Abstract
Transplantation of allogeneic organs and tissues represents a lifesaving procedure for a variety of patients affected with end-stage diseases. Although current immunosuppressive therapy prevents early acute rejection, it is associated with nephrotoxicity and increased risks for infection and neoplasia. This stresses the need for selective immune-based therapies relying on manipulation of lymphocyte recognition of donor antigens. The passenger leukocyte theory states that allograft rejection is initiated by recipient T cells recognizing donor major histocompatibility complex (MHC) molecules displayed on graft leukocytes migrating to the host’s lymphoid organs. We revisited this concept in mice transplanted with allogeneic skin, heart, or islet grafts using imaging flow cytometry. We observed no donor cells in the lymph nodes and spleen of skin-grafted mice, but we found high numbers of recipient cells displaying allogeneic MHC molecules (cross-dressed) acquired from donor microvesicles (exosomes). After heart or islet transplantation, we observed few donor leukocytes (100 per million) but large numbers of recipient cells cross-dressed with donor MHC (>90,000 per million). Last, we showed that purified allogeneic exosomes induced proinflammatory alloimmune responses by T cells in vitro and in vivo. Collectively, these results suggest that recipient antigen-presenting cells cross-dressed with donor MHC rather than passenger leukocytes trigger T cell responses after allotransplantation.
INTRODUCTION
The inflammatory immune response leading to allograft rejection is initiated through the activation of recipient T cells recognizing donor antigens in the host’s secondary lymphoid organs. T cell allorecognition is known to occur via two mechanisms: the “direct” and “indirect” pathways. Direct allorecognition involves the interaction of T cells with intact allogeneic major histocompatibility complex (MHC) molecules displayed on donor bone marrow–derived cells called “passenger leukocytes,” which are thought to leave the graft shortly after its placement (1). The direct alloresponse is polyclonal in that it involves up to 10% of the entire T cell repertoire (2). Alternatively, indirect allorecognition involves T cell recognition of peptides derived from donor MHC and minor histocompatibility antigens, which are processed and presented on host MHC by recipient antigen-presenting cells (APCs) (3). Differing from direct allorecognition, the indirect alloresponse is oligoclonal because it results from the activation of a limited set of T cell clones recognizing a few dominant alloantigen determinants (4, 5). Either direct or indirect alloresponse can trigger acute rejection of skin allografts (6). However, we have reported that direct but not indirect alloreactivity elicits acute rejection of vascularized solid transplants (7). On the other hand, indirect alloresponse is believed to mediate chronic organ allograft rejection by promoting alloantibody production, a phenomenon characterized by progressive graft tissue fibrosis and blood vessel occlusion (8, 9).
The passenger leukocyte theory was originally proposed by G. D. Snell in 1957, and the term was coined by Elkins and Guttman in 1968 (10, 11). This concept was substantiated by observations in rodents transplanted with allografts whose leukocytes had been depleted or replaced (10, 12–16). At the same time, studies of Barker and Billingham (17, 18) of skin allografts placed on vascularized but alymphatic skin pedicles demonstrated the role of afferent lymphatics in the rejection process. Together, these studies suggested that, right after transplantation, donor leukocytes [mainly dendritic cells (DCs)] leave skin allografts through lymphatic vessels and activate naïve T cells in regional lymph nodes (LNs) just as they do after a skin infection. However, to our knowledge, the presence of these passenger leukocytes in the LNs of skin-grafted mice was never formally demonstrated.
Subsequent studies emphasized the role of graft leukocytes in the alloresponse to vascularized solid organ transplants. First, kidney allografts from donors reconstituted with a bone marrow matched to the recipient showed prolonged survival (19). Likewise, renal allografts initially placed in a recipient for a few days (parked) and then retransplanted in a second host matched to the first one enjoyed long-term survival (20). This was attributed to the replacement of donor by host leukocytes. In support of this view, adoptive transfer of the second host with donor-type DCs restored rapid rejection of these renal transplants (21). Similar observations were made with cardiac allografts (22, 23). In this model, donor passenger leukocytes were identified and found in the recipient’s spleen rather than in LNs, suggesting that they had migrated through blood rather than through lymphatic vessels (24). This difference was attributed to the fact that, unlike skin grafts, heart transplants were vascularized at the time of their placement.
In skin-grafted mice, lymphatic vessels are severed during surgery and fully reconnected only 5 to 7 days after transplantation (25, 26). This infers that donor leukocytes cannot traffic to host LNs and activate T cells right after transplantation. At the same time, allospecific T cell responses can be detected in recipient LNs as early as 2 days after transplantation (27). This suggested that donor MHC presentation and subsequent T cell activation might not be initiated by LN-infiltrating donor passenger leukocytes but through a different mechanism. To test this, we revisited the passenger leukocyte theory in skin-grafted mice using imaging flow cytometry, a highly sensitive imaging technology combining flow cytometry and microscopic visualization of individual cells. After placement of fully allogeneic B6 skin grafts onto BALB/c mice, we found no donor leukocytes in host lymphoid organs examined from day 1 to day 15 after transplantation. In contrast, as early as 12 hours after skin grafting, high numbers of recipient cells coated with microvesicles exhibiting donor MHC molecules were observed in recipient LNs. By day 7, most of these recipient cells expressed donor MHC molecules on their membrane alongside with their own MHC, a phenomenon we hereafter refer to as allo-MHC cross-dressing. The importance of allo-MHC cross-dressing in allorecognition was further studied in vascularized organ transplant models. Contrary to nonvascularized skin grafts, a few donor cells (100 to 200 cells per million) were detected in recipient lymphoid organs of heart-transplanted mice. Yet, much higher numbers of recipient cells harboring donor MHC (>90,000 cells per million) were found in the host spleen. Last, we showed that purified donor microvesicles (exosomes) and recipient cells cross-dressed with donor MHC could activate alloreactive T cells both in vivo and in vitro. Thus, early T cell activation by recipient APCs cross-dressed with donor MHC antigens rather than donor passenger leukocytes is likely to initiate acute allograft rejection.
RESULTS
Activation of allospecific T cells occurs as early as 2 days after transplantation in skin-grafted mice
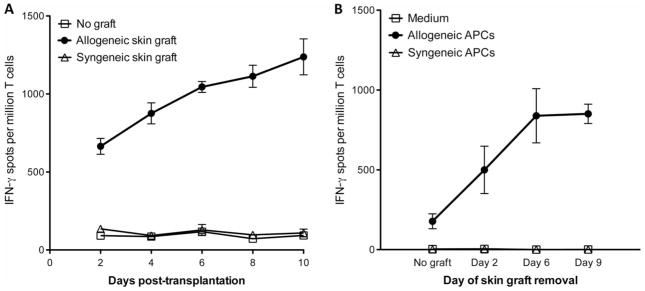
Seminal studies by McKhann and Berrian (27) showed that mice become sensitized as early as 2 days after placement of skin allografts. This prompted us to evaluate the actual frequency of proinflammatory T cells activated at different time points after skin transplantation. LN T cells of C57Bl/6 (B6, H-2b) mice recipient of a BALB/c (H-2d) fully allogeneic skin graft (mean survival time, 9 ± 2 days) were collected at different time points after transplantation. Next, these cells were tested by enzyme-linked immunospot (ELISPOT) for their proinflammatory interferon-γ (IFN-γ) cytokine secretion when exposed to allogeneic stimulator cells (direct allorecognition). As shown in Fig. 1A, substantial numbers of IFN-γ–secreting T cells were detected as early as day 2 after skin grafting. To confirm this observation, we conducted another set of experiments in which we removed skin grafts at different time points after their placement and we measured the T cell response at day 10 after transplantation (peak of the T cell response). The presence of skin grafts for 2 days only was sufficient to trigger the activation of allospecific T cells (Fig. 1B). Therefore, in skin-grafted mice, T cell alloresponses are induced in recipient LNs much before reconnection of lymphatic vessels, which takes 7 days (25, 26).
Fig. 1. Kinetics of T cell alloresponses after transplantation of allogeneic skin grafts.
(A) C57Bl/6 (B6, H-2b) mice received a skin graft from a fully allogeneic BALB/c (H-2d) or a syngeneic B6 mouse. At different time points after transplantation, recipient LN T cells were isolated and cultured in vitro for 48 hours with donor irradiated spleen cells. (B) B6 mice were transplanted with a skin patch from a BALB/c mouse. Skin allografts were removed at different time points after transplantation. Recipient LN T cells were isolated at day 10 after transplantation and cultured for 48 hours with medium or with either allogeneic (BALB/c) or syngeneic (B6) spleen cells (APCs). In (A) and (B), the frequencies of IFN-γ–producing cells were measured by ELISPOT. The results are expressed as numbers of IFN-γ spots per million T cells ± SD (triplicate wells) isolated from mouse LNs collected and pooled from three to five mice. The results are representative of three separate experiments. The P values obtained with analysis of variance (ANOVA) comparing T cell responses after syngeneic versus allogeneic graft (A) or APCs (B) were P = 0.0005 and P = 0.0001.
Donor-derived vesicles but not intact leukocytes carry allogeneic MHC molecules to recipient lymphoid organs after skin transplantation
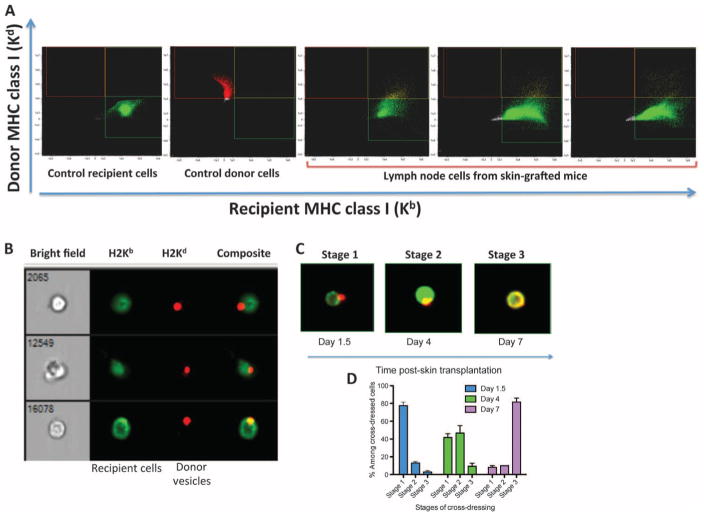
Next, we investigated whether the induction of T cell alloresponse correlated with the presence of donor leukocytes in recipient LNs of skin-grafted mice. To test this, we used imaging flow cytometry, which allows microscopic visualization of each single cell of a population selected through flow cytometry. Scanning of recipient LNs, spleen, and thymus [from day 0.5 (12 hours) to day 15 after transplantation] was conducted. To distinguish between recipient and donor cells, we incubated the cells with anti-recipient MHC class I Kb antibodies and anti-donor MHC class I Kd bound to fluorescein isothiocyanate (FITC) and allophycocyanin, respectively. Control staining of B6 or BALB/c cells showed no cross-reactivity with these antibodies (Fig. 2A). Experiments using cells mixed at defined ratios showed reliable detection of one donor cell out of 500,000 recipient cells (fig. S1). No donor cells were detected at any given time point after skin grafting (Fig. 2A). In turn, we detected substantial numbers (>1500 cells per million) of recipient cells positive for both self (Kd) and allogeneic MHC class I (Kb) MHC antigens (Fig. 2A). Microscopic examination of these double-positive cells (Kd+Kb+) at 12 and 36 hours after transplantation revealed that they were recipient LN cells coated with donor MHC class I+ vesicles (Fig. 2B). Most double-positive cells were initially found in graft-draining LNs, suggesting that donor vesicles had trafficked via lymphatics (Table 1). Nevertheless, some recipient cells coated with donor vesicles were also detected in the spleen, in nondraining LNs, and even in the recipient’s thymus (Table 1). Progressively, most double-positive cells (>80% by day 7 after transplantation) exhibited allogeneic MHC class I on their membrane, a phenomenon known as cross-dressing (Fig. 2, C and D).
Fig. 2. Detection of donor leukocytes in LNs of skin-grafted mice.
LN cells (ipsilateral axillary and brachial LNs) from naïve B6 and BALB/c mice as well as B6 mice recipient of a BALB/c skin allograft were collected at different time points after transplantation. The cells were stained with anti-MHC class I Kb antibodies and anti-MHC class I Kd bound to FITC and allophycocyanin, respectively. The presence of recipient (Kb+) and donor (Kd+) cells was assessed using flow imaging with low-speed fluidics at ×40 magnification. Data analysis was performed using IDEAS 6.1 image processing and statistical analysis software (Amnis, EMD Millipore). (A) Plots obtained with control recipient cells, control donor cells, and cells from three different transplanted mice tested at day 2 after transplantation. The results are representative of eight mice per group tested in two separate experiments. (B) Representative microscopic analysis of double-positive cells (Kb+/Kd+) observed in (A). The bright field represents the actual optical image of the cells (×40 magnification). The other columns show the fluorescence of cells. (C) Representative images of double-positive cells (stages 1 to 3) obtained at different time points after transplantation. Stage 3 corresponds to recipient leukocytes cross-dressed with donor MHC. (D) Percentages of recipient leukocytes cross-dressed with donor MHC (stage 3) among double-positive cells found in LNs of skin-grafted mice examined individually at different time points after transplantation ± SEM. The results are representative of six mice tested individually at each time point. P values using unpaired Student’s t test: stage 1: d1.5 versus d4, P = 0.0001; d1.5 versus d7, P = 0.0001; d4 versus d7, P = 0.0022; stage 2: d1.5 versus d4, P = 0.0001; d1.5 versus d7, P = 0.0051; d4 versus d7, P = 0.0001; stage 3: d1.5 versus d4, P = 0.1701 [not significant (NS)]; d1.5 versus d7, P = 0.0001; d4 versus d7, P = 0.0001.
Table 1. Frequencies of donor leukocytes and recipient cells cross-dressed with donor after skin transplantation.
Draining (ipsilateral) and nondraining (contralateral) LN cells (axillary and brachial LNs) and the spleen and thymus from naïve B6 and BALB/c mice as well as B6 mice recipient of a BALB/c skin allograft were collected at different time points after transplantation. The cells were stained with anti-MHC class I Kb antibodies and anti-MHC class I Kd bound to FITC and allophycocyanin, respectively. The presence of recipient (Kb+) and donor (Kd+) cells was assessed using flow imaging with low-speed fluidics at ×40 magnification. The frequencies are expressed as the number of donor cells or recipient cells cross-dressed with allogeneic MHC molecules per million cells collected for each lymphoid organ. The results are representative of four to six mice tested individually. nd, not determined.
| Days after skin grafting | Draining LNs | Nondraining LNs | Spleen | Thymus |
|---|---|---|---|---|
| Number of donor leukocytes (per million cells) | ||||
| 0.5 | 0 | 0 | 0 | 0 |
| 1.5 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 | 0 |
| Number of cross-dressed cells (per million cells) | ||||
| 0.5 | 377 ± 141 | 270 ± 46 | 150 ± 29 | nd |
| 1.5 | 760 ± 74 | 607 ± 18 | 463 ± 48 | 330 |
| 4 | 1107 ± 207 | 870 ± 40 | 587 ± 70 | 430 |
| 7 | 1653 ± 359 | 710 ± 121 | 433 ± 15 | 200 |
| 15 | 367 ± 367 | 220 ± 82 | 227 ± 64 | nd |
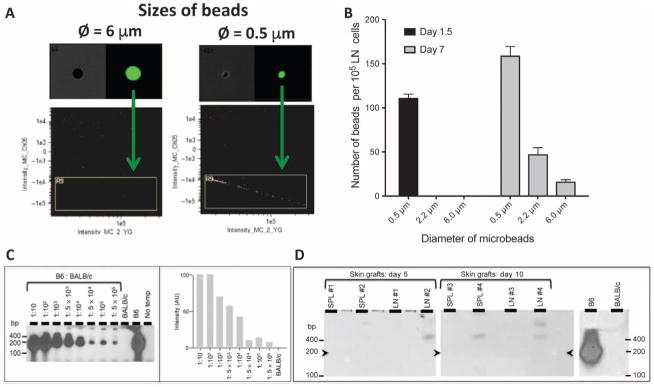
Next, we compared the trafficking to LNs of beads of different sizes (with diameters from 0.5 to 6 μm) injected into the bed of syngeneic skin grafts. At 36 hours after transplantation, only beads of the smallest subcellular size (0.5 μm) were found in the draining LNs (Fig. 3, A and B). In turn, 7 days after skin grafting, at a time when lymphatic connections have been restored (25, 26), recipient LNs also contained some beads with diameters of 2.2 and 6 μm. These results were consistent with our imaging flow cytometry results showing that, within the first few days after skin grafting, donor microvesicles but not intact cells can migrate out skin grafts via lymphatic vessels (Fig. 2).
Fig. 3. Detection of donor mRNA in lymphoid organs of skin-grafted mice and trafficking of microbeads injected in skin grafts.
Fluorescent beads of either subcellular size (diameter, 0.5 and 2.2 μm) or of cellular size (diameter, 6 μm) were injected into the bed site of syngeneic skin grafts placed in B6 mice. The presence of beads in draining LNs (ipsilateral axillary and brachial LNs) was evaluated at different time points after transplantation. (A) Representative plots obtained at 36 hours after transplantation with beads of either 6-μm or 0.5-μm diameter. (B) Distribution of beads of different diameters observed in LNs of skin-grafted mice at 36 hours or day 7 after transplantation. The results are representative of six to seven mice tested individually ± SEM. P values using unpaired Student’s t test: d1.5 size 0.5 μm versus 2.2 μm, P = 0.0001; d1.5 size 0.5 μm versus 6 μm, P = 0.0001; d1.5 size 2.2 μm versus 6 μm, P = 1 (NS); d7 size 0.5 μm versus 2.2 μm, P = 0.0001; d7 size 0.5 μm versus 6 μm, P = 0.0001; d7 size 2.2 μm versus 6 μm, P = 0.0026. (C) Left panel: Combinations of 1 × 105 splenocytes from Kb+ (B6) and Kb− (BALB/c) were prepared according to indicated B6-to-BALB/c ratios. RT-PCR was performed on complementary DNA (cDNA) derived from 250 ng of individual RNA. The amplified products were run on agarose gels and transferred on nitrocellulose filters that were hybridized with a 32P-labeled Kb-specific probe lighting up a 191–base pair (bp)–long fragment (arrowheads). Controls were BALB/c and B6 cDNA alone and no cDNA (no template). Right panel: Quantitative scans of the autoradiograph from the left panel. Signal intensities were corrected according to the amount of template loaded (estimated by RT-PCR amplification of the actin genes; not shown). Signal intensities are represented in arbitrary units (AU). (D) Kb-specific RT-PCR analysis was conducted as in (C) on splenocytes (SPL) and LNs from BALB/c mice, recipient of allogeneic B6 skin grafts. Cells were isolated at days 5 and 10 after transplantation. Positive and negative controls were splenocytes from B6 and BALB/c mice, respectively. The three sections presented were on the same series of gels run under identical conditions.
To further establish the early migration of donor vesicles and not intact cells, we prepared total RNA from the spleen and LNs of BALB/c mice at days 2, 5, and 10 after grafting of a B6 allogeneic skin patch. The presence of recipient Kd and donor Kb gene transcripts was evaluated using reverse transcription polymerase chain reaction (RT-PCR) (as described in Materials and Methods). On the basis of cell mixture assays, the sensitivity of Kb detection was 1 B6 cell among 5 × 105 BALB/c cells (Fig. 3C). No allogeneic Kb transcripts were detected at any given time point after transplantation, a result reinforcing the view that donor passenger leukocytes do not infiltrate secondary lymphoid organs of these skin-grafted mice (Fig. 3D).
In skin-grafted mice, allo-MHC cross-dressed cells are recipient DCs and B cells
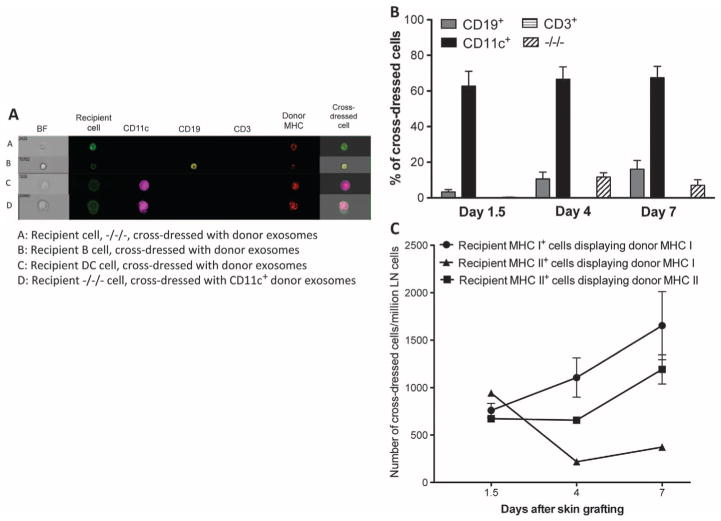
We then investigated the phenotype of allo-MHC cross-dressed cells found in the lymphoid organs of skin-grafted mice using imaging flow cytometry (Fig. 4A). As shown in Fig. 4B, most of these cells (60 to 70%) were CD11c+ DCs, whereas 10 to 15% were CD20+ B lymphocytes and none were CD3+ T cells. About 10% of cross-dressed cells showed no expression of CD3, CD20, or CD11c, awaiting further characterization. Therefore, most recipient cells cross-dressed with donor MHC after skin grafting were bone marrow–derived MHC class II+ leukocytes (DCs and B cells), which are professional APCs.
Fig. 4. Phenotype of recipient cells cross-dressed with donor MHC after skin transplantation.
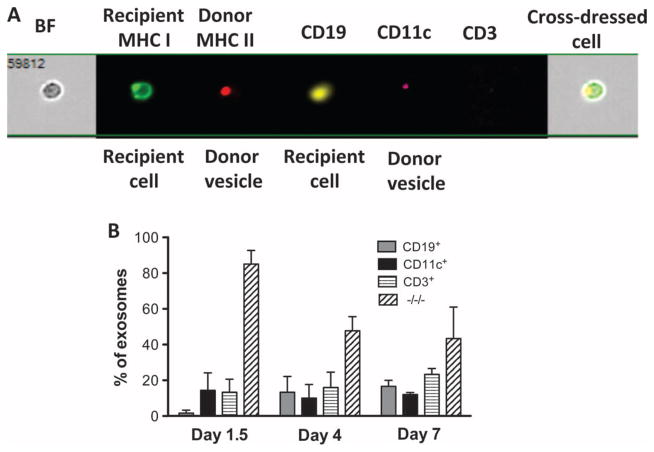
LN cells (ipsilateral axillary and brachial LNs) from naïve B6 and BALB/c mice as well as B6 mice recipient of a BALB/c skin allograft were collected at different time points after transplantation. Recipient cells (H-2Kb+) cross-dressed with donor MHC class I Kd were stained with the following fluorochrome-bound antibodies: anti–CD3–Pacific Blue (for T cells), anti–CD19-PE (phycoerythrin) (for B cells), and anti–CD11c-PECy7 (for DCs). (A) Representative images of double-positive cells (Kb+/Kd+) labeled with these antibodies. The bright field (BF) represents the actual optical image of the cells (×40 magnification). (B) Frequency of each leukocyte subset among cross-dressed cells. (C) Frequencies of recipient MHC class I+ or class II+ cells cross-dressed with either donor MHC class I (Kb) or II (Ab) per million LN cells. The results are representative of five mice per group, tested individually at each time point. P values using ANOVA: d1.5, recipient MHC II + donor MHC I versus recipient MHC II + donor MHC II, P = 0.5546 (NS); d4, recipient MHC II + donor MHC I versus recipient MHC II + donor MHC II, P = 0.0001; d7, recipient MHC II + donor MHC I versus recipient MHC II + donor MHC II, P = 0.0001.
There is preferential expression of donor MHC class II molecules on cross-dressed cells
We measured the frequency of recipient cells cross-dressed with donor MHC class I (Kb) or II (Ab) in the draining LNs at different time points after skin grafting. As shown in Fig. 4C, the number of recipient MHC class I+ cells displaying donor MHC class I almost doubled during the course of rejection (800 to 1500 per million cells). At day 1.5 after transplantation, most of the recipient cells were MHC class II+ cells cross-dressed with donor MHC class I and II, a result consistent with their professional APC nature (Fig. 4B). Progressively, the number of recipient MHC class II+ cells displaying donor MHC class II increased, whereas those expressing donor MHC class I decreased. This result confirms previous studies showing preferential transfer of MHC class II molecules between leukocytes (28). It might reflect the fact that vesicles such as exosomes are enriched in endosomes, which contain MHC class II proteins (29). Yet, it is noteworthy that a few recipient MHC class II− cells (15%) also acquired allogeneic MHC class II expression. The nature of these cells and their ability to activate allospecific T cells remain to be investigated.
Donor vesicles trafficking from skin allografts express CD11c, CD20, and CD3
We examined the expression of different leukocyte markers on donor vesicles present in the draining LNs of skin-grafted mice using imaging flow cytometry (Fig. 5A). About 30 to 50% of vesicles carrying donor MHC molecules also expressed CD3, CD11c, or CD20, a result showing that they derived from T cells, DCs, or B cells (Fig. 5B). The origin of the remaining donor-derived vesicles remains unclear.
Fig. 5. Phenotypic characterization of donor vesicles present in LNs of skin-grafted mice.
LN cells (ipsilateral axillary and brachial LNs) from B6 mice recipient of a BALB/c skin allograft were collected at different time points after transplantation. The donor vesicles were stained with anti-recipient MHC class I (anti-Kb bound to FITC), anti-donor MHC class II (anti-Ad bound to APC), anti–CD19-PE, anti–CD11c-PRCy7, and anti–CD3–Pacific Blue antibodies. (A) Representative images of cells and donor vesicles present on cross-dressed cells labeled with these antibodies. The bright field represents the actual optical image of the cells (×40 magnification). (B) Frequencies of donor vesicles displaying CD19, CD11C, or CD3 or none of these markers measured at different time points after skin grafting. The results are representative of five mice tested individually at each time point. The P values (using ANOVA) were as follows: d1, P = 0.0001; d4, P = 0.0004; and d7, P = 0.031.
Cross-dressed cells and passenger leukocytes can be detected in recipient lymphoid organs after heart and islet transplantation
Another set of experiments evaluated the relevance of donor passenger leukocytes and allo-MHC cross-dressed cells in BALB/c mice transplanted with a B6 heart (mean survival time, 11 ± 3 days). In contrast to skin-grafted mice, low but significant numbers of donor leukocytes (100 to 200 per million cells) were observed in draining and nondraining LNs as well as in the spleen of cardiac-transplanted mice (Table 2). However, the frequency of these cells declined markedly from day 1 to day 7 after transplantation. One day after cardiac transplantation, we also found more than 2000 per million recipient cells cross-dressed with donor MHC in the host spleen (Table 2). This number actually increased to reach more than 90,000 cross-dressed cells per million spleen cells at day 7 after transplantation, whereas only less than 500 cells per million were present in the LNs (Table 2). Almost all cross-dressed cells were B cells, whereas a few cross-dressed DCs and T cells were also found in the spleen and LNs, respectively (fig. S2). After transplantation of pancreatic islets in the B6-BALB/c combination, we detected a few donor passenger leukocytes (10 to 30 cells per million) but 10 times more cross-dressed cells in LNs and in the spleen of recipients examined at day 4 after transplantation (Table 2).
Table 2. Frequencies of donor leukocytes and recipient cells cross-dressed with donor after heart and islet transplantation.
Draining (ipsilateral) and nondraining (contralateral) LN cells (axillary and brachial LNs) and the spleen from naïve B6 and BALB/c mice as well as B6 mice recipient of a BALB/c skin allograft were harvested at days 1, 4, and 7 after heart transplantation and at day 4 after pancreatic islet transplantation. The cells were stained with anti-MHC class I Kb antibodies and anti-MHC class I Kd bound to FITC and allophycocyanin, respectively. The presence of recipient (Kb+) and donor (Kd+) cells was assessed using flow imaging with low-speed fluidics at ×40 magnification. The frequencies are expressed as the number of donor cells or recipient cells cross-dressed with allogeneic MHC molecules per million cells collected for each lymphoid organ. The results are representative of four to six mice tested individually.
| Days after grafting | Heart transplants
|
Pancreatic islet transplants
|
||||
|---|---|---|---|---|---|---|
| Draining LNs | Nondraining LNs | Spleen | Draining LNs | Nondraining LNs | Spleen | |
| Number of donor leukocytes (per million cells) | ||||||
|
| ||||||
| 1 | 190 ± 83 | 120 ± 60 | 84 ± 9 | — | — | — |
|
| ||||||
| 4 | 87 ± 42 | 12 ± 10 | 13 ± 9 | 53 ± 34 | 30 ± 6 | 53 ± 22 |
|
| ||||||
| 7 | 40 ± 17 | 20 ± 6 | 63 ± 43 | — | — | — |
|
| ||||||
| Number of cross-dressed cells (per million cells) | ||||||
|
| ||||||
| 1 | 657 ± 153 | 703 ± 240 | 28,333 ± 4,810 | — | — | — |
|
| ||||||
| 4 | 2,566 ± 1,103 | 662 ± 275 | 44,000 ± 3,050 | 413 ± 195 | 350 ± 29 | 416 ± 23 |
|
| ||||||
| 7 | 2,030 ± 450 | 521 ± 161 | 92,330 ± 15,930 | — | — | — |
Allospecific T cells are activated by cross-dressed cells in vitro and in vivo
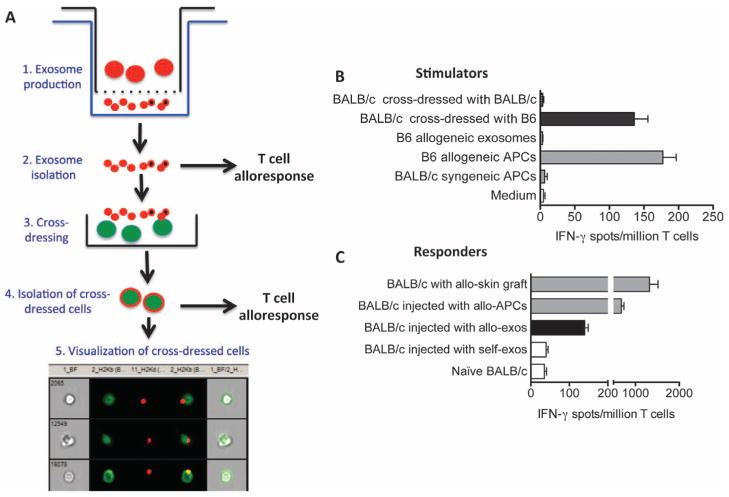
We investigated whether allogeneic exosomes could induce a direct T cell alloresponse in vitro or in vivo either on their own or after cross-dressing of APCs. Exosomes were generated in vitro from activated B6 spleen cells and isolated and counted, as described in Materials and Methods. To prepare cross-dressed cells, we cultured B6 exosomes (5 × 108) with 108 BALB/c splenocytes for 4 days. Cross-dressed cells were then isolated on an affinity column composed of beads coated with anti-MHC class I Kb-PE antibodies. The number and purity of cross-dressed cells were ascertained using imaging flow cytometry, as shown in Fig. 6A. In a first set of experiments, BALB/c T cells (5 × 105 cells per well) were cultured with B6 allogeneic exosomes or BALB/c cells cross-dressed with either allogeneic (H-2b) or control syngeneic (H-2d) MHC (5 × 105 cells per well) for 48 hours. The frequencies of activated T cells producing IFN-γ were measured using an ELISPOT assay. Only cells cross-dressed with allogeneic MHC (H-2b) induced a response, which was similar to that observed after stimulation with allogeneic B6 APCs (Fig. 6B). Conversely, B6 allogeneic exosomes alone and BALB/c cells cross-dressed with syngeneic exosomes failed to activate BALB/c T cells in vitro (Fig. 6B).
Fig. 6. Activation of T cells by allogeneic exosomes and cells cross-dressed with allogeneic MHC.
(A) Procedure used to generate allogeneic exosomes and cells cross-dressed with allogeneic MHC molecules. Steps 1 and 2: Exosomes were isolated and quantified (using ExoQuick kits) from the lower compartments of Transwell plates whose upper compartments contained B6 spleen cells stimulated for 4 to 5 days with CD40 and interleukin-4 (IL-4). Step 3: To obtain cross-dressed cells, BALB/c spleen cells (15 × 106 cells/ml) were cultured for 5 days along with B6-derived exosomes (1.9 × 109). Step 4: BALB/c cells cross-dressed with allogeneic H-2b exosomes were isolated using an affinity column composed of beads coated with anti–Kb-PE antibodies. Step 5: The number and purity of cross-dressed cells were evaluated using imaging flow cytometry. (B) In vitro activation of T cells with exosomes or cross-dressed cells. BALB/c T cells (5 × 105 cells per well) were cultured with B6 allogeneic exosomes or BALB/c cells cross-dressed with allogeneic (H-2b) or control syngeneic (H-2d) MHC (5 × 105 cells per well) for 48 hours. The frequencies of activated T cells producing IFN-γ were measured using ELISPOT. (C) BALB/c mice were injected intraperitoneally with allogeneic (B6) or syngeneic (self, BALB/c) exosomes (2 × 108 to 2 × 109 vesicles). Fourteen days later, spleen T cells from these mice as well as control naïve mice were collected and stimulated in vitro with irradiated allogeneic B6 APCs for 72 hours. The frequency of T cells secreting IFN-γ was measured using ELISPOT. BALB/c mice recipient of a B6 skin graft or injected with B6 spleen cells were tested as positive controls. In (B) and (C), the results are expressed as the number of IFN-γ–producing spots per million T cells ± SD obtained from 13 mice tested individually. The P values obtained with unpaired t test comparing T cell responses after syngeneic versus allogeneic exosome stimulation for (B) and (C) were P = 0.0003 and P = 0.0029, respectively.
Last, to examine the immunogenicity of allo-MHC molecules present on exosomes, we injected BALB/c mice intraperitoneally with 2 × 108 to 2 × 109 allogeneic (B6) or syngeneic (BALB/c) exosomes. Fourteen days later, spleen cells were collected and cultured for 72 hours with B6 irradiated APCs (0.5 × 106 cells per well) and tested for their IFN-γ secretion as readout of T cell activation. Allogeneic but not syngeneic exosomes induced a potent IFN-γ response, although less vigorous than that obtained with T cells from BALB/c mice injected with allogeneic B6 spleen cells or transplanted with a B6 skin patch (Fig. 6C). Therefore, allogeneic exosomes can induce potent direct alloresponses by T cells in vivo.
DISCUSSION
The passenger leukocyte theory from 1957 states that alloantigen presentation after skin transplantation occurs in a fashion similar to that described after skin infection or injury, in which cutaneous DCs capture antigens and traffic via lymphatics to regional LNs where they activate naïve T cells. This concept is based on (i) experiments involving the replacement or depletion of leukocytes from skin grafts (13) and (ii) the observation that Langerhans cells and other DCs migrate out of skin explants placed in culture (30). At the same time, the role of lymphatics in allosensitization is supported by the observations of Billingham and Silvers (31) that skin allografts undergo long-term survival when placed in vascularized but alymphatic sites (immune-privileged) such as the brain or cheek pouch. Reinforcing this view, Barker and Billingham (17) and Tilney and Gowans (32) showed that skin allografts placed on a piece of recipient’s skin, which had been lifted while still connected through blood vessels (pediculated skin flap), underwent longer survival. Together, these seminal studies demonstrated the role of graft leukocytes and lymphatic vessels in the initiation of T cell alloimmunity in skin-grafted rodents. Recent studies by Celli et al. (33) using two-photon microscopy revealed the rapid disappearance of DCs from skin allografts but did not reveal their presence in recipient draining LNs. To our knowledge, although many studies documented the role of graft APCs in T cell allosensitization, their actual presence in the LN draining skin allografts was never formally proven.
Our study shows the absence of donor leukocytes in the LNs and spleen of mice transplanted with a fully allogeneic skin allograft. The notion that donor DCs leave skin grafts through lymphatics right after transplantation and activate recipient T cells is counterintuitive given that these vessels are severed during surgery. Reestablishment of lymphatic connections (via neolymphangiogenesis and inosculation) after skin grafting requires 5 to 7 days (25, 26), whereas T cell responses are already detectable 2 days after transplantation. However, one should keep in mind that severed lymphatics in a cutaneous wound remain open for as long as 48 hours, allowing for material introduced in the wound to pass into the lymphatics through their gaping ends (34). This is confirmed by our observation that microbeads injected into the skin graft bed found their way to draining LNs. In 1971, Hall (15) mentioned that “debris rather than cells are found in the lymph after skin grafting,” an observation that prefigured our finding. Trafficking conditions for cells are presumably different at days 5 to 7 after skin grafting, once lymphatic connections have been restored. At this time, it is conceivable that some donor DCs could migrate out of skin transplants via lymphatics as suggested by the presence of macrobeads of cellular size in draining LNs at day 7 after injection (Fig. 2). Yet, we did not detect any donor cells in recipient LNs at day 7 or later after skin transplantation. We surmise that this might be due to their elimination by the host’s immune system. Anti-donor effector T cells become activated as early as 2 days after skin grafting (Fig. 1). In further support of this view, Garrod et al. (35) recently reported that donor DCs, in a fully mismatched setting, are quickly killed by host natural killer cells. Therefore, in an MHC-mismatched combination, donor cutaneous DCs may be eliminated before lymphatic connections are reestablished and consequently never get to migrate to draining LNs.
Our results do not disprove the original observations made in skin-grafted mice but provide a novel interpretation of the mechanisms by which T cell allorecognition is triggered. Although allogeneic MHC molecules are present in the recipient’s LNs, they are not displayed on donor skin DCs but on vesicles produced by these MHC class II+ leukocytes. We surmise that immediately after skin transplantation, donor vesicles released in the graft bed leak through open severed ends of the lymphatic vessels and traffic passively to regional LNs. Thus, these donor vesicles represent the exclusive source of donor MHC for T cell activation. On the basis of these principles, it is likely that donor MHC cross-dressing of recipient cells represents the driving force behind initiation of T cell responses, leading to acute rejection of skin allografts. In our study, donor vesicles were not able to activate allospecific T cells in vitro on their own. This confirms previous studies showing that allo-MHC cross-dressed cells but not purified allogeneic exosomes activate T cells in vitro (36, 37). This is apparently not due to lack of costimulation molecules on these vesicles, because we detected the presence of CD40, CD80, and CD86 on many donor exosomes isolated from skin-transplanted mice (fig. S3) and therefore cannot rule out their ability to stimulate T cells in vivo.
The results obtained in heart-transplanted mice differ from those of skin-grafted mice in two aspects: (i) A few (100 to 200 cells per million) donor leukocytes were detected in the recipient lymphoid organs, and (ii) most of the cross-dressed cells were found in the spleen and not in LNs. The overwhelming numbers of donor cells and vesicles in the recipient spleen presumably reflect their migration through blood rather than through lymphatic vessels. Unlike skin grafts, heart transplants are vascularized at the time of their placement. This assertion is corroborated by studies from Larsen and others (24) showing that donor MHC class II+ cells traffic from heart transplants preferentially to the host spleen. This process is believed to occur through reverse transendothelial migration across the vascular endothelium rather than passage through an LN into efferent lymph. However, the existence of such reverse leukocyte transmigration through blood vessels has never been established. It is intriguing that, in contrast to previous reports, the donor leukocytes detected in the spleen of our recipients of cardiac allografts were mainly lymphocytes instead of DCs. This raises the possibility that the cells detected in this study were derived from residual donor blood contained in the heart graft rather than APCs transmigrating from its parenchyma. Because of their very low frequencies and rapid elimination by the host immune system (35), it is unlikely that these cells play a critical role in the induction and maintenance of T cell alloresponses. Conversely, we observed 500 to 1000 times higher frequencies of allo-MHC cross-dressed cells, which increased markedly over the course of graft rejection. Thus, we surmise that antigen presentation by allo-MHC cross-dressed cells is the essential pathway of T cell activation after transplantation.
Here, donor exosomes isolated from activated allogeneic cells did not activate T cells in vitro on their own. However, we cannot exclude that donor MHC+ vesicles produced after transplantation, which express CD40 and CD80/86 co-stimulation receptors, could activate recipient T cells in vivo in an inflammatory environment. Additionally, we have not investigated whether these exosomes could trigger indirect alloresponses by T cells. It is also noteworthy that we still ignore the origin of a sizable proportion of donor exosomes, which did not express DC as well as lymphocyte receptors. It is possible that these vesicles were derived from endothelialor even graft parenchymal cells. Whether or not these vesicles could influence alloimmunity once donor passenger leukocytes have vanished represents an important question to be investigated.
Forty years ago, T cells were shown to acquire surface immunoglobulin molecules from B cells (38) and antigens from macrophages (39). It is now clear that intercellular transfer of proteins and microRNA occurs regularly through cell-cell contact and via microvesicles, which are either secreted or exchanged via nanotubes (40). There is a body of evidence indicating that this process is crucial in the initiation and regulation of immunity to microbes and tumors (40). The transfer of MHC molecules between hematopoietic cells was first reported by Frelinger et al. (41). Acquired peptide-MHC complexes can remain on APCs for as long as 2 days after transfer, providing ample opportunity for T cell activation (42). DCs that have acquired allogeneic MHC proteins in vitro via cell-cell contact can activate alloreactive T cells in vitro via a mechanism sometimes referred to as “semi-direct allorecognition” (28, 43, 44). Recent studies have documented the transfer of MHC class I and II molecules between recipient and donor DCs after solid organ and bone marrow transplantation (45–47). Together with our study, this shows that donor MHC cross-dressing of recipient APCs represents a general phenomenon in transplantation. In addition to its contribution to acute graft rejection, continuous transfer of donor MHC to recipient professional APCs via exosomes produced by endothelial and graft parenchymal cells could be involved in the perpetuation of direct and amplification of indirect alloresponses by T cells causing chronic graft rejection. On the other hand, exosomes, including microvesicles isolated from regulatory T cells, have been implicated in the suppression of inflammatory responses and allograft tolerance (36, 48–50). A better understanding of the mechanisms underlying donor vesicle production and allo-MHC cross-dressing of APCs after transplantation will help us design novel strategies to manipulate immune responses associated with rejection or tolerance.
MATERIALS AND METHODS
Study design
Mice were purchased from the Jackson Laboratory and maintained at Massachusetts General Hospital (MGH) animal facilities under specific pathogen–free conditions. All animal care and handling were performed according to institutional and National Institutes of Health (NIH) guidelines and were under the approval of the MGH Institutional Animal Care and Use Committee. All experiments included three to six mice per group and were repeated at least three times.
Transplantation procedures
Conventional (nonprimarily vascularized) full-thickness skin allografts (1 cm by 1 cm) were placed on the back of the recipients. Vascularized heterotopic cardiac transplantation was performed as described elsewhere (7). Transplanted hearts were monitored daily by palpation through the abdominal wall. Heartbeat intensity was graded on a scale of 0 (no palpable impulse) to 4 (strong impulse). Rejection was defined by the loss of palpable cardiac contractions and verified by autopsy and pathological examination.
Before islet transplantation, diabetes was induced in recipient mice with streptozotocin (200 mg/kg, intraperitoneally; Sigma-Aldrich) and was defined as blood glucose levels >300 mg/dl for at least two consecutive days. Donor islets were isolated by collagenase digestion (Liberase TL, Roche) and then separated by discontinuous Euro-Ficoll gradients (densities: 1.11, 1.096, and 1.066) from the pancreatic digest. Four hundred to 500 islets were transplanted into the renal sub-capsular space of diabetic recipients. A functioning graft was defined as a nonfasting blood glucose level of <200 mg/dl, and rejection was diagnosed at a blood glucose level of >200 mg/dl for at least two consecutive days. Mice were monitored at least twice per week by measuring blood glucose until the mice were killed. Nephrectomy was performed to rule out recovery of native islet function in mice that remained normoglycemic after 100 days.
Imaging flow cytometry
Leukocytes were obtained from recipients’ thymus, spleen, as well as draining LNs (ipsilateral axillary and brachial LNs for skin grafts and para-aortic for heart transplants) and nondraining LNs (contralateral inguinal LNs for skin grafts and brachial LNs for heart transplants) at different time points after skin, heart, or pancreatic islet transplantation. The cells were labeled with fluorescent antibodies directed against H-2Kb (AF6-88.5, BioLegend), H-2Kd (SF1-1.1, BioLegend), I-Ab (AF6-120.1, BioLegend), I-Ad (AMS-32.1, eBioscience), CD3e (145-2C11, BD Biosciences), CD20 (AISB12, eBioscience), and CD11c (N418, BioLegend). Acquisition of cells was performed with the ImageStream instrument (Amnis, EMD Milipore), using low-speed fluidics, at ×40 magnification. Data analysis was performed using IDEAS 6.1 image processing and statistical analysis software (Amnis, EMD Millipore). Leukocytes (3 × 105) were analyzed per organ and time point. Analysis gates were based on aspect ratio, area, and the root mean square of the rate of change of the image intensity profile of the bright-field image for each event. With this strategy, events that corresponded to noncellular events or debris, as well as out-of-focus images, were gated out of the analysis (less than 3% of acquired events). Subsequent gates were based on the intensity of the fluorescence associated to each event and its morphological distribution in the cell.
Preparation of exosomes and in vitro cross-dressing of cells
Twenty-four million mouse BALB/c spleen cells were isolated and cultured for 4 to 5 days in AIM V medium containing 10% exosome-free fetal calf serum along with CD40 (5 μg/ml) and mouse recombinant IL-4 (5 μg/ml) in the upper compartment of Transwell culture plates (0.40-μm pores) at 37°C and 5% CO2, as described elsewhere (51). The exosomes that have diffused to the lower compartment of the Transwell plate were purified and quantified using an exosome isolation kit (ExoQuick-TC, System Biosciences Inc.). The absence of intact cells in the exosome preparation was ascertained using imaging flow cytometry. Next, B6 spleen cells (15 × 106 cells/ml) were cultured for 5 days along with BALB/c-derived exosomes (1.9 × 109) at 37°C and 5% CO2. Last, the frequency of B6 cells cross-dressed with allogeneic MHC class I Kd was evaluated using imaging flow cytometry, as indicated above.
ELISPOT assays
Direct and indirect alloresponses by T cells were measured as previously described (52). Briefly, 96-well ELISPOT plates (Polyfiltronics) were coated with an anti-cytokine capture monoclonal antibody in sterile phosphate-buffered saline (PBS) overnight. On the day of the experiment, the plates were washed twice with sterile PBS, blocked for 1.5 hours with PBS containing 1% BSA, and then washed three times with sterile PBS. Responder cells or purified T cells were added to wells previously filled with either intact donor cells (direct response) or syngeneic APCs together with donor sonicates (indirect response) and cultured for 24 hours at 37°C and 5% CO2. After washing, biotinylated anti-lymphokine detection antibodies were added overnight as described elsewhere. The plates were developed using 800 μl of 3-amino-9-ethylcarbazole (Pierce, 10 mg dissolved in 1 ml of dimethyl formamide) mixed in 24 ml of 0.1 M sodium acetate (pH 5.0) plus 12 μl of H202. The resulting spots were counted and analyzed on a computer-assisted ELISA spot image analyzer (Cellular Technology Limited).
RT-PCR analyses
Kb-specific RT-PCR analysis was conducted on splenocytes and LNs from B6 mice, recipient of allogeneic BALB/c skin grafts. Cells were isolated at days 5 and 10 after transplantation. Positive and negative controls were splenocytes from B6 and BALB/c mice, respectively. RT-PCR was performed on cDNA derived from 250 ng of individual RNA. The amplified products were run on agarose gels and transferred on nitrocellulose filters that were hybridized with a 32P-labeled Kb-specific probe lighting up a 191-bp-long fragment. Controls were BALB/c and B6 cDNA alone and no cDNA (no template). Signal intensities were corrected according to the amount of template loaded (estimated by RT-PCR amplification of the actin genes) (fig. S4). Signal intensities are represented in arbitrary units.
Statistics
All statistical analyses were performed using StatView software (Abacus Concepts Inc.). P values were calculated using ANOVA and paired t test. P values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
Funding: This study was supported by NIH grants R21 AI117466 (to G.B.) and RO1 AI091930 (to R.A.). Research was supported by the Amnis ISx located in the Department of Pathology Flow and Image Cytometry Core facility, which received support for the purchase of the instrument using NIH Shared Instrumentation grant 1S10OD012027-01A1.
Footnotes
Author contributions: J.M. designed and performed the experiments. P.C.-B., J.T.P., and M.H.B.-M. prepared the exosomes and performed in vitro and in vivo T cell activation with exosomes. C.L. and S.G. performed the PCR analyses. J.I.K. and J.F.M. performed the islet transplants. M.U. and R.A. performed the heart transplants. G.T. supervised the ELISPOT assays. G.B. designed and supervised the experiments and wrote the manuscript with contributions from all authors.
Competing interests: The authors declare that they have no competing financial interests.
REFERENCES AND NOTES
- 1.Lechler R, Lombardi G. Structural aspects of allorecognition. Curr Opin Immunol. 1991;3:715–721. doi: 10.1016/0952-7915(91)90102-7. [DOI] [PubMed] [Google Scholar]
- 2.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: New answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 3.Benichou G, Takizawa PA, Olson CA, McMillan M, Sercarz EE. Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection. J Exp Med. 1992;175:305–308. doi: 10.1084/jem.175.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benichou G, Fedoseyeva E, Lehmann PV, Olson CA, Geysen HM, McMillan M, Sercarz EE. Limited T cell response to donor MHC peptides during allograft rejection. Implications for selective immune therapy in transplantation. J Immunol. 1994;153:938–945. [PubMed] [Google Scholar]
- 5.Liu Z, Sun YK, Xi YP, Hong B, Harris PE, Reed EF, Suciu-Foca N. Limited usage of T cell receptor V beta genes by allopeptide-specific T cells. J Immunol. 1993;150:3180–3186. [PubMed] [Google Scholar]
- 6.Auchincloss H, Jr, Lee R, Shea S, Markowitz JS, Grusby MJ, Glimcher LH. The role of “indirect” recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice. Proc Natl Acad Sci USA. 1993;90:3373–3377. doi: 10.1073/pnas.90.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kant CD, Akiyama Y, Tanaka K, Shea S, Connolly SE, Germana S, Winn HJ, LeGuern C, Tocco G, Benichou G. Primary vascularization of allografts governs their immunogenicity and susceptibility to tolerogenesis. J Immunol. 2013;191:1948–1956. doi: 10.4049/jimmunol.1202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee RS, Yamada K, Houser SL, Womer KL, Maloney ME, Rose HS, Sayegh MH, Madsen JC. Indirect recognition of allopeptides promotes the development of cardiac allograft vasculopathy. Proc Natl Acad Sci USA. 2001;98:3276–3281. doi: 10.1073/pnas.051584498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Cook HT, Lechler RI. Loss of direct and maintenance of indirect alloresponses in renal allograft recipients: Implications for the pathogenesis of chronic allograft nephropathy. J Immunol. 2001;167:7199–7206. doi: 10.4049/jimmunol.167.12.7199. [DOI] [PubMed] [Google Scholar]
- 10.Snell GD. The homograft reaction. Annu Rev Microbiol. 1957;11:439–458. doi: 10.1146/annurev.mi.11.100157.002255. [DOI] [PubMed] [Google Scholar]
- 11.Elkins WL, Guttmann RD. Pathogenesis of a local graft versus host reaction: Immunogenicity of circulating host leukocytes. Science. 1968;159:1250–1251. doi: 10.1126/science.159.3820.1250. [DOI] [PubMed] [Google Scholar]
- 12.Billingham RE. The passenger cell concept in transplantation immunology. Cell Immunol. 1971;2:1–12. doi: 10.1016/0008-8749(71)90022-0. [DOI] [PubMed] [Google Scholar]
- 13.Steinmuller D. Passenger leukocytes and the immunogenicity of skin allografts. J Invest Dermatol. 1980;75:107–115. doi: 10.1111/1523-1747.ep12521331. [DOI] [PubMed] [Google Scholar]
- 14.Barker CF, Billingham RE. Immunologically competent passenger cells in mouse skin. Transplantation. 1972;14:525–527. doi: 10.1097/00007890-197210000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Hall JG. Studies of the cells in the afferent and efferent lymph of lymph nodes draining the site of skin homografts. J Exp Med. 1967;125:737–754. doi: 10.1084/jem.125.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wachtel SS, Silvers WK. The role of passenger leukocytes in the anomalous survival of neonatal skin grafts in mice. J Exp Med. 1972;135:388–404. doi: 10.1084/jem.135.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker CF, Billingham RE. Skin homografts in vascularized skin pedicles in guinea pigs. Surg Forum. 1966;17:480–482. [PubMed] [Google Scholar]
- 18.Barker CF, Billingham RE. The role of afferent lymphatics in the rejection of skin homografts. J Exp Med. 1968;128:197–221. doi: 10.1084/jem.128.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guttmann RD, Santos GW, Lindquist RR. Acceptance of renal allografts in rat bone marrow chimeras. Transplantation. 1971;12:408–409. doi: 10.1097/00007890-197111000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Lechler RI, Batchelor JR. Immunogenicity of retransplanted rat kidney allografts. Effect of inducing chimerism in the first recipient and quantitative studies on immunosuppression of the second recipient. J Exp Med. 1982;156:1835–1841. doi: 10.1084/jem.156.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krasinskas AM, Eiref SD, McLean AD, Kreisel D, Gelman AE, Popma SH, Moore JS, Rosengard BR. Replacement of graft-resident donor-type antigen presenting cells alters the tempo and pathogenesis of murine cardiac allograft rejection. Transplantation. 2000;70:514–521. doi: 10.1097/00007890-200008150-00020. [DOI] [PubMed] [Google Scholar]
- 23.Pietra BA, Wiseman A, Bolwerk A, Rizeq M, Gill RG. CD4 T cell-mediated cardiac allograft rejection requires donor but not host MHC class II. J Clin Invest. 2000;106:1003–1010. doi: 10.1172/JCI10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scothorne RJ. Lymphatic repair and the genesis of homograft immunity. Ann N Y Acad Sci. 1958;73:673–675. doi: 10.1111/j.1749-6632.1959.tb40843.x. [DOI] [PubMed] [Google Scholar]
- 26.Barker CF, Billingham RE. Analysis of local anatomic factors that influence the survival times of pure epidermal and full-thickness skin homografts in guinea pigs. Ann Surg. 1972;176:597–604. doi: 10.1097/00000658-197211000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKhann CF, Berrian JH. Transplantation immunity: Some properties of induction and expression. Ann Surg. 1959;150:1025–1031. doi: 10.1097/00000658-195912000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, Lechler RI. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173:4828–4837. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 29.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Billingham RE, Silvers WK. Studies on the conservation of epidermal specificities of skin and certain mucosas in adult mammals. J Exp Med. 1967;125:429–446. doi: 10.1084/jem.125.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tilney NL, Gowans JL. Host sensitization by alymphatic skin allografts in the rat. Surg Forum. 1970;21:512–514. [PubMed] [Google Scholar]
- 33.Celli S, Albert ML, Bousso P. Visualizing the innate and adaptive immune responses underlying allograft rejection by two-photon microscopy. Nat Med. 2011;17:744–749. doi: 10.1038/nm.2376. [DOI] [PubMed] [Google Scholar]
- 34.McMaster PD, Hudack SS. The participation of skin lymphatics in repair of the lesions due to incisions and burns. J Exp Med. 1934;60:479–501. doi: 10.1084/jem.60.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrod KR, Liu FC, Forrest LE, Parker I, Kang SM, Cahalan MD. NK cell patrolling and elimination of donor-derived dendritic cells favor indirect alloreactivity. J Immunol. 2010;184:2329–2336. doi: 10.4049/jimmunol.0902748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morelli AE. The immune regulatory effect of apoptotic cells and exosomes on dendritic cells: Its impact on transplantation. Am J Transplant. 2006;6:254–261. doi: 10.1111/j.1600-6143.2005.01197.x. [DOI] [PubMed] [Google Scholar]
- 37.Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 38.Hudson L, Sprent J. Specific adsorption of IgM antibody onto H-2-activated mouse T lymphocytes. J Exp Med. 1976;143:444–449. doi: 10.1084/jem.143.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bona C, Robineaux R, Anteunis A, Heuclin C, Astesano A. Transfer of antigen from macrophages to lymphocytes. II. Immunological significance of the transfer of lipopolysaccharide. Immunology. 1973;24:831–840. [PMC free article] [PubMed] [Google Scholar]
- 40.Brown K, Fidanboylu M, Wong W. Intercellular exchange of surface molecules and its physiological relevance. Arch Immunol Ther Exp. 2010;58:263–272. doi: 10.1007/s00005-010-0085-y. [DOI] [PubMed] [Google Scholar]
- 41.Frelinger JA, Neiderhuber JE, David CS, Shreffler DC. Evidence for the expression of Ia (H-2-associated) antigens on thymus-derived lymphocytes. J Exp Med. 1974;140:1273–1284. doi: 10.1084/jem.140.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177:6018–6024. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- 43.Smyth LA, Herrera OB, Golshayan D, Lombardi G, Lechler RI. A novel pathway of antigen presentation by dendritic and endothelial cells: Implications for allorecognition and infectious diseases. Transplantation. 2006;82:S15–S18. doi: 10.1097/01.tp.0000231347.06149.ca. [DOI] [PubMed] [Google Scholar]
- 44.Russo V, Zhou D, Sartirana C, Rovere P, Villa A, Rossini S, Traversari C, Bordignon C. Acquisition of intact allogeneic human leukocyte antigen molecules by human dendritic cells. Blood. 2000;95:3473–3477. [PubMed] [Google Scholar]
- 45.Harper SJF, Ali JM, Wlodek E, Negus MC, Harper IG, Chhabra M, Qureshi MS, Mallik M, Bolton E, Bradley JA, Pettigrew GJ. CD8 T-cell recognition of acquired alloantigen promotes acute allograft rejection. Proc Natl Acad Sci USA. 2015;112:12788–12793. doi: 10.1073/pnas.1513533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markey KA, Koyama M, Gartlan KH, Leveque L, Kuns RD, Lineburg KE, Teal BE, MacDonald KPA, Hill GR. Cross-dressing by donor dendritic cells after allogeneic bone marrow transplantation contributes to formation of the immunological synapse and maximizes responses to indirectly presented antigen. J Immunol. 2014;192:5426–5433. doi: 10.4049/jimmunol.1302490. [DOI] [PubMed] [Google Scholar]
- 47.Brown K, Sacks SH, Wong W. Extensive and bidirectional transfer of major histocompatibility complex class II molecules between donor and recipient cells in vivo following solid organ transplantation. FASEB J. 2008;22:3776–3784. doi: 10.1096/fj.08-107441. [DOI] [PubMed] [Google Scholar]
- 48.Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. MHC class II+ exosomes in plasma suppress inflammation in an antigen-specific and Fas ligand/Fas-dependent manner. J Immunol. 2007;179:2235–2241. doi: 10.4049/jimmunol.179.4.2235. [DOI] [PubMed] [Google Scholar]
- 49.Pêche H, Renaudin K, Beriou G, Merieau E, Amigorena S, Cuturi MC. Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. Am J Transplant. 2006;6:1541–1550. doi: 10.1111/j.1600-6143.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- 50.Agarwal A, Fanelli G, Letizia M, Tung SL, Boardman D, Lechler R, Lombardi G, Smyth LA. Regulatory T cell-derived exosomes: Possible therapeutic and diagnostic tools in transplantation. Front Immunol. 2014;5:555. doi: 10.3389/fimmu.2014.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saunderson SC, Schuberth PC, Dunn AC, Miller L, Hock BD, MacKay PA, Koch N, Jack RW, McLellan AD. Induction of exosome release in primary B cells stimulated via CD40 and the IL-4 receptor. J Immunol. 2008;180:8146–8152. doi: 10.4049/jimmunol.180.12.8146. [DOI] [PubMed] [Google Scholar]
- 52.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.