Abstract
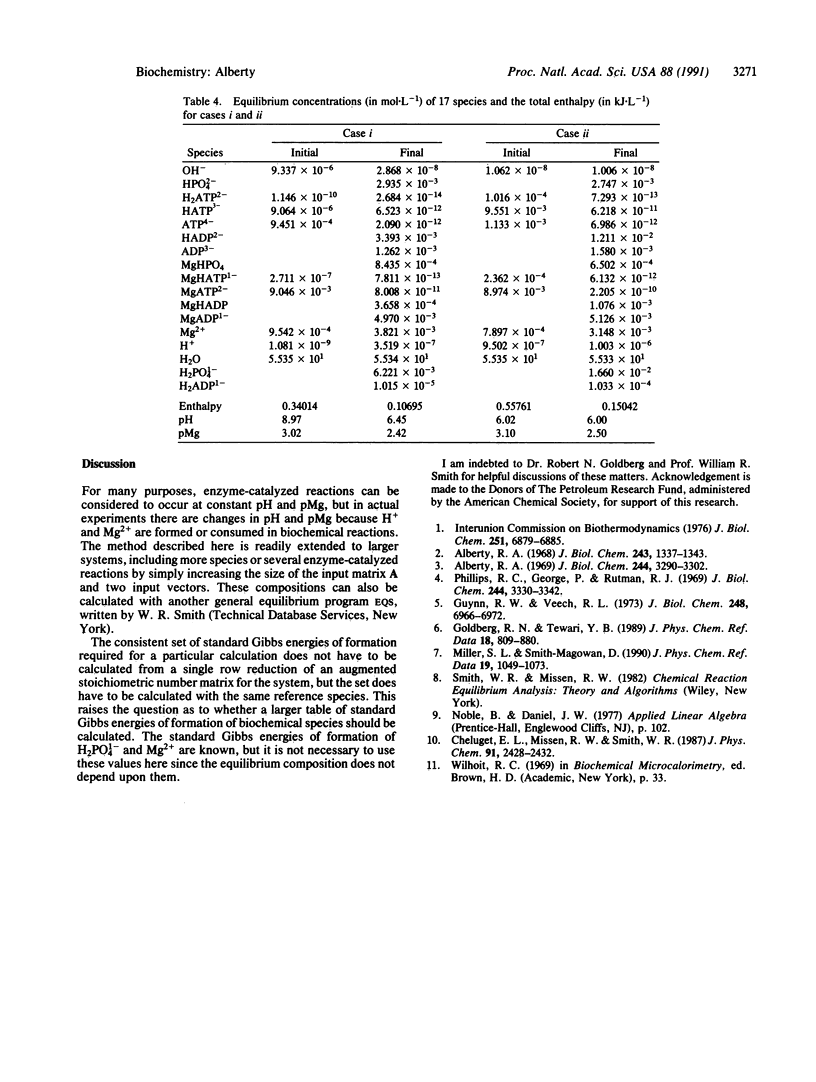
Equilibrium compositions of solutions of biochemical species can be calculated by use of general equilibrium computer programs that minimize the Gibbs energy. The standard Gibbs energies of formation and standard enthalpies of formation of the species in a biochemical system can be calculated by Gaussian reduction of the augmented transpose of the stoichiometric number matrix for the system. The conservation matrix, which is also needed for the calculation of the equilibrium composition, can be obtained in two ways. The hydrolysis of adenosine 5'-triphosphate in solutions containing magnesium ions can be treated by considering 17 species. The equilibrium composition and enthalpy are calculated before and after adding ATPase. This makes it possible to calculate DeltapH, DeltapMg, and the heat of reaction when ATPase is added.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A. Effect of pH and metal ion concentration on the equilibrium hydrolysis of adenosine triphosphate to adenosine diphosphate. J Biol Chem. 1968 Apr 10;243(7):1337–1343. [PubMed] [Google Scholar]
- Alberty R. A. Standard Gibbs free energy, enthalpy, and entropy changes as a function of pH and pMg for several reactions involving adenosine phosphates. J Biol Chem. 1969 Jun 25;244(12):3290–3302. [PubMed] [Google Scholar]
- Guynn R. W., Veech R. L. The equilibrium constants of the adenosine triphosphate hydrolysis and the adenosine triphosphate-citrate lyase reactions. J Biol Chem. 1973 Oct 25;248(20):6966–6972. [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic data for the hydrolysis of adenosine triphosphate as a function of pH, Mg2+ ion concentration, and ionic strength. J Biol Chem. 1969 Jun 25;244(12):3330–3342. [PubMed] [Google Scholar]



