Abstract
Survivors of childhood cancer are at risk of long-term adverse effects and late effects of the disease and/or its treatment. In response to national recommendations to improve evidence-based follow-up care, a web-based support system for clinical decision making, the Passport for Care (PFC), was developed for use at the point of care to produce screening recommendations individualized to the survivor. To date, the PFC has been implemented in over half of the nearly 200 clinics affiliated with the Children's Oncology Group across the USA. Most clinician users report that the PFC has been integrated into clinic workflows, and that it fosters improved conversations with survivors about the potential late effects a survivor might experience and about the screening and/or behavioural interventions recommended to improve health status. Furthermore, clinicians using the PFC have indicated that they adhered more closely to follow-up care guidelines. Perspectives on the challenges encountered and lessons learned during the development and deployment of the PFC are reviewed and contrasted with other nationwide approaches to the provision of guidance on survivor follow-up care; furthermore, the implications for the care of childhood cancer survivors are discussed.
Introduction
Between 2003 and 2006 in the USA, the Institute of Medicine (IOM), the President's Cancer Panel, and the Centers for Disease Control and Prevention (CDC) issued a series of reports focusing on the quality of care for cancer survivors in the USA.1–4 Acknowledging advances that have been made in the treatment of cancer, these reports contained similar recommendations targeted at meeting the needs and challenges of individuals completing cancer therapy and entering the realm of the ‘cancer survivor’.1–4 Cancer survivors embark on a life-long journey over the course of which continued vigilance is required to protect their health (Box 1),5 including follow-up assessments for late effects associated with cancer and cancer therapies. Among other recommendations, these reports highlighted the need to develop guidelines for survivorship care, to provide survivors with end-of-treatment summaries, and to create evidence-based follow-up care plans for all cancer survivors.1–4
In this Perspectives article, we describe the experience obtained and the lessons learned through collaborative efforts that integrated guideline development with concurrent decision support initiatives and that led to the creation of the Passport For Care (PFC) for childhood cancer survivors.6 The PFC is a web-based platform for storing childhood cancer survivor treatment summaries, and can dynamically generate individualized survivor care plans, along with other educational resources, for use at the point of care. In addition, we explore insights, drawn from recent experience in adult cancer, related to interest in and the use of treatment summaries and survivor care plans for supporting clinical decisions.
Childhood cancer—a success story
Childhood cancer represents a compelling story of medical success. In the 1950s, few children survived childhood cancer.7 By contrast, at present, >80% of paediatric patients with cancer treated with modern therapy survive for 5 years or more.8 Although cancer remains the leading disease-related cause of death in children in the USA and other developed nations, survival rates continue to improve;9 in 2010, nearly 380,000 childhood cancer survivors were recorded in the US population, with projections indicating that the prevalence of survivors could increase to 0.5 million by 2020.10
Unfortunately, childhood cancer survivors are at substantial risk of late, and potentially long-term, adverse effects of cancer treatment. These adverse events can involve major organ systems, such as the cardiac, endocrine, gastrointestinal, genitourinary, musculoskeletal, neurological, and pulmonary systems, and can affect skeletal maturation and growth, cognitive and emotional development, psychological and psychosocial functioning, and sexual development, fertility and reproduction. Cancer survivors are also at an increased risk of secondary neoplasms.10 Adverse effects can manifest soon after cancer therapy and persist, or appear many years later.10–12 By 30 years after diagnosis, almost 75% of all childhood cancer survivors will experience at least one late treatment effect with adverse consequences for their health and/or quality of life;13–15 in four out of 10 survivors, these late effects of treatment are severe, disabling, or life-threatening.13–15 The high prevalence of wide-ranging late effects of treatment and/or secondary tumours in childhood cancer survivors highlights the need for guideline-informed survivor follow-up care.
Focusing on survivorship
Guideline development
The Children's Oncology Group (COG),16 supported by the National Cancer Institute (NCI), represents the world's largest organization devoted to clinical trials and research focused on childhood and adolescent cancer. In response to a request from the IOM to develop guidelines to standardize long-term follow-up care for paediatric cancer survivors,1,4 the COG undertook an effort to review and critically summarize the medical literature and develop risk-based, exposure-related clinical practice guidelines to follow-up care for survivors of childhood cancer.17 The COG Late Effects Committee and Nursing Discipline undertook this project collaboratively through the efforts of multidisciplinary teams—comprising health-care professionals working in nursing, paediatric oncology, radiation oncology, and other clinical specialties and disciplines, including patient advocacy and behavioural health.17 Draft versions of recommendations for screening and follow-up assessments were reviewed, refined, and scored in iterative cycles by these multidisciplinary panels of experts in the late effects associated with paediatric cancer.17 Using a modified version of the National Comprehensive Cancer Network ‘Categories of Consensus’ system,18,19 the panels scored the strength of scientific data linking particular therapeutic exposures with specific late complications and determined the appropriateness of screening recommendations based on expert scorers’ collective clinical experience.17 Accompanying survivor-education materials (‘Health Links’), developed by the COG Nursing Discipline, were reviewed by medical experts and patient advocates before being finalized.20
‘Version 1.0’ of the COG Long-term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers (the COG Guidelines) was released in September 2003,17 with planned ongoing review and updating involving 10 multidisciplinary COG task forces. Task force recommendations, sent to a 25-member Long-Term Follow-Up Guidelines Core Working Committee, are evaluated and scored before changes to the extant guidelines are made. This content management approach has resulted in update releases in 2006 (Version 2.0), 2008 (Version 3.0), and 2014 (Version 4.0).21
Survivor-care plans
Major barriers—relating to survivors, health-care practitioners, or the health-care system—can impede provision of evidence-based follow-up screening services to childhood cancer survivors (Box 2; Supplementary Box 1).22–32 To address these barriers, the IOM recommended, among other approaches, the development and use of treatment summaries and survivor care plans.1,4 Survivor and clinician perspectives on the use of treatment summaries and survivor care plans are provided in the following discussion.
Survivor perspectives
Cancer survivors are concerned about the transition and coordination of care between their oncologists and their primary-care physicians (PCPs), and have expressed uncertainty about the roles of PCPs in their follow-up care.33 In addition, the expectations of survivors and professional caregivers might not always be in alignment.34–36 Among survivors of breast cancer, one study documented concerns related to their PCPs’ knowledge of the late effects of cancer therapies (59%), cancer follow-up care (50%), and treatment of the adverse effects of cancer or cancer therapies (41%).37 Other reports have indicated positive responses to receipt of care plans that support transitions from acute care to long-term survivorship care for persons treated for breast cancer,38–42 colorectal cancer,42–44 gynaecological cancer,45–47 and cancers acquired in adulthood, in general.48–51 Survivors view survivorship care plans as valuable tools in facilitating survivor–physician communication and physician–physician communication relating to individualized health risks and approaches to managing such risks.38,41,42 The perspectives of childhood cancer survivors regarding survivorship care plans, and particularly their value in addressing health issues and maintaining optimal health, are not well documented, although one study reported that one-page care summaries were “well received” by childhood cancer survivors.52
Primary-care physician perspectives
Evidence from surveys indicate that PCPs are concerned about their own readiness to assume responsibility for follow-up care of survivors of both adult and childhood cancer. With regard to adult cancer survivors, in a survey conducted by Bober et al.,53 PCPs reported inadequate access to treatment histories (36%). In that study, 92% of PCPs also reported being unfamiliar with the IOM report on survivorship.53 Another study found that only 52% of the PCPs surveyed were comfortable conducting follow-up surveillance for cancer recurrence, and only 43% felt that they were following standard guidelines for tumour surveillance in cancer survivors.54 Furthermore, approximately half of PCPs who responded to the survey by Bober et al.53 described themselves as unprepared to evaluate or manage late effects, and expressed enthusiasm for survivor-care plans or similar products; more than 90% welcomed practice guidelines in print or online, >95% wanted descriptions of survivors’ diagnoses and treatment summaries, and a similar percentage desired individualized recommendations for late-effect management of cancer survivors.53
Similarly, with regard to the care of survivors of childhood cancer, 72% of general internists reported never receiving a treatment summary (although over half reported caring for at least one cancer survivor).30 In general, the internists reported feeling ‘somewhat uncomfortable’ in caring for survivors of Hodgkin lymphoma, acute lymphoblastic leukaemia, and osteosarcoma and ‘somewhat unfamiliar’ with the available surveillance guidelines;30 in a case vignette, most did not recommend appropriate surveillance for late adverse events or cancer recurrence.30 Treatment summaries and access to surveillance guidelines were viewed by the general internists surveyed as the most-useful resources in caring for childhood cancer survivors.30
Care plan adoption
Evidence indicates that the use of or the intent to use survivor care plans is increasing. In a national survey of care programmes for survivors of adult cancer, 45% of programmes reported using survivor care plans, with higher usage reported for survivors of certain tumour types.55,56 Furthermore, 78% of survey respondents reported that they do or will use treatment summaries and survivor care plans,55 motivated in part by the 2015 cancer-centre accreditation requirements of the American College of Surgeons Commission on Cancer.57 At present, evidence is early, limited, and mixed regarding the effectiveness of survivor care plans in changing clinician behaviour and survivor outcomes.40,58
The Passport for Care
To address the needs of childhood cancer survivors and clinicians who provide care—and to respond to national recommendations1–4—investigators at the Baylor College of Medicine, Texas Children's Cancer Center (TCCC), and the COG merged parallel initiatives. The COG developed guidelines for survivor care, whereas the Baylor College of Medicine and TCCC investigators developed the PFC application system. The PFC represents an ongoing collaboration between these groups. The PFC has been funded through philanthropy and grants, and philanthropic partners are currently interested in providing long-term support.
Description of the PFC
The PFC is a web-based clinical-support tool for clinical care providers and the survivors of paediatric, adolescent, and young-adult cancer, which is provided free of charge.6 On the basis of the survivor's clinical characteristics and treatment history, the PFC uses rules based on the COG guidelines on late effects to generate individualized recommendations for follow-up screening and to select educational materials relevant to the specific survivor.59 The PFC provides the following resources: a treatment summary; an individualized care plan for clinicians, including potential late effects, and associated risk factors and screening recommendations; the full COG guidelines together with scores indicating the strength of the supporting evidence for and the appropriateness of the recommendation; surveillance guidelines for secondary and other malignancies; references for each guideline linked to abstracts in the MEDLINE database; and select educational information relevant to the survivor (Health Links), regarding potential late effects, overall treatment risks, and healthy lifestyle behaviours (Figure 1). At present the PFC is not integrated with guidelines for other chronic conditions, and the management of such conditions is beyond the scope of the current tool, which is focused specifically on the late effects of cancer. In summary, the PFC is designed to foster clinician–survivor conversations and decision making, with the overall goals of enhancing screening and long-term follow-up care, and ultimately to improve health outcomes.
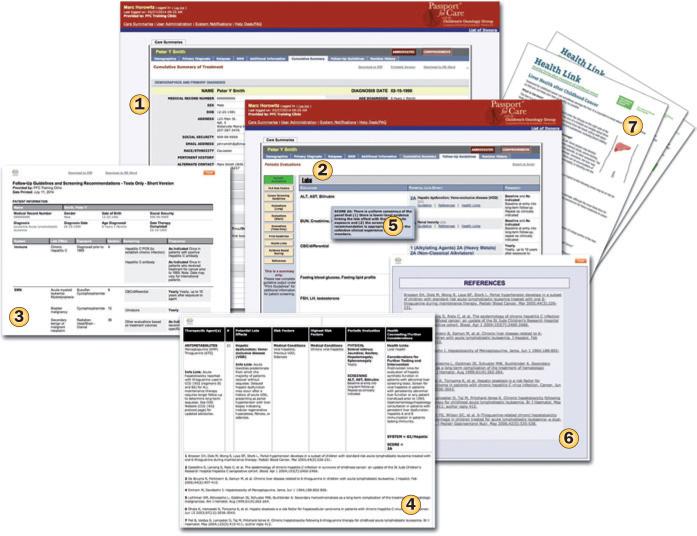
Figure 1.
Illustrative examples of PFC pages. The screen shots show examples of a treatment summary (1); a care plan that a user can access online (2) or via an output suitable for printing (3); specific guidelines (4), with evidence rating (5) and references for each guideline linked to MEDLINE abstracts (6); and downloadable survivor education resources (Health Links; 7). This online tool thus provides valuable resources aimed at ensuring survivors and their clinicians are adequately informed of the potential late effects, and individualized recommendations for screening and monitoring. To expand the view of the screenshots, please see the article online. Abbreviation: PFC, Passport for Care.
In general, clerical or clinical staff members (such as, nurses and nurse practitioners) enter treatment information into the PFC before the survivor's visit to the clinic; entry of chart-abstracted information is usually a 5–10-minute process once the data have been retrieved from records. The entered treatment data drives the PFC algorithms in extracting guidelines relevant to and individualized for survivors based on their treatment exposures and personal characteristics. The PFC provides multiple output options for the user. For example, clinicians with greater familiarity with survivorship follow-up care may select an output option summarizing only the recommended tests and/or screening protocols; others might prefer more detailed options, which include a comprehensive listing of items to be explored in considering the survivor history, findings to be aware of when conducting the physical examination, and recommended tests and other assessments to be ordered for the survivor. Output formats are available that are suitable for use online, or for printing and/or electronic dissemination—including recording in an electronic medical record.
Experience in a large clinic indicates that often the clinician is able to review the PFC data before the survivor's appointment, and can therefore order the tests recommended by the PFC in advance. Using the PFC interface, the clinician can also review the references, abstracts, and level of evidence for the guidelines proposed as well as the risk factors for development of a specific potential late adverse effect. Clinics can also provide the survivor with a treatment summary, a plan for follow-up care, and relevant educational materials on potential late effects, as well as guidance on follow-up screening for such events. Many clinics use the PFC to generate information for the survivor's PCP or referring doctor (see ‘User assessment of the PFC’ section). At this point, whether the PFC has changed clinical work flows remains unclear, and any effects might differ among clinics depending on their prior use of treatment summaries and COG guidelines; these aspects are the subject of ongoing study.
Thus, the PFC provides information to facilitate a survivor's transition from acute cancer care to long-term follow-up care. The tool was designed to accommodate the needs of clinicians with varying levels of familiarity with survivorship care issues. The strategy was to develop and deploy the PFC initially in COG-affiliated clinics, at which >90% of paediatric patients with cancer in the USA are treated, therefore representing a setting in which treatment summary data can be captured. Leveraging this large treatment network provides opportunities to generate care plans for many childhood cancer survivors throughout the USA. Furthermore, the PFC enables users to store the survivor treatment summaries and automatically generate survivor care plans using the latest guidelines when needed for reference. This approach also facilitates modification of the treatment summary and survivor care plan should the survivor experience a relapse or subsequent malignancy.
Because the guideline database and PFC application is managed centrally, changes to the guidelines or other resources (such as survivor education materials) can be made in one location and survivor care plans updated automatically for relevant survivors. Alerts to clinicians are provided if more-immediate attention is required for particular survivors. The PFC is updated with each guideline version release by the COG; version 4.0 of the COG guidelines was released by the COG on 15th September 2014,21 and the updated recommendations were simultaneously deployed in the PFC.
These important features differ from two other national initiatives—focused on survivors of adult cancers—that aim to disseminate care plans widely via information technology. One of these initiatives, the LIVESTRONG Care Plan,60 also a web-based application, was developed by the LIVESTRONG foundation in collaboration with Penn Medicine's OncoLink.61,62 This tool uses the responses to specific questions, which can be answered either by survivors or their clinicians, to generate a care plan that can be printed or saved as a portable document format (PDF) file.60 No survivor data is stored (for example, in a treatment summary) and, therefore, updating or reviewing the plan requires re-entry of all of the survivor information.60 By contrast, the second initiative also for adult cancer survivors, Journey Forward (a partnership involving the National Coalition for Cancer Survivorship; the University of California, Los Angeles [UCLA] Cancer Survivorship Center; the Oncology Nursing Society; WellPoint; and Genentech),63 offers downloadable software that can be used locally to record the survivor's treatment summary and create a care plan with recommendations for follow-up care and surveillance using templates for care plans specifically for breast, colon and lung cancer, and lymphoma, or a generic adult cancer template.64 Journey Forward reports that their Survivorship Care Plan Builder represents an adaptation of the ASCO Chemotherapy Treatment Plan and Summary Templates, and surveillance guidelines.65,66 The treatment summaries and care plans generated using the Journey Forward software can be shared in print or electronically.
Access to personal health information (PHI) is associated with important security concerns. In the LIVESTRONG Care Plan system, no PHI is stored. This approach reduces security vulnerability; however, the data must be re-entered each time a user wishes to examine the care plan or when guidelines change. The Journey Forward approach stores survivor information locally on the personal computers on which the software is used and/or on shared-network drives, which could potentially introduce distributed security vulnerabilities across all installations of the software in clinics and offices. The distributed nature of the Journey Forward system also requires that each installation of the software/templates (for example, in each clinic) be updated as templates/guidelines change. By contrast, the PFC stores PHI in a central, encrypted database, which provides easy access to authorized users for generating updated care plans as the treatment history changes or guidelines are revised. The PFC also enables the sending of alerts to clinicians of affected survivors when important guideline changes occur. The central repository used in the PFC also permits security resources to be focused in one location. One potential disadvantage of using such a central repository is that, should security be compromised, the number of records at risk is greater. In addition, use of a central repository containing PHI by the PFC requires that participating institutions complete agreements about how the data is to be stored, accessed, owned, and secured (Box 3; Supplementary Box 2).
PFC deployment
Multiple stakeholders were consulted on an ongoing basis throughout the PFC development and deployment process. Included among the stakeholders were childhood cancer survivors and their parents or guardians; survivorship experts; guideline-development groups; specialty and primary-care clinicians; clinical support staff; experts in information technology; specialists in risk management, security and legal aspects; and state and national policymakers. These stakeholders were engaged using various methods (as proved most suitable for the individuals) including focus groups, working-group meetings, interviews, usability testing, surveys, online user feedback, and training or user-support sessions.
During development of the PFC, the decision rules were tested individually and also with fictional treatment summaries and survivor cases to ensure alignment of the generated care plans with published guidelines. Pilot testing was conducted in select clinics to examine potential barriers to PFC use and integration into workflow (for example, the data-entry time needed, demands placed on clinicians, and possible expenditure of staff resources). The pilot tests provided user feedback that enabled iterative refinement of prototype models before widespread deployment of the PFC, which began in 2009.59
Deployment of the PFC has been accompanied by the successful enrolment of clinics and their survivors. By early 2014, 102 clinics had enrolled over 13,600 survivors in the PFC programme (Figure 2 depicts enrolment in the PFC from 2009 through to the end of 2013). The sites that have deployed the PFC are geographically dispersed and range from large medical centres serving >1,000 cancer survivors to medium-sized clinics that see fewer survivors, as well as smaller clinics that serve only a small number of survivors annually. Another 50 clinics are currently at some stage of implementation of the PFC initiative (D. G. Poplack, unpublished data).
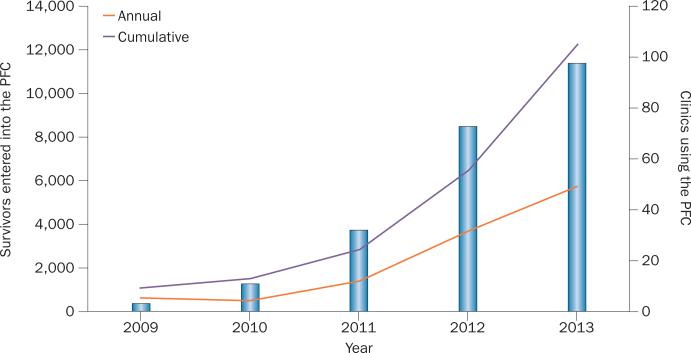
Figure 2.
Growth in PFC enrolment. The graph illustrates the annual and cumulative number of survivors enrolled in the PFC (left axis and lines). The annual number of survivors enrolled is increasing considerably each year, and in 2013 the cumulative number of survivors registered was in excess of 12,000. The number of clinics using the PFC is also presented (right axis and bars), and reveals uptake of the PFC by clinics is also increasing each year, with approximately 100 centres across the USA using the tool. Abbreviation: PFC, Passport for Care.
However, nine institutions declined to participate; five had internal systems established to support decision making, two had legal barriers relevant to storage of data off-site, and two declined without providing reasons (D. G. Poplack, unpublished data). Although no clinic cited staff resources as a reason for not deploying the PFC, one might anticipate that small clinics might be reluctant to pursue deployment if they were not currently devoting staff resources to the preparation of the treatment summaries required for application of the COG guidelines.
User assessment of the PFC
In early 2013, for purposes of ongoing improvement, the PFC evaluation team and clinical experts in survivor care, together with the PFC leadership team, developed a brief questionnaire that was used to examine the use and user experiences of the PFC (D. G. Poplack, unpublished data). The 17-item questionnaire was constructed by selecting items from a longer questionnaire that was developed and pilot tested with survivorship experts to promote content validity. The longer questionnaire had been administered to clinicians in multiple survivor clinics and adjusted based on empirical data to improve item wording, response options, and item-scaling characteristics. To minimize response bias, the questionnaire was uploaded to a standalone website unrelated to the PFC, and all data were collected anonymously.
Of the 70 clinics surveyed, 45 participated in the survey (that is, a 64% response rate) yielding a total of 84 completed questionnaires averaging 1.9 responses per clinic. The represented clinics varied in size, geographical location, and percentage of survivors to patients with cancer served. Respondents included physicians (43.0%), nurses (26.9%), nurse practitioners (15.1%), research coordinators (5.4%), research associates (4.3%), social workers (2.2%), physician assistants (1.1%), and others (2.2%). All of those surveyed were asked to answer questions based on their individual use of the PFC within their specific clinical roles.
A key finding was that 82% of respondents reported adhering more closely to guidelines on late-effects screening and ordering of recommended tests/labs when using the PFC. Furthermore, 83% of survey respondents viewed the PFC as useful in fostering additional or improved conversations with survivors about late effects, screening approaches, and/or healthy behaviours, with 79% indicating that the PFC enabled the sharing of more comprehensive information with survivors. Entering data into the PFC was found to be ‘easy’ by 78% of the respondents for whom the item was applicable. In fact, 74% of the individuals indicated that they were using the PFC in at least 75% of their survivor visits (Figure 3). To date, of the 189 COG clinics in the USA, 119 have completed the necessary paper work for implementation, 103 clinics have enrolled survivors and, on the basis of data from the PFC log files, 75% of sites with a survivor enrolled have accessed the PFC multiple times in the past 6 months (D. G. Poplack, unpublished data).
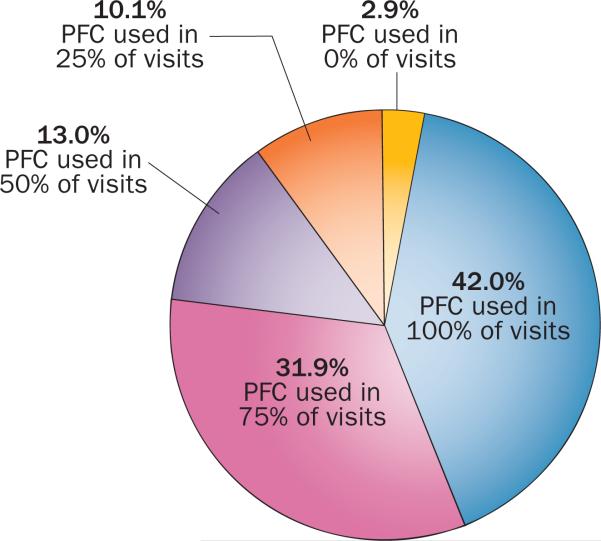
Figure 3.
Clinic respondent estimates of the percentage of cancer survivors for whom the PFC is used, based on a survey by the PFC developers (D. G. Poplack, unpublished data). The graph shows that a large proportion of clinics (73.9%) enrolled in the PFC programme use the tool in 75% or more of visits by cancer survivors. Almost all of the clinics (around 97%) enrolled in the PFC programme use the tool in 25% or more visits by survivors, with only 2.9% not using the tool during any consultations with survivors. Abbreviation: PFC, Passport for Care.
Moreover, 93% of those surveyed described the PFC as ‘well integrated’ or ‘partially integrated’ into their clinical workflow, in contrast to 7% for whom the PFC was ‘not at all integrated.’ The main purposes of the PFC for survivor care among those respondents indicating at least partial integration of the PFC into their clinical workflow included determining potential late adverse effects and appropriate approaches to screening, generation of follow-up recommendations and treatment summaries for survivors, among others (Figure 4).
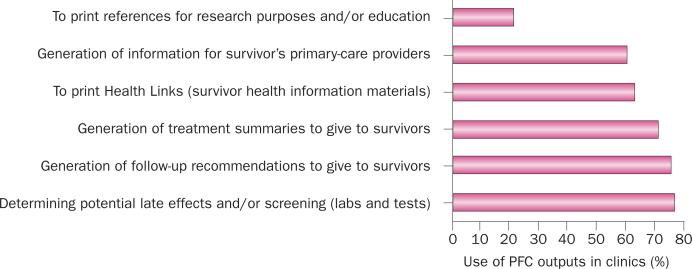
Figure 4.
Breakdown of uses of the PFC. The graph depicts the main uses of PFC outputs in cancer-survivor care in clinics that described the PFC as ‘well integrated’ or ‘partially integrated’ into clinic workflow in a survey by the PFC developers (D. G. Poplack, unpublished data), representing 93% of clinic respondents. The data demonstrate that the main applications of the PFC resources are determination of the potential late effects of cancer and recommended screening protocols, and generation of information for primary-care providers, as well as the generation of health outputs to inform and educate the survivors (follow-up recommendations, treatment summaries, and health information resources). Abbreviation: PFC, Passport for Care.
Importantly, the survey respondents indicated high levels of satisfaction with the tool: 90% satisfied (60% ‘very satisfied’ and 30% ‘generally satisfied’), 8% neutral, and 3% dissatisfied. Recommendations for improving the PFC focused on guideline changes (for example, to decrease the number of follow-up tests); PFC integration with electronic health records (EHRs); provision of direct access to survivors; and additional interface features and functionalities. More generally, the feedback comments received from clinicians and clinical staff members who have used the PFC since its availability have generally been very favourable, with a number of clinicians describing specific ways in which their clinical practices have been improved. Box 4 lists examples of the comments received from health-care providers obtained via the survey as well as concurrent interviews of PFC users from diverse survivor clinics in which the PFC has been made available. Users have indicated that the PFC is helpful when transitioning survivors to primary-care or adult-care services, simplifies adherence to guidelines, saves time in the compilation of late effects, and is well received by survivors owing to the provision of treatment summaries and educational information (COG Health Links). Studies of the reception of the PFC among survivors are ongoing.
Challenges facing the PFC
At the time when the PFC project was initiated, a variety of medical record systems (for example, paper-based systems, rudimentary electronic systems used for billing, and sophisticated systems with clinical alerts) were in use. Prior to passage of the Health Information Technology for Economic and Clinical Health (HITECH) Act of 2009,67 adoption of EHRs was proceeding slowly in the USA; EHR vendors had limited incentive to address issues relating to interoperability for data sharing and exchange.68 In addition to the diversity in medical record systems, the PFC developers anticipated other challenges related to differences in the following areas between clinics: size and support available; care-provider familiarity with survivorship care; technology infrastructure; staff familiarity with and availability to abstract charts and enter treatment summaries; clinical workflows; user-training requirements; and policy and procedural issues, such as local security approval processes.
Despite the numerous challenges anticipated, relatively few of these barriers posed major impediments to PFC use with the exception of policy and procedural issues related to security concerns. There are several possible explanations for the limited number of major barriers encountered. First, the PFC uses a web-based platform that requires minimal technical infrastructure (a computer with Internet access) and training. Presumably this approach contributed to the observation that many COG clinics have been able to integrate the PFC into their clinic workflow. Second, continuing stakeholder engagement, including user feedback obtained during development, beta testing, and training, resulted in application refinements that met the needs of many stakeholders. Third, it is possible that only those clinics with few barriers chose to participate. However, other barriers do exist. For example, although the web-based platform accommodates clinics with a wide variety of medical record systems, barriers to more-complete integration of the PFC across proprietary EHR platforms remain (such as, automated abstracting of survivor treatment information from the EHR).
The PFC—lessons learned
In Box 3 (and Supplementary Box 2) we have summarized, using the eight-dimension Sittig–Singh sociotechnical model for health information technology evaluation,69 lessons learned from the PFC development and deployment experiences. In addressing user needs across the eight dimensions, three overarching lessons emerged.
Chief among the knowledge gained was the importance of early and continuing stakeholder engagement in defining and refining the requirements, goals, pathways, features, interface, workflows, and implementation of the PFC. Our experience using ongoing stakeholder engagement to improve the PFC aligns with that previously and articulately summarized in implementation strategies for clinical decision support.70
The second key lesson learned centred on the development of solutions to problems related to diverse interinstitutional policies and procedures (for example, issues of data ownership, content usage, data storage, security, and liability; Box 3 and Supplementary Box 2). Early in the rollout phase of the PFC programme, negotiating and executing each inter institutional agreement extended over months. To improve control and streamline the agreement process the PFC team assembled multidisciplinary groups of experts in risk management, security, health policy and law, information technology, and project management; created standard documents and agreements specifying roles, rights, ownership, responsibilities, and security issues; and developed workflows for tracking and executing clinical enrolment agreements through institutions. This standardized approach reduced the time spent by the PFC team on enrolment to days or a few weeks, and proved workable for the overwhelming majority of clinics interested in implementing the PFC.
A third area in which understanding increased, perhaps unsurprisingly, was that continuing and escalating resource investments would be required by the PFC team associated with evolving security requirements. These include the security measures mandated (in the USA) by the 1996 Health Insurance Portability and Accountability Act (HIPAA) Administrative Simplification Regulations,71 and the Federal Information Security Management Act (FISMA) of 2002.72 With the current PFC architecture (that is, the use of a central data repository) the PFC team has managed the hosting and security costs without burdening participating clinics.
Future considerations
Empowering survivors
Early in the development and deployment of the PFC, focus was placed on the enrolment of those centres with internal access to treatment records and those in which transition from acute treatment to survivorship care could be facilitated by the PFC, specifically COG-affiliated clinics. However, in settings in which recommended follow-up services are not available, many other survivors are receiving either limited follow-up care or no care.73 Fewer than one in five survivors report receiving guidance on how to reduce the long-term risks associated with the cancer treatment they have received or have participated in discussions about ordering relevant screening tests.74 For these survivors, lack of access to treatment summaries and follow-up recommendations considerably limits their ability to manage their own care.1–4,10,20,27,75,76
For this reason, the PFC system is being expanded, with a web-based ‘survivor PFC portal’ scheduled for launch in the autumn of 2014, which will provide survivors with electronic access to PFC resources whenever needed. The survivor PFC portal will enable survivors to view, via a computer or mobile platform, their treatment summaries, follow-up survivor care plans, relevant survivor education material, and other information tailored to their individualized risks of late effects. Furthermore, it will be possible for survivors to share treatment summaries and care plans with their health-care provider.
This survivor PFC portal will offer the potential to empower survivors by offering them increased control over their recommended follow-up care and by providing the ability to share information with PCPs and other caregivers who might not otherwise have access to this information. This opportunity will require systems of care that will enable survivors not currently in survivor follow-up clinics to obtain access to their treatment summaries and enrol in the survivor PFC. Approaches to providing such systems of care—involving survivor navigators, for example—are currently under study.
Evidence for acceptance and use of patient portals in the management of disease is mounting, with patients reporting that such resources are “...removing barriers to communication, reducing hassles, maximizing convenience, providing a sense of control and independence, reducing anxiety, and providing reassurance.”77 Furthermore, the growth in mobile phone (including Smartphones) use among minority and disadvantaged groups, who are now increasingly using mobile phones to access the internet might bode well for reaching and serving populations of survivors who previously had limited or no access to health-care information, let alone long-term follow-up services.78–80
However, intensifying efforts to engage and support all childhood cancer survivors, particularly those ‘lost to follow-up’, will likely require a variety of interventions at all levels—that is, strategies aimed at survivors, the clinicians (oncologists and PCPs) who provide their care, the health-care systems (for example, access to ‘patient navigators’ who are trained to provide support and guidance throughout a patient's cancer care), and general models of care used therein.76,81,82 Investigations are needed to determine which interventions and additional tools most effectively improve outcomes important to survivors, such as quality of life. That a single tool or technology, including the PFC, will be sufficient to meet the diverse needs of survivors is unlikely; multiple complementary approaches will be needed.
In the USA, two recent policy changes might positively affect survivorship outcomes. Firstly, the passage of the Patient Protection and Affordable Care Act in 2010,83 an initiative designed to increase access to health insurance, could have important implications for the continuing care of survivors of paediatric cancer.84 Secondly, the accrediting body for cancer centres, the American College of Surgeon's Commission on Cancer, added standards requiring that, by 2015, cancer centres have systems to provide survivors with comprehensive care summaries and follow-up plans upon completion of treatment.85 These two policies hold the potential to remove important barriers to follow-up care for cancer survivors.
Global perspective
The focus of this article reflects perspectives primarily based on the experience in the USA, although several inter national sites have also enrolled in the PFC. International cooperation will become increasingly important to accommodate the needs of cancer survivors worldwide. To meet the needs of childhood cancer survivors worldwide, the International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG) was assembled in 2010,86 with the aim of establishing a common vision and integrated strategy for the surveillance of late effects of cancer or its treatment in survivors of childhood, adolescent and young-adult cancer throughout the world. The IGHG collaboration aims to reduce duplication of effort, optimize the use of expertise, and enhance research opportunities through harmonization of currently available recommendations for clinical practice.87–91 International guideline harmonization, drawing on standard-setting conventions from several organizations, is already underway and offers the potential for continuity of survivor follow-up care globally informing the future directions of initiatives such as the PFC.86,87,92–95
PFC as an education and research tool
The PFC, with a portal for clinicians and soon one for survivors, provides education at the point of care for clinicians, survivors, and parents regarding key medical issues in survivorship follow-up care. In addition, the PFC potentially offers opportunities for reaching and engaging survivors, family members, and clinicians to work collaboratively with researchers to answer questions important to survivors. With many thousands of survivors enrolled in the PFC, participating institutions will have a unique opportunity to collaborate in conducting studies across a wide variety of topics in survivorship. A governance infrastructure to facilitate institutional review board (IRB)-approved research using the PFC across multiple institutions is in development. In this regard the NCI-funded Childhood Cancer Survivor Study96 experience offers a model of successful governance and processes for interinstitutional research collaboration.
Evolving technology
In developing the PFC, challenges were posed by the continuing use of paper-based record systems, the lack of interoperability and data exchange across EHRs, and disparate systems used for record archiving. These experiences have informed the current approaches to treatment summary creation and delivery of the decision support, which use a web-based platform. However, the recording and use of patients’ medical data and health information is evolving in the USA and globally. In the USA, the 2009 HITECH Act67 created financial incentives for health-care providers to adopt EHRs and meet requirements for ‘meaningful use’ of clinical data.97 The definition of ‘meaningful use’ reflects an intended move from recording systems that are mainly used for billing and coding purposes to approaches that “improve quality, safety, efficiency, and reduce disparities; engage patients and family; improve care coordination and population and public health; maintain privacy and security of patient health information”.98 Early evidence indicates that EHR adoption has increased since the HITECH Act was passed.99,100 As part of the initiatives associated with meaningful use of health information, the Office of the National Coordinator for Health Information Techno logy is also leading efforts to promote the exchange of health information (for example, sharing of health information among the consumer, health-care providers, pharmacies, laboratories, and others) across health information technology systems.101 Improved information exchange will in turn facilitate, among other efforts, the integration of clinical decision support applications such as the PFC into EHRs and/or delivering functionality to EHRs, improving communications, clinical workflow, and lowering the barriers to use.102–108
Conclusions
Herein, we have described our experiences and perspectives on the successful creation of a web-based clinical decision support system, the PFC, designed to support the needs of childhood cancer survivors for life-long, evidence-guided follow-up care, as articulated in national policy recommendations.1–4 Our initial efforts focused on deployment of the tool in COG-affiliated clinics, where most patients with paediatric cancer in the USA are treated and transitions from acute care to survivorship begin. Chief among the lessons learned was the importance of early and continuing stakeholder engagement.
In looking to the future, we identified several opportunities for expanding the potential utility of the PFC. Important among these are (in the clinical arena) establishing direct survivor access to tools supporting clinical decisions as one part of intensified efforts to reach not only those transitioning from treatment to survivorship, but also those ‘lost to follow-up’ at the completion of therapy, and (in the research arena) applying decision-support tools such as the PFC as a research resource supporting wide-ranging investigations to advance the evidence base on the effectiveness of survivorship services.
Initiatives in decision support, such as the PFC, are likely to contribute to transforming the experience of cancer survivorship, which has been likened to beginning a trackless journey without a map in a vehicle of questionable reliability (Box 1), into one in which survivors realize greater control, confidence, and satisfaction in charting their life-long course.
Supplementary Material
Box 1 | The experience of survivorship, the requirement for ongoing support, and the PFC.
The following quote from a publication by Cantrell and Conte5 provides an analogy illustrating that, for survivors, the reality of experiencing survivorship is one of unknowns: “Picture yourself being told you must take a trip. You are given a general destination, but not a certain end point to your journey. You are provided a car, but are unsure as to whether or not it is capable of making the trip. You have no map, no directions. You also do not know the conditions of the road, or whether or not you will encounter adverse weather conditions or detours.”
This analogy highlights the requirement to provide information and support for cancer survivors throughout their lives. The PFC is a free-of-charge, web-based clinical support tool for survivors of childhood cancer and their health-care providers. The PFC provides select information on late effects of cancer and its treatment, overall treatment risks, and healthy lifestyle behaviours that are relevant to the survivors. The tool can also be used to generate recommendations for individualized follow-up screening and accompanying survivor educational materials to support clinician–survivor discussions during decision making, and provides other relevant data including treatment summaries, Children's Oncology Group Guidelines and associated references.
Abbreviation: PFC, Passport for Care.
Box 2 | Examples of barriers to effective care for the late effects of childhood cancer*.
Barriers to the effective provision of care for the late effects of childhood cancer can relate to survivor, care-provider, and health-care domains.
Survivor issues
■ Lack of knowledge and/or understanding of treatment history22–24
■ Lack of trust in their provider25
Care-provider issues
■ Lack of knowledge about late effects of childhood cancers28–30
■ Inability to devote sufficient time to the assessment of late effects during care visits31
Health-care system issues
■ Lack of coverage under patients’ health insurance and, therefore, inability to fund the care required10,32
■ Problems or communication breakdowns in referral networks10,25
■ Lack of professional training opportunities for care givers10,25,30
*An expanded version of this Box, discussing each barrier in turn, as well as the opportunities they create with regard to the Passport for Care programme is provided in Supplementary Box 1.
Box 3 | Lessons learned from implementing the PFC*.
Hardware and software
■ Clinical-decision-support project (software): proved feasible to provide both recommendations for evidence-based follow-up screening and surveillance, and survivor-specific educational resources to users with diverse needs and technological infrastructures
Clinical content
■ Content and technical development: simultaneous engagement of guideline authors and the developers of the clinical-decision tools at project initiation would facilitate and accelerate tool development
■ Content management: clinical-decision support requires an ongoing commitment to and processes for evidence synthesis, review, and updating
■ Content updates: the public release of guideline revisions and PFC updates should be coordinated to avoid potential confusion among user communities
Human–computer interface
■ User-centred design: iterative feedback from stakeholders informed development and enabled developers to accommodate a wide range of user needs
■ Web-based platform: a web-based platform proved feasible for addressing the diverse working and technical environments of users
People
■ Training and support: early and ongoing stakeholder engagement in software development lead to simplified and effective approaches to training
Workflow and communication
■ Treatment summaries: developing and/or capturing treatment summaries for use in the PFC posed several challenges; additional data-entry time was recognized as a potential barrier to adoption of the PFC, and the option to use an abbreviated form when time was limited was developed for use in patients without relapse or second malignancies
■ Workflow adjustments: in response to user input describing diverse workflow needs, the individualized survivor-screening recommendations were made available with varying levels of detail and in differing formats
■ Communications: some guideline revisions affecting follow-up care necessitated the development of alerts to clinical users
Organizational policies and procedures
■ Enrolment: deployment to approximately 100 institutions proved feasible, but required addressing differences in organizational policies and procedures at the different enrolling institutions
External rules, regulations, and pressures
■ Security: a multidisciplinary team was required to manage the external rules and regulations, through document standardization, and a formal review and agreement execution process
■ Pressures: continuing security investments are needed
System measurement and monitoring
■ Systems monitoring 24 h per day, 7 days per week: required for ongoing system assessment for security vulnerabilities, and to ensure quality of service and availability for clinic users
■ Informing survivors: surveys revealed that the PFC better informs survivors109
*On the basis of the eight-dimension Sittig–Singh sociotechnical model for evaluation of health information technology.69 An expanded version of this Box is provided in Supplementary Box 2.
Abbreviation: PFC, Passport for Care.
Box 4 | Examples of user comments on the PFC.
■ “Eliminates human error as long as data correctly entered. Dramatic improvement in time to review and prepare for upcoming visits.” Physician
■ “It is an excellent tool [that allows one] to be more organized in looking after survivors, to make sure one pays attention to all the important things and does not forget all the aspects that need to be considered to deliver better patient care.” Physician
■ “... it has allowed me not to have to make the clinical decision of what long-term follow-up [is needed] because it is already going to be given to them. I may say, ‘I’m only going to do general screening at these times because of cost, but if we need to do further testing we will.’ And I think it has increased the information I give to them. It has actually lessened preparation time because it automatically generates the summary for you.” Nurse Practitioner
■ “I think it's fantastic. [The PFC-based resources] provide the family with so much information in a very succinct manner, and they just love it. I love it because it makes my job so much easier, and I've become much more efficient in preparing for my patients, as well as during my interaction with them, and examining them—all of that.” Nurse Practitioner
■ “...you had this huge amount of information that you had to weed through to find the late effects. It's like ‘night and day’ as far as the amount of time now [spent] preparing for my patients, and preparing for their visits, as well as ordering all of the blood work, or tests, or whatever we’re ordering—[these are all] so much smoother and easier to do with the Passport for Care.” Nurse Practitioner
■ “I think overall that some of the COG guidelines are requesting too many tests too often—but that is not PFC's fault.” Physician
■ “Note: neither PFC nor COG seems to include [follow-up] issues raised by the genetics or biology of the malignancy itself...” Physician
Abbreviations: COG, Children's Oncology Group; PFC, Passport of Care
Acknowledgements
D.G.P., M.F., and M.E.H. acknowledge funding support from the Cancer Prevention and Research Institute of Texas (CPRIT; grants PP100090 and PP130070 to D.G.P., PP100090 and PP130070 to M.F., and PP100090 and PP130070 to M.E.H.), the Alex's Lemonade Stand Foundation, the Ronald McDonald House Charities (RMHC), and the Lance Armstrong Foundation, Young Texans Against Cancer, and SurvivorVision. The work of W.L. and M.M.H. is funded in part by grants from the NIH National Cancer Institute (NCI; U10 CA098543 to W.L. and U10 CA098543 to M.M.H.). In addition, the involvement of W.L. and M.M.H. in the Children's Oncology Group (COG) Long-Term Follow-Up Guidelines is partially supported by the NCI COG Chair's Grant (U10 CA098543; Principal Investigator: P. C. Adamson). The authors wish to acknowledge J. E. King at the Baylor College of Medicine in the Center for Collaborative and Interactive Technologies, who contributed to survey development and analysis, and some drafting of the manuscript, and Q. W. Smith, also at the Baylor College of Medicine in the Center for Collaborative and Interactive Technologies, who contributed to literature searches and summaries, as well as some drafting and editing.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
D.G.P., M.F., S.B. and M.E.H. made substantial contributions to researching data for the article. All authors contributed substantially to writing and review/editing of the manuscript before submission. D.G.P., M.F., and M.E.H. contributed to discussion of content.
Supplementary information is linked to the online version of the paper at www.nature.com/nrclinonc.
References
- 1.Institute of Medicine (US) and National Research Council (US) National Cancer Policy Board . In: Childhood Cancer Survivorship: Improving Care and Quality of Life. Hewitt M, Weiner SL, Simone JV, editors. National Academies Press; 2003. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . A National Action Plan for Cancer Survivorship: Advancing Public Health Strategies. The Centers for Disease Control and Prevention and The Lance Armstrong Foundation; 2004. [Google Scholar]
- 3.President's Cancer Panel . In: 2003–2004 Annual Report. Reuben S, editor. Vol. 112. NIH National Cancer Institute; 2004. [Google Scholar]
- 4.National Cancer Policy Board (US) Committee on Cancer Survivorship: Improving Care and Quality of Life . In: From Cancer Patient to Cancer Survivor: Lost in Transition. Hewitt ME, Greenfield S, Stovall E, editors. National Academies Press; 2006. [Google Scholar]
- 5.Cantrell MA, Conte TM. Between being cured and being healed: the paradox of childhood cancer survivorship. Qual. Health Res. 2009;19:312–322. doi: 10.1177/1049732308330467. [DOI] [PubMed] [Google Scholar]
- 6.Texas Children's Cancer and Hematology Centers Passport for Care® Application [online] 2014 http://txch.org/cancer-center/ long-term-survivor-program/passport-for-care/
- 7.O'Leary M, Krailo M, Anderson JR, Reaman GH. Children's Oncology Group. Progress in childhood cancer: 50 years of research collaboration, a report from the Children's Oncology Group. Semin. Oncol. 2008;35:484–493. doi: 10.1053/j.seminoncol.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER Cancer Statistics Review, 1975–2010 [online] 2013 http://seer.cancer.gov/csr/1975_2010/
- 9.National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Childhood Cancer by Site: Incidence, Survival and Mortality [online] 2013 Section 28. http://seer.cancer.gov/csr/1975_2010/results_merged/sect_28_childhood_cancer.pdf.
- 10.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat. Rev. Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kremer LC, van der Pal HJ, Offringa M, van Dalen EC, Voute PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann. Oncol. 2002;13:819–829. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- 12.Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol. Biomarkers Prev. 2006;15:2020–2026. doi: 10.1158/1055-9965.EPI-06-0414. [DOI] [PubMed] [Google Scholar]
- 13.Oeffinger KC, Hudson MM. Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J. Clin. 2004;54:208–236. doi: 10.3322/canjclin.54.4.208. [DOI] [PubMed] [Google Scholar]
- 14.Oeffinger KC, et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong GT, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J. Clin. Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Children's Oncology Group Children's Oncology Group—The World's Childhood Cancer Experts: About Us [online] 2014 http://www.childrensoncologygroup.org/index.php/about.
- 17.Landier W, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J. Clin. Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network NCCN Categories of Evidence and Consensus [online] 2014 http://www.nccn.org/professionals/physician_gls/categories_of_consensus.asp.
- 19.Winn RJ, Botnick WZ. The NCCN Guideline Program: a conceptual framework. Oncology (Williston Park) 1997;11:25–32. [PubMed] [Google Scholar]
- 20.Eshelman D, et al. Facilitating care for childhood cancer survivors: integrating Children's Oncology Group Long-Term Follow-up Guidelines and Health Links in clinical practice. J. Pediatr. Oncol. Nurs. 2004;21:271–280. doi: 10.1177/1043454204268875. [DOI] [PubMed] [Google Scholar]
- 21.Children's Oncology Group Long-Term Follow-Up Guidelines for Childhood, Adolescent & Young Adult Cancers [online] 2014 http://www.survivorshipguidelines.org/
- 22.Kadan-Lottick NS, et al. Childhood cancer survivors' knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA. 2002;287:1832–1839. doi: 10.1001/jama.287.14.1832. [DOI] [PubMed] [Google Scholar]
- 23.Bashore L. Childhood and adolescent cancer survivors' knowledge of their disease and effects of treatment. J. Pediatr. Oncol. Nurs. 2004;21:98–102. doi: 10.1177/1043454203262754. [DOI] [PubMed] [Google Scholar]
- 24.Caprino D, Wiley TJ, Massimo L. Childhood cancer survivors in the dark. J. Clin. Oncol. 2004;22:2748–2750. doi: 10.1200/JCO.2004.07.153. [DOI] [PubMed] [Google Scholar]
- 25.Freyer DR, Brugieres L. Adolescent and young adult oncology: transition of care. Pediatr. Blood Cancer. 2008;50:1116–1119. doi: 10.1002/pbc.21455. [DOI] [PubMed] [Google Scholar]
- 26.Eshelman-Kent D, et al. Cancer survivorship practices, services, and delivery: a report from the Children's Oncology Group (COG) Nursing Discipline, Adolescent/Young Adult, and Late Effects Committees. J. Cancer Surviv. 2011;5:345–357. doi: 10.1007/s11764-011-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freyer DR. Transition of care for young adult survivors of childhood and adolescent cancer: rationale and approaches. J. Clin. Oncol. 2010;28:4810–4818. doi: 10.1200/JCO.2009.23.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson TO, Friedman DL, Meadows AT. Childhood cancer survivors: transition to adult-focused risk-based care. Pediatrics. 2010;126:129–136. doi: 10.1542/peds.2009-2802. [DOI] [PubMed] [Google Scholar]
- 29.Henderson TO, Hlubocky FJ, Wroblewski KE, Diller L, Daugherty CK. Physician preferences and knowledge gaps regarding the care of childhood cancer survivors: a mailed survey of pediatric oncologists. J. Clin. Oncol. 2010;28:878–883. doi: 10.1200/JCO.2009.25.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suh E, et al. General internists' preferences and knowledge about the care of adult survivors of childhood cancer. Ann. Intern. Med. 2014;160:11–17. doi: 10.7326/M13-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sima JL, Perkins SM, Haggstrom DA. Primary care physician perceptions of adult survivors of childhood cancer. J. Pediatr. Hematol. Oncol. 2014;36:118–124. doi: 10.1097/MPH.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keegan TH, et al. Medical care in adolescents and young adult cancer survivors: what are the biggest access-related barriers? J. Cancer Surviv. 2014;8:282–292. doi: 10.1007/s11764-013-0332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hudson SV, et al. Adult cancer survivors discuss follow-up in primary care: ‘not what I want, but maybe what I need’. Ann. Fam. Med. 2012;10:418–427. doi: 10.1370/afm.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J. Clin. Oncol. 2009;27:2489–2495. doi: 10.1200/JCO.2008.20.3232. [DOI] [PubMed] [Google Scholar]
- 35.Absolom K, et al. Follow-up care for cancer survivors: views of the younger adult. Br. J. Cancer. 2009;101:561–567. doi: 10.1038/sj.bjc.6605213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung WY, Neville BA, Earle CC. Associations among cancer survivorship discussions, patient and physician expectations, and receipt of follow-up care. J. Clin. Oncol. 2010;28:2577–2583. doi: 10.1200/JCO.2009.26.4549. [DOI] [PubMed] [Google Scholar]
- 37.Mao JJ, et al. Delivery of survivorship care by primary care physicians: the perspective of breast cancer patients. J. Clin. Oncol. 2009;27:933–938. doi: 10.1200/JCO.2008.18.0679. [DOI] [PubMed] [Google Scholar]
- 38.de Bock GH, et al. Patient's needs and preferences in routine follow-up after treatment for breast cancer. Br. J. Cancer. 2004;90:1144–1150. doi: 10.1038/sj.bjc.6601655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burg MA, Lopez ED, Dailey A, Keller ME, Prendergast B. The potential of survivorship care plans in primary care follow-up of minority breast cancer patients. J. Gen. Intern. Med. 2009;24(Suppl. 2):S467–S471. doi: 10.1007/s11606-009-1012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grunfeld E, et al. Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. J. Clin. Oncol. 2011;29:4755–4762. doi: 10.1200/JCO.2011.36.8373. [DOI] [PubMed] [Google Scholar]
- 41.Blinder VS, et al. Patient perspectives on breast cancer treatment plan and summary documents in community oncology care: a pilot program. Cancer. 2013;119:164–172. doi: 10.1002/cncr.27856. [DOI] [PubMed] [Google Scholar]
- 42.Sprague BL, et al. Patient satisfaction with breast and colorectal cancer survivorship care plans. Clin. J. Oncol. Nurs. 2013;17:266–272. doi: 10.1188/13.CJON.17-03AP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baravelli C, et al. The views of bowel cancer survivors and health care professionals regarding survivorship care plans and post treatment follow up. J. Cancer Surviv. 2009;3:99–108. doi: 10.1007/s11764-009-0086-1. [DOI] [PubMed] [Google Scholar]
- 44.Faul LA, et al. Survivorship care planning in colorectal cancer: feedback from survivors & providers. J. Psychosoc. Oncol. 2012;30:198–216. doi: 10.1080/07347332.2011.651260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papadakos J, et al. Informational needs of gynecologic cancer survivors. Gynecol. Oncol. 2012;124:452–457. doi: 10.1016/j.ygyno.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 46.Jones JM, et al. Experiences of care delivery: endometrial cancer survivors at end of treatment. Gynecol. Oncol. 2012;124:458–464. doi: 10.1016/j.ygyno.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 47.Brothers BM, Easley A, Salani R, Andersen BL. Do survivorship care plans impact patients' evaluations of care? A randomized evaluation with gynecologic oncology patients. Gynecol. Oncol. 2013;129:554–558. doi: 10.1016/j.ygyno.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on post-treatment cancer care: qualitative research with survivors, nurses, and physicians. J. Clin. Oncol. 2007;25:2270–2273. doi: 10.1200/JCO.2006.10.0826. [DOI] [PubMed] [Google Scholar]
- 49.Mayer DK, Gerstel A, Leak AN, Smith SK. Patient and provider preferences for survivorship care plans. J. Oncol. Pract. 2012;8:e80. doi: 10.1200/JOP.2011.000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salz T, Oeffinger KC, McCabe MS, Layne TM, Bach PB. Survivorship care plans in research and practice. CA Cancer J. Clin. 2012 doi: 10.3322/caac.20142. http://dx.doi.org/10.3322/caac.20142. [DOI] [PMC free article] [PubMed]
- 51.Kent EE, et al. Health information needs and health-related quality of life in a diverse population of long-term cancer survivors. Patient Educ. Couns. 89:345–352. doi: 10.1016/j.pec.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spain PD, et al. Response to a treatment summary and care plan among adult survivors of pediatric and young adult cancer. J. Oncol. Pract. 2012;8:196–202. doi: 10.1200/JOP.2011.000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bober SL, et al. Caring for cancer survivors: a survey of primary care physicians. Cancer. 2009;115:4409–4418. doi: 10.1002/cncr.24590. [DOI] [PubMed] [Google Scholar]
- 54.Nissen MJ, et al. Views of primary care providers on follow-up care of cancer patients. Fam. Med. 2007;39:477–482. [PubMed] [Google Scholar]
- 55.Birken SA, Deal AM, Mayer DK, Weiner BJ. Determinants of survivorship care plan use in US cancer programs. J. Cancer Educ. doi: 10.1007/s13187-014-0645-7. http://dx.doi.org/10.1007/s13187-014-0660-8. [DOI] [PMC free article] [PubMed]
- 56.Birken SA, Deal AM, Mayer DK, Weiner BJ. Following through: the consistency of survivorship care plan use in United States cancer programs. J. Cancer Educ. doi: 10.1007/s13187-014-0628-8. http://dx.doi.org/10.1007/s13187-014-0628-8. [DOI] [PMC free article] [PubMed]
- 57.American College of Surgeons: Commission on Cancer Cancer Program Standards 2012, Version 1.2.1: Ensuring Patient-Centered Care [online] 2014 http://www.facs.org/cancer/coc/programstandards2012.html.
- 58.Oeffinger KC, et al. Increasing rates of breast cancer and cardiac surveillance among high-risk survivors of childhood Hodgkin lymphoma following a mailed, one-page survivorship care plan. Pediatr. Blood Cancer. 2011;56:818–824. doi: 10.1002/pbc.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horowitz M, Fordis M, Krause S, McKellar J, Poplack D. Passport for Care: implementing the survivorship care plan. J. Oncol. Prac. 2009;5:110–112. doi: 10.1200/JOP.0934405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LIVESTRONG Care Plan. Penn Medicine's OncoLink [online] 2014 http://www.livestrong careplan.org/
- 61.LiveStrong LiveStrong Foundation: We're Grabbing Cancer by the Horns [online] 2014 http://www.livestrong.org/
- 62.The Perelman Center for Advanced Medicine The University of Pennsylvania. OncoLink: Cancer Resources for Patients and Healthcare Professionals [online] 2014 http://www.oncolink.org/
- 63.National Coalition for Cancer Survivorship About Journey Forward [online] 2014 http://www.journeyforward.org/about-journey-forward.
- 64.National Coalition for Cancer Survivorship Journey Forward: Frequently Asked Questions [online] 2014 http://www.journeyforward.org/faq-page#t114n118.
- 65.Hausman J, Ganz PA, Sellers TP, Rosenquist J. Journey forward: the new face of cancer survivorship care. J. Oncol. Pract. 2011;7:e50s–e56s. doi: 10.1200/JOP.2011.000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hausman J, Ganz PA, Sellers TP, Rosenquist J. Journey forward: the new face of cancer survivorship care. Am. J. Manag. Care. 2011;17:e187–e193. [PubMed] [Google Scholar]
- 67.US Department of Health and Human Services, Office of the National Coordinator for Health Information Technology HITECH Act [online] 2013 http://www.healthit.gov/policy-researchers-implementers/hitech-act-0.
- 68.Blumenthal D. Health information technology: what is the federal government's role? Commonwealth Fund; 2006. [Google Scholar]
- 69.Sittig DF, Singh H. A new sociotechnical model for studying health information technology in complex adaptive healthcare systems. Qual. Saf. Health Care. 2010;19(Suppl. 3):i68–i74. doi: 10.1136/qshc.2010.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Osheroff JA, et al. Improving Outcomes with Clinical Decision Support: An Implementer's Guide. 2nd edn HIMSS; 2012. [Google Scholar]
- 71.US Department of Health and Human Services The Health Insurance Portability and Accountability Act of 1996 (HIPAA) Administrative Simplification Regulations [online] 2013 http://www.gpo.gov/fdsys/pkg/FR-2013-01-25/pdf/2013-01073.pdf.
- 72.US Government Printing Office Federal Information Security Management Act of 2002 [online] 2014 http://csrc.nist.gov/drivers/documents/FISMA-final.pdf.
- 73.McCabe MS, et al. American Society of Clinical Oncology statement: achieving high-quality cancer survivorship care. J. Clin. Oncol. 2013;31:631–640. doi: 10.1200/JCO.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nathan PC, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J. Clin. Oncol. 2008;26:4401–4409. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oeffinger KC, Wallace WH. Barriers to follow-up care of survivors in the United States and the United Kingdom. Pediatr. Blood Cancer. 2006;46:135–142. doi: 10.1002/pbc.20614. [DOI] [PubMed] [Google Scholar]
- 76.Landier W. Survivorship care: essential components and models of delivery. Oncology (Williston Park) 2009;23:46–53. [PubMed] [Google Scholar]
- 77.Britto MT, Hesse EA, Kamdar OJ, Munafo JK. Parents' perceptions of a patient portal for managing their child's chronic illness. J. Pediatr. 2013;163:280–281. e1–e2. doi: 10.1016/j.jpeds.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 78.Kim H, Zhang Y. Proceedings of the 5th Information Interaction in Context Symposium. ACM; 2014. pp. 255–258. [Google Scholar]
- 79.Williamson R, et al. Predictors of successful use of a web-based healthcare document storage and sharing system for pediatric cancer survivors: Cancer SurvivorLink™. J. Cancer Surviv. 2014;8:355–363. doi: 10.1007/s11764-014-0346-6. [DOI] [PubMed] [Google Scholar]
- 80.Chou WY, Liu B, Post S, Hesse B. Health-related Internet use among cancer survivors: data from the Health Information National Trends Survey, 2003–2008. J. Cancer Surviv. 2011;5:263–270. doi: 10.1007/s11764-011-0179-5. [DOI] [PubMed] [Google Scholar]
- 81.Friedman DL, Freyer DR, Levitt GA. Models of care for survivors of childhood cancer. Pediatr. Blood Cancer. 2006;46:159–168. doi: 10.1002/pbc.20611. [DOI] [PubMed] [Google Scholar]
- 82.Viswanathan M, et al. Models of Cancer Survivorship Care (Agency for Healthcare Research and Quality. 2014 [PubMed] [Google Scholar]
- 83.US Government Printing Office Public Law 111–148: The Patient Protection and Affordable Care Act [online] 2010 http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf.
- 84.Mueller EL, Park ER, Davis MM. What the Affordable Care Act means for survivors of pediatric cancer. J. Clin. Oncol. 2014;32:615–617. doi: 10.1200/JCO.2013.52.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.American College of Surgeons. Commission on Cancer Cancer Program Standards 2012: Ensuring Patient-Centered Care--Version 1.2.1. 2012 [online], http://www.facs.org/cancer/coc/programstandards2012.pdf.
- 86.International Guideline Harmonization Group for Late Effects of Childhood Cancer A collaboration to optimize care for childhood, adolescent & young adult cancer survivors [online] 2014 http://www.ighg.org/
- 87.Kremer LC, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr. Blood Cancer. 2013;60:543–549. doi: 10.1002/pbc.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scottish Intercollaegiate Guidelines Network . SIGN 132—Long Term Follow Up of Survivors of Childhood Cancer: A National Clinical Guideline. Scottish Intercollaegiate Guidelines Network; 2013. [Google Scholar]
- 89.Stichting Kinderoncologie Nederland (SKION) SKION: Over Ons [online] 2014 https://www.skion.nl/
- 90.Children's Cancer and Leukaemia Group Caring for Children: Curing Their Cancer [online] 2014 http://www.cclg.org.uk/
- 91.Children's Oncology Group Long-term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, Version 3.0--October 2008; Appendix 1, Materials for Clinical Application of LTFU Guidelines Children's Oncology Group [online] 2008 http://www.survivorshipguidelines.org.
- 92.AGREE Collaboration Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual. Saf. Health Care. 2003;12:18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gibbons RJ, Smith S, Antman E. American College of Cardiology; American Heart Association. American College of Cardiology/American Heart Association clinical practice guidelines: part I: where do they come from? Circulation. 2003;107:2979–2986. doi: 10.1161/01.CIR.0000063682.20730.A5. [DOI] [PubMed] [Google Scholar]
- 94.Atkins D, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. In: Graham R, et al., editors. Clinical practice guidelines we can trust. National Academies Press; 2011. [PubMed] [Google Scholar]
- 96.Children's Oncology Group . Children's Oncology Group Nursing Discipline Clinical Practice Subcommittee/Survivorship in collaboration with the Late Effects Committee. In: Landier W, editor. Establishing and Enhancing Services for Childhood Cancer Survivors: Long-Term Follow-Up Program Resource Guide. Children's Oncology Group; 2007. [Google Scholar]
- 97.CMS, Centers for Medicare and Medicaid Services Meaningful Use [online] 2013 http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Meaningful_Use.html.
- 98.Office of the National Coordinator for Health Information Technology EHR Incentives & Certification: Meaningful Use Definition & Objectives [online] 2014 http://www.healthit.gov/providers-professionals/ehr-implementation-steps/step-5-achieve-meaningful-use.
- 99.Office of the National Coordinator for Health Information Technology EHR Incentives & Certification: How to Attain Meaningful Use [online] 2014 http://www.healthit.gov/providers-professionals/how-attain-meaningful-use.
- 100.Wright A, et al. Early results of the meaningful use program for electronic health records. N. Engl. J. Med. 2013;368:779–780. doi: 10.1056/NEJMc1213481. [DOI] [PubMed] [Google Scholar]
- 101.Office of the National Coordinator for Health Information Technology Health Information Exchange (HIE): Standards & Interoperability [online] 2014 http://healthit.gov/providers-professionals/standards-interoperability.
- 102.Committee on Improving the Quality of Cancer Care: Addressing the Challenges of an Aging Population; Board on Health Care Services; Institute of Medicine . In: Delivering High-quality Cancer Care: Charting a New Course for a System in Crisis. Levit L, Balogh E, Nass S, Ganz PA, editors. The National Academies Press; 2013. [PubMed] [Google Scholar]
- 103.Burton LC, Anderson GF, Kues IW. Using electronic health records to help coordinate care. Milbank Q. 2004;82:457–481. doi: 10.1111/j.0887-378X.2004.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O'Malley AS. Tapping the unmet potential of health information technology. N. Engl. J. Med. 2011;364:1090–1091. doi: 10.1056/NEJMp1011227. [DOI] [PubMed] [Google Scholar]
- 105.Cimino JJ, Jing X, Del Fiol G. Meeting the electronic health record “meaningful use” criterion for the HL7 infobutton standard using OpenInfobutton and the Librarian Infobutton Tailoring Environment (LITE). AMIA Annu. Symp. Proc. 2012;2012:112–120. [PMC free article] [PubMed] [Google Scholar]
- 106.Mandl KD, Kohane IS. Escaping the EHR trap—the future of health IT. N. Engl. J. Med. 2012;366:2240–2242. doi: 10.1056/NEJMp1203102. [DOI] [PubMed] [Google Scholar]
- 107.Miriovsky BJ, Shulman LN, Abernethy AP. Importance of health information technology, electronic health records, and continuously aggregating data to comparative effectiveness research and learning health care. J. Clin. Oncol. 2012;30:4243–4248. doi: 10.1200/JCO.2012.42.8011. [DOI] [PubMed] [Google Scholar]
- 108.Cimino JJ, et al. Practical choices for infobutton customization: experience from four sites. AMIA Annu. Symp. Proc. 2013;2013:236–245. [PMC free article] [PubMed] [Google Scholar]
- 109.King J, et al. Evaluating the clinical utility of the Passport for Care, an online decision-support system for managing survivors of childhood cancer [online] 2012 http://www.speakerready.com/abstracts/CPRIT/admin/detail_simple.php?height=520&width=600&abstract_id=380.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.