 2008;350:27–34. doi: 10.1016/j.ijpharm.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Zhang HM, Chen SR, Cai YQ, Richardson TE, Driver LC, Lopez-Berestein G, et al. Signaling mechanisms mediating muscarinic enhancement of GABAergic synaptic transmission in the spinal cord. Neuroscience. 2009;158:1577–1588. doi: 10.1016/j.neuroscience.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katas H, Alpar HO. Development and characterisation of chitosan nanoparticles for siRNA delivery. J Control Release. 2006;115:216–225. doi: 10.1016/j.jconrel.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng A, Li M, Liang Y, Wang Y, Wong L, Chen C, et al. Stem-loop RT-PCR quantification of siRNAs in vitro and in vivo. Oligonucleotides. 2009;19:203–208. doi: 10.1089/oli.2008.0176. [DOI] [PubMed] [Google Scholar]
- 19.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]