Abstract
Importance
Sex is a variable that is poorly controlled for in clinical research.
Objective
Determine if sex bias exists in human surgical clinical research, determine if data are reported and analyzed using sex as an independent variable, and identify specialties where the greatest and least sex biases exist.
Design
Review and data abstraction from published peer-reviewed manuscripts.
Setting
All original peer-reviewed manuscripts published in 2011 and 2012 in Annals of Surgery, American Journal of Surgery, JAMA Surgery, Journal of Surgical Research, and Surgery.
Main Outcome Measures
Study type, location, number and sex of subjects, sex matching, and inclusion of sex-based reporting, statistical analysis, and discussion of data.
Results
Of 2,347 articles reviewed, 1,668 included human subjects. After excluding 365 articles, 1,303 manuscripts remained: 17 (1%) included only males, 41 (3%) included only females, 1,020 (78%) included males and females, and 225 (17%) did not document the sex of the subjects. While females represent over 50% of the total number of subjects included, considerable variability existed with the number of male, female, and unspecified subjects included among the journals, between US domestic and international studies, and between single versus multi-center studies. For manuscripts included in the study, only 38% reported these data by sex, 33% analyzed these data by sex, and 23% included a discussion of sex-based results. Sex matching of the subjects included in the research was poor, with only 18% of the studies matching the inclusion of both sexes by 80%. Upon analysis of the different surgical specialties, a wide variation in sex-based inclusion, matching, and data reporting existed, with colorectal surgery having the best matching of males and females and cardiac surgery having the worst.
Conclusion
Our data show that sex bias exists in human surgical clinical research. Few studies included men and women equally, less than one-third performed data analysis by sex, and there was wide variation in inclusion and matching of the sexes among the specialties and the journals reviewed. Because clinical research serves as the foundation for evidence-based medicine, it is imperative that this disparity be addressed so that therapies benefit both sexes.
INTRODUCTION
In 1977, the Food and Drug Administration (FDA) barred women of “childbearing potential” from participating in clinical research until adequate safety and efficacy information could be developed during animal and early clinical studies.2 This act, along with perceived challenges with recruiting women, subsequently resulted in the poor inclusion of women in clinical trials.3,4 In addition, female subjects have been historically regarded as suboptimal research subjects due to the estrous cycle and inherent reproductive differences from males, even though data exist to refute this assumption.5,6 Due to the low enrollment of women in clinical trials, the National Institutes of Health (NIH) Revitalization Act was introduced and signed into law in 1993. This law mandated the inclusion of women as subjects in clinical research funded by the NIH. However, recent studies have shown that the inclusion of women in clinical trials has only marginally improved.7,8,9
The equal inclusion of men and women in clinical trials is important as exemplified by the drug zolpidem (AmbienR), a sleep aid medication. Zolpidem was originally approved by the FDA in 1992. In the New Drug Application submitted to the FDA, it was documented that the peak drug concentration after administration was 45% higher in females, yet the drug was approved for the same dose in both males and females.10 In 2013, it was uncovered that some women taking zolpidem were involved in automobile accidents the next morning.11 Investigation revealed that women metabolized the drug more slowly than men.12 This led to a label change approved by the FDA in May 2013, recommending that women take half the dose as men.13 It is currently unknown how many other drugs may be metabolized differently by sex, or simply work differently in males and females. However, the Government Accountability Office (GAO) performed an assessment of adverse events by sex and reported that 8 out of 10 drugs removed from the market by the FDA were due to adverse events in women.14 It is well known that males and females experience health and disease differently, metabolize and respond to some drugs differently, respond to medical devices differently, and can have different outcomes following medical interventions.15–24
Surgical research is not immune to this problem. We recently demonstrated that significant sex bias exists in surgical biomedical research.25 Based on this study and pressure from the advocacy community, the NIH announced that sex must be considered as a variable in all NIH funded studies beginning January 2016.26–28 It remains unknown if the same sex bias exists in human surgical clinical research. Males and females can have different postoperative outcomes, complication rates, and re-admission rates, so it is important to know if this problem of sex bias is pervasive in surgery.29–33 Adequately controlling for sex as a variable with inclusion, data reporting, and data analysis is important as data derived from clinical research serve as the foundation for evidence-based medicine. Thus, the objective of this study was to determine if sex bias exists in human surgical clinical research, and to identify areas where the greatest and least sex biases exist. We hypothesize that males and females are not included in surgical clinical research in equal numbers, and that data are not reported or analyzed using sex as an independent variable.
METHODS
Data Abstraction
Data were collected as previously described by Yoon et al.25 All original manuscripts published from January 1, 2011 through December 31, 2012 in the top five ranked American non-specialty surgical journals which publish internationally and across specialties were reviewed. These journals include Annals of Surgery, American Journal of Surgery, JAMA Surgery, Journal of Surgical Research, and Surgery. Only original peer-reviewed research articles were included in the study. Articles excluded from data abstraction were review articles, letters to the editor, case reports etc. Of the original peer-reviewed research articles, articles were further excluded if they included any animal or cell data, the number of included subjects was zero or not stated, or the article studied anatomically sex-specific disease (e.g. ovarian, testicular, prostate etc.).
Variables abstracted
The following data were abstracted: type of study (i.e. animal, cell, or human), single or multi-center study, domestic or international study, whether the manuscript studied a sex specific disease by anatomic criteria such as prostate, ovarian, cervical etc., the number and sex of each subject studied (if specified), and presence of sex-based data reporting. When stratifying for sex-based reporting, articles were assessed for sex-based reporting of data, analysis of data by sex, and inclusion of sex-based results in the discussion section. Finally, surgical sub-specialty responsible for each study was noted. Specialties in which <10 articles were published over the 2-year study period were marked as “other”.
Matching of included subjects by sex
The degree of sex matching for the subjects included within each study was calculated. The number of included males and females was considered and the lesser number of subjects (male or female) in an individual study was defined as the numerator, and the greater number of subjects (male or female) was defined as the denominator for individual studies. The percent matching of males and females included as subjects was calculated as the ratio of the numerator/denominator multiplied by 100. For example, a 100% match of male and females would include 50 males and 50 females as subjects. The equation would equal 50/50 × 100 = 100%. A 50% matching of male and female subjects would include 50 males and only 25 females, with the equation equal to 25/50 × 100 = 50%.
Statistical analysis
Chi-squared tests were employed to examine differences between publications by journal which stated or did not state sex, to compare the numbers of males, females, and unspecified sex subjects in the studies presented in each journal, between domestic and international manuscripts, and between manuscripts published by the different specialties. The differences in the distribution of sex matched subjects, sex-based reporting, analysis, and discussion of the data by specialty were also assessed using a chi-squared test. Significance was assumed for p< 0.05. Analyses were conducted using SAS version 9.4.
RESULTS
Sex of human subjects is often unstated
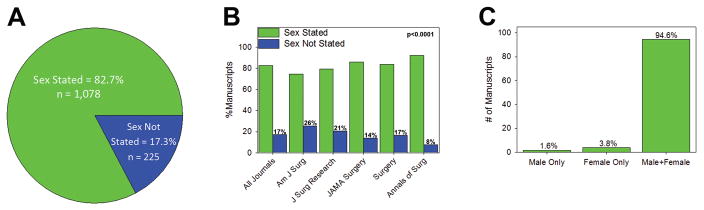
In total, 2,347 publications were reviewed among all five surgery journals. Of these, 1,668 (71%) included human subjects. 365 publications were excluded because they included animals or cells, studied a sex-specific disease, or did not report the number of subjects included. Of the 1,303 manuscripts reviewed (Table 1), 1,078 (83%) stated the sex of subjects included in the study, while 225 (17%) did not state the sex of subjects included (Figure 1A). While the pattern of this finding was consistent among all five journals (Figure 1B), the distribution of these differences were statistically significant. The American Journal of Surgery had the most subjects with the sex not stated (26%) while Annals of Surgery had the least (8%, p<0.0001). Of the 1,078 manuscripts that stated the sex of the subjects, 17 (1.6%) were male only studies, 41 (3.8%) were female only studies, and 1,020 (94.6%) included males and females (Figure 1C, p<0.0001). These data were similar between US domestic and international studies. Of the 771 US domestic publications that stated the sex of the subjects, 1.6% were male only, 4.3% were female only, and 94.1% included males and females. Of the 532 international publications that stated the sex of the subjects, 1.5% were male only, 3.1% were female only, and 95.4% included males and females.
Table 1.
Characteristics of all articles included across journals, centers, and specialties.
| # Manuscripts (%) | |
|---|---|
| Total | 1303 |
| Journal | |
| American Journal of Surgery | 392 (30.1%) |
| Journal of Surgical Research | 116 (8.9%) |
| JAMA* Surgery | 256 (19.6%) |
| Surgery | 266 (20.4%) |
| Annals of Surgery | 273 (21.0%) |
| Single & Multi Center | |
| Single Center | 916 (70.3%) |
| Multi Center | 387 (29.7%) |
| Domestic & International | |
| Domestic | 771 (59.2%) |
| International | 532 (40.8%) |
| Surgical Specialty | |
| Bariatric | 33 (2.5%) |
| Breast | 56 (4.3%) |
| Cardiac | 17 (1.3%) |
| Colorectal | 137 (10.5%) |
| Endocrine | 126 (9.7%) |
| General Surgery | 205 (15.7%) |
| Pediatric | 34 (2.6%) |
| Surgical Education/Training | 98 (7.5%) |
| Surgical Oncology | 250 (19.2%) |
| Surgery – Unspecified | 72 (5.5%) |
| Thoracic | 39 (3.0%) |
| Transplant | 49 (3.8%) |
| Trauma/Critical Care | 118 (9.1%) |
| Vascular | 42 (3.2%) |
| Other** | 27 (2.1%) |
JAMA = Journal of the American Medical Association.
Other includes biotech/biodesign (n=4), burn (n=2), neurosurgery (n=6), orthopedic surgery (n=2), healthcare disparities/EMR/epidemiology studies (n=8), otolaryngology/HNS (n=2), plastic surgery (n=1), urology (n=2).
Figure 1. Sex of Subjects Included.
Number and percent of manuscripts which state the sex of the human subjects included in (A) all manuscripts and (B) by journal. The distribution of rates of sex stated/not stated was different between journals (p<0.0001). (C) Number and percent of manuscripts that include male only, female only, and male and female subjects.
Sex-based reporting, analysis, and discussion of data
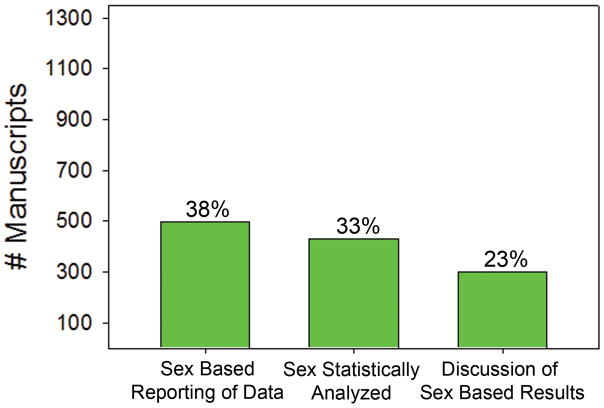
Of all studies included, only 38% reported data separately for male and female subjects, only 33% performed statistical analysis on data collected by sex, and only 23% of articles addressed sex-based results in the discussion section (Figure 2). These data were similar between domestic (37%, 32% and 23% respectively) and international studies (40%, 35%, and 23% respectively). Between specialties, these data were highly variable. For example, endocrine surgery, surgical oncology, colorectal, and thoracic surgery were the highest performers in sex-based data reporting, analysis, and discussion of the data (Supplemental Table 1, p<0.0001). Breast, bariatric, and cardiac surgery were the lowest performers in sex-based data reporting, analysis, and discussion of the data (p<0.0001).
Figure 2. Sex-Based Data Reporting.
(A) Percent of manuscripts that reported the data by sex. (B) Percent of manuscripts that statistically analyzed the data by sex. (C) Percent of manuscripts that included a discussion of the results by sex.
Disparity exists with the absolute number of male and female subjects studied
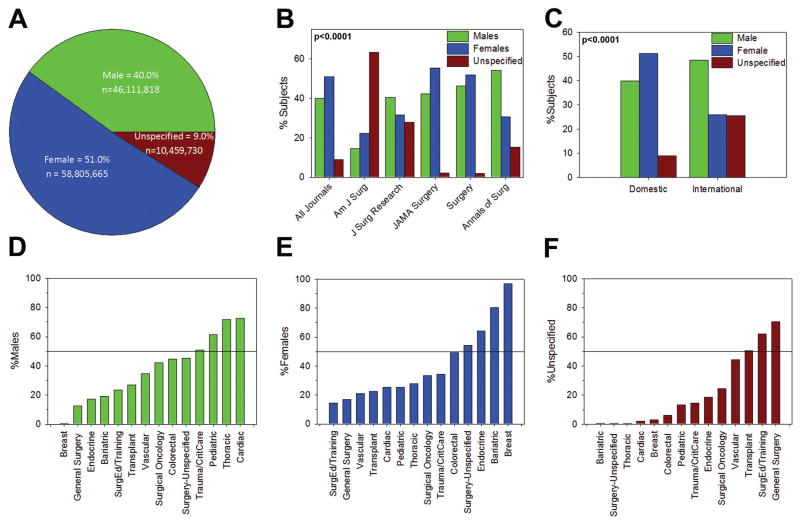
In total, over two years and among the five journals studied, 115,377,213 human subjects were included in the published manuscripts. There were 46,111,818 (40%) males, 58,805,665 (51%) females, and 10,459,730 (9%) unspecified subjects (Figure 3A). After excluding manuscripts on which veterans and surgical trainees were the subjects, the sex of subjects included were similar: 40% male, 51% female, and 9% unspecified. An analysis of manuscripts publishing on cardiac and thyroid disease, which are known to be more prominent in women, revealed that the sex of subjects included were 25% male, 59% female, and 16% unspecified. The total number of males, females, and unspecified subjects between journals was highly variable (Figure 3B, p<0.0001). For example, there were 15%, 22%, and 63% males, females, and unspecified subjects, respectively, in the American Journal of Surgery, but 46%, 52%, and 2% males, females, and unspecified subjects, respectively, in Surgery. Similarly, differences were detected between US domestic and international studies. In US domestic studies there were 40%, 51%, and 9% male, female, and unspecified subjects, respectively, while in international studies there were 48%, 26%, and 26% male, female, and unspecified subjects, respectively (Figure 3C, p<0.0001). In single-center studies, there were 46%, 51%, and 3% male, female, and unspecified subjects, respectively, while in multi-center studies there were 39%, 51%, 10% male, female, and unspecified subjects, respectively, (p < 0.0001).
Figure 3. Number of Male and Female Subjects Included.
Absolute number of male, female, and unspecified subjects included in (A) all manuscripts, (B) by journal, (C) in US domestic and international studies. Distribution of included males, females, and unspecified subjects between journals and between domestic and international studies was different (p<0.0001). (D) Number of male subjects included by specialty. (E) Number of female subjects included by specialty. (F) Number of unspecified subjects included by specialty. Distribution of included males, females, and unspecified subjects was different by specialty (p<0.0001).
Disparity in inclusion of subjects by sex exists between surgical specialties
There was inconsistency between surgical subspecialties in the number of male, female, and unspecified subjects included in the studies. Articles from trauma/critical care, pediatric, thoracic, and cardiac surgery had greater than 50% male subjects (Figure 3D, p<0.0001). Surgery-unspecified, endocrine, bariatric, and breast surgery had greater than 50% female subjects (Figure 3E, p<0.0001). Transplant, general surgery, and articles regarding surgical education and training had greater than 50% of unspecified subjects (Figure 3F, p<0.0001). Furthermore, there was a significant difference in overall distribution of male, female, and unspecified subjects by specialty (p<0.0001).
Studies do not match included subjects by sex
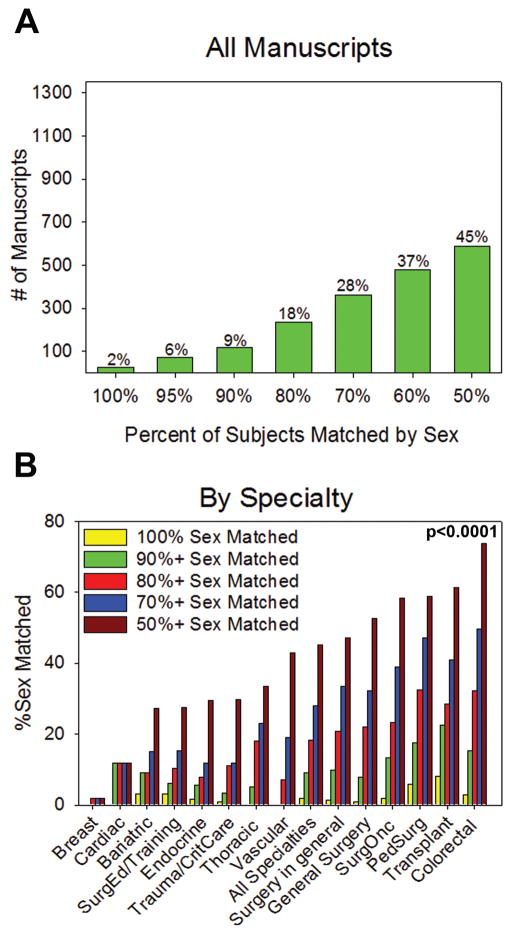
Among articles which included both males and females, only 2% of articles matched the sex of included subjects by 100% (Figure 4A). Only 45% of articles matched included male and female subjects by 50%. In comparing specialties, breast and cardiac surgery did not match any studies by 100%. Examination of the specialties that matched inclusion of males and females by at least 50% revealed that colorectal (p<0.0001), transplant (p<0.0001), pediatric surgery (p<0.0001), and surgical oncology (p<0.0001) contained the highest number of studies, while breast (p=0.0003), cardiac (p<0.0001), bariatric (p<0.0001) and surgical education (p<0.0001) contained the lowest number of studies. There was a significant difference in the overall distribution of 50% or greater matching across all specialties (p< 0.0001) (Figure 4B).
Figure 4. Sex Matching of Subjects.
(A) Degree of sex matching in all manuscripts and (B) by specialty. There is a statistically significant difference in distribution of 50% or greater matching across all specialties (p<0.0001).
DISCUSSION
In this manuscript we show that significant sex bias exists in human surgical clinical research. Most importantly, of the manuscripts reviewed only approximately one-third of manuscripts statistically analyzed and reported the data by sex. These data were consistent in comparing domestic and international studies, and after excluding surgical education and VA studies, the latter of which is predominantly male. Furthermore, the sex of the subjects included in the research was not stated in 17% of published peer-reviewed studies. Of those manuscripts that stated the sex of the subjects, 95% included both males and females. While females represent over 50% of the total number of subjects included in the clinical research studies, considerable variability existed with the number of male, female, and unspecified subjects included among the journals, between US domestic and international studies, and between single versus multi-center studies. The proportions of male, female, and unspecified subjects was unchanged with the exclusion of studies conducted at the VA and on surgical trainees. There was also wide variability in the number of male, female, and unspecified subjects included among the surgical specialties. Finally, sex matching of the subjects included in the research is practiced in less than half of the peer-review publications reviewed using a very liberal 50% matching criteria. Thus, the results of this study confirm our hypothesis that males and females are not included in human surgical clinical research in equal numbers, and that data are analyzed and discussed using sex as an independent variable in less than one-third of surgical clinical research studies
To our knowledge this is the largest and most comprehensive study to examine sex bias in human surgical clinical research. However, there are a few studies in other disciplines that examine sex bias in clinical research. The data presented in our study shows an improvement compared to the data published by Kwiatkowski et al who showed that in cancer trials more men (59.8%) were included than women.34 Meinert et al showed that for clinical trials published in United States journals from 1966–1998 the percent of subjects by sex included: males and females (55%), males only (12%), females only (11%), and unspecified sex (21%).7 Vidaver et al surveyed the medical literature from 1995–2000 and showed that less than 20–30% (depending on the year) of the studies analyzed data by sex.35 Similarly, Blauwet et al showed that sex specific reporting of data was only 37% in general medical journals and 23% in cardiovascular journals, while Geller et al showed that outcomes were not reported by sex in 75% of federally funded randomized clinical trials published in 2009.9,36 Finally, in a study of the orthopedic literature, Hettrich et al showed an increase in sex-specific analysis from 19% to 30% from 2000 to 2010.37 These data are remarkable given that many diseases have a clear female prevalence.38–44 Of note, our results are consistent with the US GAO report to the NIH in October 2015 which revealed that more females than males were included in NIH-funded clinical research from 2004–2015. In addition, our data on the lack of sex-based reporting and analysis is consistent with the US GAO’s report and the Institute of Medicine’s 2010 report on healthcare research. These later reports showed that despite the NIH Revitalization act of 1993 and increased female enrollment in clinical trials, sex-based reporting and analysis of results remains an area of disparity.45,46 Despite good overall inclusion of females in human surgical clinical research, we were surprised to find the low rate of matching of subjects with regards to sex. Furthermore, we were surprised to find that the sex of the subjects included was still not reported in over 17% of peer-reviewed studies published in 2011 and 2012. Finally, the wide variation in sex-based data reporting and analysis between surgical specialties was surprising in that some specialties included sex-based data reporting and analysis in over 50% of published studies, while some specialties included sex-based data reporting and analysis in less than 10% of published studies. Together, our data imply that sex disparity exists in human surgical clinical research in many ways despite the government mandate of female inclusion in NIH-funded clinical trials.
Implications of these findings are numerous. First, drugs, therapies, and devices may be developed that are effective for only one sex.47 Second, for therapies and drugs that are reported to have an overall low efficacy in men and women when the data is aggregated, the therapy or drug may be abandoned; however, that therapy or drug may have greater efficacy in one sex versus the other. This would only be known if sex-based analysis and reporting of the data was performed. For example, the Human Papilloma Virus vaccine is much more effective in women versus men.48,49 But, in aggregate, the efficacy is low. If sex-based reporting of the data was not conducted, this therapy that is effective at preventing cervical papillomas in women may not have been developed. Third, therapies may be developed that have undesirable side effects in the opposite sex. For example, the odds of an adverse drug reaction in women is 50% greater than in men, women are more likely to be hospitalized due to an adverse drug reaction, and 80% of the drugs removed from the market by the FDA were due to undesirable side effects in women.14,50,51 Thus, while it is important to aggregate data of both males and females, performing independent data analysis and reporting for both males and females can yield discoveries leading to valuable contributions to the health and wellbeing of males or females independently.
We acknowledge and understand that there are criticisms to including both sexes and accounting for sex differences in clinical research. 52–55 However, our recommendations are for an FDA mandate that requires drugs, devices, and new therapies to be tested equally in male and female subjects prior to market approval. Drugs which have been recalled due to adverse effects in one sex should be tested independently in both sexes as these drugs may be candidates for re-release with different dosing parameters for each sex. Research funding agencies such as the NIH, National Science Foundation, Department of Defense, the Veterans Affairs Administration, etc. should mandate researchers to match inclusion of males and females in clinical research, and report results independently for males and female subjects so that sex may be examined as in independent variable. Journal editors should require authors to include the sex of all subjects studied in published literature and require sex-based reporting, analysis, and discussion of data. In support of these views, the IOM recently published guidelines for sex-specific reporting of research.56 Finally, government monitoring of sex-based inclusion of subjects, sex as an independent variable, and sex-based data reporting should be required, especially for research conducted using government funds. Such practices have been adopted by other countries and the U.S. should follow suit.57
Limitations exist within this study. Our study design was intended to capture a representative sampling of surgical clinical research, not a complete analysis of all surgical clinical research. The scope of this study was limited to manuscripts published in only five surgery journals over a two year period. These journals publish mainly on general surgery topics so there was a paucity of manuscripts from certain surgical sub-specialties such as neurological surgery, urology, orthopedic surgery, and plastic surgery. There was no inclusion or specification regarding the difference between sex and gender, and this study focused solely on the differences between phenotypic male and female sex.58 Furthermore, these are observational data intended to highlight differences in inclusion, reporting, and analysis between sexes, and are not corrected for specific disease prevalence. Data capture for funding source was not consistent among the manuscripts; thus, we were not able to consistently and reliably discern if the research was funded and if it was funded by the NIH versus industry. Lastly, within the manuscripts analyzed for this study there were too few randomized controlled trials to develop meaningful conclusions; thus, we are conducting a separate study analyzing sex bias in all clinical trials registered with clinicaltrials.gov over a defined time period.
In conclusion, this study shows that sex bias exists in human surgical clinical research. Few publications included men and women equally, less than one-third performed data analysis by sex, and there was wide variation in inclusion and matching of the sexes between the specialties and the journals reviewed. Because clinical research serves as the foundation for evidence-based medicine, it is imperative that this disparity be addressed because therapies and practice derived from such studies may be specific to only one sex.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Mrs. Lynnette Dangerfield for her administrative and editorial assistance. We would also like to acknowledge that the author NM is partially supported by a National Institutes of Health Grant (2T32HL094293-06).
References
- 1.Klein SL, Schiebinger L, Stefanick ML, et al. Opinion: Sex inclusion in basic research drives discovery. Proc Natl Acad Sci U S A. 2015;112(17):5257–5258. doi: 10.1073/pnas.1502843112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health Education and Welfare. General considerations for the clinical evaluation of Drugs. 1977. (Publication No. (FDA) 77-3040) [Google Scholar]
- 3.Kim AM, Tingen CM, Woodruff TK. Sex bias in trials and treatment must end. Nature. 2010;465(7299):688–689. doi: 10.1038/465688a. [DOI] [PubMed] [Google Scholar]
- 4.Woodruff TK. Sex, equity, and science. Proc Natl Acad Sci U S A. 2014;111(14):5063–5064. doi: 10.1073/pnas.1404203111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117(1–2):1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Hughes RN. Sex does matter: comments on the prevalence of male-only investigations of drug effects on rodent behaviour. Behav Pharmacol. 2007;18(7):583–589. doi: 10.1097/FBP.0b013e3282eff0e8. [DOI] [PubMed] [Google Scholar]
- 7.Meinert CL, Gilpin AK, Unalp A, Dawson C. Gender representation in trials. Control Clin Trials. 2000;21(5):462–475. doi: 10.1016/s0197-2456(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 8.Pinnow E, Sharma P, Parekh A, Gevorkian N, Uhl K. Increasing participation of women in early phase clinical trials approved by the FDA. Womens Health Issues. 2009;19(2):89–93. doi: 10.1016/j.whi.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Blauwet LA, Hayes SN, McManus D, Redberg RF, Walsh MN. Low rate of sex-specific result reporting in cardiovascular trials. Mayo Clin Proc. 2007;82(2):166–170. doi: 10.4065/82.2.166. [DOI] [PubMed] [Google Scholar]
- 10.Gordin MD. U.S. Food and Drug Administration New Drug Application 199–08. 1991. [Google Scholar]
- 11.Logan BK, Couper FJ. Zolpidem and driving impairment. J Forensic Sci. 2001;46(1):105–110. [PubMed] [Google Scholar]
- 12.Farkas RH, Unger EF, Temple R. Zolpidem and driving impairment--identifying persons at risk. N Engl J Med. 2013;369(8):689–691. doi: 10.1056/NEJMp1307972. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA approves new label changes and dosing for zolpidem products and a recommendation to avoid driving the day after using Ambien CR. 2013. [Google Scholar]
- 14.Heinrich J. GAO-01-286R Drugs Withdrawn From Market. United States General Accounting Office; 2001. [Google Scholar]
- 15.Anderson GD. Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health (Larchmt) 2005;14(1):19–29. doi: 10.1089/jwh.2005.14.19. [DOI] [PubMed] [Google Scholar]
- 16.Arnold AP, Lusis AJ. Understanding the sexome: measuring and reporting sex differences in gene systems. Endocrinology. 2012;153(6):2551–2555. doi: 10.1210/en.2011-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ung CY, Lam SH, Zhang X, et al. Inverted expression profiles of sex-biased genes in response to toxicant perturbations and diseases. PLoS One. 2013;8(2):e56668. doi: 10.1371/journal.pone.0056668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oza NM, Baveja S, Tedrow U. Bridging the gender gap in atrial fibrillation. Expert Rev Cardiovasc Ther. 2015;13(3):317–323. doi: 10.1586/14779072.2015.1002466. [DOI] [PubMed] [Google Scholar]
- 19.Conrotto F, D’Ascenzo F, Salizzoni S, et al. A gender based analysis of predictors of all cause death after transcatheter aortic valve implantation. Am J Cardiol. 2014;114(8):1269–1274. doi: 10.1016/j.amjcard.2014.07.053. [DOI] [PubMed] [Google Scholar]
- 20.Hess CN, McCoy LA, Duggirala HJ, et al. Sex-Based Differences in Outcomes After Percutaneous Coronary Intervention for Acute Myocardial Infarction: A Report From TRANSLATE-ACS. Journal of the American Heart Association. 2014;3(1) doi: 10.1161/JAHA.113.000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiramoto JS, Katz R, Ix JH, et al. Sex differences in the prevalence and clinical outcomes of subclinical peripheral artery disease in the Health, Aging, and Body Composition (Health ABC) study. Vascular. 2014;22(2):142–148. doi: 10.1177/1708538113476023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh MN. Risk of Obstructive Sleep Apnea: Sex Matters. Circulation. 2015;132(14):1305–1306. doi: 10.1161/CIRCULATIONAHA.115.018694. [DOI] [PubMed] [Google Scholar]
- 23.Woodruff TK, Kibbe MR, Paller AS, Turek FW, Woolley CS. Commentary: “Leaning in” to support sex differences in basic science and clinical research. Endocrinology. 2014;155(4):1181–1183. doi: 10.1210/en.2014-1068. [DOI] [PubMed] [Google Scholar]
- 24.Gall SL, Donnan G, Dewey HM, et al. Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology. 2010;74(12):975–981. doi: 10.1212/WNL.0b013e3181d5a48f. [DOI] [PubMed] [Google Scholar]
- 25.Yoon DY, Mansukhani NA, Stubbs VC, Helenowski IB, Woodruff TK, Kibbe MR. Sex bias exists in basic science and translational surgical research. Surgery. 2014;156(3):508–516. doi: 10.1016/j.surg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Woodruff TK, Green S, Paller A, et al. Sex-based biomedical research policy needs an implementation plan. Womens Health (Lond Engl) 2015;11(4):449–452. doi: 10.2217/WHE.15.28. [DOI] [PubMed] [Google Scholar]
- 27.Clayton JA. Studying both sexes: a guiding principle for biomedicine. FASEB J. 2015 doi: 10.1096/fj.15-279554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institutes of Health Office of Extramural Research. Consideration of Sex as a Biological Variable in NIH-funded Research. 2015. [Google Scholar]
- 29.Wendling P. Women dogged by unplanned readmissions after aortic surgery. 2015. [Google Scholar]
- 30.Sutton JM, Wima K, Wilson GC, et al. Factors associated with 30-day readmission after restorative proctocolectomy with IPAA: a national study. Dis Colon Rectum. 2014;57(12):1371–1378. doi: 10.1097/DCR.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 31.Moghavem N, Morrison D, Ratliff JK, Hernandez-Boussard T. Cranial neurosurgical 30-day readmissions by clinical indication. J Neurosurg. 2015;123(1):189–197. doi: 10.3171/2014.12.JNS14447. [DOI] [PubMed] [Google Scholar]
- 32.Otto W, May M, Fritsche HM, et al. Analysis of sex differences in cancer-specific survival and perioperative mortality following radical cystectomy: results of a large German multicenter study of nearly 2500 patients with urothelial carcinoma of the bladder. Gend Med. 2012;9(6):481–489. doi: 10.1016/j.genm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Schmid M, Shariat SF, Soave A, Engel O, Fisch M, Rink M. Contemporary gender-specific outcomes in Germany after radical cystectomy for bladder cancer. Curr Urol Rep. 2014;15(6):409. doi: 10.1007/s11934-014-0409-2. [DOI] [PubMed] [Google Scholar]
- 34.Kwiatkowski K, Coe K, Bailar JC, Swanson GM. Inclusion of minorities and women in cancer clinical trials, a decade later: Have we improved? Cancer. 2013;119(16):2956–2963. doi: 10.1002/cncr.28168. [DOI] [PubMed] [Google Scholar]
- 35.Vidaver RM, Lafleur B, Tong C, Bradshaw R, Marts SA. Women subjects in NIH-funded clinical research literature: Lack of progress in both representation and analysis by sex. Journal of Womens Health & Gender-Based Medicine. 2000;9(5):495–504. doi: 10.1089/15246090050073576. [DOI] [PubMed] [Google Scholar]
- 36.Geller SE, Koch A, Pellettieri B, Carnes M. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: have we made progress? J Womens Health (Larchmt) 2011;20(3):315–320. doi: 10.1089/jwh.2010.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hettrich CM, Hammoud S, LaMont LE, Arendt EA, Hannafin JA. Sex-specific Analysis of Data in High-impact Orthopaedic Journals: How Are We Doing? Clin Orthop Relat Res. 2015;473(12):3700–3704. doi: 10.1007/s11999-015-4457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jellinger KA. The enigma of mixed dementia. Alzheimers Dement. 2007;3(1):40–53. doi: 10.1016/j.jalz.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Office on Women’s Health US Department of Health and Human Services. Lupus Fact Sheet. 2012. [Google Scholar]
- 41.Centers for Disease Control and Prevention. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation--United States, 2010–2012. MMWR Morb Mortal Wkly Rep. 2013;62(44):869–873. [PMC free article] [PubMed] [Google Scholar]
- 42.Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol. 2014;35(3):97–104. doi: 10.1016/j.it.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Danska JS. Sex matters for mechanism. Sci Transl Med. 2014;6(258):258fs240. doi: 10.1126/scitranslmed.3009859. [DOI] [PubMed] [Google Scholar]
- 44.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2(3):119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 45.Institute of Medicine. Women’s Health Research: Progress, Pitfalls, and Promise. Washington (DC): 2010. [Google Scholar]
- 46.U.S. Government Accountability Office. NATIONAL INSTITUTES OF HEALTH: Better Oversight Needed to Help Ensure Continued Progress Including Women in Health Research. 2015. [Google Scholar]
- 47.Check Hayden E. Sex bias blights drug studies. Nature. 2010;464(7287):332–333. doi: 10.1038/464332b. [DOI] [PubMed] [Google Scholar]
- 48.Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 49.Perkins RB, Legler A, Hanchate A. Trends in Male and Female Genital Warts Among Adolescents in a Safety-Net Health Care System 2004–2013: Correlation With Introduction of Female and Male Human Papillomavirus Vaccination. Sex Transm Dis. 2015;42(12):665–668. doi: 10.1097/OLQ.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 50.Zopf Y, Rabe C, Neubert A, et al. Women encounter ADRs more often than do men. Eur J Clin Pharmacol. 2008;64(10):999–1004. doi: 10.1007/s00228-008-0494-6. [DOI] [PubMed] [Google Scholar]
- 51.Tharpe N. Adverse drug reactions in women’s health care. J Midwifery Womens Health. 2011;56(3):205–213. doi: 10.1111/j.1542-2011.2010.00050.x. [DOI] [PubMed] [Google Scholar]
- 52.Bailey KR. Reporting of sex-specific results: a statistician’s perspective. Mayo Clin Proc. 2007;82(2):158. doi: 10.4065/82.2.158. [DOI] [PubMed] [Google Scholar]
- 53.Prins MH, Smits KM, Smits LJ. Methodologic ramifications of paying attention to sex and gender differences in clinical research. Gend Med. 2007;4(Suppl B):S106–110. doi: 10.1016/s1550-8579(07)80051-9. [DOI] [PubMed] [Google Scholar]
- 54.Bolon B. Gender agenda: sex bias can be justified in animal research. Nature. 2010;466(7302):28. doi: 10.1038/466028d. [DOI] [PubMed] [Google Scholar]
- 55.Overemphasizing Sex Differences as a Problem. 2015. Gendered Innovations. [Google Scholar]
- 56.IOM (Institute of Medicine) Sex-specific reporting of scientific research: A workshop summary. The National Academies Press; 2012. [PubMed] [Google Scholar]
- 57.Zeeb H, Spallek J, Jahn I. Gender agenda: positive steps taken in Germany. Nature. 2010;466(7304):315. doi: 10.1038/466315b. [DOI] [PubMed] [Google Scholar]
- 58.Klinge I. Gender perspectives in European research. Pharmacol Res. 2008;58(3–4):183–189. doi: 10.1016/j.phrs.2008.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.