Abstract
Purpose
Postsurgical endophthalmitis is a sight-threatening problem. We introduce a simple approach by using a single application of thermoresponsive controlled-release microspheres, loaded with moxifloxacin, to prevent bacterial endophthalmitis in a rabbit endophthalmitis prevention model.
Methods
We separated 24 rabbits into 3 treatment groups in which topical drop treatment was placed onto the conjunctival cul-de-sac: (1) a single drop of controlled-release microspheres containing moxifloxacin, (2) a single drop of controlled-release microspheres without moxifloxacin, and (3) multiple topical treatment with moxifloxacin alone every 15 minutes for 1 hour. All rabbits were challenged, 1 hour after microspheres drop placement and immediately after the fifth topical dose of moxifloxacin, with anterior chamber injections of Staphylococcus aureus. Rabbits in the topical moxifloxacin group also were treated after challenge and four additional times over the next 24 hours. After 24 hours, the rabbits were clinically evaluated for endophthalmitis and the animals were euthanized to culture for intraocular S. aureus. The treatment groups were compared statistically for bacterial endophthalmitis.
Results
No eyes had endophthalmitis, based on clinical presentation and/or positive culture, in the groups with controlled-release microspheres loaded with moxifloxacin (0/8, 0%) or multiple drops of topical moxifloxacin (0/8, 0%). In contrast, 8 of 8 eyes (100%; P = 0.0001), had endophthalmitis among eyes treated with controlled-release microspheres drops without moxifloxacin.
Conclusion
A single drop of controlled-release microspheres loaded with moxifloxacin was successful in preventing endophthalmitis. Further clinical studies will be required to confirm the full potential of controlled-release anti-infective loaded microspheres to prevent endophthalmitis.
Translational Relevance
This study presents a simple method of prophylaxis to prevent postsurgical endophthalmitis.
Keywords: prophylaxis, endophthalmitis, Staphylococcus aureus, drug delivery, microspheres
Introduction
Cataract surgery is the most common ocular surgery in adults with potential of infection. For cataract surgery, off-label prophylactic use of topical anti-infectives is part of the standard of care for prevention of postoperative endophthalmitis. Topical prophylaxis involves instillation of anti-infective drops multiple times before and after surgery. Patient compliance can be low due to a number of factors, one of which is the inability to instill the drops successfully.1 Traditional eye drops also suffer from extremely low bioavailability, with an estimated 5% or less of administered drug able to penetrate the cornea.2 Controlled release technology can effectively decrease the dosing frequency, thereby improving adherence rates, and increase bioavailability through localized administration.3,4 Cataract patients may benefit from a controlled-release formulation that is placed once in the eye to provide the necessary anti-infective without the repetitive chore of instilling an anti-infective at regular intervals.
Several groups have investigated such systems, typically using degradable polymeric materials with various routes of administration, including topical drops or gels,5,6 ocular insets, or contact lenses,7,8 and subconjunctival or sub-Tenon injection.9,10 Reformulated topical drops using mucoadhesive agents or gels tend to provide shorter windows of drug release,11 which would necessitate additional instillation by the patient during the postoperative period. In general, longer drug release times require larger and potentially uncomfortable inserts, or more invasive methods to accommodate the degrading depot as it releases the entrapped drug. Further, injected or implanted materials may require surgical explantation in the case of adverse events.
Fedorchak et al.12 developed and tested a combined thermoresponsive hydrogel/microsphere eye drop that is administered similarly to a traditional eye drop, but forms a pliable, nondegradable depot after exposure to body temperature in the conjunctival cul de sac (available in the public domain at http://www.google.com/patents/WO2014138085A1?cl=en) The hydrogel material is loaded with controlled-release microspheres capable of delivering a wide range of ocular drugs for varying lengths of time, as with preliminary studies with the glaucoma drug brimonidine, which demonstrate efficacy in vivo over 28 days.12
The goal of this study was to adapt this ocular drug delivery system to release the anti-infective moxifloxacin over a shorter period of time and at higher concentrations than previously tested. This controlled-release eye drop would be placed into the conjunctival cul de sac and ideally provide prophylaxis against bacterial endophthalmitis, thus eliminating the need for multiple topical anti-infective drops.
We hypothesized that controlled-release microspheres containing moxifloxacin, when placed onto the conjunctival cul-de-sac of rabbits before bacterial challenge, will prevent endophthalmitis when challenged with an anterior chamber injection of Staphylococcus aureus. This will be tested by comparing endophthalmitis prevention with standard of care topical anti-infective therapy and controlled-release microspheres that release no anti-infective.
Methods
Overview
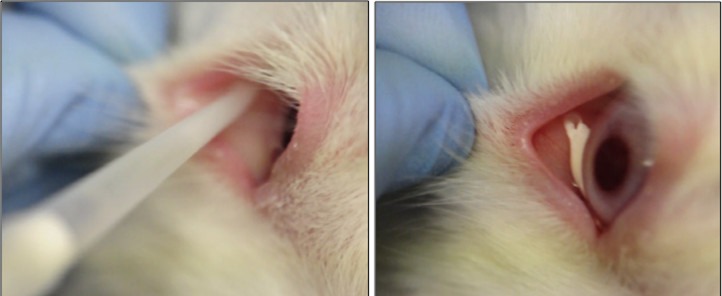
A single 0.1 mL drop of thermoresponsive controlled-release microspheres containing moxifloxacin was placed onto the inferior conjunctival cul de sac of the right eyes of rabbits to prevent S. aureus endophthalmitis (Fig. 1). Before instillation, the microspheres suspension was drawn slowly using a pipette fitted with standard pipet tips and allowed 5 to 10 seconds extra to avoid any air in the tip. If the tip clogged, the tip was moved to dislodge the clot and more was drawn. It was important to avoid and disperse clumps. A week before placement, the nictitating membranes were removed from these eyes. The rabbits treated with controlled-release microspheres loaded with moxifloxacin were compared to two groups: (1) controlled-release microspheres loaded with deionized (DI) water (blank) and (2) a group of rabbits that received topical 0.5% moxifloxacin. All rabbits were challenged, 1 hour after controlled-release microspheres drop placement and immediately after the fifth topical dose of 0.5% moxifloxacin, with anterior chamber injections of S. aureus. Rabbits in the topical 0.5% moxifloxacin group also were treated immediately after challenge and four additional times over the next 24 hours. After 24 hours, the rabbits were evaluated clinically for endophthalmitis and the animals were euthanized to detect the presence of viable intraocular S. aureus.
Figure 1.
Instillation and placement of a thermoresponsive controlled-release microspheres eye drop into the rabbit conjunctival cul de sac.
Controlled-Release Microspheres Fabrication and In Vitro Characterization
All materials and reagents were obtained from Sigma-Aldrich Corp. (St. Louis, MO) unless otherwise specified. Microspheres containing moxifloxacin were prepared using a standard double emulsion procedure with a target release window of 24 hours to correspond to the termination point of the in vivo study.12 First, 4 mL of dichloromethane as the continuous phase was used to dissolve 200 mg of poly(lactic-co-glycolic acid; ester terminated 50:50 lactide:glycolide, molecular weight [MW] 7–17 kDa, viscosity 0.16–0.24 dL/g). A volume of 250 μL of 24 mg/mL moxifloxacin hydrochloride in DI water was added as the dispersed phase for the first emulsion, which was formed using 10 seconds of probe sonication at 20% amplitude. Blank microspheres were fabricated using the same methods, with DI water only as the inner aqueous phase. The primary emulsion was homogenized for 1 minute at 7000 rpm (Silverson L4RT-A) in an aqueous 2% poly(vinyl alcohol [PVA]) solution (MW ∼25 kDa; Polysciences, Inc., Warminster, PA). The final double emulsion was added to a 1% PVA solution being stirred at a rate of 600 rpm for 3 hours before four consecutive centrifugation and washing steps. The washed microsphere suspension was frozen in liquid nitrogen and lyophilized for a minimum of 48 hours and subsequently stored at −20°C until use.
Free microspheres containing moxifloxacin were qualitatively analyzed for surface morphology and average diameter using scanning electron microscopy (JEOL 6335F Field Emission SEM; JEOL USA, Inc., Peabody, MA). Moxifloxacin released in vitro from the microspheres was determined using known masses of dry microspheres suspended in sufficient volumes of Dulbecco's phosphate buffered saline (DPBS plus calcium and magnesium; Thermo Fisher Scientific, Waltham, MA) to maintain sink-like conditions. These samples were incubated at 37°C in between releasate collection. To collect the releasate, microspheres containing moxifloxacin were centrifuged for 5 minutes at 1000 rpm and the supernatant removed for moxifloxacin quantification via plate reader (SpectraMax M4; Molecular Devices, Sunnyvale, CA) absorbance at 292 nm (linear range, 1–100 μg/mL). Absorbance measurements from blank microspheres were subtracted at each time point to account for background signal.
The thermoresponsive hydrogel carrier for the solid drops containing moxifloxacin was synthesized via aqueous free radical polymerization. Briefly, 2.1 mL of a 1:20 (vol/vol) solution of poly(ethylene glycol; MW 200 Da) and DI water was prepared. A mass of 0.1 g n-isopropylacrylamide (NIPAAm) was added and subsequently vortexed before adding 30 μL ammonium persulfate (APS, 0.1 mg/mL in DI water) and 5 μL of tetramethylethylenediamine (TEMED). This solution, which yields approximately 1 mL of final material, was mixed and stored at 4°C for 24 hours before washing five times in DI water. The lower critical solution temperature (LCST) at which phase transition occurs was measured via plate reader absorbance for n = 3 samples (SpectraMax M4; Molecular Devices). For the microspheres containing moxifloxacin used in vivo, the liquid hydrogel was presoaked in a 24 mg/mL aqueous solution of moxifloxacin hydrochloride as an analog to the five prophylactic drops administered in the positive control group.
Microsphere Drop Placement
The controlled-release microspheres were prepared immediately before placement using 100 mg of poly (lactic-co-glycolic) acid microspheres, blank or moxifloxacin-loaded, added to 0.5 mL of liquid gel precursor. The mixing was performed gently with a sterile pipet tip to avoid air bubbles and to maintain a homogeneous suspension. The controlled-release microspheres drops were created in the liquid state, but became a solid mass after exposure to body temperature (± 37°C; thermoresponsive) in the conjunctival cul de sac.
Each rabbit received one 0.1 ml of the controlled release microspheres drop in the right eye, as shown in Figure 1. Prior to instillation, the microspheres suspension was drawn slowly using a pipettor fitted with standard pipet tips and allowed 5—10 seconds extra to avoid any air in the tip. If the tip clogged, the tip was moved to dislodge the clot and more was drawn. It was important to avoid and disperse clumps.
For microspheres drop placement, the pipet tip was placed deep in the inferior fornix. A drop was placed smoothly and evenly with the acceptance of some air bubbles. The plunger was fully depressed to the second stop. A pipet tip and/or lowering of the eyelid were used to adjust the shape/placement of the drop. The microsphere drop was placed centrally or somewhat temporally, but not nasally if possible.
The microsphere drop would have been removed if there were any issues with the placement or if some of the drop did not stay in the fornix. Removal would be accomplished by rolling a clean cotton swab along the inner eyelid. Additionally, stubborn pieces of the microsphere drop would be flushed from conjunctiva with saline. The conjunctiva would be blotted thoroughly before instilling another microsphere drop.
Experimental Design and Protocol
The present study conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and was approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC Protocol #14125011, “Endophthalmitis prophylaxis with controlled-release of antibiotic from an ocular hydrogel”). A one eye-design using the right eye was chosen for 24 rabbits. The New Zealand white rabbits (0.95–1.4 kg) were purchased from Charles River's Oakwood Research Facility, Oxford, MI.
The nictitating membranes that partially cover the surface of the eye were removed with a cauterizing tool to cut the tissue and minimize bleeding. The rabbits were anesthetized intramuscularly with 40 mg/kg of ketamine (Ketathesia; Henry Schein Animal Health, Dublin, OH) and 4 mg/kg of xylazine (AnaSed Injection; Lloyd Laboratories, Shenandoah, IA), and topical 0.5% proparacaine (Proparacaine Hydrochloride Ophthalmic Solution; USP, Sandoz, Princeton, NJ) before removal. Following the procedure, one topical drop of the antibiotic/steroid combination 0.3% tobramycin/0.1% dexamethasone ophthalmic (Falcon Pharmaceuticals, Fort Worth, TX) was immediately administered and repeated once daily for the next 4 days to prevent infection and limit inflammation. The rabbits were treated with one dose of 1.5 mg/kg of ketoprofen (Ketofen; Zoetis, Inc., Kalamazoo, MI) immediately after resection.
One week after nictitating membrane removal, a clinical endophthalmitis isolate of S. aureus (E253; moxifloxacin minimal inhibitory concentration [MIC] = 1.0 μg/mL) was subcultured on trypticase soy agar supplemented with 5% sheep blood (Becton, Dickinson and Company, Sparks, MD) and incubated overnight at 37°C in 6% CO2. The isolate was part of a clinical bank of isolates that were deidentified and saved for antibiotic validation testing. The S. aureus isolate was suspended in sterile trypticase soy broth to a 0.5 McFarland Standard that approximates 1 × 108 colony-forming units per milliliter (CFU/mL) of bacteria. This concentration was appropriately diluted in sterile trypticase soy broth to provide the inoculum of 50,000 (5.0 × 104) CFU/eye in 25 μl. Colony counts were performed on the inoculum to determine the actual number of CFU inoculated.
The 24 rabbits were divided into 3 groups: (1) controlled-release microspheres containing moxifloxacin, (2) controlled-release microspheres without moxifloxacin, and (3) topical 0.5% moxifloxacin (Vigamox; Alcon, Fort Worth, TX). Before instillation of the microspheres drops in Groups 1 and 2, the rabbits were anesthetized with intramuscular injections of 40 mg/kg of ketamine and 4 mg/kg of xylazine. The rabbits in Group 3 were treated topically with one drop of Vigamox in the right eye at 60, 45, 30, and 15 minutes, and immediately before S. aureus anterior chamber challenge. All rabbits were systemically anesthetized before bacterial challenge.
The controlled-release microspheres drops (moxifloxacin and blank) were placed into the right conjunctivas of groups 1 and 2 at 60 minutes before bacterial inoculation, and group 3 was administered topical drops.
The right eye anterior chambers of the rabbits were inoculated by injection near the central dome of the cornea with 25 μL containing approximately 50,000 (5.0 × 104) CFU of S. aureus following topical anesthesia with 2 drops of proparacaine.
Immediately after challenge, the right eyes of the group 3 rabbits were topically treated with 1 drop of Vigamox, and over the next 24 hours with four additional drops.
All rabbits were intramuscularly administered a dose of ketoprofen (1.5 mg/kg) for pain after bacterial challenge.
At 24 hours after anterior chamber challenge, all rabbit eyes were examined grossly and with a slit-lamp by a board certified ophthalmologist trained as a corneal specialist (AM). The eyes were graded in a masked fashion for clinical signs of endophthalmitis (hypopyon, iritis, anterior chamber cells, anterior chamber flare, and fibrin) using a grading scale based on increasing severity (0 = normal, 0.5 = trace, 1.0 = mild, 2.0 = moderate, 3.0 = severe). An eye with an “anterior chamber score” of 3.0 or more was considered to have clinical endophthalmitis.2,3 Using the same grading scale, conjunctiva/scleral injection, limbal injection, and corneal infiltration also were evaluated.
After the clinical examinations, the rabbits were euthanized with an overdose of intravenous Euthasol (Virbac AH, Inc., Fort Worth, TX) following systemic anesthesia.
Aqueous humor taps were performed on the inoculated eyes by inserting 23-gauge needles attached to 1 mL syringes into the anterior chambers at the limbus and removing approximately 0.15 mL of fluid. A portion of each aqueous humor sample was immediately plated onto trypticase soy agar supplemented with 5% sheep blood and into a tube of Mueller-Hinton broth (Remel; Thermo Fisher Scientific). Similarly, vitreous humor taps and cultures were performed by inserting a 23-gauge needle attached to a 1 mL syringe into the posterior chamber approximately 4 mm from the limbus and removing approximately 0.2 to 0.3 mL of fluid.
After 48 hours at 37°C, the agar and liquid media were monitored for positive growth of S. aureus to determine the presence of endophthalmitis. Any positive S. aureus culture from the aqueous or vitreous humor samples was considered positive for endophthalmitis.
Analysis
Clinical presentation and/or positive bacterial growth determined the presence of endophthalmitis. The “anterior chamber scores” of the three treatment groups were analyzed nonparametrically with Kruskal-Wallis statistics (True Epistat, Richardson, TX). Differences in the number of eyes with endophthalmitis based on “anterior chamber scores” ≥ 3.0 and/or positive bacterial growth in the aqueous or vitreous humor samples among the three groups were analyzed using Fisher Exact testing (True Epistat). Differences between conjunctiva/scleral injection, limbal injection, and corneal infiltration were determined with the Mann-Whitney U test (Minitab, State College, PA).
Results
In vitro characterization studies were performed on the hydrogel and microsphere materials before in vivo validation of the solid-based drug delivery system. Scanning electron microscope images of the moxifloxacin-loaded microspheres can be seen in Figure 2. The microspheres containing moxifloxacin were confirmed to have a nonporous surface morphology with an estimated average diameter below 10 μm. Moxifloxacin release was measured from the drug-loaded microspheres (Fig. 3) using drug-free microspheres as a control for background signal. The resulting release kinetics show near-complete drug release within 24 hours at a level of 0.28 μg moxifloxacin per mg microspheres. The LCST of the hydrogel retention matrix was measured to be approximately 33.5°C and the phase transition was determined to be reversible.
Figure 2.
A scanning electron microscope image of moxifloxacin-loaded microspheres.
Figure 3.
Cumulative moxifloxacin release (μg) per mg of moxifloxacin-loaded microspheres from 0 to 60 minutes.
Colony counts of S. aureus injected into the anterior chamber of the two experiments were determined to be 4.39 × 104 and 4.5 × 104 CFU, respectively.
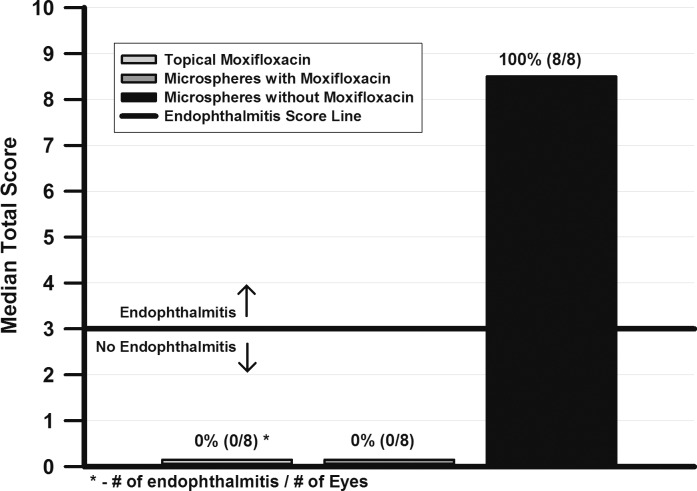
Figure 4 details the clinical “anterior chamber scores” and the number of eyes per total with endophthalmitis present after treatment with the controlled-release microspheres drops and topical therapy with 0.5% moxifloxacin. Kruskal-Wallis analysis (P < 0.05) determined that the median “anterior chamber scores” of eyes treated with controlled-release microspheres drops loaded with moxifloxacin (0.0) or topical 0.5% moxifloxacin (0.0) were less than eyes treated with microspheres drops without moxifloxacin (median = 8.5).
Figure 4.
The “anterior chamber scores” and the number of endophthalmitis present after treatment with controlled-release microspheres and topical therapy.
Clinically, there were no corneal infiltrates in the rabbits given topical moxifloxacin or with eyes treated with controlled-release microspheres loaded with moxifloxacin (grade = 0, normal). All eyes treated with microspheres drops without moxifloxacin demonstrated corneal infiltrates (median grade = 2.0, moderate severity). The conjunctival/scleral and limbal injections were graded as mild (median = 1.0) in the topically-treated group, and trace (median = 0.5) in the rabbits treated with controlled-release microspheres loaded with moxifloxacin. There was no significant difference between the treatment groups (P = 0.21, Mann-Whitney)
The numbers of eyes per total with endophthalmitis, based on “anterior chamber score” and/or positive S. aureus culture from the aqueous and/or vitreous, were less (P = 0.001, FE) for eyes treated with controlled-release microspheres loaded with moxifloxacin (0/8, 0%) and topical 0.5% moxifloxacin (0/8, 0%), than for eyes treated with microspheres drops without moxifloxacin (8/8, 100%).
Overall, treatments with either topical 0.5% moxifloxacin or controlled-release microspheres drops loaded with moxifloxacin were successful in preventing endophthalmitis in all eyes, whereas, all eyes treated with microspheres drops without moxifloxacin had endophthalmitis.
Discussion
Endophthalmitis after cataract surgery continues to be a problem. This emphasizes the need for new and effective approaches to prevent endophthalmitis. Topical antibiotics to sterilize the ocular surface and/or provide intraocular anti-infective concentrations,13–17 povidone iodine to decrease the flora of the eyelids,18,19 and intracameral antibiotics to eliminate any invading bacteria20–22 may have decreased the incidence of endophthalmitis, but these interventions have not completely eliminated this potentially devastating infection.
A controlled-release eye drop delivered over time as a topical antibiotic delivery system may be advantageous in preventing endophthalmitis by eliminating the need for frequent topical anti-infective drops instilled by patients before and after surgery. This could be instilled by the clinician, thereby minimizing patient compliance concerns. Drug release from highly porous hydrogels occurs very quickly relative to the microspheres [Hoare and Kohane, available in the public domain at http://www.sciencedirect.com/science/article/pii/S0032386108000487], and thus, the prolonged effect is presumed to be a result of the drug-loaded microspheres.
In our study, thermoresponsive controlled-release microspheres containing moxifloxacin, placed in the cul de sac of rabbits one hour before anterior chamber challenge of moxifloxacin-susceptible S. aureus, prevented the formation of endophthalmitis in the rabbit endophthalmitis prevention model.2,3 This one-time treatment was as effective as topical treatment every 15 minutes over 1 hour before bacterial challenge and 5 times over the next 24 hours (10 total drops of 0.5% moxifloxacin). It was demonstrated previously that 10 total drops were necessary to completely prevent endophthalmitis in this model.13
We have demonstrated the “Proof of Principle” that thermoresponsive controlled-release microspheres containing moxifloxacin appear to be a viable alternative to topical antibiotic drops for the prevention of endophthalmitis by S. aureus. The present study describes a simple approach that could enhance endophthalmitis prophylaxis and take patient compliance out of the equation. Clinical studies would need to confirm ocular tolerance and anti-infective efficacy in the future use of controlled-release microspheres containing anti-infectives following a thorough preclinical evaluation.
Acknowledgments
Presented in part at the 2016 ARVO meeting in Seattle, WA (MVF), and has been submitted for presentation to the 2016 AAO in Chicago, IL (AM).
Supported by The Charles T. Campbell Ophthalmic Microbiology Laboratory; NIH grants P30-EY08098 and RO1-A1085570 (R.M.S.), the Eye and Ear Foundation of Pittsburgh; and unrestricted funds from Research to Prevent Blindness Inc.
The authors have no current “Conflict of Interests” to disclose for the completion of this study as determined by the Office of Research, University of Pittsburgh, Pittsburgh, PA, USA.
Disclosure: A. Mammen, None; E.G. Romanowski, None; M.V. Fedorchak, None; D.K. Dhaliwal, None; R.M. Shanks, None; R.P. Kowalski, None
References
- 1. An JA,, Kasner O,, Samek DA,, Lévesque V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J Cataract Refract Surg. 2014. ; 40: 1857–1861. [DOI] [PubMed] [Google Scholar]
- 2. Zhang W,, Prausnitz MR, Edwards A. Model of transient drug diffusion across cornea. J Controlled Release. 2004. ; 99: 241–258. [DOI] [PubMed] [Google Scholar]
- 3. Kimura H,, Ogura Y. Biodegradable polymers for ocular drug delivery. Ophthalmologica. 2001. ; 215: 143–155. [DOI] [PubMed] [Google Scholar]
- 4. Kearns VR,, Williams RL. Drug delivery systems for the eye. Expert Rev Med Devices. 2009. ; 6: 277–290. [DOI] [PubMed] [Google Scholar]
- 5. Mehanna MM,, Elmaradny HA,, Samaha MW. Mucoadhesive liposomes as ocular delivery system: physical, microbiological, and in vivo assessment. Drug Dev Ind Pharm. 2010. ; 36: 108–118. [DOI] [PubMed] [Google Scholar]
- 6. Al-Kassas RS,, El-Khatib MM. Ophthalmic controlled release in situ gelling systems for ciprofloxacin based on polymeric carriers. Drug Deliv. 2009. ; 16: 145–52. [DOI] [PubMed] [Google Scholar]
- 7. Patel UL,, Chotai NP,, Nagda CD. Design and evaluation of ocular drug delivery system for controlled delivery of gatifloxacin sesquehydrate: In vitro and in vivo evaluation. Pharm Dev Technol. 2012. ; 17: 15–22. [DOI] [PubMed] [Google Scholar]
- 8. Ciolino JB,, Hoare TR,, Iwata NG,, et al. A drug-eluting contact lens. Invest Ophthalmol Vis Sci. 2009. ; 50: 3346–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cardillo JA,, Paganelli F,, Melo LA, Jr,, et al. Brazilian Ocular Pharmacology and Pharmaceutical Technology Research Group. Subconjunctival delivery of antibiotics in a controlled-release system: a novel anti-infective prophylaxis approach for cataract surgery. Arch Ophthalmol. 2010. ; 128: 81–87. [DOI] [PubMed] [Google Scholar]
- 10. Paganelli F,, Cardillo JA,, Melo LA, Jr,, et al. Brazilian Ocular Pharmacology and Pharmaceutical Technology Research Group. A single intraoperative sub-tenon's capsule injection of triamcinolone and ciprofloxacin in a controlled-release system for cataract surgery. Invest Ophthalmol Vis Sci. 2009. ; 50: 3041–3047. [DOI] [PubMed] [Google Scholar]
- 11. Guo Q,, Aly A,, Schein O,, et al. Moxifloxacin in situ gelling microparticles-bioadhesive delivery system. Results Pharma Sci. 2012. ; 25: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fedorchak MV,, Conner IP,, Medina CA,, et al. 28-day intraocular pressure reduction with a single dose of brimonidine tartrate loaded microspheres. Exp Eye Res. 2014. ; 125: 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kowalski RP,, Romanowski EG,, Mah FS,, et al. Topical prophylaxis with moxifloxacin prevents endophthalmitis in a rabbit model. Am J Ophthalmol. 2004. ; 138: 33–37. [DOI] [PubMed] [Google Scholar]
- 14. Kowalski RP,, Romanowski EG,, Shanks RMQ,, Mah FS. The comparison of fluoroquinolones to non-fluoroquinolone antibacterial agents for the prevention of endophthalmitis in a rabbit model. J Ocul Pharmacol Ther. 2012. ; 28: 604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim DH,, Stark WJ,, O'Brien TP,, Dick JD. Aqueous penetration and biological activity of moxifloxacin 0.5% ophthalmic solution and gatifloxacin 0.3% solution in cataract surgery patients. Ophthalmology. 2005. ; 112: 1992–1996. [DOI] [PubMed] [Google Scholar]
- 16. Solomon R,, Donnenfeld ED,, Perry HD,, et al. Penetration of topically applied gatifloxacin 0.3%, moxifloxacin 0.5%, and ciprofloxacin 0.3% into the aqueous humor. Ophthalmology. 2005. ; 112: 466–469. [DOI] [PubMed] [Google Scholar]
- 17. Ciula TA,, Starr MB,, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology. 2002. ; 109; 13–24. [DOI] [PubMed] [Google Scholar]
- 18. Speaker MG,, Menikoff JA. Prophylaxis of endophthalmitis with topical povidine iodine. Ophthalmology. 1991. ; 98: 1769–1775. [DOI] [PubMed] [Google Scholar]
- 19. Chronister DR,, Kowalski RP,, Mah FS,, Thompson PP. An independent in vitro comparison of povidone-iodine and sterilid. J Ocular Pharm Ther. 2010. ; 26: 277–280. [DOI] [PubMed] [Google Scholar]
- 20. Barry P,, Seal DV,, Gettinby G,, et al. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery. J Cataract Refract Surg. 2006. ; 32: 407–410. [DOI] [PubMed] [Google Scholar]
- 21. Romero P,, Mendez I,, Salvat M,, et al. Intracameral cefazolin as prophylaxis against endophthalmitis in cataract surgery. J Cataract Refrac Surg. 2006. ; 32: 438–441. [DOI] [PubMed] [Google Scholar]
- 22. Garat M,, Moser CL,, Martin-Baranera M,, et al. Prophylactic intracameral cefazolin after cataract surgery. Endophthalmitis risk reduction and safety results in a 6-year study. J Cataract Refrac Surg. 2009. ; 35: 637–642. [DOI] [PubMed] [Google Scholar]