Abstract
Background
Individually, heart failure (HF) and Alzheimer Disease (AD) are severe threats to population health, and their potential coexistence is an alarming prospect. In addition to sharing analogous epidemiological and genetic profiles, biochemical characteristics, and common triggers, we recently recognized common molecular and pathological features between the 2 conditions. Whereas cognitive impairment has been linked to HF through perfusion defects, angiopathy, and inflammation, whether patients with AD present with myocardial dysfunction, and if the 2 conditions bear a common pathogenesis as neglected siblings is unknown.
Objectives
Here we investigated if amyloid beta (Aβ) protein aggregates are present in the hearts of patients with a primary diagnosis of AD, affecting myocardial function.
Methods
We examined myocardial function in a retrospective cross-sectional study from a cohort of AD patients and age-matched controls. Imaging and proteomics approaches were used to identify and quantify Aβ deposits in AD heart and brain specimens compared to controls. Cell shortening and calcium transients were measured on isolated adult cardiomyocytes.
Results
Echocardiographic measurements of myocardial function suggest that patients with AD present with an anticipated diastolic dysfunction. As in the brain, Aβ40 and Aβ42 are present in the heart, and their expression is increased in AD.
Conclusions
Here we provide the first report of the presence of compromised myocardial function and intramyocardial deposits of Aβ in AD patients. Our findings depict a novel biological framework in which AD may be viewed either as a systemic disease or as a metastatic disorder leading to heart, and possibly multiorgan failure. AD and HF are both debilitating and life-threatening conditions, affecting enormous patient populations. Our findings underline a previously dismissed problem of a magnitude that will require new diagnostic approaches and treatments for brain and heart disease, and their combination.
Keywords: Amyloidosis, Cardiomyopathy, Dementia, Heart Failure, Protein Aggregates
Heart failure (HF) and Alzheimer’s disease (AD) are age-dependent diseases that are growing worldwide. HF claims 36% of cardiovascular deaths, with an aging prevalence growth of 4% to 9% from 60 to 80 years of age, and AD is the fifth most common cause of death in patients 65 years of age and older (1). Epidemiological evidence indicates that HF shares risk factors with dementing processes, and clinical studies link cardiovascular diseases and dementia through analogous genetic and biochemical profiles, and common triggers (2–8). Additionally, a number of more recent discoveries suggest a closer common pathogenesis between the 2 conditions. These include the discovery that protein aggregates deposit in the myocardium of patients affected by idiopathic dilated cardiomyopathy (iDCM) (9), and that such deposits are biochemically akin to those found in AD (10). Moreover, genetic variants in the same gene associated with early-onset AD (presenilin = PSEN) were reported in familial (11) and sporadic cases (9) of iDCM. Thus, whether these conditions are causally linked or part of a multiorgan syndrome, their potential coexistence raises an alarming prospect with people living longer.
Even though “cardiogenic dementia” was first postulated nearly 4 decades ago (12) and numerous studies have identified HF as a risk factor for AD (13,14), it is unknown whether AD affects myocardial function and if the 2 maladies share a common pathogenesis. The prevailing belief is that major determinants of the heart-to-head connection are compromised blood flow to the brain, amyloid, or atherosclerotic angiopathy (15–17). The cognitive decline from low brain perfusion has been shown early in pre-symptomatic AD, whereas increasing blood flow to the brain improves AD symptoms (15). Whether the opposite is true, namely compromised heart function in patients affected by AD, in the absence of other underlying cardiovascular disease, is unknown.
A pathological hallmark of AD is the accumulation of amyloid deposits in the form of extracellular plaques (18) (composed of the amyloid precursor protein [APP] proteolytic fragments [Aβ]) (19,20) in the brain parenchyma, causing neuritis and neuronal cell death (21). Abnormal cleavage of APP (22) leads to an amyloidogenic pathway, generating pathological Aβ fragments.
Here, we investigated whether Aβ amyloid accumulates in the hearts of AD patients, affecting organ function. We found that: in vivo myocardial and in vitro cardiomyocyte function are compromised in AD patients; Aβ40 and Aβ42 are both present in the myocardium, and are increased in the hearts of AD patients. These findings, in combination with our previous report of the toxic effect of Aβ pre-amyloid oligomers (PAOs) on cardiomyocytes (9), suggest Aβ amyloid as a novel pathogenesis for myocardial dysfunction.
Methods
Detailed methods are available in the Online Appendix.
Human subjects
A cohort of AD cases and controls was selected from the Beth Israel Deaconess Medical Center clinical database to determine in vivo myocardial function in AD. Fresh heart and brain specimens from a separate cohort of patients with clinical diagnoses of AD and controls were used for in vitro pathological and functional studies.
Tissue samples
Myocardial tissue samples were fixed in 4% paraformaldehyde or 2% glutaraldehyde for imaging. Frozen myocardial and brain samples were used for imaging and molecular tests. Fresh tissue was used to isolate adult left ventricular (LV) cardiomyocytes (23,24).
Imaging
Brain sections were stained for amyloid fibers with Thioflavin S or silver stain, and for PAO by immunohistochemistry using structural antibodies (A11). Transmission electron microscopy (TEM) was used to visualize the fibers in the myocardium. Immunogold TEM with structural antibodies against Aβ42-PAO (VIA antibodies, recognizing the last 3 amino acids Val40-Ile41-Ala42 of Aβ42) (25) were used to identify Aβ in the myocardial deposits.
Molecular tests
Immunoblotting and enzyme-linked-immunosorbent assays (ELISA) were used to identify and quantify Aβ peptides in heart and brain tissue.
Cardiomyocyte function
Adult LV cardiomyocytes were isolated by enzymatic digestion (24,26). Contractility and calcium (Ca2+) transients were measured using a video edge-detection and dual-excitation system (Ion-Optix, Westwood, Massachusetts) (27).
Results
Diastolic function is reduced in AD
Reduced blood flow to the brain from low cardiac output has been linked to cognitive impairment in HF. Here we analyzed if, conversely, myocardial function is abnormal in AD. We studied a cohort of AD patients with the clinical diagnosis or diagnostic work-up of AD, in the absence of other underlying conditions affecting myocardial function (including history of coronary artery disease [CAD], previous myocardial infarction, hypertension, primary or secondary amyloidosis, dilated/hypertrophic cardiomyopathy, endocarditis, chemotherapy, or radiotherapy). Cases and controls were matched by age/sex/ethnicity with a 1:2 ratio (Table 1A, Online Table 1, and Online Figure 1).
Table 1.
Clinical Characteristics of AD Patients and Pathological Specimen Cases
| A. Clinical Characteristics of AD Cohort Cases | ||
|---|---|---|
| Feature | Control (n = 35) | AD (n = 22) |
| Median age, yrs (IQR) | 78 (67–82.5) | 79 (73.5–83.8) |
| Male sex – number (%) | 17 (48.6) | 11 (50) |
| Caucasian ethnicity – number (%) | 35 (100) | 22 (100) |
| Medical history – number (%) | ||
| Diabetes | 2 (6.3) | 1 (4.5) |
| Dyslipidemia | 5 (15.6) | 8 (36.4) |
| Neoplasia | 10 (31.3) | 7 (31.8) |
| High blood pressure readings | 4 (12.5) | 0 of 22 (0) |
| Atrial fibrillation | 7 (21.9) | 3 (13.6) |
| Cerebrovascular accidents | 1 (3.1) | 2 (9.1) |
| Medications – number (%) | ||
| Aspirin | 12 (37.5) | 11 (50) |
| NSAIDs | 0 (0) | 2 (9.1) |
| Oral anticoagulants | 9 (28.1) | 0 (0) |
| Antiaggregant agents | 0 (0) | 2 (9.1) |
| Statins | 8 (25) | 7 (31.8) |
| Antiarrhythmic agents | 5 (15.6) | 0 (0) |
| Digoxin | 4 (12.5) | 0 (0) |
| β-blockers | 13 (40.6) | 1 (4.5) |
| Calcium-channel blockers | 2 (6.3) | 1 (4.5) |
| ACE inhibitors | 2 (6.3) | 1 (4.5) |
| ARBs | 1 (3.1) | 0 (0) |
| Diuretic agents | 6 (18.8) | 0 (0) |
| Levothyroxine | 7 (21.9) | 7 (31.8) |
| Any AchE inhibitors | 0 (0) | 7 (31.8) |
| Donepezil | 0 (0) | 5 (22.7) |
| Rivastigmine | 0 (0) | 1 (4.5) |
| Galantamine | 0 (0) | 1 (4.5) |
| Memantine | 0 (0) | 4 (18.2) |
| SSRI/SNRI | 3 (9.4) | 6 (27.3) |
| Vitamin E | 1 (3.1) | 4 (18.2) |
| B. Clinical Characteristics of AD Pathological Specimen Cases | |||||||
|---|---|---|---|---|---|---|---|
| Age (yrs) | Ethnicity/Sex | Cause of Death | Medical History | Therapy | Time of Harvest | Heart Weight (g) | Gross Pathology* |
| 91 | CM | Cardiac arrest secondary to pneumonia | AD, hypertension, cerebrovascular accident, NIDDM, cataract | N/A | 7 h post-brain death | N/A | N/A |
| 58 | CF | Anoxia, cardiopulmonary arrest | AD, cognitive impairment | Inotropes, PPIs, amiodarone, insulin, NSAIDs, antibiotics | 135 h post- brain death | 304 | LonD 11 cm, LatD 9.4 cm, VD 4 cm, LVAWT 12 mm, LVPWT 12 mm, LVLWT 14 mm, SWT 15 mm, RVWT 5 mm |
| 84 | CF | Acute respiratory failure | Hypertension, TIA, hypercholesterolemia, pneumonia, anemia, osteoporosis, GERD | Memantine, SSRI, ASA, heparin, PPIs, diuretic agents, NSAIDs | 7 h postmortem | N/A | N/A |
| 70 | CF | Intracranial hemorrhage | Diabetes, hypothyroidism | N/A | 28 h post- brain death | 313 | LVAWT 11 mm, LVPWT 9 mm, LVLWT 12 mm, RVWT 5 mm |
Demographic features, clinical presentation, and drug prescriptions recorded at the time of cardiac ultrasound.
Clinical data from AD and age/sex/ethnicity-matched control patients.
ACE = angiotensin-converting enzyme; AchE = acetylcholinesterase; ARB = angiotensin-receptor blocker; ASA = acetylsalicylic acid; CF = Caucasian female; CM = Caucasian male; GERD = gastroesophageal reflux disease; LatD = lateral dimension; LonD = longitudinal dimension; LVAWT = left ventricle anterior wall thickness; LVLWT = left ventricle lateral wall thickness; LVPWT = left ventricle posterior wall thickness; N/A = not available; NIDDM = noninsulin-dependent diabetes mellitus; NSAID = nonsteroidal anti-inflammatory drug; PPI = proton pump inhibitor; RVWT = right ventricle wall thickness; SSRI = selective serotonin reuptake inhibitors; SSRI/SNRI = selective serotonin/serotonin-norepinephrine reuptake inhibitors; SWT = septal wall thickness; TIA = transient ischemic attack; VD = vertical dimension.
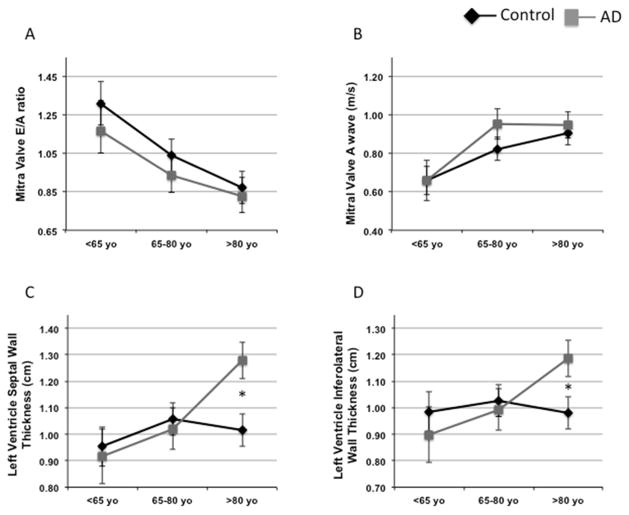
Clinical assessment of myocardial function (Online Table 2) did not show any significant difference between AD cases and controls. Because AD is an age-related disease, we then performed a linear regression analysis and adjusted the associations between AD status and cardiac function for age. We found that age predicts a reduction in diastolic function by mitral valve E/A ratio (MVE/A) (Online Table 3A), as previously shown (28). We then performed exploratory analyses of means and standard errors of the mean by age category and AD status. Cases and controls were divided into 3 age groups (<65, 65 to 80, and >80 years of age). The Student t-test analysis, as well as the estimates obtained by ordinary least squares of our linear regression model (Online Table 3B), showed no significant differences between AD and MVE/A ratio. Notably, diastolic function did not differ in the third tertiles of age in AD versus controls (MVE/A ratio 0.82 ± 0.08 vs. 0.86 ± 0.08). However, a correlation between AD and anticipated decline of myocardial function was present in the first age tertile (1.20 ± 0.18 vs. 1.34 ± 0.12) (Figure 1A). This appeared to be better explained by defects in myocardial compliance because such a pattern was not shown for the atrial component (A) of the MVE/A (Figure 1B). Furthermore, we also used a linear regression F-test (p < 0.05), which showed that the pattern we observed was consistent with poorer diastolic function at earlier ages among the AD participants, conforming with the pathogenesis of AD as a disease of anticipated aging.
Figure 1. Crude Means of Cardiac Ultrasound Parameters for Groups and Age Ranges Show Worse Myocardial Function in the AD Clinical Cohort Compared With Controls.
Crude means of cardiac ultrasound parameters for age tertiles and groups corresponding to the linear regression. The top left panel shows how the value of the MVE/A ratio is lower in younger AD subjects and progressively overlaps with the controls at advanced ages. The mean value of the MVE/A ratio of AD patients in the first tertile appears intermediate between the value of the first and the second tertile of controls. An analogous pattern is shown for AD patients in the second tertile (A). (B) Notably, the atrial (A) component alone showed no differences in all groups, suggesting that the LV compliance is the main contributor to the diastolic dysfunction in AD patients. (C and D) show the increased LV septal and inferior wall thickness, respectively, in the older AD subjects. *p < 0.05. Black diamonds: controls; gray squares: AD cases. Aβ = amyloid beta; AD = Alzheimer’s disease; LV = left ventricle/ventricular; MVE/A = mitral valve E/A ratio.
AD status was also associated with increased LV wall thickness, as shown in other infiltrative diseases, including classical cardiac amyloidosis. However, the difference was significant only in elderly subjects (left ventricle septal wall thickness [LVSWT] 1.12 ± 0.05 vs. 1.01 ± 0.04; p < 0.05 left ventricle infero-laretal wall thickness (LVILT) 1.07 ± 0.05 vs. 1.00 ± 0.03; p < 0.05), but not in the first age tertile, and therefore not a possible cause of the anticipated diastolic defect found in AD subjects younger than 65 years of age (Figures 1C and 1D). Similarly, aortic valve peak velocity was increased only in older AD subjects (2.44 ± 0.41 vs. 1.51 ± 0.14; p < 0.05), suggesting it as a putative cause of wall thickening at later time points, but, again, unlikely to be a determinant of early diastolic impairment. The analysis of individual cases showed that 4 AD patients had high values of peak aortic velocity, indicating a stochastic occurrence of associated reduced aortic valve compliance in a few older cases.
Clinical assessment of the patient population underlined the role of beta-blockade in protection from cognitive dysfunction
Associated diseases were similarly distributed, with an equivalent number of cases with the diagnosis of diabetes, arrhythmia (particularly atrial fibrillation, which often occurs in the aging population), and neoplasms (Online Table 4). Interestingly, only 1 AD case was on beta-blocker therapy. Instead, about one-third of controls were on such treatment. Beta-blocker therapy has been associated with reduced risk of AD (29). Although beta-blockade may improve diastolic function, skewing our results, it does so when it achieves a significant reduction in heart rate, which was unchanged in our control versus AD cases. However we performed ANOVA to account for this variable, and separated the controls in 2 groups on the basis of the presence or absence of beta-blocker therapy. We found that there was no difference in diastolic function, as measured by MVE/A, between the 2 therapeutic regimen groups (Online Table 5).
AD tissue samples
The definitive diagnosis of AD versus other forms of dementia can only be made at pathological and histological examination. Therefore we collected samples of heart and, when possible, brain tissue from 4 cases with a medical history of AD from which the patients were maintained in respiratory support (Table 1B). This stringent criterion is required to avoid postmortem false positive depositions of protein aggregates, but made the procurement of such samples extremely rare. We were therefore limited to the following few, but highly selected and significant cases: 1) a 58-year-old Caucasian woman with a history of cognitive impairment for 10 years and diagnosis of AD for 7 years; 2) a 70-year-old Caucasian woman with a clinical diagnosis of dementia. In this case, the clinical diagnosis of AD was confirmed at pathology by CERAD (Consortium to Establish a Registry for AD) plaque score C and Braak tangle score IV (30,31); 3) an 84-year-old Caucasian woman with clinical diagnosis of AD for 3 years. The heart from this case was removed 7 h and 30 min postmortem (labeled as tissue donor); 4) 91-year-old Caucasian man with clinical diagnosis of AD.
Structural staining identified Aβ in the myocardium
Neuritic plaques are distinctive histological lesions required for the diagnosis of AD. We therefore imaged brain and heart tissue from the 4 pathological specimens of AD compared to controls.
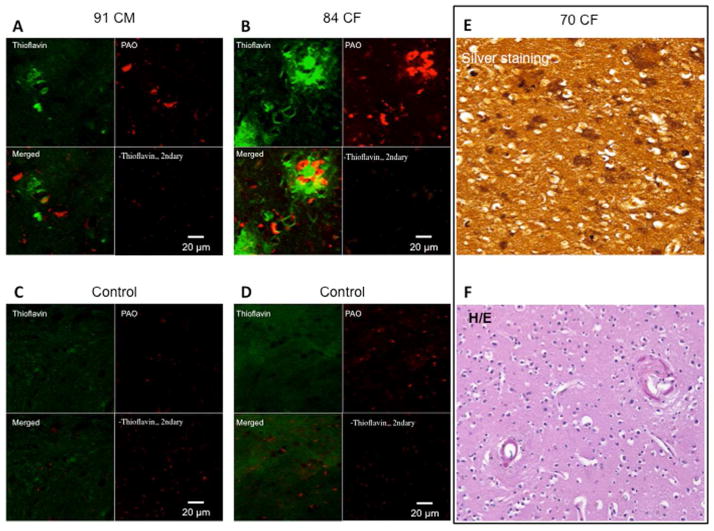
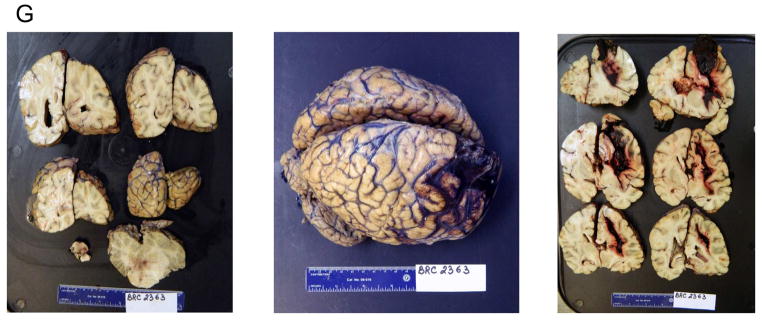
First, we verified the presence of Aβ for the diagnosis of AD in the brain specimens by immunostaining with structural A11 antibodies (32,33) and staining with Thioflavin S (34), which revealed the presence of pre-amyloid and amyloid deposits, respectively (Figures 2A and 2B). The brain of the 70-year-old woman showed plaques by Hirano silver staining (Figures 2E and 2F), and the typical global atrophy at gross pathology (Figure 2G).
Figure 2. Aβ is present in AD Brain Specimens at Histopathology.
Immunohistochemistry images of brain plaques stained with Thioflavin S and structural anti-oligo A11 antibodies in the brain of the (A) 91-year-old man and (B) the 84-year-old woman. The images shows minimal overlap of the A11 anti-oligo antibodies with the fibrillar mature plaques, as described by Kayed et al. (33) (C–D) Control brains. (E) Silver staining of the occipital cortex of the 70-year-old woman, showing amyloid plaques and amyloid angiopathy by Hirano silver staining. (F) Hematoxylin-eosin staining for the brain structure of the 70-year-old woman. Magnification 200×. (G) Gross pathology of the brain of the 70-year-old woman. The middle image shows the entire brain, with the hemorrhagic frontal lobe of the right hemisphere. The left and right images show the brain sections of the posterior and anterior halves of the brain, respectively. The left hemisphere appears hypotrophic, a common feature of AD. Magnification 100×. CF = Caucasian female; CM = Caucasian male; H/E = hematoxylin and eosin; PAO = pre-amyloid oligomer. Other abbreviations as in Figure 1.
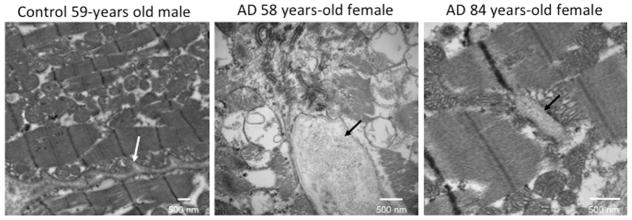
In the heart, small intracellular or extracellular amyloid deposits cannot be clearly visualized by traditional staining due to the high background generated by the birefringent sarcomeres. Therefore, we imaged the hearts by TEM and identified fibrillar deposits in AD myocardial specimens (Figure 3, Online Figure 2). However, the fibrillar-rich content of interstitial tissue in the myocardium also hinders the recognition of pre-amyloid and amyloid deposits. Therefore, to identify the pathological inclusions within the normal extracellular fibrillar matrix, we used immunogold TEM on the specimens from the oldest and the youngest cases. We used anti-Aβ42 antibodies and/or Aβ42-PAO structural VIA antibodies. Positive VIA immunogold staining was detected within the muscle fibers and the interstitial tissue (Figure 4). These data indicate that inclusions of pathological Aβ are present in the myocardium of AD patients. In contrast, the signal for nonoligomerized Aβ42 was below the threshold of the signal noise; therefore, we cannot infer an evaluation of the presence of nonoligomerized form of the peptide using this method.
Figure 3. Transmission Electron Microscopy Identified Myocardial Aggregates.
Representative transmission electron micrographs of myocardium from the 58- and 84-year-old female donors, showing interstitial aggregates (black arrows). The white arrow indicates the interstitial tissue in the normal heart to distinguish it from the aggregates indicated by the black arrows in the diseased hearts. Images of the full set of samples are in Online Figure 2. AD = Alzheimer’s disease.
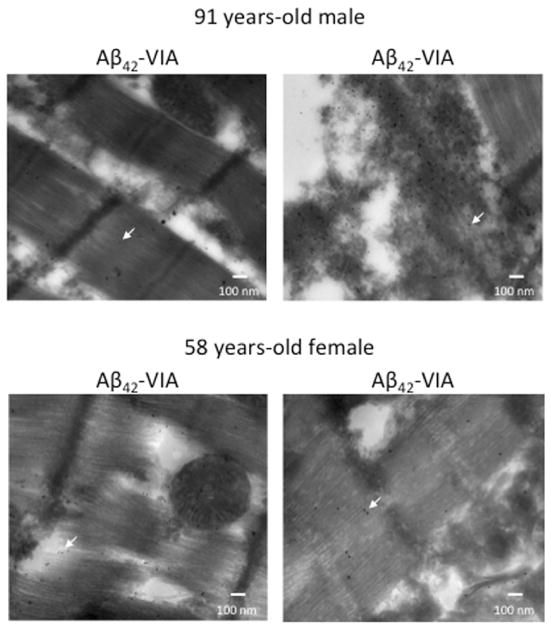
Figure 4. Aβ-PAO Can Be Detected in the Myocytes and Interstitial tissue.
Gold-immunolabeled electron micrographs of the myocardium from the oldest and youngest AD donors. Anti-Aβ42 antibodies were conjugated with 10-nm gold beads and structural antibodies against Aβ42-PAO (VIA) were conjugated with 15-nm gold beads. The arrows indicate representative gold-labeled beads. The images show the presence of positive staining for the Aβ42-PAO detected by the VIA antibodies, but minimal to no staining for the nonoligomerized Aβ42 inside and outside the myocytes. Abbreviations as in Figures 1 and 2.
Molecular tests identified both Aβ40 and Aβ42 in the myocardium
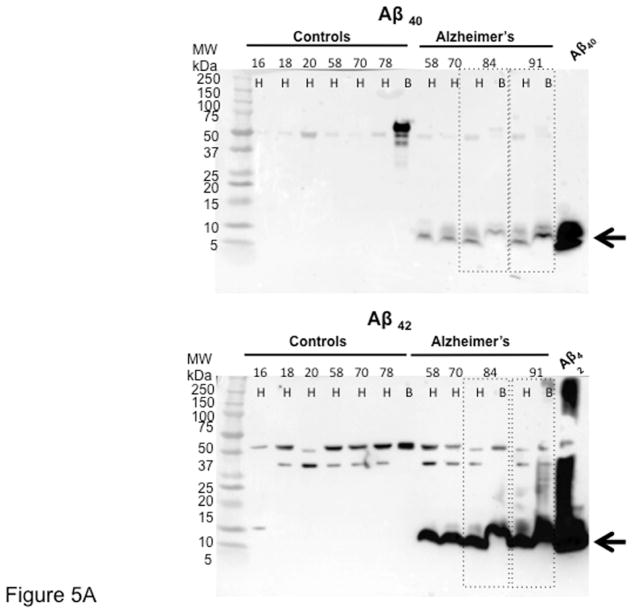
The presence of both Aβ peptides (Aβ40 and Aβ42) was confirmed and quantified by immunoblotting and ELISA. Given the difference of only 2 amino acids between Aβ40 and Aβ42, we first determined the antibody specificity by dot blot. We confirmed that each antibody uniquely recognizes the corresponding peptide (Online Figure 3). By immunoblotting, we then found that both Aβ peptides (MW ≅5 to 10 kDa) are present in the heart and brain of AD patients (Figure 5A and Online Figure 4).
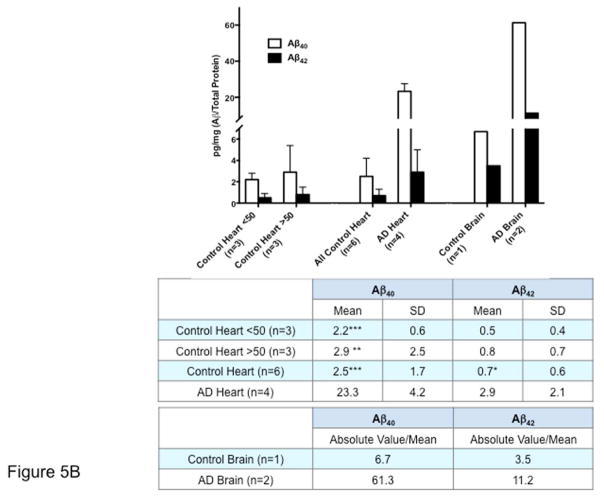
Figure 5. Aβ is Increased in AD Hearts.
(A) Immunoblotting of AD and young and age/sex/ethnicity-matched control hearts and brains. Samples are ordered by age, from younger to older. The blot demonstrated the presence of both Aβ40 (upper panel) and Aβ42 (lower panel) in the hearts and brains of AD patients. Aβ fragments of multiple molecular weights (≅30, 40, 50 kDa) could be detected both in the heart and the brain. Of note, both Aβ40 and Aβ42 high molecular weight bands were also present in old cases without diagnosis of AD, and also in young patients, although at lower levels, as previously described in the brain. (B) Quantification of the levels of Aβ40 and Aβ42 in AD and donor samples by ELISA, showing increased levels of both fragments in the hearts and brains of patients affected by AD. Controls were divided by age ≥ or ≤50 years. Student t-test: *p < 0.05, **p < 0.01 ***p < 0.005 compared with AD. A 1-way analysis of variance (ANOVA) was also performed in all groups, followed by Bonferroni post hoc analysis. ANOVA was significant for Aβ40 (p < 0.0001) and Aβ42 (p < 0.05). Post-test for Aβ40 was significant (p < 0.001) by age group comparisons, whereas p = 0.074 for all groups for Aβ42. The Levene test was performed for equal variances. A larger sample size may be necessary to establish the significance of the differences. The axis of ordinates is separated to better visualize the control values. Values indicate pg/mg Aβ/total protein. Abbreviations as in Figure 1.
ELISA confirmed that Aβ40 and Aβ42 are expressed in the heart, although at lower levels than the brain. In both organs, Aβ40 and Aβ42 levels were increased in the tissue from AD patients compared with controls (Figure 5B). These data further indicate that, similar to the brain, Aβ is present in the myocardium and is increased in AD.
Cardiomyocytes display relaxation defects
To test whether the relaxation defect identified clinically is solely due to interstitial deposits and extracellular matrix stiffness, or if cardiomyocyte function is also abnormal in AD, we measured contractility and Ca2+ transients in LV cardiomyocytes isolated from the 58-year-old AD heart, compared to cardiomyocytes isolated from 68- and 56-year-old female control hearts (Online Figure 5). AD cardiomyocytes displayed slower velocities of relaxation (R50 [time to 50% relaxation]: 0.272 vs. 0.238) and prolonged Ca2+ transients (time-to-peak [TTP]: 0.544 vs. 0.151; R90: 0.328 vs. 0.084) compared with controls, indicating a defect in Ca2+ homeostasis (23,26,27,35). No statistical calculations were performed because we only measured 3 cardiomyocytes from 1 AD case. Although we cannot infer a role for circulating Aβ versus a primary defect in AD cardiomyocytes, it is possible that cardiomyocytes and extracellular infiltrates may combine to determine the overall functional defect in AD hearts.
Discussion
Dilated cardiomyopathy (DCM) and HF have been recently added to the growing list of age-related proteinopathies (9,36), of which AD is one of the founders. DCM shares several risk factors with dementing disorders, including AD, and clinical evidence links DCM and AD through analogous epidemiological, biochemical, and genetic profiles (2,4). Here, we provide novel evidence that the heart and brain can be affected within the same clinical picture. We found that diastolic dysfunction is an early defect in patients affected by AD, and that intramyocardial deposits of Aβ are present in the myocardium of these patients. Because Aβ-PAOs affect cell function in the heart as in the brain (9,37), it is possible that they may contribute to the observed myocardial dysfunction.
Head-to-heart connection in AD: a multifaceted mechanism
HF has been found to be a risk factor for both vascular dementia and AD (13,38), and cognitive impairment has been found in patients with HF and correlates with its severity (13,14). This relationship has been attributed to reduced blood flow to the brain and diffuse amyloid angiopathy (13,15,39,40). The latter is also regarded as the reverse link between myocardial functional decline in AD and aging (38,41). Furthermore circulating Aβ40 was shown to worsen vascular atherosclerosis, and to predict disease progression and cardiovascular mortality in patients with established CAD (42,43). Here, we tested whether other mechanisms link cardiac dysfunction to the brain disorder in the absence of any known causes of compromised cardiovascular function. This possibility was suggested by our recent discoveries of: 1) protein aggregates in iDCM (9); 2) common genetic mutations in AD and iDCM (9); and 3) common components of the AD and iDCM aggregates (10). Thus, low perfusion and amyloid angiopathy may not be the sole contributors to the heart-to-brain connection, and the possibility that patients with AD would present with myocardial dysfunction was a reasonable hypothesis. Although the small number of cases for a clinical assessment limits the statistical significance of our findings, our data suggest that AD patients may also present with compromised diastolic cardiac function, raising the awareness of a growing clinical hazard.
Anticipated diastolic dysfunction occurs in patients with clinical diagnosis of AD
Reduced diastolic function is a normal event in myocardial aging. Although AD has a close correlation with age, and diastolic function in AD progressively worsen with age, we found that AD patients displayed worse values at younger ages compared with controls, asymptotically approaching the values of old-age controls, and supporting the definition of the AD phenotype as anticipated aging. Larger datasets are, however, necessary to unequivocally establish a direct link between AD and myocardial dysfunction. Although the pathogenesis of diastolic dysfunction is complex, it is a common feature associated with myocardial hypertrophy. In our AD cohort, a significant thickening of the left ventricular walls was observed only in the oldest individuals, and therefore was unlikely to be the cause of the observed diastolic dysfunction in the younger cases. This would most likely be due to the occurrence of aortic stenosis in elderly AD cases. Thus, neither hypertrophy nor aortic valve stenosis can be the underlying cause of the diastolic dysfunction observed in the younger AD cohort. Other mechanisms linked to the pathogenesis of AD may account for the observed changes, including the accumulation of Aβ in the myocardium.
Aβ inclusions are present in myocardial tissue in AD
Deterioration of cognitive functions characterizes a number of dementing processes, including “senile dementia.” AD instead defines a distinct condition of “presenile” dementia for which the definitive diagnosis can only be made at pathology. In our clinical cohort, in 1 case who was deceased, the diagnosis was also confirmed by gross pathology and histological analysis. Three cases presented with positive family history for AD. Although these data strengthen the confidence in the diagnosis of our cohort of AD patients, we further tested whether, in fact, AD pathology extends outside the brain by determining the presence of Aβ in cardiac muscle tissue samples harvested from a separate cohort of deceased AD patients.
Aβ fragments generated from abnormal cleavage of APP (Online Figure 6) (22) are among the main constituents of senile plaque and cerebrovascular deposits in AD. Aβ aggregates typically deposit not only in the brain, but also in the submeningeal vasculature in patients affected by AD, causing damage to the vascular wall (44). Such deposits have been attributed to either basolateral-to-apical transport of soluble Aβ or disruption of the blood-brain barrier and Aβ leakage into the bloodstream, as shown in human and mouse models of AD (45,46). Flowing in the bloodstream, Aβ may deposit in distal organs and vessels, causing other forms of non-neurological amyloid pathology, as has been reported in the skeletal muscle, skin, kidney, lung, and intestine of AD patients (47), but never in myocardial tissue.
Indeed, deposition of amyloid, secondary to the pathology of other organs, is a common mechanism causing cardiac amyloidosis (e.g., caused by accumulation of light chain or transthyretin) (48). Furthermore, organ damage due to circulating amyloid-prone proteins has also been described in diabetes, where amylin, secreted together with insulin, causes amyloid deposits not only in the pancreas, but also at distance, in the heart and brain, causing AD and DCM (49,50). Thus, the deposition of amyloid fibers in the heart from circulating Aβ peptides, leading to cardiac dysfunction in AD patients, is a likely event. However, a significant amount of Aβ was identified in the peripheral circulation and platelets, worsening CAD. Thus, it is possible that accumulation of Aβ in the heart compromises myocardial function in patients with AD, identifying a more profound pathogenic root for the brain-to-heart connection, in addition to the effect of Aβ on vascular atherosclerosis or to other mechanisms linked to amyloidosis, including neurohormonal activation and inflammation. This possibility is supported by our previous data indicating a similar mechanism of toxicity of PAOs on cardiomyocytes (9), as demonstrated in neurons (37). Additionally, amyloid proteins, including Aβ, have been shown to directly activate specific proinflammatory and necrotic responses (inflammasome and necrosome). Thus, Aβ may also affect myocardial function through the activation of the newly discovered functional amyloid signaling (51).
Myocardial dysfunction in AD: a possible metastatic or systemic process
Various pathogenic mechanisms can be invoked to explain our findings: either Aβ fragments may deposit in the heart as in other distal tissues, recreating the amyloid pathology as a metastatic disease; or a common genetic, biochemical, and molecular profile and common triggers affect both organs as a systemic disease; or both (Central Illustration). Although the genotype was not available to exclude the presence of known AD mutations, our cohort of AD cases was predominantly sporadic, suggesting the absence of Mendelian genetic mutations, but not excluding predisposing genetic variants or polymorphisms.
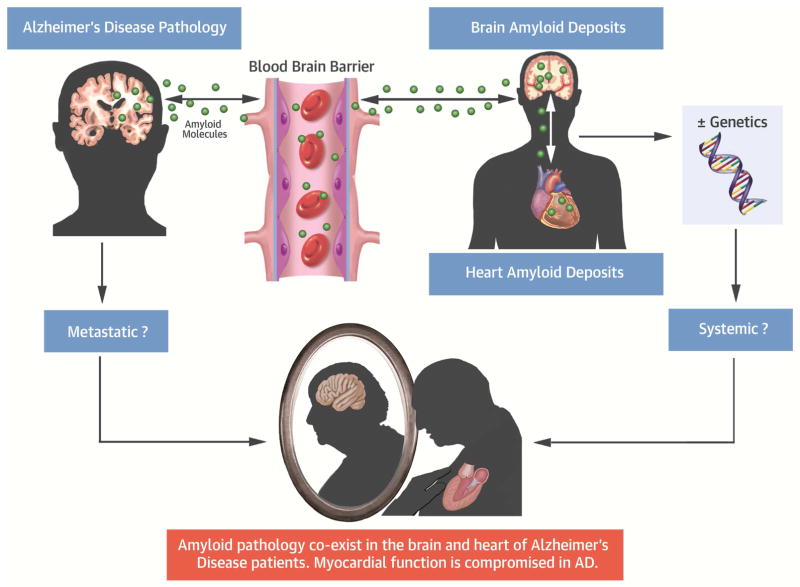
Central Illustration. Myocardial Aβ Amyloid Deposits in Alzheimer Disease.
Aβ aggregates extend outside the central nervous system to the myocardium in AD. Patients with a diagnosis of AD present with clinical signs of a functional defect. Therefore, in the context of increasing life expectancy, these findings raise an alarming threat for public health, as they suggest that the 2 maladies may coexist, either sharing a common etiology or as phenotypes of a multiorgan syndrome. Aβ = amyloid beta; AD = Alzheimer’s disease; BBB = blood-brain barrier.
Limitations
We acknowledge the following limitations of this study.
The number of samples available for the clinical, pathological, and molecular biology assessment is limited. However, obtaining heart specimens from patients under respiratory support is extremely difficult, and we were fortunate to be able to obtain such specimens. Furthermore, case reports are important to raise the awareness of a potential clinical problem. The results from epidemiological cohorts generally have wide confidence intervals. Therefore, we cannot infer temporality on the basis of the cross-sectional nature of our validation cohort. However, the results of our analysis support the possibility of differences in cardiac outcomes by AD status. We also acknowledge the measurements of cardiomyocyte function were obtained only from 3 myocytes of the youngest case. Isolating adult human cardiomyocytes is challenging, and even more so from hearts with amyloid inclusions, limiting the technical success of the procedure.
The diagnosis of AD is anatomopathological. It is obtained from the following brain regions: the middle frontal gyrus (Brodmann area [BA]8/9); superior temporal gyrus (BA22/21); inferior parietal lobule (BA39/40); occipital cortex, including 1 visual (BA17/18); 1 motor/sensory cortex (BA4/1/2/3); and the hippocampus/entorhinal cortex. In our study, the brain samples were obtained from the cortex. However, given the mentioned difficulties in obtaining such highly-selected heart and brain samples, and because, at the late symptomatic stage, Aβ pathology spreads outside the hippocampus and entorhinal cortex, the cortical regions we studied may be representative for the pathological diagnosis of AD. Furthermore, the diagnosis of 71-year-old case of AD followed the Aging-Alzheimer’s Association guidelines, and the heart of this case featured the same myocardial pathological changes as the other cases.
A detailed clinical assessment of cardiac function for the cases from which the specimens were obtained was not always available. Instrumental evaluation (e.g., echocardiography for myocardial function) is not performed in patients found unsuitable for organ explant, such as those with AD, due to age. Other causes of cardiac dysfunction were excluded in the cases we collected. Larger studies are, however, mandatory to assess the presence and type of cardiomyopathy in patients with AD, as well as to determine the impact and mechanisms.
We acknowledge that the E/E’ ratio is a more accurate index of diastolic function by Doppler echocardiography. However, the clinical data were obtained retrospectively, and this measurement is not part of the routine clinical diagnostic assessment. Future prospective research studies directed to assess diastolic function will be required. Those may include cardiac magnetic resonance imaging.
Conclusions
Overall, our data underscore the importance of recognizing the pathological involvement of the heart in AD, in addition to the known involvement of other organs (18,24–26). The clinical burden of AD has notably increased over the last decade, due to the aging of the population and the development of more effective therapies, prolonging lives. The increase in prevalence and improved survival of AD patients would lead to an expansion of the already high prevalence of cardiovascular disease, including CAD and now HF. As the heart is exposed to the free circulatory flow of aggregate-prone peptides, and no barrier segregates the organ, the involvement of this “nonimmunoprivileged” organ may carry a worse prognosis, as is the case with cardiac AL amyloidosis (52). If AD and HF alone are severe health threats, their combination will represent a clinical challenge requiring an interdisciplinary approach to patient management and treatment.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
The relationship between AD and HF is mediated not only by abnormalities of tissue perfusion, neurohormonal activation, and inflammation, but also by factors that directly affect the functional properties of the myocardium.
TRANSLATIONAL OUTLOOK
Further studies are needed to define the dysfunctional cardiac phenotype observed in patients with AD, characterize proteomics and biomarkers common to the cardiomyopathies, and brain pathology that accompany aging, and explore the impact of therapeutic approaches to each disease on outcomes related to the other.
Abbreviations and Acronyms
- AD
Alzheimer’s disease
- CAD
coronary artery disease
- CF
Caucasian female
- CM
Caucasian male
- ELISA
enzyme-linked immunosorbent assay
- HF
heart failure
- iDCM
idiopathic dilated cardiomyopathy
- LV
left ventricle
- MVE/A
mitral valve E/A ratio
- PAO
pre-amyloid oligomer
Footnotes
Disclosures: This work was supported by grants National Institutes of Health, NHLBI R01HL098468 and the AHA 14IRG18980028 to Dr. del Monte, and by an Italian Society of Cardiology fellowship to Dr. Luciani. Dr. Luciani’s fellowship from the Italian Society of Cardiology is with the contribution of Merck. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Institute on Aging. [Accessed September 20, 2016];Alzheimer’s Disease Progress Report 2014–2015: Advancing Research Toward a Cure. 2015 Available at: https://www.nia.nih.gov/alzheimers/publication/2014-2015-alzheimers-disease-progress-report/introduction.
- 2.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreira PI, Nunomura A, Honda K, et al. The Key Role of Oxidative Stress in Alzheimer’s Disease. In: Ali Qureshi G, Hassan Parvez S, editors. Oxidative Stress and Neurodegenerative Disorders. Amsterdam, The Netherlands: Elsevier; 2007. pp. 267–82. [Google Scholar]
- 4.Moreira PI, Smith MA, Zhu X, et al. Oxidative stress and neurodegeneration. Ann N Y Acad Sci. 2005;1043:545–52. doi: 10.1196/annals.1333.062. [DOI] [PubMed] [Google Scholar]
- 5.Tan MS, Yu JT, Tan L. Bridging integrator 1 (BIN1): form, function, and Alzheimer’s disease. Trends Mol Med. 2013;19:594–603. doi: 10.1016/j.molmed.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Nicot AS, Toussaint A, Tosch V, et al. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet. 2007;39:1134–9. doi: 10.1038/ng2086. [DOI] [PubMed] [Google Scholar]
- 7.Cermakova P, Eriksdotter M, Lund LH, et al. Heart failure and Alzheimer’s disease. J Intern Med. 2015;277:406–25. doi: 10.1111/joim.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debette S, Bauters C, Leys D, et al. Prevalence and determinants of cognitive impairment in chronic heart failure patients. Congest Heart Fail. 2007;13:205–8. doi: 10.1111/j.1527-5299.2007.06612.x. [DOI] [PubMed] [Google Scholar]
- 9.Gianni D, Li A, Tesco G, et al. Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation. 2010;121:1216–26. doi: 10.1161/CIRCULATIONAHA.109.879510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian K, Gianni D, Balla C, et al. Cofilin-2 phosphorylation and sequestration in myocardial aggregates: novel pathogenetic mechanisms for idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2015;65:1199–214. doi: 10.1016/j.jacc.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li D, Parks SB, Kushner JD, et al. Mutations of presenilin genes in dilated cardiomyopathy and heart failure. Am J Hum Genet. 2006;79:1030–9. doi: 10.1086/509900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardiogenic dementia. Lancet. 1977;1:27–8. [PubMed] [Google Scholar]
- 13.Román GC. Vascular dementia prevention: a risk factor analysis. Cerebrovasc Dis. 2005;20(Suppl 2):91–100. doi: 10.1159/000089361. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MB, Mather PJ. A review of the association between congestive heart failure and cognitive impairment. Am J Geriatr Cardiol. 2007;16:171–4. doi: 10.1111/j.1076-7460.2007.06563.x. [DOI] [PubMed] [Google Scholar]
- 15.de la Torre JC. Vascular basis of Alzheimer’s pathogenesis. Ann N Y Acad Sci. 2002;977:196–215. doi: 10.1111/j.1749-6632.2002.tb04817.x. [DOI] [PubMed] [Google Scholar]
- 16.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–66. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagust W. Untangling vascular dementia. Lancet. 2001;358:2097–8. doi: 10.1016/S0140-6736(01)07230-0. [DOI] [PubMed] [Google Scholar]
- 18.Divry P. Etude histochimique des plaques séniles. J Belge Neurol Psychiatr. 1927;9:649–57. [Google Scholar]
- 19.Allsop D, Landon M, Kidd M. The isolation and amino acid composition of senile plaque core protein. Brain Res. 1983;259:348–52. doi: 10.1016/0006-8993(83)91273-8. [DOI] [PubMed] [Google Scholar]
- 20.Masters CL, Simms G, Weinman NA, et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–9. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castellani RJ, Rolston RK, Smith MA. Alzheimer disease. Dis Mon. 2010;56:484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–9. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harding SE, MacLeod KT, Jones SM, et al. Contractile responses of myocytes isolated from patients with cardiomyopathy. Eur Heart J. 1991;12(Suppl D):44–8. doi: 10.1093/eurheartj/12.suppl_d.44. [DOI] [PubMed] [Google Scholar]
- 24.del Monte F, Kaumann AJ, Poole-Wilson PA, et al. Coexistence of functioning beta 1- and beta 2-adrenoceptors in single myocytes from human ventricle. Circulation. 1993;88:854–63. doi: 10.1161/01.cir.88.3.854. [DOI] [PubMed] [Google Scholar]
- 25.Bodani RU, Sengupta U, Castillo-Carranza DL, et al. Antibody against small aggregated peptide specifically recognizes toxic Aβ-42 oligomers in Alzheimer’s disease. ACS Chem Neurosci. 2015;6:1981–9. doi: 10.1021/acschemneuro.5b00231. [DOI] [PubMed] [Google Scholar]
- 26.Harding SE, Jones SM, O’Gara P, et al. Isolated ventricular myocytes from failing and non-failing human heart; the relation of age and clinical status of patients to isoproterenol response. J Mol Cell Cardiol. 1992;24:549–64. doi: 10.1016/0022-2828(92)91843-t. [DOI] [PubMed] [Google Scholar]
- 27.del Monte F, Harding SE, Schmidt U, et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–11. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Yasar S, Xia J, Yao W, et al. Ginkgo Evaluation of Memory (GEM) Study Investigators. Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo Evaluation of Memory Study. Neurology. 2013;81:896–903. doi: 10.1212/WNL.0b013e3182a35228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biancalana M, Koide S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta. 2010;1804:1405–12. doi: 10.1016/j.bbapap.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kayed R, Glabe CG. Conformation-dependent anti-amyloid oligomer antibodies. Meth Enzymol. 2006;413:326–44. doi: 10.1016/S0076-6879(06)13017-7. [DOI] [PubMed] [Google Scholar]
- 34.Puchtler H, Sweat Waldrop F, Meloan SN. Application of thiazole dyes to amyloid under conditions of direct cotton dyeing: correlation of histochemical and chemical data. Histochemistry. 1983;77:431–45. doi: 10.1007/BF00495799. [DOI] [PubMed] [Google Scholar]
- 35.Gwathmey JK, Copelas L, MacKinnon R, et al. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61:70–6. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- 36.Willis MS, Patterson C. Proteotoxicity and cardiac dysfunction--Alzheimer’s disease of the heart? N Engl J Med. 2013;368:455–64. doi: 10.1056/NEJMra1106180. [DOI] [PubMed] [Google Scholar]
- 37.Demuro A, Mina E, Kayed R, et al. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 38.Qiu C, Winblad B, Marengoni A, et al. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med. 2006;166:1003–8. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 39.Luchsinger JA, Reitz C, Honig LS, et al. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–51. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sauvé MJ, Lewis WR, Blankenbiller M, et al. Cognitive impairments in chronic heart failure: a case controlled study. J Card Fail. 2009;15:1–10. doi: 10.1016/j.cardfail.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 41.de Bruijn RF, Portegies ML, Leening MJ, et al. Subclinical cardiac dysfunction increases the risk of stroke and dementia: the Rotterdam Study. Neurology. 2015;84:833–40. doi: 10.1212/WNL.0000000000001289. [DOI] [PubMed] [Google Scholar]
- 42.Janelidze S, Stomrud E, Palmqvist S, et al. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci Rep. 2016;6:26801. doi: 10.1038/srep26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stamatelopoulos K, Sibbing D, Rallidis LS, et al. Amyloid-beta (1–40) and the risk of death from cardiovascular causes in patients with coronary heart disease. J Am Coll Cardiol. 2015;65:904–16. doi: 10.1016/j.jacc.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 44.Cummings BJ, Head E, Afagh AJ, et al. Beta-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol Learn Mem. 1996;66:11–23. doi: 10.1006/nlme.1996.0039. [DOI] [PubMed] [Google Scholar]
- 45.Ujiie M, Dickstein DL, Carlow DA, et al. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–70. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 46.Pitschke M, Prior R, Haupt M, et al. Detection of single amyloid beta-protein aggregates in the cerebrospinal fluid of Alzheimer’s patients by fluorescence correlation spectroscopy. Nat Med. 1998;4:832–4. doi: 10.1038/nm0798-832. [DOI] [PubMed] [Google Scholar]
- 47.Kuo YM, Kokjohn TA, Watson MD, et al. Elevated Aβ42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AβPP metabolism. Am J Pathol. 2000;156:797–805. doi: 10.1016/s0002-9440(10)64947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quarta CC, Kruger JL, Falk RH. Cardiac amyloidosis. Circulation. 2012;126:e178–82. doi: 10.1161/CIRCULATIONAHA.111.069195. [DOI] [PubMed] [Google Scholar]
- 49.Despa S, Margulies KB, Chen L, et al. Hyperamylinemia contributes to cardiac dysfunction in obesity and diabetes: a study in humans and rats. Circ Res. 2012;110:598–608. doi: 10.1161/CIRCRESAHA.111.258285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson K, Barisone GA, Diaz E, et al. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann Neurol. 2013;74:517–26. doi: 10.1002/ana.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parry TL, Melehani JH, Ranek MJ, et al. Functional amyloid signaling via the inflammasome, necrosome, and signalosome: new therapeutic targets in heart failure. Front Cardiovasc Med. 2015;2:25. doi: 10.3389/fcvm.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quarta CC, Solomon SD, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129:1840–9. doi: 10.1161/CIRCULATIONAHA.113.006242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.