Abstract
Background
It has been suggested that Schistosoma infection may be associated with Plasmodium falciparum infection or related reduction in haemoglobin level, but the nature of this interaction remains unclear. This systematic review synthesized evidence on the relationship of S. haematobium or S. mansoni infection with the occurrence of P. falciparum malaria, Plasmodium density and related reduction in haemoglobin level among children in sub-Saharan Africa (SSA).
Methodology/Principal findings
A systematic review in according with PRISMA guidelines was conducted. All published articles available in PubMed, Embase, Cochrane library and CINAHL databases before May 20, 2015 were searched without any limits. Two reviewers independently screened, reviewed and assessed all the studies. Cochrane Q and Moran’s I2 were used to assess heterogeneity and the Egger test was used to examine publication bias. The summary odds ratio (OR), summary regression co-efficient (β) and 95% confidence intervals (CI) were estimated using a random-effects model. Out of 2,920 citations screened, 12 articles (five cross-sectional, seven prospective cohort) were eligible to be included in the systematic review and 11 in the meta-analysis. The 12 studies involved 9,337 children in eight SSA countries. Eight studies compared the odds of asymptomatic/uncomplicated P. falciparum infection, two studies compared the incidence of uncomplicated P. falciparum infection, six studies compared P. falciparum density and four studies compared mean haemoglobin level between children infected and uninfected with S. haematobium or S. mansoni. Summary estimates of the eight studies based on 6,018 children showed a higher odds of asymptomatic/uncomplicated P. falciparum infection in children infected with S. mansoni or S. haematobium compared to those uninfected with Schistosoma (summary OR: 1.82; 95%CI: 1.41, 2.35; I2: 52.3%). The increase in odds of asymptomatic/uncomplicated P. falciparum infection among children infected with Schistosoma remained significant when subgroup analysis was conducted for S. haematobium (summary OR: 1.68; 95%CI: 1.18, 2.41; I2: 53.2%) and S. mansoni (summary OR: 2.15; 95%CI: 1.89, 2.46: I2: 0.0%) infection. However, the density of P. falciparum infection was lower in children co-infected with S. haematobium compared to those uninfected with Schistosoma (summary-β: -0.14; 95% CI: -0.24, -0.01; I2: 39.7%). The mean haemoglobin level was higher among children co-infected with S. haematobium and P. falciparum than those infected with only P. falciparum (summary-mean haemoglobin difference: 0.49; 95% CI: 0.04, 0.95; I2: 66.4%)
Conclusions/Significance
The current review suggests S. mansoni or S. haematobium co-infection may be associated with increased prevalence of asymptomatic/uncomplicated P. falciparum infection in children, but may protect against high density P. falciparum infection and related reduction in haemoglobin level.
Author Summary
A clear understanding of the epidemiology of malaria during Schistosoma co-infection is essential to inform decisions on appropriate control strategies for schistosomiasis and malaria in SSA. In this systematic review and meta-analysis, we synthesized evidence on the nature of relationship of S. haematobium and S. mansoni infection with the prevalence/incidence of P. falciparum infection, density of the parasite and related reduction in haemoglobin level among children in SSA. We searched all published articles available in PubMed, Embase, Cochrane library and CINAHL databases before May 20, 2015 without any language restriction. We found five cross-sectional and seven prospective cohort studies eligible to be included in the systematic review, and 11 of these studies were included in the meta-analysis. A summarized analysis of the study findings showed that S. haematobium and S. mansoni infection is associated with an increased odds of asymptomatic/uncomplicated P. falciparum infection. However, density of P. falciparum infection decreased and haemoglobin level increased during S. haematobium co-infection.
Introduction
Malaria and schistosomiasis are common in tropical and sub-tropical areas, causing high burden of morbidity and mortality, particularly in children [1,2]. In 2015, about 214 million people were infected and 438,000 estimated to have died globally due to malaria [1]. Additionally, more than 261 million people required preventive treatment for schistosomiasis and close to 200,000 are estimated to die due to this disease annually [2]. About 90% of the malaria deaths and 90% of those who require treatment for schistosomiasis live in sub-Saharan Africa (SSA), with children being the most affected group [1,2]. Plasmodium falciparum (P. falciparum) is responsible for most malaria cases and deaths due to the disease in SSA [3–5]. Likewise, two schistosome species, Schistosoma mansoni (S. mansoni) and S. haematobium are responsible for almost all of schistosomiasis cases in SSA [6]. S. mansoni causes intestinal and hepatic schistosomiasis and S. haematobium causes urogenital schistosomiasis [6]. Both Schistosoma spp. cause inflammation that leads to anaemia, growth stunting or cognitive impairment [6].
Humans infected with Plasmodium species that cause malaria can manifest a wide range of symptoms that vary from asymptomatic infection to severe complications resulting in death [6–8]. Severe malaria complications such as cerebral malaria, respiratory failure, acute renal failure or severe anaemia usually occur when unimmune individuals get infected with P. falciparum [7]. On the other hand, people living in regions where there is stable malaria transmission will usually show common symptoms such as fever, chills, fatigue, malaise when infected with Plasmodium spp. [8,9]. Still some immune individuals infected with Plasmodium may not develop fever, chills or other acute clinical symptoms of malaria, or may show symptoms intermittently but not severe enough to require attention from a health care provider [8].
Schistosoma co-infection can affect the development of Plasmodium infection related symptoms by altering the immune function [10]. Distributions of Plasmodium and Schistosoma species overlap in most of SSA, resulting in high rates of co-infection [11]. Based on the immunological findings in murine models and human subjects, it is hypothesized that there is a down-regulating effect of Schistosoma on the immune system of individuals, which in turn, may affect the course of other intracellular infections like Plasmodium [10]. However, research on the course of P. falciparum infection and related outcomes during Schistosoma co-infection has generated contradictory findings. While some studies report increased odds of P. falciparum infections and/or malaria-related complications associated with S. haematobium or S. mansoni co-infections [12–14], others reported lower incidence and density of P. falciparum infection in children with S. haematobium infection [15,16]. Some studies reported lack of statistically significant association between S. mansoni or S. haematobium and the risk of P. falciparum infection [17,18]. The differences in study design, age groups, and outcomes may have blurred attempts to reach clear conclusions.
In order to reduce adverse impact of schistosomiasis on child health, it is recommended that children living in endemic regions be treated with praziquantel [19]. However, if co-infection with schistosomiasis may reduce children’s susceptibility to the more severe form of P. falciparum malaria, then such treatment might have unwanted consequences. Thus, a clear understanding of the epidemiology of malaria during Schistosoma co-infection is essential to inform decisions on appropriate control strategies for schistosomiasis and malaria in SSA.
Two previous reviews examining helminth and malaria co-infection, partially addressed the issue of Schistosoma and malaria co-infection in the general population based on studies conducted in various regions of the world [20,21]. However, the current systematic review and meta-analysis was undertaken to quantify the odds of asymptomatic/uncomplicated P. falciparum infection, parasite density and P. falciparum malaria-related reduction in haemoglobin level in Schistosoma co-infected children in SSA.
Methods
The protocol for this systematic review and meta-analysis was conducted following the PRISMA guidelines [22] (S1 Table). It would have been preferable to register this study with PROSPERO [23, 24], an international registry of systematic reviews, from the onset to minimize the potential for reporting bias. However, we did not decide to seek publication until after the review had been conducted. The aim of PROPERO is to minimize reporting bias, particularly if there have been major changes to methods which could potentially introduce biases through increased knowledge of potentially eligible studies leading to the narrowing of objectives or the addition of new outcome measures. Fortunately, we did not make changes on the methods for conducting this review after we had developed the protocol.
Eligibility criteria
All epidemiological studies except case studies which reported prevalence or incidence of P. falciparum infection and/or Plasmodium density stratified by the presence or absence of S. haematobium or S. mansoni infection among children living in SSA were included. Studies that reported immunology of P. falciparum malaria and S. haematobium or S. mansoni co-infection in the general population were also included if they reported the prevalence or incidence of P. falciparum infection in children separately. Unpublished studies, conference abstracts, protocol, gray literature, review protocols, studies that involved only adults or pregnant women, animal or in vitro studies, and studies conducted outside of SSA were excluded after screening the titles and abstracts. Studies were additionally excluded after full text review when children infected with Schistosoma were co-infected with soil transmitted helminths or if they lacked epidemiologic data on P. falciparum and Schistosoma co-infection.
Outcome measures
The primary outcomes were prevalence/incidence of P. falciparum infection. Asymptomatic/uncomplicated P. falciparum malaria was defined as microscopic confirmation of the Plasmodium parasite in blood without clinical evidence (signs or symptoms) of severe malaria [25]. Seven studies included in this review did not clearly differentiate cases based on the clinical stages of malaria as asymptomatic and/or symptomatic. Although three studies indicated malaria cases as asymptomatic and symptomatic, results on P. falciparum and Schistosoma co-infection was not done on basis of the clinical stages of malaria. Hence, this review did not make any distinction between asymptomatic and uncomplicated P. falciparum malaria while estimating the nature of association of Schistosoma co-infection with P. falciparum malaria.
Secondary outcomes included asexual stage (ring forms) Plasmodium density per microliter of blood, haemoglobin levels per deciliter of blood and anaemia. Anaemia was defined as haemoglobin level below the cut-off values defined by WHO: 11.0 g/dl for children 6–59 months; 11.5 g/dl for children 5–11 years; 12.0 g/dl for children 12–14 years [26]. S. haematobium and S. mansoni infections were confirmed in all studies by urine filtration and Kato Katz techniques, respectively.
Search methods for identification of studies
Two authors (AD and DD) independently conducted a search in Pubmed, Embase, Cochrane Library, CINAHL databases using keywords: malaria OR Plasmodium OR “Plasmodium falciparum” OR “Plasmodium vivax” in combination with helminth OR Schistosoma OR “Schistosoma mansoni” OR “Schistosoma haematobium” (S2 Table) for articles published before May 20, 2015. The search was limited to humans, but no limits were made on language. References of some related reviews [10,11,20,21] and African Online Journals database were also searched for relevant studies. Following exclusion of duplicates; abstracts and titles of 2,149 papers were screened for eligibility criteria and 45 were chosen for full text evaluation. Any discrepancies in the choice of articles being included in the review were resolved by third reviewer adjudication. However, there was a very low degree of discrepancy between the two authors in the choice of articles for the review.
Data collection
Information about the author, study area, study design, sample size, age range, Schistosoma species investigated, prevalence of P. falciparum and Schistosoma co-infection, diagnosis techniques and the main findings on prevalence/incidence and density of P. falciparum infection and mean haemoglobin level/ prevalence of anaemia were abstracted and entered into an excel sheet by two authors independently. Any discrepancies were resolved by consensus between the two authors. There was a very low degree of discrepancy between the two authors data that were extracted from the articles.
Quality and bias assessment
Quality and risk of bias of the studies was evaluated using the effective public health practice project [27]. The quality of the studies was assessed on the basis of selection of the study participants, study design, confounder, blinding, data collection methods and withdrawals and drop-outs comparability.
Study synthesis
Heterogeneity was assessed using Cochrane Q (Chi-square) and Moran’s I2 (Inconsistency) using STATA software (Version 11, Texas, USA) [28]. Publication bias was evaluated using a funnel plot and statistical significance was assessed by the Egger test (bias if p<0.1) [29]. Sub-group analyses were conducted for S. mansoni and S. haematobium. Odds ratio, relative risk, regression coefficients, mean differences along with the 95% confidence intervals were used as effect measures. When studies did not report 95% CI for mean differences in Plasmodium density or haemoglobin level, we estimated it using p-values as suggested by Altman and Bland [30]. The 95% CI for mean difference in haemoglobin level between children co-infected with S. haematobium and P. falciparum and those infected with only P. falciparum for the studies by Deribew et al. [31] and Ateba-Ngoa et al. [32] was estimated using the mean and standard deviations values. The mean and standard deviation of haemoglobin levels for Ateba-Ngoa et al. [32] was estimated from the median and interquartile range based on the formula suggested by Wan et al. [33]. A random effects model was used to estimate the summary Mantel-Haenszel odds ratio of P. falciparum infection, among children infected with Schistosoma and those uninfected with Schistosoma. A random effects model was also used to estimate the summary regression coefficients and mean differences of P. falciparum density among children infected with S. haematobium and those uninfected with Schistosoma.
Results
Search results and study characteristics
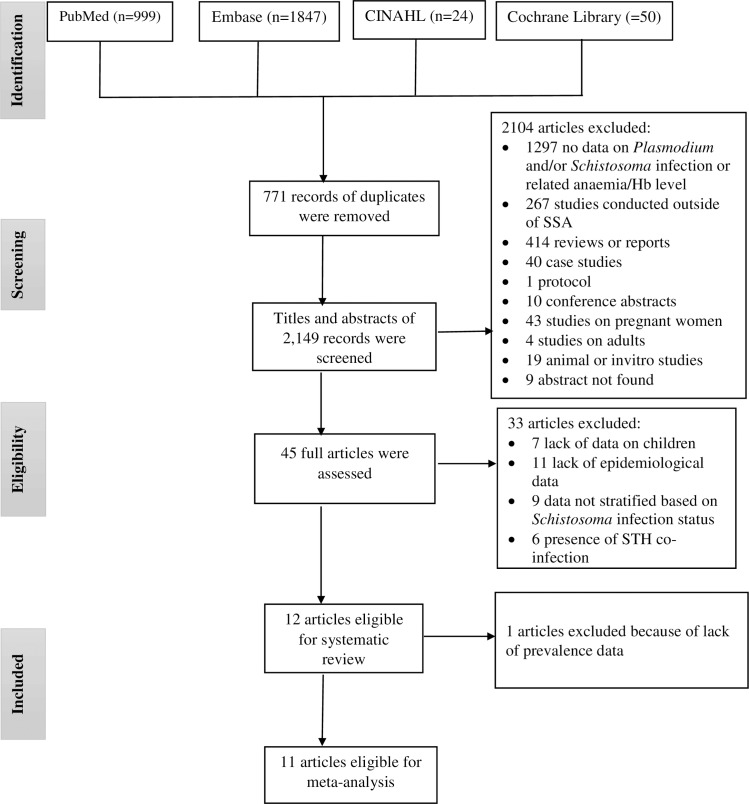
A total of 2,920 citations were identified from PubMed (n = 999), Embase (n = 1,847), Cochrane library (n = 50) and CINAHL (n = 24), of which 771 articles were found to be duplicates. All relevant articles identified from reviews on malaria and helminth co-infection [10,11,20,21] and African Journal database were similar with the articles found from the four databases. Of the 2,149 articles screened; 2104 articles were excluded after reading the titles and abstracts. Of the 45 full-text articles reviewed, 33 were excluded. A total of 12 articles were considered for the systematic review, of which 11 were included in the meta-analysis (Fig 1).
Fig 1. PRISMA diagram.
Flow chart for study selection. Hb: Haemoglobin; SSA: sub-Saharan Africa; STH: Soil-transmitted helminths
The characteristics of the 12 studies with 9,337 subjects included in this review are summarized in Table 1. Five studies were cross-sectional and seven were prospective cohorts. Nine studied S. haematobium and P. falciparum co-infection, and three studied S. mansoni and P. falciparum co-infection. Eight studies compared the odds of asymptomatic/uncomplicated P. falciparum infection and six studies compared P. falciparum density between children infected and uninfected with Schistosoma. Four studies compared mean haemoglobin level/prevalence of anaemia between children co-infected with P. falciparum and Schistosoma and those infected with only P. falciparum. Two studies reported uncomplicated P. falciparum infection, three studies reported both asymptomatic and uncomplicated P. falciparum infection, but seven studies did not clearly differentiate cases based on the clinical stages of malaria as asymptomatic and/or uncomplicated.
Table 1. Characteristics of the included studies.
| Reference | Study area (years of the survey) | Sample size (Age range) | Study Design | Schistosoma species | Outcomes Reported | Prevalence of ‘Sh’ or ‘Sm’ & malaria co-infection | The magnitude of outcomes in those co-infected with ‘Sh’ or ‘Sm’ &Pf compared to those with only Pf | Diagnostic Techniques |
|---|---|---|---|---|---|---|---|---|
| Ateba-Ngoa et al. 2015 | Gabon (2011) | 125 (6–16 years) | CS | Sh | 1. Pf prevalence 2. Hb level |
26% | 1. Similar 2. Higher, mean Hb difference:0.7; 95% CI: 0.21, 1.19 |
Malaria: PCR. Sh: Urine filtration, 3 different day samples. Stools not checked |
| Briand et al. 2005 | Senegal (2001 & 2002 | 523 (3–15 years) | PC | Sh | Pf density | Not given | Lower in children with light intensity ‘Sh’ compared to children without Schistosoma (β: -0.34, 95% CI: −0.85, −0.10) | Malaria: microscope. Sh: Urine filtration, only1 sample. Other helminth: Kato Katz. Adjusted for age, sex, season and level of exposure |
| Courtin et al. 2011 | Senegal (2003) | 234 (6–16 years) | PC | Sh | 1. Pf prevalence 2. Pf density |
34% | 1. Similar, OR: 1.62; 95% CI: 0.94, 2.80 2. Similar |
Malaria: microscope. Sh: Urine filtration,1 sample. Stools not checked |
| Deribew et al. 2013 | Ethiopia (2008) | 387 (6–23 month) | CS | Sh | 1. Pf prevalence 2. Anaemia prevalence 3. Mean Hb |
2.84% | 1. Higher, OR: 2.8; 95% CI: 1.21, 6.5 2. Higher, OR: 10.12; 95% CI: 1.47, 69.94 3. Similar, |
Malaria: microscope. Sh: Urine filtration, 1 sample. Stools not checked |
| Doumbo et al.2014 | Mali (2011 & 2012) | 616 (3 month to 25 Years) | PC | Sh | 1. Pf prevalence 2. Pf risk(hazard ratio) 3. Pf density |
8.5% | 1. Higher, OR: 3.23; 95% CI: 1.76, 5.90 2. Similar 3. Heavy Sh negatively associated with Pf density but light Sh did not associate with Pf density |
Malaria: microscopy & PCR. Sh: Urine filtration. Stools, 2 sample |
| Florey et al. 2012 | Kenya (2005) | 223 (8 to 17 years) | CS | Sh | Pf prevalence | 31.8% | Higher, adjusted OR: 1.79; 95% CI: 1.32, 2.44 | Malaria: PCR. Sh: Urine filtration, 2 samples on different day. Stool not checked. Adjusted for water contact, night activity, bed net use, distance from water |
| Kabatereine et al. 2011 | Uganda (2009 & 2010) | 5000 (Mean age = 12.5 years) | CS | Sm | Pf prevalence | 23.5% | Higher, OR: 2.16; 95% CI: 1.89, 2.47 | Malaria: microscope. Sm: Kato Katz |
| Lyke et al. 2005 | Mali (2002 & 2003) | 654 (4–14 years) | PC | Sh | 1. Pf incidence 2. Pf density 3. Hb level |
1. Lower in children ages 4 to 8 2. Lower in children ages 4 to 8 3. Similar |
Malaria: microscope. Sh: Urine filtration (Sh), 2 to 3 samples. Other helminths: Kato Katz | |
| Mazigo et al. 2010 | Tanzania (2009) | 400 (8–16 years) | CS | Sm | Pf prevalence | 6% | Higher, OR: 2.1; 95% CI: 1.03, 4.26 | Malaria: microscopy. Sm: Kato Katz |
| Lemaitre et al. 2014 | Senegal (2001 to 2003) | 178 (5–13 years) | PC | Sh | Pf density | lower in children with light intensity ‘Sh’ compared to children without Schistosoma (β−0.28; 95% CI: -0.52, -0.04) | Malaria: microscopy. Sm: Kato Katz. Sh: Urine filtration. Adjusted for Sex, age, village, season | |
| Sangweme et al. 2010 | Zimbabwe(2004 & 22005) | 485 (6–17 years) | PC | Sh | 1. Pf prevalence 2. Pf density 3. mean Hb 4.Anaemia prevalence |
18.8% | 1. Similar 2. Lower but not significant,Sexual stage ‘Pf’ density was higher in children infected than uninfected with ‘Sh’ 3. Similar 4. Similar |
Malaria: microscopy. Sh: Urine filtration, 3 samples on different day. Other helminths: Kato Katz, 3 sample on different day. Only few cases were infected with Sm |
| Sokhna et al. 2004 | Senegal (1998) | 512 (6–15 years) | PC | Sm | Pf incidence | 13.3% | Higher in children with heavy Sm egg load compared to those without helminth (RR: 2.24, 95% CI: 1.20, 4.20) | Malaria: microscope. Sh: Urine filtration. Other helminth: Kato Katz |
CS: Cross-sectional; CI: Confidence interval; Hb: Haemoglobin; OR: Odds ratio; Pf: Plasmodium falciparum; RR: Relative risk; Sh: Schistosoma haematobium; Sm: Schistosoma manosni; STH: Soil-transmitted helminths; PC: Prospective cohort; PCR: polymerase chain reaction
Longitudinal studies by Sangweme et al. [34], Courtin et al. [35] and Doumbo et al. [36] did not report incidence, hence the prevalence data reported in these studies during the baseline surveys were used when estimating the summary-odds of P. falciparum infection in children infected and uninfected with S. haematobium. However, Sokhna et al. [37] reported incidence of P. falciparum infection in S. mansoni infected children, the study was thus excluded from the meta-analysis.
Schistosoma haematobium and Plasmodium falciparum infection
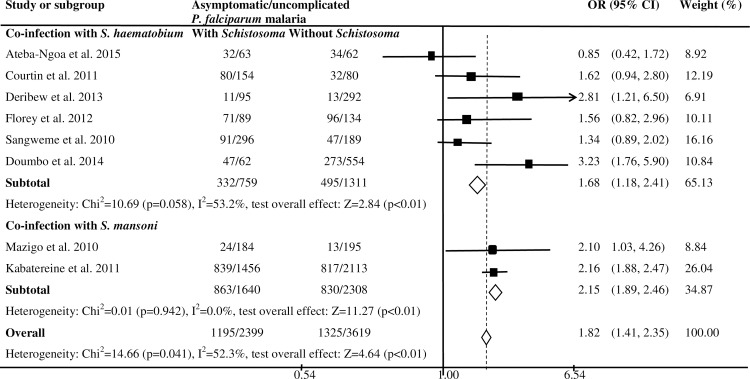
Six studies examined the nature of the relationship of S. haematobium infection with the odds of asymptomatic/uncomplicated P. falciparum infection (Fig 2). A cross-sectional study in Ethiopia [31] and a prospective cohort study in Mali [36] showed increased odds of asymptomatic/uncomplicated P. falciparum infection among children infected with S. haematobium compared to children uninfected with S. haematobium. A cross-sectional study in Kenya also showed higher odds of asymptomatic/uncomplicated P. falciparum infection in children infected with S. haematobium compared to those uninfected with S. haematobium after adjusting for the effects of water contact, night activity, bednet use and distance from water (adjusted OR: 1.79; 95% CI: 1.32, 2.44) [38]. Although the difference was not statistically significant, prospective cohort studies in Zimbabwe [34] and Senegal [35] showed higher odds of asymptomatic/uncomplicated P. falciparum infection in children infected with S. haematobium compared to those who were not infected with S. haematobium. However, a cross-sectional study in Gabon showed similar odds of asymptomatic/uncomplicated P. falciparum infection in children infected with S. haematobium and those uninfected with S. haematobium [32]. The overall estimates based on six studies showed higher odds of asymptomatic/uncomplicated P. falciparum infection in children infected with S. haematobium than those uninfected with S. haematobium (summary OR: 1.68; 95%CI: 1.18, 2.41; I2: 53.2%) [31,32, 34–36, 38]. However, longitudinal studies in Mali showed similar risk of uncomplicated P. falciparum infection in children infected and uninfected with S. haematobium [16,36]. Age-stratified analysis of the data in the study by Lyke et al. [16], showed association of S. haematobium infection with fewer episodes of P. falciparum infection in children of ages 4 to 8 years, but the association was no longer present in children aged 9 to 14 years. Additionally, the study by Doumbo et al. [36] showed association of baseline co-infection with S. haematobium and P. falciparum with reduced risk of febrile P. falciparum infection.
Fig 2. Forest plot showing the difference in the prevalence of asymptomatic/uncomplicated P. falciparum malaria between children infected with S. haematobium or S. mansoni and those not infected with Schistosoma in SSA.
Schistosoma mansoni and Plasmodium falciparum infection
A cross-sectional study of 5,000 children in Uganda showed higher odds of uncomplicated P. falciparum infection among children infected than those uninfected with S. mansoni [39]. Another cross-sectional study in Tanzania documented increased odds of asymptomatic/uncomplicated P. falciparum malaria among children infected with S. mansoni than children without S. mansoni infection [40]. A summary analysis based on the two studies [39,40] showed higher odds of asymptomatic/uncomplicated P. falciparum infection among children infected than those not infected with S. mansoni (summary OR: 2.15; 95%CI: 1.89, 2.46; I2: 0.0%). Similarly, a prospective cohort study in Senegal showed increased risk of uncomplicated P. falciparum infection in children with S. mansoni egg load >1000 eggs/gram as compared to those uninfected with Schistosoma (RR: 2.24, 95% CI: 1.20, 4.20) [37]. However, this association was not seen in children with moderate intensity S. mansoni egg load (100 to 399 eggs/gram). The overall estimates based on eight studies [31, 32, 34–36, 38–40] showed higher odds of asymptomatic/uncomplicated P. falciparum infection in children infected with S. mansoni or S. haematobium as compared to those uninfected with Schistosoma (summary OR: 1.82; 95%CI: 1.41, 2.35; I2: 52.3%). There was no publication bias detected in the meta-analysis (Egger test = -1.89; p = 0.11), which included eight studies (S1 Fig). Subgroup analysis showed that the increase in odds of asymptomatic/uncomplicated P. falciparum infection among children infected with Schistosoma was significant for studies which used microscopy for the diagnosis of Plasmodium infection (summary OR: 1.91; 95%CI: 1.52, 2.40; I2: 31.8%) and those conducted in East African region (summary OR: 1.91; 95%CI: 1.51, 2.42; I2: 31.7%). However, significant association was not seen between asymptomatic/uncomplicated P. falciparum infection and Schistosoma among studies which used polymerase chain reaction (PCR) for the diagnosis of Plasmodium infection and those conducted in the West African region (S2 Fig).
Schistosoma infection and Plasmodium falciparum density
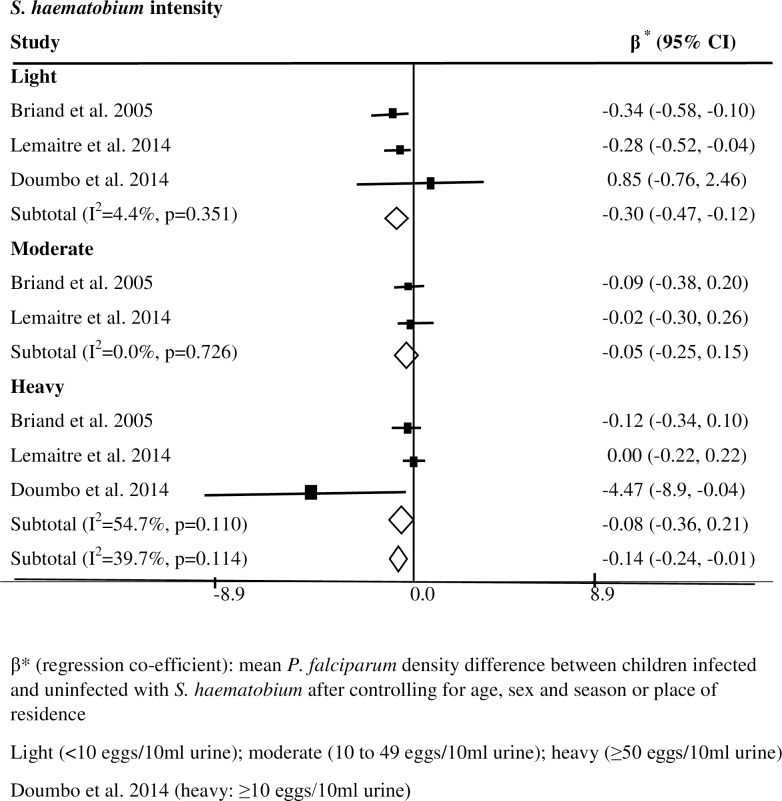
Out of the 12 studies included in this review, six prospective cohort studies examined the effect of S. haematobium infection on the density of P. falciparum infection. Among these six studies, two reported lower P. falciparum density among children with low intensity of S. haematobium infection (<10 eggs/10ml urine) as compared to those uninfected with Schistosoma [15,41]. A study in Mali showed lower P. falciparum density in children with heavy intensity S. haematobium infection compared to those uninfected with Schistosoma [36]. A summary analysis based on the three studies showed significantly lower P. falciparum density in children infected with S. haematobium as compared to those uninfected with Schistosoma (summary β = -0.14; 95% CI = -0.24, -0.01; I2 = 39.7%) [15, 36, 41]; the difference was greater in children with low intensity S. haematobium infection (summary β = -0.30; 95% CI = -0.47, -0.12; I2 = 4.4%) (Fig 3). A study in Mali also showed significantly lower P. falciparum density among children aged 4 to 8 years with low-intensity S. haematobium infection compared to those uninfected with S. haematobium, however this difference in P. falciparum density was not significant (p = 0.19) when data were analyzed without stratifying by age and intensity of S. haematobium infection (5521 vs.6761) [16]. Although the difference was not statistically significant, the density of P. falciparum infection tended to be lower in children infected with S. haematobium (mean = 1764) compared to those uninfected with S. haematobium (mean = 2509), irrespective of the intensity of S. haematobium infection (p = 0.4) [34]. In contrast, a study in Senegal showed lack of significant difference in mean P. falciparum densities in infected children (mean = 626) compared to those uninfected (mean = 444) with S. haematobium but data were not stratified based on intensity of S. haematobium infection (p = 0.56) [35]. Based on a summary analysis of the data in the three studies [16,34,35], P. falciparum density tended to be lower in children infected with S. haematobium than those uninfected with the parasite (mean difference = -924.2, 95% CI = -2151.8, 303.4; I2 = 0.0). The differences in some of the aforementioned studies in mean P. falciparum densities among children with moderate or heavy (>10 eggs/10ml urine) intensity of S. haematobium infection compared to those not infected with the parasite were not significant [15,16,41]. The significant protective effects were largely seen in children with light intensity of S. haematobium infection.
Fig 3. Effect of S. haematobium infection on P. falciparum density in children in SSA.
Co-infection with Schistosoma and Plasmodium falciparum and haemoglobin level
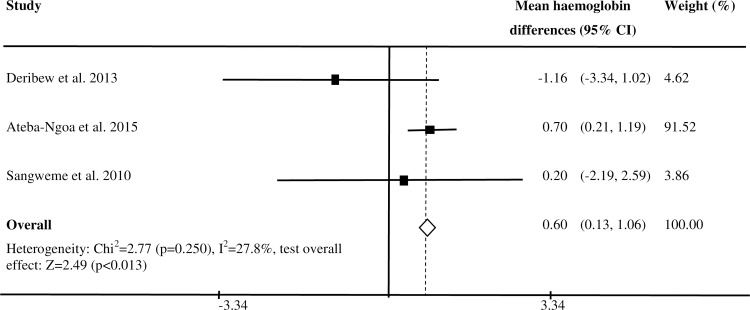
Out of 12 studies included in this review, four studies reported findings on mean haemoglobin level among children co-infected with P. falciparum and S. haematobium and those infected with only P. falciparum. A study in Gabon reported higher haemoglobin level among children co-infected with S. haematobium and P. falciparum than those infected with only P. falciparum (mean haemoglobin difference = 0.7; 95% CI = 0.21, 1.19) [32]. However, three studies in Mali, Ethiopia and Zimbabwe showed similar mean haemoglobin levels in children co-infected with S. haematobium and P. falciparum and those infected with only P. falciparum [16,31,34]. A summary estimate based on three studies [31, 32,34] showed higher mean haemoglobin level in children co-infected with S. haematobium and P. falciparum than those infected with only P. falciparum (summary mean haemgolobin level difference = 0.49; 95% CI: 0.04, 0.95; I2: 66.4%) (Fig 4).Among the four studies, two studies reported the odds of anaemia among children co-infected with P. falciparum and S. haematobium and those infected with only P. falciparum. The study in Ethiopia reported increased odds of anaemia [31], but the study in Zimbabwe [34] reported similar odds of anaemia in children co-infected with S. haematobium and P. falciparum as compared to those infected with only P. falciparum. All the four studies reported similar mean haemoglobin level in children infected with S. haematobium, and those uninfected with S. haematobium and P. falciparum [16, 31, 32,34].
Fig 4. Mean haemoglobin differences between children co-infected with S. haematobium and asymptomatic/uncomplicated P. falciparum and those infected with only P. falciparum.
Quality of the studies
Table 2 summarizes the quality of the studies included in this review in terms of selection bias, study design, confounders, blinding, data collection methods, withdrawals and dropouts. The majority of the studies showed strong quality in data collection methods. The quality of most studies included in this review was moderate in terms of design (prospective cohort), control of selection bias and blinding. Overall rating based on the six criteria showed that two studies were strong quality, six studies were of moderate quality and four studies were of weak quality. None of the studies were excluded from the review because of quality issues.
Table 2. Assessment of the quality of the studies included in the review based on Effective Public Health Practice Project: Quality assessment tool for quantitative studies.
| No. | Author, Year | SelectionBias | Study Design | Confounders | Blinding | Data Collection Methods | Withdrawals and Drop-Outs | Final Rating |
|---|---|---|---|---|---|---|---|---|
| 1 | Ateba-Ngoaet al., 2015 | 2 | 3 | 3 | 2 | 1 | NA | 2 |
| 2 | Briand et al. 2005 | 2 | 2 | 1 | 2 | 1 | 1 | 2 |
| 3 | Courtin et al. 2011 | 2 | 2 | 3 | 2 | 1 | 3 | 3 |
| 4 | Deribew et al. 2013 | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 5 | Doumbo et al. 2014 | 1 | 2 | 1 | 2 | 1 | 1 | 1 |
| 6 | Florey et al. 2012 | 3 | 3 | 2 | 2 | 1 | NA | 3 |
| 7 | Kabatereineet al. 2011 | 2 | 3 | 2 | 2 | 2 | NA | 2 |
| 8 | Lyke et al. 2005 | 3 | 2 | 1 | 2 | 1 | 1 | 2 |
| 9 | Mazigo et al. 2010 | 2 | 3 | 3 | 2 | 1 | NA | 3 |
| 10 | Lemaitre et al. 2014 | 2 | 2 | 1 | 2 | 1 | 3 | 2 |
| 11 | Sangweme et al. 2010 | 1 | 2 | 2 | 2 | 1 | 3 | 2 |
| 12 | Sokhna et al. 2004 | 2 | 2 | 2 | 2 | 1 | 1 | 1 |
1 = strong 2 = moderate 3 = weak NA = not applicable
Discussion
In the present systematic review of 12 studies based on 9,337 children in eight SSA countries, a meta-analysis of 11 studies confirmed increased odds of asymptomatic/uncomplicated P. falciparum infection among children infected with S. haematobium or S. mansoni as compared to those uninfected with Schistosoma in SSA. However, lower P. falciparum density was associated with S. haematobium co-infection, particularly when the intensity of S. haematobium infection was low. The finding of a higher prevalence of asymptomatic/uncomplicated P. falciparum infection among children infected with Schistosoma could be due to the occurrence of common social or environmental factors increasing the susceptibility of individuals to infection with both parasite groups [11]. The increased prevalence of asymptomatic/uncomplicated P. falciparum infection may result from both increased infection duration and/or increased incidence of the parasite. Increased incidence and infection duration could result from immune responses against chronic Schistosoma infection down regulating the effective response against P. falciparum infection [10]. Hence, P. falciparum parasites could persist for longer duration in children with chronic Schistosoma infection. P. falciparum sporozoites could have increased survival in liver, which is immunologically affected by S mansoni egg granulomas [10]. Children co-infected with S. mansoni may thus be more susceptible to P. falciparum infection. Another hypothesis is that anaemia associated with Schistosoma infection may cause hyperventilation of carbon dioxide and increased lactates, making the infected individuals more attractive for mosquitoes and thus increasing their risk for Plasmodium infection [42].
However, the finding of lower P. falciparum density among children with low intensity S. haematobium infection could be due to an imbalance in Th1/Th2 immune response, which might modulate the proportion of cytophylic antibodies that control Plasmodium parasitemia [10]. Low intensity S. haematobium may thus promote an effective Th1 immune response that will also have a protective effect against P. falciparum. In contrast, increased intensity S. haematobium infections may enhance a Th2 immune response that alter the protective Th1 response.
Studies included in this review showed varying effects of Schistosoma infection on the risk of acquiring P. falciparum infection [16, 36, 37]. This could be due to varying relationships of Schistosoma and P. falciparum infection based on the age of the host [16] and intensity of Schistosoma infection [16, 37] or due to small number of cases with insufficient power to detect statistically significant differences [36]. Indeed, the studies included in this review showed similar risk of acquiring uncomplicated P. falciparum infection in children infected and uninfected with S. haematobium when data were analyzed without stratifying by study participants’ ages or Schistosoma infection intensity [16, 36, 37]. However, the risk of acquiring uncomplicated P. falciparum infection and the density of parasitemia varied with the S. haematobium or S. mansoni infection status when data were analyzed stratified by age of the study participants or intensity of Schistosoma infection. For example, the risk of P. falciparum infection was higher among children with S. mansoni egg load >1000 eggs/per gram compared to children without S. mansoni, but this association was not seen when the analysis was restricted to children with moderate intensity S. mansoni egg load (100 to 399 eggs/per gram) [37]. Lyke et al. [16] showed delayed time to first clinical episode of malaria, fewer episodes, and lower levels of parasitemia in S. haematobium infected children aged 4 to 8 years compared to children uninfected with S. haematobium; this protective effect was not seen in children aged 9 to 14 years. Importantly, the decrease in incidence of P. falciparum infection and density of the parasite as well as the increased time to first clinical P. falciparum infection was more pronounced in children excreting low numbers of S. haematobium eggs than those excreting heavy S. haematobium eggs [16]. Doumbo et al [36] found a lower risk of asymptomatic/uncomplicated P. falciparum infection in children who were co-infected with S. haematobium and P. falciparum at baseline, but S. haematobium mono-infection at baseline (seen only in 23 children) was not significantly associated with protection from P. falciparum infection.
The summary estimates of the three studies included in this review showed a higher mean haemoglobin level in children co-infected with S. haematobium and P. falciparum than in those infected with only P. falciparum [31, 32, 34]. This result could reflect the immunological effects of Plasmodium infection on erythropoiesis and haemoglobin levels through interferon (IFN)-γ and tumor necrosis factor (TNF)-ᾳ activation [43]. Chronic Schistosoma infection stimulates anti-inflammatory cytokines interleukin (IL)-10 and Transforming growth factor (TGF)-β, which down-regulate (IFN)-γ and TNF-ᾳ [10]. Hence, Schistosoma infection may attenuate the haemoglobin reduction due to immunological effects of Plasmodium. However, in some studies included in this review, the findings of a lack of significant difference in haemoglobin levels between children co-infected with S. haematobium and P. falciparum and those infected with only P. falciparum could reflect different clinical stages of Plasmodium infection and/or low intensity Schistosoma infections. While chronic low Plasmodium parasitaemia affect haemoglobin through dyserythropoiesis, the effect of acute initial Plasmodium infection on haemoglobin is mainly the result of hemolysis [11]. Moreover, the effect of Schistosoma infection on the immunological modulation of Plasmodium related dyserythropoiesis could be low when the intensity of infection is light.
Public health implications
The findings of this review suggest that treatment of children in SSA for schistosomiasis may reduce the risk of asymptomatic/uncomplicated P. falciparum infection. Hartgers and Yazdanbakhsh, [10] suggested that praziquantel treatment may boost antimalarial immune response by reducing the down-regulating effect of Schistosoma [10]. However, the reasons why studies reported lower Plasmodium density among children with light [15, 16, 41], heavy [36] or any [34] intensity S. haematobium infection remains unclear. In addition, the impact of Schistosoma infection on risk of acquiring P. falciparum infection and severity of the disease is poorly understood. Thus, the question of whether the present mass treatment of children living in SSA against schistosomiasis could have some detrimental impact through more severe P. falciparum infection cannot be definitely answered based on this review. Mass treatment programs generally focus only on the control of schistosomiasis among school-age children in SSA. Since malaria and schistosomiasis frequently co-exist in school-age children and schistosomiasis appears to be associated with increased odds of P. falciparum infection, collaboration among control programs for both infections and other services for young children might be advantageous.
Strengths and limitations
This is the first meta-analysis to study association of P. falciparum and Schistosoma co-infection conducted and reported according to the PRISMA guidelines [22]. Studies included in the meta-analysis did not show publication bias. The summary estimates of P. falciparum density in children infected with S. haematobium compared with those uninfected with Schistosoma were adjusted estimates [15, 36, 41]. However, the present summary results in the meta-analyses of odds of asymptomatic/uncomplicated P. falciparum infection in children infected with Schistosoma were based on crude estimates of individual studies. Only one study provided adjusted estimates of the effect size [38]. Hence, the current summary estimates might have been affected by confounders that can influence the nature of relationship of Schistosoma and P. falciparum in the original studies. In addition, there was a moderate level of bias in selecting the study participants within the studies included in this review. This might have resulted in overestimation of the relationship between Schistosoma and asymptomatic/uncomplicated P. falciparum malaria in the current review. Intervention measures taken against Schistosoma or malaria in different regions before the studies could have also altered the nature of relationship between P. falciparum and Schistosoma. This could also have introduced bias into the review. Furthermore, there was a moderate level of heterogeneity among the studies examining association of P. falciparum and Schistosoma co-infection (Moran’s I2: 52.3.1%, Cochran’s Q: 14.66, p = 0.041). However, after removing one study [32], the heterogeneity decreased (Moran’s I2: 31.1%, Cochran’s Q: 8.66 p = 0.191)] and subgroup analysis further minimized the risk of heterogeneity among studies evaluating the effect of S. mansoni infection on the odds of P. falciparum infection (I2: 0.0%).
Limitations of the original studies related to the diagnosis of Plasmodium and Schistosoma infection could also affect the present results [44,45]. In addition, some studies which examined S. haematobium and P. falciparum co-infection did not examine the study participants for infection with soil-transmitted helminths. Therefore, children without S. haematobium infection may have had other helminth infections that could have further confounded the results [46,47]. Moreover, some studies included in this review did not follow WHO criteria to determine classes of intensity of Schistosoma infection. This made it difficult to clearly evaluate the potential effect of classes of intensity of Schistosoma infection on the current result. Finally, the lack of sufficient data precluded performing a meta-analysis to estimate the effect of Schistosoma co-infection on the risk of acquiring P. falciparum infection and related anaemia. We did not find any studies which examined the relationship between Schistosoma and P. vivax infection or severe malaria that were eligible in the present review.
Conclusions
The present review suggests that S. mansoni or S. haematobium co-infection may increase susceptibility of children for asymptomatic/uncomplicated P. falciparum infection. However, S. haematobium co-infection may protect against high P. falciparum density and related reduction in haemoglobin level. Findings on the effect of Schistosoma infection against the risk of P. falciparum infection are heterogeneous.
Supporting Information
(DOC)
(DOCX)
Odds ratio against standard error of odds ratio for eight studies, which compared the prevalence of asymptomatic/uncomplicated P. falciparum infection between children who were infected and uninfected with Schistosoma in SSA.
(TIF)
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.WHO. Malaria. http://www.who.int/mediacentre/factsheets/fs094/en/. Accessed January 14, 2016.
- 2.WHO. Schistosomiasis Fact sheet N°115". World Health Organization. http://www.who.int/mediacentre/factsheets/fs115/en/. Accessed October 16, 2015.
- 3.Snow RW, Omumbo JA. Malaria In: Jamison DT, Feachem RG, Makgoba MW, et al. , editors. Disease and mortality in Sub-Saharan Africa. 2nd edition Washington (DC): The World Bank; 2006. pp: 195–215. [Google Scholar]
- 4.Snow RW. Global malaria eradication and the importance of Plasmodium falciparum epidemiology in Africa. BMC Med. 2015. February; 13: 23 10.1186/s12916-014-0254-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015. September; 526(7572):207–11. 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014. September; 383: 2253–64. 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Disv. 2012. May; 4(1): e2012026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect. 2013. June; 11(6):623–39. [DOI] [PubMed] [Google Scholar]
- 9.Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, O'Meara W, et al. Asymptomatic malaria: a chronic and debilitating infection that should be treated. PLoS Med. 2016. January;13(1):e1001942 10.1371/journal.pmed.1001942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartgers FC, Yazdanbakhsh M. Co-infection of helminths and malaria: modulation of the immune responses to malaria. Parasite Immunol. 2006. October; 28(10):497–506. 10.1111/j.1365-3024.2006.00901.x [DOI] [PubMed] [Google Scholar]
- 11.Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, Snow RW, et al. Epidemiology of Plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg. 2007. December;77(6):88–98. [PMC free article] [PubMed] [Google Scholar]
- 12.Faye B, Ndiaye JL, Tine RC, Lô AC, Gaye O. Interaction between malaria and intestinal helminthiasis in Senegal: influence of the carriage of intestinal parasites on the intensity of the malaria infection. Bull Soc Pathol Exot. 2008. December;101(5):391–4. [DOI] [PubMed] [Google Scholar]
- 13.Degarege A, Legesse M, Girmay M, Animute A, Erko B. Malaria and related outcomes in patients with intestinal helminths: A cross-sectional study. BMC Infect Dis. 2012. November; 9;12:291 10.1186/1471-2334-12-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurlimann E, Yapi RB, Houngbedji CA, Schmidlin T, Kouadio BA, Silue KD, et al. The epidemiology of polyparasitism and implications for morbidity in two rural communities of Cote d'Ivoire. Parasite Vect. 2014. February; 7: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briand V, Watier L, Le Hesran J, Garcia A, Cot M. Co-infection with Plasmodium falciparum and Schistosoma haematobium: Protective effect of schistosomiasis on malaria in Senegalese children? Am J Trop Med Hyg. 2005. June;72(6):702–7. [PubMed] [Google Scholar]
- 16.Lyke KE, Dicko A, Dabo A, Sangare L, Kone A, Coulibaly D, et al. Association of Schistosoma haematobium infection with protection against acute plasmodium falciparum malaria in malian children. Am J Trop Med Hyg. 2005. December;73(6):1124–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Bejon P, Mwangi TW, Lowe B, Peshu N, Hill AV, Marsh K. Helminth infection and eosinophilia and the risk of Plasmodium falciparum malaria in 1- to 6-year-old children in a malaria endemic area. PLoS Negl Trop Dis. 2008. February; 2(2): e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooker SJ, Pullan RL, Gitonga CW, Ashton RA, Kolaczinski JH, Kabatereine NB, et al. Plasmodium-helminth coinfection and its sources of heterogeneity across east Africa. J Infect Dis. 2012. March; 205(5):841–52. 10.1093/infdis/jir844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. World Health Organ Tech Rep Ser. 2002;912:i–vi, 1–57. [PubMed] [Google Scholar]
- 20.Nacher M. Interactions between worms and malaria: good worms or bad worms? Malar J. 2011. September;12(10):259 10.1186/1475-2875-10-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adegnika A.A., Kremsner PG. Epidemiology of malaria and helminth interaction: a review from 2001 to 2011. Curr Opin HIV AIDS. 2012. May;7(3):221–4. 10.1097/COH.0b013e3283524d90 [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. BMJ. 2009. July;339:b2700 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Systematic Reviews. 2012. February; 1:2 10.1186/2046-4053-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NHS. International Prospective Register of Systematic Reviews (PROSPERO). National Institute for Health Research. http://www.crd.york.ac.uk/PROSPERO/. Accessed November 2, 2016.
- 25.WHO. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000. April;94 Suppl 1:S1–90. [PubMed] [Google Scholar]
- 26.WHO. Iron deficiency anaemia: assessment, prevention and control, a guide for programme managers Geneva: WHO; 2001. [Google Scholar]
- 27.Effective Public Health Practice Project. Quality assessment tool for quantitative studies. Hamilton, ON: Effective Public Health Practice Project. Available from: http://www.ephpp.ca/index.html. Accessed December 20, 2015.
- 28.Stata Corp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP
- 29.Hayashino Y, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. Epidemiol. 2005. November;15(6):235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altman D G, Bland JM. How to obtain the confidence interval from a P value BMJ 2011. August; 343:d2090. [DOI] [PubMed] [Google Scholar]
- 31.Deribew K, Tekeste Z, Petros B. Urinary schistosomiasis and malaria associated anemia in Ethiopia. Asian Pac J Trop Biomed. 2013. April;3(4):307–10. 10.1016/S2221-1691(13)60068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ateba-Ngoa U, Adegnika AA, Zinsou JF, Kassa Kassa RF, Smits H, Massinga-Loembe M, et al. Cytokine and chemokine profile of the innate and adaptive immune response of Schistosoma haematobium and Plasmodium falciparum single and co-infected school-aged children from an endemic area of Lambarene, Gabon. Malar J. 2015. February;14:94 10.1186/s12936-015-0608-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014. December 19;14:135 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sangweme DT, Midzi N, Zinyowera-Mutapuri S, Mduluza T, Diener-West M, Kumar N. Impact of Schistosoma infection on Plasmodium falciparum malariometric indices and immune correlates in school age children in Burma valley, Zimbabwe. PLoS Negl Trop Dis. 2010. November; 4(11):e882 10.1371/journal.pntd.0000882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Courtin D, Djilali-Saiah A, Milet J, Soulard V, Gaye O, Migot-Nabias F, et al. Schistosoma haematobium infection affects Plasmodium falciparum-specific IgG responses associated with protection against malaria. Parasite Immunol. 2011. February;33(2):124–31. 10.1111/j.1365-3024.2010.01267.x [DOI] [PubMed] [Google Scholar]
- 36.Doumbo S, Tran TM, Sangala J, Li S, Doumtabe D, Kone Y, et al. Co-infection of long-term carriers of Plasmodium falciparum with Schistosoma haematobium enhances protection from febrile malaria: a prospective cohort study in Mali. PLoS Negl Trop Dis. 2014. September;8(9):e3154 10.1371/journal.pntd.0003154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokhna C, Le Hesran JY, Mbaye PA, Akiana J, Camara P, Diop M, et al. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar J. 2004. November; 15;3:43 10.1186/1475-2875-3-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Florey LS, King CH, van Dyke MK, Muchiri EM, Mungai PL, Zimmerman PA, et al. Partnering parasites: Evidence of synergism between heavy Schistosoma haematobium and Plasmodium species infections in Kenyan children. PLoS Negl Trop Dis. 2012. July; 6(7): e1723 10.1371/journal.pntd.0001723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kabatereine BA, Standley JC, Sousa-Figueiredo CJ, Fleming MF, Stothard RJ, Talisuna A, et al. Integrated prevalence mapping of Schistosomiasis, soil-transmitted helminthiasis and Malaria in Lakeside and Island Communities in Lake Victoria, Uganda. Parasite Vect. 2011. December; 134: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazigo HD, Waihenya R, Lwambo NJS, Mnyone LL, Mahande AM, Seni J, et al. Co-infections with Plasmodium falciparum, Schistosoma mansoni and intestinal helminths among schoolchildren in endemic areas of northwestern Tanzania. Parasites Vect. 2010. May; 3(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemaitre M, Watier L, Briand V, Garcia A, Le Hesran JY, Cot M. Coinfection with Plasmodium falciparum and Schistosoma haematobium: Additional evidence of the protective effect of schistosomiasis on malaria in Senegalese children. Am J Trop Med Hyg. 2014. February; 90(2):329–334. 10.4269/ajtmh.12-0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nacher M. Worms and malaria: blind men feeling the elephant? Parasitolo. 2008;135:861–8. [DOI] [PubMed] [Google Scholar]
- 43.McDevitt MA, Xie J, Gordeuk V, Bucala R. The anemia of malaria infection: role of inflammatory cytokines. Curr Hematol Rep. 2004. March; 3(2):97–106. [PubMed] [Google Scholar]
- 44.Stete K, Krauth SJ, Coulibaly JT, Knopp S, Hattendorf J, Müller I, et al. Dynamics of Schistosoma haematobium egg output and associated infection parameters following treatment with praziquantel in school-aged children. Parasit Vectors. 2012. December; 5:298 10.1186/1756-3305-5-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Degarege A, Medhin G, Teklehaymanot T, Legesse M, Erko B. Day-to-day fluctuation of circulating cathodic antigen levels in urine and faecal egg counts in children infected with Schistosoma mansoni in Ethiopia. BMC Infect Dis. 2014. April; 14:210 10.1186/1471-2334-14-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Degarege A, Veledar E, Degarege D, Erko B, Nacher M , Madhivanan P. Plasmodium falciparum and soil-transmitted helminths co-infection among children in sub-Saharan Africa: systematic review and meta-analysis. Parasit Vectors. 2016. June; 9:344 10.1186/s13071-016-1594-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Degarege A, Erko B. Epidemiology of Plasmodium and helminth coinfection and possible reasons for heterogeneity. Biomed Res Int. 2016; 2016 March;3083568. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Odds ratio against standard error of odds ratio for eight studies, which compared the prevalence of asymptomatic/uncomplicated P. falciparum infection between children who were infected and uninfected with Schistosoma in SSA.
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.