Abstract
Dendritic cells (DCs) are a critical component of anti-tumor immunity due to their ability to induce a robust immune response to antigen (Ag). Alcohol was previously shown to reduce DC ability to present foreign Ag and promote pro-inflammatory responses in situations of infection and trauma. However the impact of alcohol exposure on generation of an anti-tumor response, especially in the context of generation of an immune vaccine has not been examined. In the clinic, DC vaccines are typically generated from autologous blood, therefore prior exposure to substances such as alcohol may be a critical factor to consider regarding the effectiveness in generating an immune response. In this study we demonstrate for the first time that ethanol differentially affects DC and tumor Ag-specific T cell responses depending on sex. Signaling pathways were found to be differentially regulated in DC in females compared to males and these differences were exacerbated by ethanol treatment. DC from female mice treated with ethanol were unable to activate Ag-specific cytotoxic T cells (CTL) as shown by reduced expression of CD44, CD69, and decreased production of granzyme B and IFNγ. Furthermore, although FOXO3, an immune suppressive mediator of DC function, was found to be upregulated in DC from female mice, ethanol related suppression was independent of FOXO3. These findings demonstrate for the first time differential impacts of alcohol on the immune system of females compared to males and may be a critical consideration for determining the effectiveness of an immune based therapy for cancer in patients that consume alcohol.
Keywords: Anti-tumor immunity, sex, alcohol, dendritic cell, cytotoxic T cell
Introduction
The impact of alcohol on cancer risk remains inconclusive due to seemingly conflicting data in several reports (Allen et al., 2009; Brandon-Warner, Walling, Schrum, & McKillop, 2012; Fowke, Howard, Andriole, & Freedland, 2014; Garcia-Lavandeira, Ruano-Ravina, & Barros-Dios, 2016; Gong et al., 2009; Meadows & Zhang, 2015; Newcomb et al., 2013; F. Zhang, Zhu, Meadows, & Zhang, 2015). These conflicting reports may be partially due to the study of different types of cancer, such as prostate, liver, breast, lung and melanoma, but also are due in large part to direct effects of alcohol on the immune system. The immune system, especially the adaptive immune response, is critical for recognition and destruction of tumor cells. Cytotoxic T cells (CTL) recognize tumor associated antigens by major histocompatibility complex (MHC) I mechanisms which trigger release of Granzyme B, IFNγ and promote lytic responses to destroy tumor cells. Alcohol has been shown to have dramatic effects on the adaptive immune response including inhibiting T and B cell development (H. Zhang, Zhu, Zhang, & Meadows, 2015). Alcohol was also found to have substantial effects on macrophages (Goral, Choudhry, & Kovacs, 2004) and in DC populations harvested from ethanol fed mice (Heinz & Waltenbaugh, 2007; Lau, Abe, & Thomson, 2006). However, studies have not examined the impact of alcohol on the efficacy of an immune therapy which includes dendritic cell (DC) activation and function of tumor antigen (Ag) specific T cells. Alcohol can have dramatic effects on the immune cells directly or mediate changes in the tumor microenvironment (TME) that lead to immune tolerance in favor of immune activation. For example, increases in indolamine 2–3-dideoxygenase (IDO), while considered pro-inflammatory in some instances, is immune suppressing in the TME. IDO catabolizes tryptophan and leads to inhibition of T cell proliferation and activation (Platten, von Knebel Doeberitz, Oezen, Wick, & Ochs, 2014; Xiao, Liu, & Link, 2004). Additionally, alcohol can up-regulate signaling pathways which can be detrimental in the generation and activation of immune responses providing protection from tumor development such as MAPK, AKT and SGK (Ebert et al., 2016; Goral et al., 2004; Ho et al., 2012; Huang et al., 2015; Morelli et al., 2010). Given the recent rise in use and promise of immune therapies for cancer, this may be an important aspect to consider when deciding on an appropriate treatment regimen (Boudewijns, Bloemendal, Gerritsen, de Vries, & Schreibelt, 2016; Linch & Redmond, 2016). In the current study, we demonstrate for the first time that ethanol has a significant influence on DC from female but not male activation and effector function of tumor-Ag specific T cells.
Methods
Experimental Mice
C57BL/6, male and female mice aged 6–8 weeks were obtained from Jackson laboratories. FOXO3−/− were bred from previously acquired generous gift from Dr. Karen Arden (UCSD). Tyrosinase related protein 2 (TRP2) Ag-specific T cells were obtained from 24H9 mice, obtained from NCI-Frederick. For in vitro studies, C57BL/6 mice were used as a source of bone marrow derived dendritic cells (BMDC) and primary DC were also purified by magnetic beads from splenocytes. Mice were housed under specific pathogen-free conditions and were treated in accordance with NIH guidelines under protocols approved by the animal care and use committee (IACUC) of Loyola University Chicago. (Maywood, IL).
Cell Isolations
TRP2 T cells were isolated by gently rolling lymph nodes harvested from 24H9 mice between frosted glass slides into PBS +2% FBS. The single cell suspension generated by this method is 99% CD8+ TRP-Ag specific T cells as 24H9 mice are on a Rag2−/− background, thus they only have CD8 cells with the specific T cell receptor (TcR) (Singh, Ji, Feigenbaum, Leighty, & Hurwitz, 2009).
Bone marrow cell differentiation
Bone marrow was taken from the tibia and fibula of 6–8 week old male or female mice and treated with AKC lysis buffer to remove red blood cells. Remaining cells were plated in complete RPMI supplemented with 20ng/mL GM-CSF (Thompson et al., 2015). Media was changed every other day for 9 days to direct differentiation of dendritic cells. After nine days of differentiation BMDCs were plated at a concentration of 1×106/ml and were treated for 3 hours with 50mM ethanol. After ethanol pre-treatment cells were stimulated with 100ng/mL LPS for 12 hours.
Ethanol treatments
Cells were plated in RPMI media + 5% FBS and left untreated or treated with ethanol at 2.5, 25 and 50mM concentrations. To avoid evaporation during treatment cells cultures were placed in plastic sealed containers with additional ethanol maintained in beaker outside of the well. (Garcia-Lavandeira et al., 2016). Absolute ethanol was purchases from Sigma-Aldrich and used for all ethanol treatments (D’Souza El-Guindy et al., 2010).
Western Blots and Immunoprecipitations
Whole cell lysates were generated from bone marrow derived DCs using lauryl-maltoside (Sigma Aldrich) immunoprecipitation buffer supplemented with protease inhibitor (Roche). Proteins were run by electrophoresis on a 4–15% gradient polyacrylamide gel and transferred to a nitrocellulose membrane (Bio-Rad) Membrane were blotted for FOXO3 using anti-FOXO3 antibody, anti-MAPK p42/44 (ERK1/2), anti-AKT, anti-AKTp308, anti-SGKp (Cell Signaling) or for β-Actin (Sigma-Aldrich) used to normalize protein loading. Densitometry was calculated using Bio-rad Image Lab software.
Flow Cytometry
Cell suspensions were blocked with Fc block, washed, and incubated with antibodies anti-CD11c, CD317 (PDCA-1), CD11b, CD4, CD8, CD80, anti-CD86 and anti-MHCII (BD Pharmingen) or anti-CD44, and anti-CD69 eBioscience for surface staining and intracellular staining was performed on cells following surface staining and treatment with fix-perm buffer on ice for 30 min for IL-12 and granzyme B, (eBioscience) (Thompson et al., 2015).
Proliferation
T cells were isolated from transgenic mice containing the TRP2 Ag T cell receptor. T cells were stimulated with control or ethanol pre-treated DC loaded with TRP2 peptide for 48 hours in RPMI supplemented with 50uM β-ME. Cultures were then stained for surface markers indicated above and CFSE dilution was measured on CD3+/CD8+ cells by Flow cytometry detection as a measure of proliferation (Thompson et al., 2015).
qRT-PCR
Cells were cultured as indicated above and DCs were harvested for mRNA purification using the Bio-Rad Aurum total RNA kit per the manufacturer’s instructions. RNA was isolated from DCs purified from tumors by RNAeasy Spin Columns (Qiagen) per manufacturer’s instructions. RNA quality was determined by analysis on an Agilent bioanalzyer 2000. PCR reactions were run on a QuantStudio 6 using a Taqman assay system with primers for IDO, IL-10, IL-6, TNF-α, β-actin, and GAPDH (Thompson et al., 2015). CT values were determined by the Applied Biosystems 7300 SDS software. Data were analyzed by the ΔΔCT method. The ΔΔCT uses the CT values of the gene of interest (GOI) and housekeeping gene (HKG). ΔCT is determined by subtracting the CT(HKG) from the CT(GOI), and then the ΔΔCT is determined by subtracting the ΔCT(control) from the ΔCT(experimental). Fold change is equal to 2(−ΔΔCt).
ELISPOT
Multiscreen plates (Millipore) were coated with 100μl of capture antibody (R&D Systems) overnight at 4°C. IFN- γ (1 × 104) purified TRP2 T cells were added to increasing concentrations of peptide loaded control or ethanol pre-treated DC (WT or FOXO3−/−). After incubation, plates were washed and processed.
Statistical Analysis
To determine statistical significance, data was analyzed using descriptive and graphical techniques. Statistical analysis for differences between groups for cytokine production or gene expression used the unpaired Student’s t test. Data generated with multi variables was transformed to their common logarithms to satisfy homogeneity of variance and normality requirements in the ANOVAs. Statistically significant differences in tumor growth was determined using the Wilcoxon rank sum test as described. With 10 mice per group, we have 80% power to detect a difference of 1.25 standard deviations across groups which is a reasonable effect size for a preclinical study of this type in genetically identical mice. All experiments were repeated 2–3 x for reproducibility.
Results
Sex has an impact on gene expression and signaling pathway activation in ethanol treated DC with or without additional stimulation
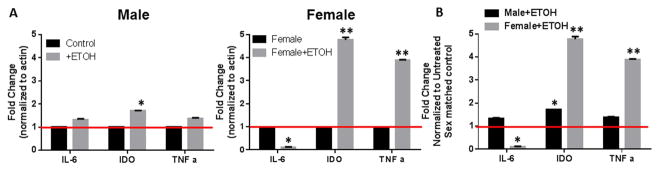
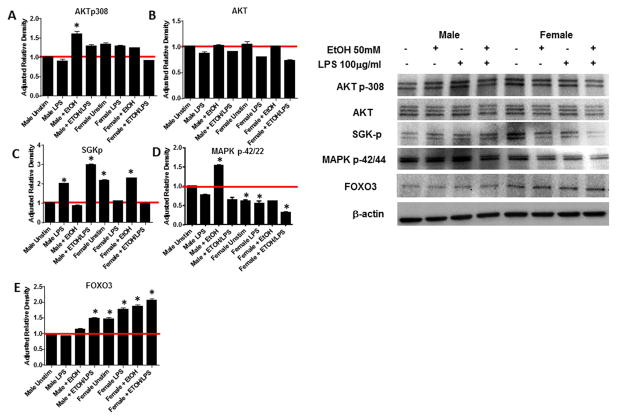
Previous reports have described ethanol to have significant impacts on immune responses during situations of infection, in response to burn injury or trauma. However, how ethanol may impact immune function in response to a self and/or tumor antigen has not been addressed. Our research has been focused on the differential regulation of anti-tumor immunity based on sex, therefore we wanted to determine whether ethanol could be a compounding factor to the efficacy of an immune therapy for the treatment of cancer. One study by Olubadewo et.al, demonstrated that ethanol had more of an impact on male diabetic mice compared to females in terms of cytokine production (Olubadewo & Spitzer, 2003). Therefore, we began our studies by harvesting bone marrow from male and female mice and generating dendritic cells (BMDC), such as those that would be used to develop a dendritic cell vaccine. BMDC were then treated with a high dose of ethanol (50mM) for 3 hrs. Cytokines (IL-6 and TNF-α) and an enzyme (IDO) important in the activation or suppression of T cells was then assessed by qRT-PCR. In the DC from male mice, ethanol treatment induced a statistically significant increase in indolamine-2, 3-dideoxygenase (IDO) a highly tolerogenic molecule to T cells. However, changes in IL-6 and TNF-α were minimal under these conditions. In contrast, DC from female mice had a significant reduction in IL-6 and a dramatic increase in both IDO and TNFα (Figure 1). Given the significant differences in cytokine expression by the male and DC from female mice after ethanol exposure, we sought to detect differences in the activation of signaling pathways that regulate these cytokines including AKT, SGK, MAPK and FOXO3. Unexpectedly, there were differences in expression of each of these factors between male and female BMDC even in the absence ethanol. Specifically, phosphorylated SKG, phosphorylated AKT and FOXO3 were all upregulated in unstimulated female BMDC compared to male (Figure 2). These differences were compounded by stimulation with lipopolysaccharide (LPS) and further modulated by ethanol treatment. Although, we were unable to perform statistical analysis across 3 different gels from 3 different experiments, which utilize 3 different sets of protein lysates, the pattern of up and down regulation of each of the signaling molecules in response to LPS and/or ethanol remained consistent. In male BMDC, phosphorylation of AKT increased in the presence of ethanol whether or not an exogenous stimulation was received whereas ethanol in the presence of LPS actually reduced phosphorylation of AKT in female BMDC (Figure 2A). Expression of total AKT was unchanged in both populations confirming that the regulation of AKT was at the phosphorylation level (Figure 2B). A similar result was observed with phosphorylation of SGK. Male BMDC up regulated SGK-p with LPS stimulation which was further upregulated by ethanol treatment, however female BMDC decreased SGK-p upon LPS stimulation which was not altered by the presence of ethanol (Figure 2C). MAPK regulation had yet another pattern of response, male BMDC increased MAPK p42/44 in response to ethanol, however female BMDC had a lower expression which was further decreased up exposure to ethanol (Figure 2D). Finally examination of FOXO3 expression demonstrated that although female BMDC expression of FOXO3 is higher than male even in unstimulated control samples, exposure to ethanol increased FOXO3 in both male and female BMDC (Figure 2E). We did not examine phosphorylation of FOXO3 in DC, as our previous study demonstrates that in DC the mechanism of action of FOXO3 is unphosphorylated FOXO3 binds and sequesters NF-κB to the cytoplasm to prevent pro-inflammatory signaling (Thompson et al., 2015). These results demonstrate that there are intrinsic differences in immune cell signaling between male and females, but these differences are exacerbated by exposure to ethanol. While it may be argued that the overall fold change in these signaling molecules is small (1.5–3 fold), the fact that phosphorylation of AKT, SGK and MAPK are all impacted by ethanol treatment and that total FOXO3, a critical molecule in immune tolerance is significantly up regulated in females upon ethanol exposure suggest that these alterations hold biological significance. Decreases in MAPK phosphorylation in female support the findings that IL-6 is highly up-regulated (Figures 1 and 2D) and (Goral et al., 2004). Increased FOXO3 expression can also be correlated to the increase in IDO expression by DC treated with ethanol (Figures 1 and 2E) and (Dejean et al., 2009). Taken together these data demonstrate that ethanol is shifting DC phenotype, especially in females from immune stimulatory to immune suppressive.
Figure 1. Ethanol has higher impact on gene expression in DC from females. BM-DC were generated from male and female mice.
DC cultures treated with 50mM ethanol (EtOH) for 3hrs. DC were then lysed and mRNA was isolated and tested by qRT-PCR for IDO, IL-10, IL-6, TNFa and housekeeping genes b-actin and GAPDH. A) Demonstrates individually male and female control samples compared to ethanol treated. B) Demonstrates ethanol treated comparing male to female. The red line represents the 0 treated control gene expression as a baseline for ratio comparison. *p<0.05, **p<0.01. Data represent the mean of 9 individual samples. Experiments were repeated a total of 3 times.
Figure 2. Ethanol differentially impacts DC signaling depending on gender.
BM-DC generated from male and female mice were cultured for 9 days and treated with 50mM ethanol (EtOH) for 3hrs followed by LPS stimulation. DC were then lysed and assessed for accumulation of protein or phosphorylation of key signaling molecules involved in stimulating a pro-inflammatory, anti-tumor immune response. Data are representative of 3 separate experiments. *p>0.05
Ethanol significantly reduces IL-12 production by DC from female but not male mice in response to tumor antigen
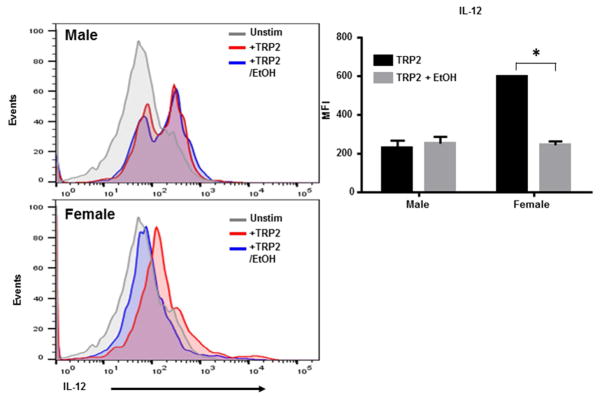
To determine the effect of ethanol on DC ability to stimulate T cells, DC surface phenotype was first examined to test for expression of MHC II and co-stimulatory molecules. DC were generated from bone marrow from male and female CD57Bl/6 mice and treated with ethanol or control media for 2 or 12 hours. DC were then fed B16 melanoma tumor lysate overnight for maturation and activation. Activated DC were then stained for expression of CD80, CD86 and MHC II and analyzed by flow cytometry. No differences were detected in regardless of the length of ethanol exposure (Supplemental Figure 1). Because T cells require 3 signals from DC for proper activation, we next sought to determine whether pro-inflammatory cytokine production important for T cell activation was altered. DC were generated and treated the same as in the previous experiment but were then tested for IL-12 production by intracellular staining. DC from male mice produced a similar level of IL-12 in response to a melanoma tumor antigen tyrosinase related protein 2 (TRP2) regardless of ethanol exposure. However, DC from female mice significantly reduced IL-12 production (Figure 3). Although DC from female mice did still produce some IL-12, because of the many differences in the signaling profiles of DC from female mice compared to the male, this level of IL-12 may not be sufficient for T cell activation.
Figure 3. Ethanol significantly reduced IL-12 production in female but not male DC in response to tumor antigen.
BM-DC generated from male and female mice were cultured for 9 days and treated with 50mM ethanol (EtOH) for 3hrs. DC were then activated by melanoma tumor lysate and tested for IL-12 production by intracellular staining. Data are presented as MFI +/− SD and are representative of 4 separate experiments. *p<0.01
Ethanol treated DC from females but not males have a reduced capacity to stimulate tumor Ag-specific T cells
We next sought to determine the full effect of DC exposure to ethanol on tumor Ag-specific T cell activation in males and females. DC were generated from bone marrow and treated with control or ethanol media for 2 or 12 hours. For full potential to stimulate Ag-specific T cells, DC were loaded with TRP2 peptides overnight. Peptide loaded DC were then co-cultured with TRP2 Ag-specific T cells labeled with CFSE for 48 hours. T cells cultures were then stained for additional surface markers, CD44 and CD69 as an indication of activation and analyzed by flow cytometry. Significant differences were detected upon examination of activation markers. TRP2 T cells stimulated with DC from male mice with or without ethanol had high expression of both CD44 and CD69 indicating they have received stimulation with their cognate antigen and were activated (Figure 4A). In contrast, DC from female mice treated with ethanol for only 2 hours had a significant reduction in CD69 expression which demonstrates a reduced activation state and DC treated for 12 hours were completely incapable of stimulating either CD44 or CD69 expression (Figure 4A). Interestingly, although CD44 expression is down and would suggest the T cells were missing appropriate Ag stimulation, as demonstrated by CFSE dilution there was no change in either DC population’s ability to stimulate T cell proliferation even after 12 hours of ethanol exposure (Figure 4B).
Figure 4. Ethanol treatment of DC does not impact tumor Ag specific T cell proliferation but does inhibit expression of activation markers in female T cells.
TRP2 Ag specific CD8+ T cells were labeled with CFSE to detect proliferation and co-cultured with peptide loaded control or ethanol pre-treated DC. T cells were assessed for A) activation markers and B) proliferation 48 hours after co-culture. Data are representative of 3 separate experiments.
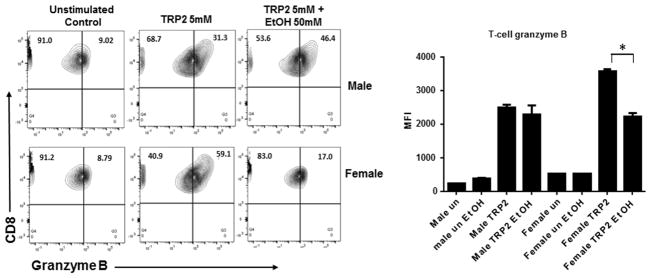
To further determine the impact of ethanol the effectiveness of an anti-tumor response, granzyme B production was measure by intracellular staining and flow cytometry. DC were generated and treated as described above. Treated DC and TRP 2 Ag-specific T cells were co-cultured for 12 hours. T cells stimulated with TRP2 loaded DC significantly upregulated granzyme B expression as expected. DC ethanol pre-treatment did not impact T cell stimulation with the DC from male mice, but did significantly reduce granzyme B by T cells stimulated with the DC from female mice (Figure 5). Similar to the IL-12 production by the DC, while these T cells do still express some granzyme B, it is likely that their full cytotoxic T cell response critical for generating anti-tumor immunity is significantly altered due to reduced effectiveness of DC exposed to ethanol.
Figure 5. Ethanol treatment of DC reduces CD8+ T cell granzyme B release in Females.
TRP2 Ag specific CD8+ cytotoxic effector T cells were co-cultured with peptide loaded DC +/− ethanol pre-treatment. T cells were assessed for granzyme B production 12 hrs after co-culture by intracellular staining and flow cytometry. Data are presented as MFI +/− SD and are representative of 2 separate experiments on 6 individual samples. *p<0.03
Ethanol reduces effectiveness of silencing FOXO3 in DC for tumor vaccine
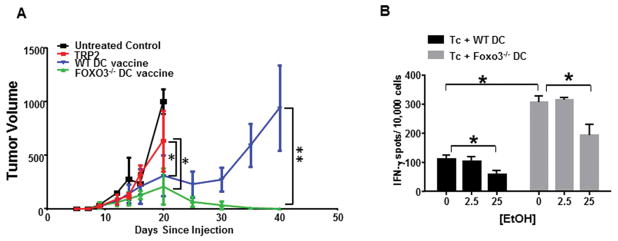
We and others have demonstrated that FOXO3 expression in DC promotes immune tolerance in tumors, in response to virus and overall T cell responses. (Dejean et al., 2009; Koorella et al., 2014; Thompson et al., 2015; Tumurbaatar et al., 2013) Because our previous studies were all in male mice (Thompson et al., 2015), we first wanted to verify administration of a FOXO3−/− DC vaccine enhanced anti-tumor immunity in female mice. Female C57Bl/6 mice with B16 tumors were 1) left untreated (black line), 2) injected I.P. with TRP2 peptide (red line), 3) given a wild type DC vaccine where DC were loaded with TRP2 (blue line) or 4) given a FOXO3−/− DC vaccine where DC were loaded with TRP2 (green line) (Figure 6A). The untreated and TRP2 treated groups succumb to tumor burden within 3 weeks of tumor injection, mice receiving the WT DC vaccine had an initial regression of tumors which rebounded approximately 30 days after injection, but mice receiving FOXO3−/− DC vaccine eradicated their tumors (Figure 6A). These data demonstrate that silencing FOXO3 in DC from female mice (similar to previous findings in male) promotes generation of an effective anti-tumor immune response.
Figure 6. Silencing FOXO3 enhances T cell response to tumor antigens but is still reduced by ethanol stimulation.
A) Female C57BL/6 mice were injected subcutaneously with B16 melanoma, 10 days later tumor bearing mice received a DC vaccine with wild type or FOXO3−/− peptide loaded DC. Tumors were measured every 3 days. Data displayed as mean +/− S.D. of 5 mice per group. *p<0.05,**p<0.01 B) TRP2 Ag specific CD8+ cytotoxic effector T cells were co-cultured with peptide loaded WT or FOXO3−/− DC +/− ethanol pre-treatment. T cells were assessed for IFNg production 24 hrs after co-culture by ELISPOT. Data are presented as the average of triplicate wells +/− SD. *p<0.02. Data are representative of 2 experiments of 6 mice per group.
Given that FOXO3 was upregulated in female BMDC compared to male even in the absence of ethanol, we sought to determine whether silencing FOXO3 could promote T cells responses when DC became exposed to ethanol. DC were generated from WT or FOXO3−/− bone marrow as previously described. BMDC’s were treated with low (2.5mM) or moderate (25 mM) doses of ethanol for 12 hrs. BMDC were then peptide loaded and used to stimulate TRP2 T cells for IFNγ production in an ELISPOT assay for 24 hrs. T cells stimulated with FOXO3−/− DC had a significantly higher production of IFNγ compared to WT DC (Figure 6B). Low dose of ethanol pre-treatment had no effect on T cells from either DC treatment group. However, DC exposed to moderate ethanol dosage resulted in reduced T cell function in both the WT and FOXO3−/− groups (Figure 6B). These data demonstrate that ethanol treatment of DC from female mice results in a reduced ability to stimulate CTL’s important for anti-tumor immunity. This mechanism is independent of FOXO3 induced immune suppression as previously described in tumor associated DC (Thompson et al., 2015).
Discussion
The option of immune therapy for the treatment of cancer is a quickly growing and changing field. Many successes have transpired in recent years including the use of check-point inhibitors such as anti-PD-1 and anti-CTLA-4 which support sustained T cell function in the tumor microenvironment (Yun, Vincelette, Green, Wahner Hendrickson, & Abraham, 2016). Additionally direct injection of immune cells such as DC vaccines and adoptive T cell transfer therapy have gained momentum in recent years (Boudewijns et al., 2016; Yang et al., 2016). However, the success rate of these therapies vary dramatically and the mechanisms regulating these variances are poorly understood.
Many studies have implicated significant impacts of ethanol on host immune responses from inhibiting T and B cell development to (Barr et al., 2016; Hemann, McGill, & Legge, 2014; Pasala, Barr, & Messaoudi, 2015; Song et al., 2002) altering cytokine production and signaling in macrophages (Huang et al., 2015; Karavitis, Murdoch, Deburghgraeve, Ramirez, & Kovacs, 2012; Qin et al., 2014; Rendon, Janda, Bianco, & Choudhry, 2012). In each case, the effect of ethanol on the immune system is varied depending on whether it is a situation of acute or chronic exposure to ethanol and is focused on immune responses to injury or foreign antigen such as LPS, CPG or viral infection. Given the diversity of effects on different immune cell populations in different settings, we hypothesized that the response to a self and/or tumor antigen may also be altered. DC generated for use as a DC vaccine are often generated from patient blood, (Baar, 1999 ), whether autologous or allogeneic these DC could be hampered in their ability to activate CTL toward effective anti-tumor immunity due to prior exposure to ethanol. In the current study, we focused on the direct effects of ethanol on dendritic cells specifically in regard to their ability to stimulate tyrosinase related protein 2, a melanoma tumor Ag-specific T cell (TRP2 T cells). Our preliminary studies on bone marrow derived DC treated with ethanol quickly identified a significant difference in DC responses to ethanol depending on whether they were generated from the bone marrow of a male or female mouse (Figure 1). Given these discrepancies all our studies were conducted side by side and the data conclusively demonstrates that ethanol has a significant impact in decreasing anti-tumor immune stimulating ability of DC from female but not male mice. It could be argued that alcohol has a more negative effect on estrogen or estrogen receptor signaling compared to testosterone and androgen receptor, however, given that these were DC generated in vitro in the absence of exogenous hormones that is unlikely. Future in vivo studies are needed to more closely address the possibility of alcohol disruption of nuclear hormone receptor activation.
In this study it was demonstrated that ethanol altered key signaling pathways in DC generated from females but not males. These changes further resulted in differential functions observed including: DC from female mice exposed to ethanol had reduced IL-12 production and failed to stimulate CTL upregulation of CD44 and CD69, and further resulted in reduced CTL granzyme B and IFNγ production. Collectively the reduction in these import effector T cell mechanisms would allow for tumor escape and growth.
Our previous work demonstrate that tumor associated DC that fail to efficiently activate CTL are regulated by a transcription factor FOXO3 which suppresses immunity by sequestering NF-κB to the cytoplasm which inhibits pro-inflammatory signaling (Thompson et al., 2015). These data led us to consider the possibility that ethanol was upregulating FOXO3 in DC to a level that because tolerance inducing. Although, we did see an increase in FOXO3 expression upon ethanol treatment, upon gene silencing FOXO3 in DC, ethanol treatment still had a damning effect on DC stimulatory ability demonstrating that the suppression of immunity by ethanol is through a FOXO3 independent mechanism. The results of this study are both biologically and statistically significant, however, it should be noted we have demonstrated these effects primarily in vitro under an acute exposure to ethanol. In the clinic it is highly unlikely that leukocytes taken from a patient to generate an immune vaccine have had a single exposure to alcohol. This is a current limitation of the study but given the important findings, future studies are needed to determine the mechanism regulating DC dysfunction induced by ethanol in females and the duration of the effect. The identification of the effects of ethanol in the female immune system may be immediately beneficial clinically as appropriate courses of treatment are considered for anti-tumor therapy.
Supplementary Material
Highlights.
Alcohol differentially stimulates dendritic cells in males and females.
Alcohol promotes reduced immune stimulatory capacity of female DC.
Female DC treated with alcohol have reduced IL-12 production.
Tumor Ag-specific T cells stimulated by female DC previously exposed to alcohol have reduced anti-tumor effector functions.
Acknowledgments
The authors would like to thank Ms. Amy Vidrine for technical assistance and Drs. Michael Nishimura, John Callaci and Mashkoor Choudhry for research discussion and manuscript feedback.
Funding Sources
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers R00CA151294 and NIH, NIAAA T32 Training Grant AA013527. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The corresponding authors was further supported by Loyola University Chicago start-up funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, Green J. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101(5):296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- Baar J. Clinical applications of dendritic cell cancer vaccines. Oncologist. 1999;4(2):140–144. [PubMed] [Google Scholar]
- Barr T, Girke T, Sureshchandra S, Nguyen C, Grant K, Messaoudi I. Alcohol Consumption Modulates Host Defense in Rhesus Macaques by Altering Gene Expression in Circulating Leukocytes. J Immunol. 2016;196(1):182–195. doi: 10.4049/jimmunol.1501527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudewijns S, Bloemendal M, Gerritsen WR, de Vries IJ, Schreibelt G. Dendritic cell vaccination in melanoma patients: from promising results to future perspectives. Hum Vaccin Immunother. 2016 doi: 10.1080/21645515.2016.1197453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon-Warner E, Walling TL, Schrum LW, McKillop IH. Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis. Alcohol Clin Exp Res. 2012;36(4):641–653. doi: 10.1111/j.1530-0277.2011.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza El-Guindy NB, Kovacs EJ, De Witte P, Spies C, Littleton JM, de Villiers WJ, … Meadows GG. Laboratory models available to study alcohol-induced organ damage and immune variations: choosing the appropriate model. Alcohol Clin Exp Res. 2010;34(9):1489–1511. doi: 10.1111/j.1530-0277.2010.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean AS, Beisner DR, Ch’en IL, Kerdiles YM, Babour A, Arden KC, … Hedrick SM. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10(5):504–513. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert PJ, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, … Mellman I. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity. 2016;44(3):609–621. doi: 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Fowke JH, Howard L, Andriole GL, Freedland SJ. Alcohol Intake Increases High-grade Prostate Cancer Risk Among Men Taking Dutasteride in the REDUCE Trial. Eur Urol. 2014;66(6):1133–1138. doi: 10.1016/j.eururo.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lavandeira JA, Ruano-Ravina A, Barros-Dios JM. Alcohol consumption and lung cancer risk in never smokers. Gac Sanit. 2016 doi: 10.1016/j.gaceta.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Gong Z, Kristal AR, Schenk JM, Tangen CM, Goodman PJ, Thompson IM. Alcohol consumption, finasteride, and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer. 2009;115(16):3661–3669. doi: 10.1002/cncr.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goral J, Choudhry MA, Kovacs EJ. Acute ethanol exposure inhibits macrophage IL-6 production: role of p38 and ERK1/2 MAPK. J Leukoc Biol. 2004;75(3):553–559. doi: 10.1189/jlb.0703350. [DOI] [PubMed] [Google Scholar]
- Heinz R, Waltenbaugh C. Ethanol consumption modifies dendritic cell antigen presentation in mice. Alcohol Clin Exp Res. 2007;31(10):1759–1771. doi: 10.1111/j.1530-0277.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- Hemann EA, McGill JL, Legge KL. Chronic ethanol exposure selectively inhibits the influenza-specific CD8 T cell response during influenza a virus infection. Alcohol Clin Exp Res. 2014;38(9):2403–2413. doi: 10.1111/acer.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KK, McGuire VA, Koo CY, Muir KW, de Olano N, Maifoshie E, … Lam EW. Phosphorylation of FOXO3a on Ser-7 by p38 promotes its nuclear localization in response to doxorubicin. J Biol Chem. 2012;287(2):1545–1555. doi: 10.1074/jbc.M111.284224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Li X, Ma Q, Xu Q, Duan W, Lei J, … Wu Z. Chronic alcohol exposure exacerbates inflammation and triggers pancreatic acinar-to-ductal metaplasia through PI3K/Akt/IKK. Int J Mol Med. 2015;35(3):653–663. doi: 10.3892/ijmm.2014.2055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Karavitis J, Murdoch EL, Deburghgraeve C, Ramirez L, Kovacs EJ. Ethanol suppresses phagosomal adhesion maturation, Rac activation, and subsequent actin polymerization during FcgammaR-mediated phagocytosis. Cell Immunol. 2012;274(1–2):61–71. doi: 10.1016/j.cellimm.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koorella C, Nair JR, Murray ME, Carlson LM, Watkins SK, Lee KP. Novel regulation of CD80/CD86-induced phosphatidylinositol 3-kinase signaling by NOTCH1 protein in interleukin6 and indoleamine 2,3-dioxygenase production by dendritic cells. J Biol Chem. 2014;289(11):7747–7762. doi: 10.1074/jbc.M113.519686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AH, Abe M, Thomson AW. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2006;79(5):941–953. doi: 10.1189/jlb.0905517. [DOI] [PubMed] [Google Scholar]
- Linch SN, Redmond WL. How do I steer this thing? Using dendritic cell targeted vaccination to more effectively guide the antitumor immune response with combination immunotherapy. J Immunother Cancer. 2016;4:31. doi: 10.1186/s40425-016-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows GG, Zhang H. Effects of Alcohol on Tumor Growth, Metastasis, Immune Response, and Host Survival. Alcohol Res. 2015;37(2):311–322. [PMC free article] [PubMed] [Google Scholar]
- Morelli C, Lanzino M, Garofalo C, Maris P, Brunelli E, Casaburi I, … Ando S. Akt2 inhibition enables the forkhead transcription factor FoxO3a to have a repressive role in estrogen receptor alpha transcriptional activity in breast cancer cells. Mol Cell Biol. 2010;30(3):857–870. doi: 10.1128/mcb.00824-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb PA, Kampman E, Trentham-Dietz A, Egan KM, Titus LJ, Baron JA, … Willett WC. Alcohol consumption before and after breast cancer diagnosis: associations with survival from breast cancer, cardiovascular disease, and other causes. J Clin Oncol. 2013;31(16):1939–1946. doi: 10.1200/jco.2012.46.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olubadewo JO, Spitzer JA. Immune response modulation in acutely ethanol-intoxicated, acutely diabetic male and female rats. Alcohol. 2003;31(3):137–147. doi: 10.1016/j.alcohol.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Pasala S, Barr T, Messaoudi I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol Res. 2015;37(2):185–197. [PMC free article] [PubMed] [Google Scholar]
- Platten M, von Knebel Doeberitz N, Oezen I, Wick W, Ochs K. Cancer Immunotherapy by Targeting IDO1/TDO and Their Downstream Effectors. Front Immunol. 2014;5:673. doi: 10.3389/fimmu.2014.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Hamilton JL, Bird MD, Chen MM, Ramirez L, Zahs A, … Makowski L. Adipose inflammation and macrophage infiltration after binge ethanol and burn injury. Alcohol Clin Exp Res. 2014;38(1):204–213. doi: 10.1111/acer.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon JL, Janda BA, Bianco ME, Choudhry MA. Ethanol exposure suppresses bone marrow-derived dendritic cell inflammatory responses independent of TLR4 expression. J Interferon Cytokine Res. 2012;32(9):416–425. doi: 10.1089/jir.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Ji Q, Feigenbaum L, Leighty RM, Hurwitz AA. Melanoma progression despite infiltration by in vivo-primed TRP-2-specific T cells. J Immunother. 2009;32(2):129–139. doi: 10.1097/CJI.0b013e31819144d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72(6):1109–1116. [PubMed] [Google Scholar]
- Thompson MG, Larson M, Vidrine A, Barrios K, Navarro F, Meyers K, … Watkins SK. FOXO3-NF-kappaB RelA Protein Complexes Reduce Proinflammatory Cell Signaling and Function. J Immunol. 2015;195(12):5637–5647. doi: 10.4049/jimmunol.1501758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumurbaatar B, Tikhanovich I, Li Z, Ren J, Ralston R, Kuravi S, … Weinman SA. Hepatitis C and alcohol exacerbate liver injury by suppression of FOXO3. Am J Pathol. 2013;183(6):1803–1814. doi: 10.1016/j.ajpath.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao BG, Liu X, Link H. Antigen-specific T cell functions are suppressed over the estrogendendritic cell-indoleamine 2,3-dioxygenase axis. Steroids. 2004;69(10):653–659. doi: 10.1016/j.steroids.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Yang F, Jin H, Wang J, Sun Q, Yan C, Wei F, Ren X. Adoptive Cellular Therapy (ACT) for Cancer Treatment. Adv Exp Med Biol. 2016;909:169–239. doi: 10.1007/978-94-017-7555-7_4. [DOI] [PubMed] [Google Scholar]
- Yun S, Vincelette ND, Green MR, Wahner Hendrickson AE, Abraham I. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: a systematic review and meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Med. 2016 doi: 10.1002/cam4.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhu Z, Meadows GG, Zhang H. Chronic alcohol consumption inhibits melanoma growth but decreases the survival of mice immunized with tumor cell lysate and boosted with alpha-galactosylceramide. Int Immunopharmacol. 2015;28(1):359–368. doi: 10.1016/j.intimp.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhu Z, Zhang F, Meadows GG. Alcohol consumption and antitumor immunity: dynamic changes from activation to accelerated deterioration of the immune system. Adv Exp Med Biol. 2015;815:313–331. doi: 10.1007/978-3-319-09614-8_18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.