Abstract
Preeclamptic women produce agonistic autoantibodies to the angiotensin II type 1 receptor (AT1-AA) and exhibit increased blood pressure (MAP), vascular sensitivity to angiotensin II (ANG II), and display a decrease in renal function. The objective of this study was to examine the renal hemodynamic changes during pregnancy in the presence of AT1-AAs with or without a slow pressor dose of ANGII. In this study MAP was elevated in all pregnant rats treated with ANG II with or without AT1-AA. Glomerular filtration rate (GFR) was reduced from 1.90 ±0.16 ml/min in normal pregnant (NP) to 1.20 ±0.08 in ANGII + AT1-AA rats. Renal blood flow (RBF) was decreased in ANGII + AT1-AA vs NP rats to 7.4 ±1.09 vs. 15.4 ±1.75 ml/min. Renal vascular resistance (RVR) was drastically increased between ANGII + AT1-AA vs NP rats (18.4 ±2.91 vs. 6.4 ±0.77 mmHg/ml/min). Isoprostane excretion was increased by a 3.5 fold in ANG II + AT1-AA vs. NP (1160±321 vs. 323±52 pg/ml). In conclusion ANG II and AT1-AA together, significantly decrease GFR by 37%, RBF by 50%, and caused a 3 fold increase in RVR and isoprostane levels vs NP rats. These data indicate the importance of AT1-AAs to enhance ANG II induced renal vasoconstriction and reduce renal function as mechanisms to cause hypertension as observed during preeclampsia.
Keywords: AT1-AA, ANGII, renal, pregnancy, preeclampsia, GFR, renal function
Introduction
Preeclampsia is a multisystemic disorder that is characterized by new onset hypertension that usually occurs in the 3rd trimester of pregnancy1, 2. It is estimated that between 3–5% of pregnant women in the USA each year have preeclampsia. Preeclampsia is the leading cause of preterm birth, morbidity, and mortality for the mother and the fetus1, 2. Women with preeclampsia have elevated levels of the angiotensin II type 1 receptor autoantibodies (AT1-AA), oxidative stress, reduced renal function, and increased ANG II sensitivity3–8. AT1-AAs in preeclampsia bind with a high affinity to the 7 amino acid sequence on the second extracellular loop of the angiotensin II type 1 (AT1) receptor, in which it activates the AT1 receptor, increases intracellular calcium levels, and stimulates activation of intracellular MAP/ERK kinase and TNFα pathways similar to ANG II9–13.
Several studies from our lab and others have demonstrated the important role of AT1-AAs in the pathophysiology of preeclampsia. In-vitro studies with vascular smooth muscle and trophoblast cells demonstrate increased tissue factor VII and tissue factor activity, reactive oxygen species (ROS), NADPH oxidase components, and activation of NFκβ when incubated with AT1-AAs13–15. Furthermore, human umbilical vein endothelial cells (HUVEC) incubated for 6 hours with the AT1-AAs drastically increased endothelin-1 (ET-1) protein expression16. In-vivo studies, in which isolated human and rodent AT1-AAs administered to rats during pregnancy, increased blood pressure, oxidative stress, renal artery resistant index, and several circulating factors associated with preeclampsia, such as sFlt-1, sEng, endothelin-1, and endothelial microparticles7, 16–22. Thus all these studies taken together confirm the critical role of AT1-AAs in causing many of the common characteristics of preeclampsia observed in women7, 16–22.
Another characteristic shared by women with preeclampsia is increased sensitivity to ANG II, demonstrated years ago by Gant et al8. In Gant’s studies, young women between ages of 13–17, with majority being Black American women, were infused with various doses of ANG II until their diastolic blood pressure increased by 20 mmHg11. In this study they found that women destined to become preeclamptic displayed an increase in blood pressure at lower concentrations of ANG II versus those that had a normal pregnancy. From this early study it was established that women with preeclampsia have increased sensitivity to ANG II from as early as 22 weeks of gestation until term11.
In rodent studies it has been shown that renal hemodynamic parameters, such as glomerular filtration rate (GFR), renal vascular resisintance (RVR), and renal blood flow (RBF) are not altered by ANG II infusion, suggesting that the renal vascularture in normal pregnant rodents, and perhaps humans, are refractory to the vasoconstrictory actions of ANG II (23) In our previous studies, the renal artery resistance index was enhanced to a greater degree with low pressor doses of ANG II and AT1-AA infused together into normal pregnant rats, thus suggesting that AT1-AA enhances the renal vasoconstriction of low dose ANG II17. Additionally, renal afferent arterioles isolated from pregnant rats subjected to non-constrictor doses of ANG II and the AT1-AA together, caused enhanced vasoconstriction compared to nonconstrictor doses of either ANGII or AT1AA alone4. Therefore, these data support the synergistic effect to occur in the kidney between ANG II and AT1-AA during pregnancy. Thus we believe the presence of AT1-AAs during preeclampsia facilitates the increase in ANG II sensitivity. Although our previous data has shown marked changes in the renal artery resistance index and constriction of renal afferent arterioles, the effect of AT1-AAs and ANG II on renal hemodynamics during pregnancy has never been examined. Therefore, the overall purpose of this study was to examine the changes in renal hemodynamics during pregnancy in the presence of AT1-AAs and/or ANGII.
Methods
Pregnant Sprague Dawley rats purchased from Harlan Sprague Dawley Inc. (Indianapolis, IN) were used in this study. Rats were housed in a temperature-controlled room (75°F) with a 12 hour light and dark cycle each day and maintained on a normal diet with free access to water. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center. All experiments performed were in accordance with the National Institutes of Health guidelines for use and care of animals.
Administration of ANG II and/or AT1-AA
On day 13 of pregnancy rats were anesthetized with isoflurane and implanted with osmotic mini-pumps (Alzet Model 2001, Durect Corp, Cupertino, CA) intraperitoneally infusing angiotensin II (ANG II) at 50 ng/kg/min and/or agonistic angiotensin II type 1 receptor auto-antibodies (AT1-AA; 1:40 dilution), previously isolated from reduced uterine pressure rats (RUPP), the rat model of preeclampsia. Pregnant Sprague Dawley rats were divided into 4 groups: Normal Pregnant (NP, n=6), Pregnant + ANG II (ANG II, n=6), Pregnant + AT1-AA (AT1-AA, n=8), and Pregnant + ANG II + AT1-AA (ANGII + AT1-AA, n= 6). The concentration of ANG II was selected, as it should not result in a rapid increase in blood pressure, but rather a slow and modest increase in blood pressure. The 1:40 dilution for the AT1-AA was chosen because it has the same bio-activity response serum from preeclamptic patients containing the AT1-AA, and it is the dose that we previously published to achieve similar levels of AT1-AAs found in circulation of PE women when infused into pregnant rats3. Furthermore this dilution of AT1-AA alone has been shown increase blood pressure and other facets of preeclampsia when infused into pregnant rats.
Renal Function Assessment
On day 19 of pregnancy, rats were subjected to terminal renal function surgeries. Briefly, pregnant rats were anesthetized with Ketamine (30 mg/Kg im) and Inactin (50 mg/Kg ip) and maintained at 37°C on a thermostatically controlled surgical table. Next, catheters were placed into the femoral artery and vein for the measurement of blood pressure and the infusion of a saline solution containing FITC-labeled inulin (2 mg/mL) for the determination of GFR. A transonic flowmeter probe was placed on the left renal artery to measure renal blood flow and the bladder was cannulated for the collection of urine. After a 45–60 minute equilibration period, a 30-minute urine and plasma collection was performed. At the end of the experiment, the plasma and urine were collected for the determination inulin concentrations by fluorescence. At the end of the experiments, the rats were euthanized, and blood and kidneys were collected and snap frozen in liquid nitrogen. All tissues and blood were stored in the −80°C freezer.
Determination of oxidative stress
Plasma isoprostanes were measured using the 8-Isoprostane EIA kit (Cayman Chemical, Ann Harbor, MI; 516351) according to the manufactures instructions. The intra and inter-assay variation in concentration (CV) for this kit were determined at multiple points on the standard curve, with CVs lower than 20%.
Statistical Analysis
All data and results are presented as means ± SE. Data were analyzed by one-way ANOVA with Bonferroni post hoc analysis or by student t-test comparing each group to normal pregnant (control) values using Graphpad Prism 6 software (GraphPad Software, La Jolla, CA). P < 0.05 was considered statistically significant.
Results
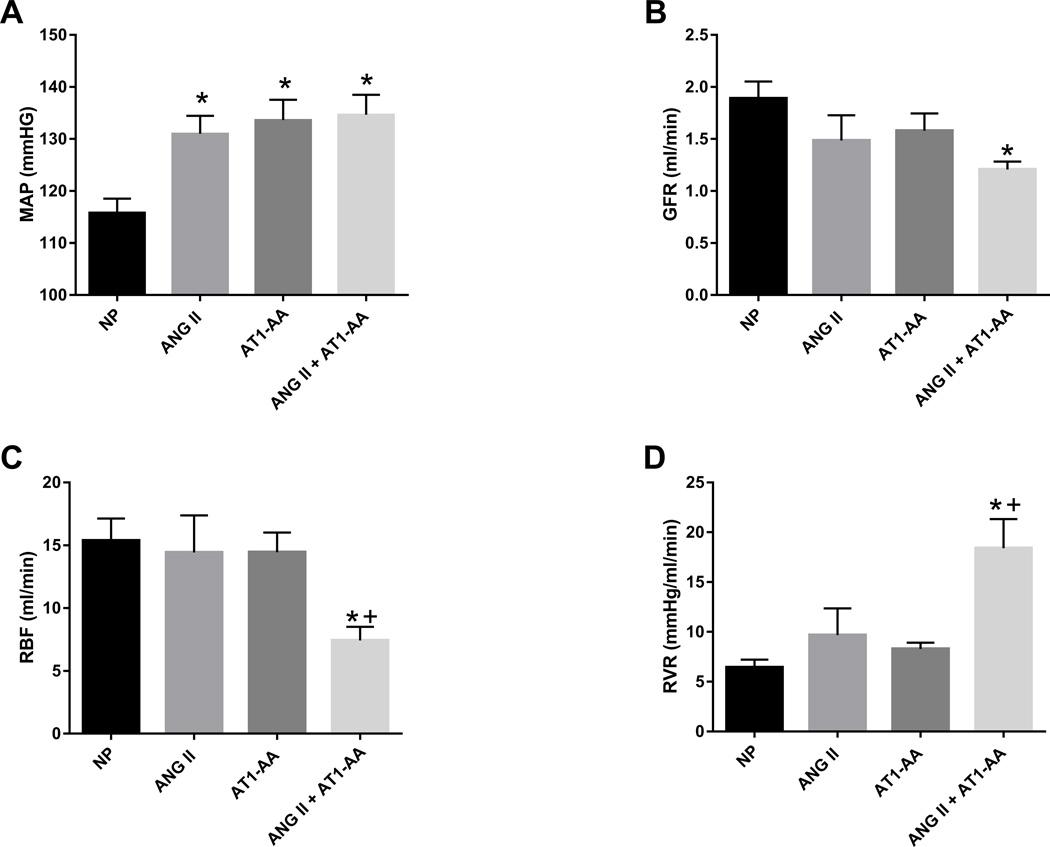
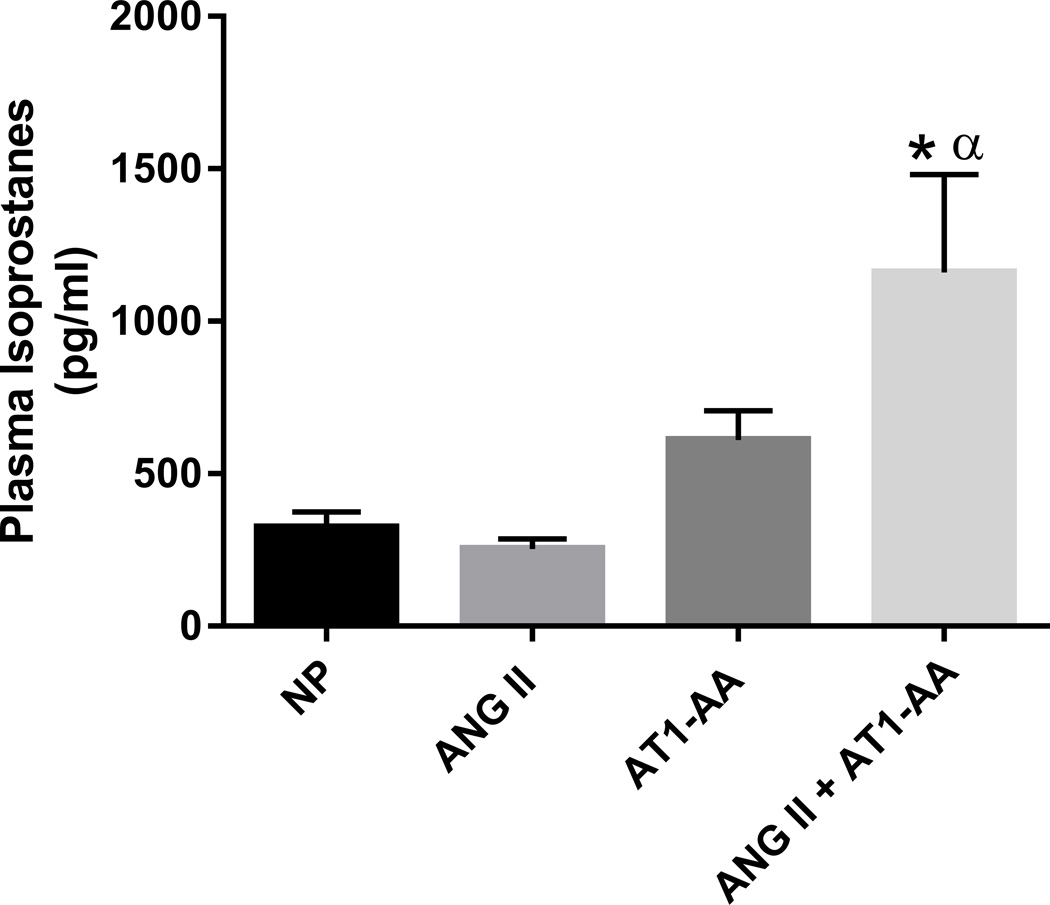
Blood pressure was elevated in pregnant rats treated with ANG II (131±3 mmHg), AT1-AA (134±4 mmHg), and ANG II + AT1-AA (135±4 mmHg) vs. normal pregnant rats (116±3 mmHg, p<0.05) (Figure 1A). As shown in previous studies17, there was no difference in blood pressure between rats administered ANG II with those receiving AT1-AA alone and both together (Figure 1A). There was a significant decrease in GFR with ANG II + AT1-AA vs. normal pregnant rats (1.21 ±0.08 vs. 1.89±0.16 ml/min, p<0.05 t-test) (Figure 1B), however, the difference in GFR between pregnant rats administered ANG II (1.49±0.24 ml/min) and the AT1-AA (1.58±0.17 ml/min) vs normal pregnant rats (1.89±0.16 ml/min) was not significant. Furthermore, treatment with ANG II + AT1-AA significantly reduced RBF compared to all other groups (7.42±1.09 ml/min), ANG II 14.42±2.96 ml/min, AT1-AA 14.44±1.58 ml/min vs normal pregnant rats 15.37±1.75 ml/min. (Figure 1C). Importantly, we detected a 3-fold increase in RVR in ANG II + AT1-AA treated rat’s vs normal pregnant (18.41±2.91 vs. 6.44±0.78 mmHg/ml/min). However there was no change in RVR between pregnant rats’ administered ANG II (9.67±2.6 mmHg/ml/min) or the AT1-AA (8.29±0.62 mmHg/ml/min) vs normal pregnant rats (6.44±0.78). (Figure 1D). In addition, isoprostane levels were 3 times higher in ANG II + AT1-AA treated pregnant rats compared to normal pregnant (1160±321 vs. 323±52 pg/ml). We did not observe any differences in plasma isoprostane levels in either ANG II or AT1-AA alone, (Figure 2).
Figure 1.
A) MAP was elevated in ANG II, AT1-AA, ANG II + AT1-AA vs. normal pregnant rats (NP). B) GFR was decreased in ANGII + AT1-AA vs. NP rats. C) RBF was decreased in ANGII + AT1-AA vs. NP rats and ANGII + AT1-AA vs. AT1-AA rats. D) RVR was drastically increased in ANG II + AT1-AA vs. NP rats. Statistical changes is MAP, RBF, and RVR were derived using one-way ANOVA with Bonferroni post hoc analysis, *p<0.05 vs NP and +p<0.05 vs. AT1-AA. No difference in GFR was observed using one-way ANOVA analysis. However, student t-test comparing ANGII + AT1-AA vs. NP rats (control) was statistically different, *p<0.05 vs NP.
Figure 2.
Isoprostane levels were increased in ANG II + AT1-AA vs. NP rats and ANG II + AT1-AA vs. ANG II rats. One-way ANOVA with Bonferroni post hoc analysis, *p<0.05 vs NP and αp<0.05 vs. ANG II.
Discussion
The initiating event of preeclampsia is thought to be reduced placental perfusion that leads to endothelial dysfunction in the maternal vasculature. However, the mechanisms linking placental ischemia with endothelial, renal, and cardiovascular pathophysiology during preeclampsia are unclear24–27. Numerous factors have been implicated in the pathogenesis of preeclampsia, including immune activation, upregulation of endothelin (ET-1), and reactive oxidative stress (ROS)2, 4, 12, 17, 27, 28. In the 1960’s human studies performed by Gant et al demonstrated that women that went on to develop preeclampsia exhibited increased vasoconstriction and blood pressure sensitivity to intravenously infused ANG II than those that went on to have normal pregnancies8. However, biochemical assessment indicates preeclamptic women have normal or lower plasma renin activity (PRA) and ANG II levels29–32.
Since the 1970’s, small advances in determining he mechanism of increased ANG II sensitivity in pregnancy induced hypertension have been made. One mechanism of increased ANG II sensitivity can be attributed to elevated levels of circulating soluble fms-like tyrosine kinase 1 (sFlt1), a circulating antiangiogenic protein, during preeclamptic pregnancies. sFlt1 is a soluble variant of VEGF receptor 1, which inhibits VEGF and placental growth factor in tissues and in circulation from binding to VEGF receptors33. VEGF receptor activation is important for normal pregnant implantation and is correlated with the severity of preeclampsia33. Overexpression studies of sFlt1 in pregnant mice induced angiotensin II sensitivity by impairing endothelial nitric oxide synthase (eNOS) phosphorylation and increasing oxidative stress.34 These alterations in NO production and bioavailability are observed in women with preeclampsia. Another component that may play a role in increased ANG II sensitivity during preeclampsia is a decrease in the G- protein regulating protein RGS535. Studies by Holobotovskyy et al., showed that pregnant RGS5 deficient mice, caused gestational hypertension via an increase in ANG II sensitivity35. This was proven by supplemental doses of ANG II given to RGS5 deficient mice, which displayed the preeclampsia-like symptoms of hypertension, proteinuria, placental pathology, and reduced birth weight of the fetuses. In summary these data suggest that reduced RGS5 in mice and women may play a critical role in increased ANG II sensitivity in preclamptic patients35.
Several years later in the 90s, Wallukat et al reported that preeclamptic women produce an autoantibody to the ANGII type 1 receptor (AT1-AA)3. After this discovery numerous research has been performed, examining the role of AT1-AAs in the pathophysiology of preeclampsia36–38. This AT1-AA binds to and activates the AT1R, thereby increasing chronotropic events in cultured cardiomyocytes, very similarly to ANGII. Our previously published data demonstrated that the AT1-AA markedly enhances the effect of ANGII potentiating the downstream signaling events, such as ET-1, ROS and vasoconstriction in renal afferent arterioles from pregnant rats17. Nevertheless these drastic changes were not associated with an even greater increase in blood pressure in rats treated with both AT1-AA and ANGII together compared to those treated with either ANG II or AT1-AA alone17. Therefore, the purpose of this study was to determine if the drastic effect in renal vasoconstriction seen previously translated to significant changes in renal function evaluated by RBF, GFR, and RVR.
Although, in concert with previous findings, blood pressure was not exacerbated, we did find that pregnant rats administered ANG II + AT1-AAs, exhibited a marked decrease in renal function when compared to NP, ANGII or AT1-AA treated groups17. The decrease in renal function in this study is characterized by a 37% decrease in GFR and a 50% decrease in RBF in pregnant rats treated with ANG II + AT1-AA compared to NP, ANG II or AT1-AA treated groups. The decrease in RBF and GFR was associated with a 3 fold increase in RVR in the ANGII + AT1-AA group compared to NP, ANG II or AT1-AA treated groups. These changes in renal hemodynamics are similar to what is observed in women with preeclampsia5.
In normal human and rodent pregnancy there is usually an increase in GFR and RBF due to a decrease in RVR in comparison to non-pregnant women or virgin rats39,40,41. The decrease in RVR observed in pregnancy is attributed to an increase in NO production during pregnancy, which causes vasodilation of the systemic and renal vasculature leading to a decrease in total peripheral resistance and RVR42,39,40,41. However, in pregnancy complications, such as preeclampsia, RVR is increased and GFR and RBF are decreased5, 43. The exact cause for these alterations in renal hemodynamics is unknown and is often linked to an increase in oxidative stress and decrease in NO bioavailability28, 42. Note that it is important to maintain these normal alterations in renal hemodynamics during pregnancy, because these changes are critical to the mortality and morbidity of the mother and the fetus5, 42, 43. In fact earlier studies done in the 1970’s by Gibson et al. showed that the lack of rise in GFR during early pregnancy is associated with women that have increased risks of unexplained stillbirths, abortions, or small for gestational age babies43. The same trends have been observed in more recent studies where there is a strong positive correlation between maternal GFR and fetal size44. Furthermore, the lack of rise in GFR during pregnancy can increase the levels of circulating uric acid during pregnancy and damage the glomerular filtration barrier, leading to an increase in proteinuria and glomerular damage45.
In the current study, the increase in RVR is consistent with our previous observations, in which there is an increase in renal artery resistance index and increase in vasoconstriction of renal afferent arterioles incubated with ANG II with AT1-AA17. In these previous studies we observed the renal artery resistance index increased with AT1-AA infusion, and was even furthered enhanced with ANGII + AT1-AA induced hypertension17. The increase in RVR observed here could be attributed to the systemic 3 fold increase in plasma isoprostane levels, a marker for oxidative stress, in rats administered ANG II and AT1-AA compared to normal pregnant rats. Previous studies in our lab were performed with tempol, a superoxide dismutase mimetic, which markedly reduced oxidative stress and decreased blood pressure in pregnant rats infused with AT1-AA7. Furthermore, treatment with tempol in ANG II + AT1-AA infused rats significantly improved RARI but not blood pressure, indicating that this may be a pathway more specific for the AT1-AA compared to ANGII.
Due to the enhanced increase in RVR and decrease in GFR with ANG II in the presence of AT1-AAs, we believe that AT1-AAs are a mechanism for increased sensitivity to ANGII during preeclampsia by increasing the AT1R interaction with ANG II. Although not directly tested in this study, one possible mechanism where by the AT1-AA can increase ANGII sensitivity is by binding to the 2nd extracellular loop of the AT1 G-coupled receptor, creating bonds between transmembrane receptor domains 2 and 7, triggering a conformational change that will increase the binding affinity for ANG II36, 46. Another mechanism is by dimerization of the AT1R with the vasodepressor bradykinin receptor (B2)47. Studies performed by AbdAlla et al, showed that women with preeclampsia have increased B2 protein amounts and that AT1/ B2 heterodimerzation is present in the platelets and the omental vessels of preeclamptic patients, thus suggesting a plausible mechanism of increase ANG II binding47,48, 49. Future studies are currently being designed to test if this exacerbated effect is by increased binding or by downstream mechanisms that may be occurring in the presence of AT1-AAs.
In conclusion, we have shown that pregnant rats administered both the ANG II and AT1-AA display a decrease renal function and oxidative stress, which may further contribute to the increase in RVR and rise in blood pressure. Our data strongly suggest that there is an enhanced responsiveness to ANG II in the presence of the AT1-AAs during pregnancy, which maybe a mechanism of increased ANGII sensitivity in preeclamptic patients. Overall this study highlights the importance of AT1-AAs in the renal pathophysiology of preeclampsia. Thus drugs targeted to specifically inhibit the binding of the AT1-AAs during pregnancy may serve as a future therapeutic for women with preeclampsia. These conclusions are in concert with studies performed by Zhou et al., where administration of the inhibitory peptide to the AT1-AA, displayed improvements in albuminuria and glomerular endotheliosis caused by human AT1-AA into pregnant mice.. These signs along with decreased renal function and hypertension are classical markers of preeclampsia22. This study in corroboration with the study by Zhou et al suggests that blockade of AT1-AA could improve renal function during preeclampsia and highlights the importance of continued research in this area.
Perspective.
Understanding the role of AT1-AAs in pathophysiology of preeclampsia is important as we search for new therapies to help these patients. This study suggests that AT1-AAs enhance the deleterious actions of ANG II during pregnancy to increase renal vascular resistance and decrease renal function. The exact mechanisms where by AT1-AAs enhance ANG II sensitivity is still unknown and will be the topic for future research. Nevertheless, we conclude that AT1-AA inhibition maybe a potential therapy for preeclamptic patients.
Novelty and Significance.
What is New?
Rats administered both AT1-AA and ANG II display an increase in blood pressure, renal vascular resistance, and circulating isoprostane levels. AT1-AAs administered with ANG II, further increased the renal vascular resistance, isoprostanes, and decreased GFR suggesting that AT1-AA’s enhance ANG II induced renal vasoconstriction during pregnancy.
What is Relevant?
This study is relevant because it shows that AT1-AAs with a slow pressor dose ANG II, significantly enhances renal vascular resistance and decrease renal function during pregnancy. This data suggest that the AT1-AA maybe a causal factor in the increased vascular response PE women had to ANGII, which was previously observed by Norman Gant.
Based on our data, AT1-AA inhibition during preeclampsia maybe a potential therapy for preeclamptic patients to reduce the endogenous effect to ANGII that may play a pathophysiological role in the disease.
Summary
In summary, this study suggests that AT1-AAs enhance the harmful actions of ANG II during pregnancy to increase renal vascular resistance, decrease renal function, and increase oxidative stress, perhaps contributing to hypertension during pregnancy. Overall this study highlights the importance of AT1-AAs in the renal pathophysiology of hypertensive disorders during pregnancy.
Acknowledgments
Funding Sources
This work was supported by NIH grants HL78147 and HL51971 and HD067541. RD is supported by the German Research Foundation (DFG, DE 631/9-1). JMW is supported by funds from the National Institutes of General Medical Sciences of the National Institutes of Health (NIGMS/NIH) Obesity, Cardiorenal and Metabolic Diseases-COBRE (P20GM104357) and the American Heart Association (12SDG9440034).
Footnotes
Conflict of Interest/ Disclosure Statement
There are no disclosures or conflicts of interest.
References
- 1.Amaral LM, Cunningham MW, Jr, Cornelius DC, LaMarca B. Preeclampsia: Long-term consequences for vascular health. Vascular Health and Risk Management. 2015;11:403–415. doi: 10.2147/VHRM.S64798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW, Jr, Wallace K, LaMarca B. The role of inflammation in the pathology of preeclampsia. Clinical Science. 2016;130:409–419. doi: 10.1042/CS20150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin at1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herse F, LaMarca B. Angiotensin ii type 1 receptor autoantibody (at1-aa)-mediated pregnancy hypertension. Am J Reprod Immunol. 2013;69:413–418. doi: 10.1111/aji.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller-Deile J, Schiffer M. Preeclampsia from a renal point of view: Insides into disease models, biomarkers and therapy. World Journal of Nephrology. 2014;3:169–181. doi: 10.5527/wjn.v3.i4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsubara K, Higaki T, Matsubara Y, Nawa A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. International Journal of Molecular Sciences. 2015;16:4600–4614. doi: 10.3390/ijms16034600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parrish MR, Wallace K, Tam Tam KB, Herse F, Weimer A, Wenzel K, Wallukat G, Ray LF, Arany M, Cockrell K, Martin JN, Dechend R, LaMarca B. Hypertension in response to at1-aa: Role of reactive oxygen species in pregnancy-induced hypertension. American Journal of Hypertension. 2011;24:835–840. doi: 10.1038/ajh.2011.62. [DOI] [PubMed] [Google Scholar]
- 8.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin ii pressor response throughout primigravid pregnancy. The Journal of Clinical Investigation. 1973;52:2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai W, Zhang W, Jin Z, Feng Y, Kuang Y, Zhi J. Angiotensin ii type i receptor agonistic autoantibody-induced apoptosis in neonatal rat cardiomyocytes is dependent on the generation of tumor necrosis factor-alpha. Acta Biochimica et Biophysica Sinica. 2012;44:984–990. doi: 10.1093/abbs/gms087. [DOI] [PubMed] [Google Scholar]
- 10.Jin Z, Wang J, Zhang W, Zhang G, Jiao X, Zhi J. Changes in cardiac structure and function in rats immunized by angiotensin type 1 receptor peptides. Acta Biochimica et Biophysica Sinica. 2011;43:970–976. doi: 10.1093/abbs/gmr096. [DOI] [PubMed] [Google Scholar]
- 11.Yang S, Zhong Q, Qiu Z, Chen X, Chen F, Mustafa K, Ding D, Zhou Y, Lin J, Yan S, Deng Y, Wang M, Zhou Y, Liao Y, Zhou Z. Angiotensin ii receptor type 1 autoantibodies promote endothelial microparticles formation through activating p38 mapk pathway. Journal of Hypertension. 2014;32:762–770. doi: 10.1097/HJH.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 12.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC, 3rd, Xia Y, Kellems RE. Antibodies from preeclamptic patients stimulate increased intracellular ca2+ mobilization through angiotensin receptor activation. Circulation. 2004;110:1612–1619. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- 13.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. At(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–2387. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 14.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. At1 receptor agonistic antibodies from preeclamptic patients stimulate nadph oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 15.Estelles A, Gilabert J, Grancha S, Yamamoto K, Thinnes T, Espana F, Aznar J, Loskutoff DJ. Abnormal expression of type 1 plasminogen activator inhibitor and tissue factor in severe preeclampsia. Thrombosis and Haemostasis. 1998;79:500–508. [PubMed] [Google Scholar]
- 16.Wenzel K, Rajakumar A, Haase H, Geusens N, Hubner N, Schulz H, Brewer J, Roberts L, Hubel CA, Herse F, Hering L, Qadri F, Lindschau C, Wallukat G, Pijnenborg R, Heidecke H, Riemekasten G, Luft FC, Muller DN, Lamarca B, Dechend R. Angiotensin ii type 1 receptor antibodies and increased angiotensin ii sensitivity in pregnant rats. Hypertension. 2011;58:77–84. doi: 10.1161/HYPERTENSIONAHA.111.171348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewer J, Liu R, Lu Y, Scott J, Wallace K, Wallukat G, Moseley J, Herse F, Dechend R, Martin JN, Jr, Lamarca B. Endothelin-1, oxidative stress, and endogenous angiotensin ii: Mechanisms of angiotensin ii type i receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension. 2013;62:886–892. doi: 10.1161/HYPERTENSIONAHA.113.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhillion P, Wallace K, Herse F, Scott J, Wallukat G, Heath J, Mosely J, Martin JN, Jr, Dechend R, LaMarca B. Il-17-mediated oxidative stress is an important stimulator of at1-aa and hypertension during pregnancy. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2012;303:R353–R358. doi: 10.1152/ajpregu.00051.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin ii type i receptor (at1-aa) in pregnant rats: Role of endothelin-1. Hypertension. 2009;54:905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G, Keiser S, Ray LF, Dechend R, Martin JN, Granger JP, LaMarca B. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin ii type i receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. American Journal of Hypertension. 2010;23:911–916. doi: 10.1038/ajh.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, Martin JN, Jr, Dechend R, Lamarca B. Activating autoantibodies to the angiotensin ii type i receptor play an important role in mediating hypertension in response to adoptive transfer of cd4+ t lymphocytes from placental ischemic rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2012;302:R1197–R1201. doi: 10.1152/ajpregu.00623.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nature Medicine. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak J, Reckelhoff J, Bumgarner L, Cockrell K, Kassab S, Granger JP. Reduced sensitivity of the renal circulation to angiotensin ii in pregnant rats. Hypertension. 1997;30:580–584. doi: 10.1161/01.hyp.30.3.580. [DOI] [PubMed] [Google Scholar]
- 24.Roberts JM, Pearson G, Cutler J, Lindheimer M Pregnancy NWGoRoHD. Summary of the nhlbi working group on research on hypertension during pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 25.Sibai BM, Caritis S, Hauth J National Institute of Child H, Human Development Maternal-Fetal Medicine Units N. What we have learned about preeclampsia. Seminars in Perinatology. 2003;27:239–246. doi: 10.1016/s0146-0005(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 26.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 27.Granger JP. Inflammatory cytokines, vascular function, and hypertension. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2004;286:R989–R990. doi: 10.1152/ajpregu.00157.2004. [DOI] [PubMed] [Google Scholar]
- 28.Pereira AC, Martel F. Oxidative stress in pregnancy and fertility pathologies. Cell Biology and Toxicology. 2014;30:301–312. doi: 10.1007/s10565-014-9285-2. [DOI] [PubMed] [Google Scholar]
- 29.Shah DM. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. American Journal of Physiology. Renal Physiology. 2005;288:F614–F625. doi: 10.1152/ajprenal.00410.2003. [DOI] [PubMed] [Google Scholar]
- 30.August P, Lenz T, Ales KL, Druzin ML, Edersheim TG, Hutson JM, Muller FB, Laragh JH, Sealey JE. Longitudinal study of the renin-angiotensin-aldosterone system in hypertensive pregnant women: Deviations related to the development of superimposed preeclampsia. American Journal of Obstetrics and Gynecology. 1990;163:1612–1621. doi: 10.1016/0002-9378(90)90639-o. [DOI] [PubMed] [Google Scholar]
- 31.Israel A, Peceno A. Renin-angiotensin-aldosterone system in pregnancy-induced hypertension. Journal of Human Hypertension. 2000;14(Suppl 1):S36–S39. doi: 10.1038/sj.jhh.1000985. [DOI] [PubMed] [Google Scholar]
- 32.Brown MA, Nicholson E, Gallery ED. Sodium-renin-aldosterone relations in normal and hypertensive pregnancy. British Journal of Obstetrics and Gynaecology. 1988;95:1237–1246. doi: 10.1111/j.1471-0528.1988.tb06812.x. [DOI] [PubMed] [Google Scholar]
- 33.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sflt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. The Journal of Clinical Investigation. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burke SD, Zsengeller ZK, Khankin EV, Lo AS, Rajakumar A, DuPont JJ, McCurley A, Moss ME, Zhang D, Clark CD, Wang A, Seely EW, Kang PM, Stillman IE, Jaffe IZ, Karumanchi SA. Soluble fms-like tyrosine kinase 1 promotes angiotensin ii sensitivity in preeclampsia. The Journal of Clinical Investigation. 2016;126:2561–2574. doi: 10.1172/JCI83918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holobotovskyy V, Chong YS, Burchell J, He B, Phillips M, Leader L, Murphy TV, Sandow SL, McKitrick DJ, Charles AK, Tare M, Arnolda LF, Ganss R. Regulator of g protein signaling 5 is a determinant of gestational hypertension and preeclampsia. Science Translational Medicine. 2015;7:290ra288. doi: 10.1126/scitranslmed.aaa5038. [DOI] [PubMed] [Google Scholar]
- 36.Balakumar P, Jagadeesh G. Structural determinants for binding, activation, and functional selectivity of the angiotensin at1 receptor. Journal of Molecular Endocrinology. 2014;53:R71–R92. doi: 10.1530/JME-14-0125. [DOI] [PubMed] [Google Scholar]
- 37.Dechend R, Muller DN, Wallukat G, Homuth V, Krause M, Dudenhausen J, Luft FC. Activating auto-antibodies against the at1 receptor in preeclampsia. Autoimmunity Reviews. 2005;4:61–65. doi: 10.1016/j.autrev.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Dechend R, Homuth V, Wallukat G, Muller DN, Krause M, Dudenhausen J, Haller H, Luft FC. Agonistic antibodies directed at the angiotensin ii, at1 receptor in preeclampsia. Journal of the Society for Gynecologic Investigation. 2006;13:79–86. doi: 10.1016/j.jsgi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney International. 1980;18:152–161. doi: 10.1038/ki.1980.124. [DOI] [PubMed] [Google Scholar]
- 40.Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney International. 1998;54:2056–2063. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham MW, Jr, Sasser JM, West CA, Baylis C. Renal redox response to normal pregnancy in the rat. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2013;304:R443–R449. doi: 10.1152/ajpregu.00496.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Podjarny E, Mandelbaum A, Bernheim J. Does nitric oxide play a role in normal pregnancy and pregnancy-induced hypertension? Nephrology, Dialysis, Transplantation : Official Publication of the European Dialysis and Transplant Association - European Renal Association. 1994;9:1527–1529. [PubMed] [Google Scholar]
- 43.Gibson HM. Plasma volume and glomerular filtration rate in pregnancy and their relation to differences in fetal growth. The Journal of Obstetrics and Gynaecology of the British Commonwealth. 1973;80:1067–1074. doi: 10.1111/j.1471-0528.1973.tb02981.x. [DOI] [PubMed] [Google Scholar]
- 44.Morken NH, Travlos GS, Wilson RE, Eggesbo M, Longnecker MP. Maternal glomerular filtration rate in pregnancy and fetal size. PloS One. 2014;9:e101897. doi: 10.1371/journal.pone.0101897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeyabalan A, Conrad KP. Renal function during normal pregnancy and preeclampsia. Frontiers in Bioscience : a Journal and Virtual Library. 2007;12:2425–2437. doi: 10.2741/2244. [DOI] [PubMed] [Google Scholar]
- 46.Inoue Y, Nakamura N, Inagami T. A review of mutagenesis studies of angiotensin ii type 1 receptor, the three-dimensional receptor model in search of the agonist and antagonist binding site and the hypothesis of a receptor activation mechanism. Journal of Hypertension. 1997;15:703–714. doi: 10.1097/00004872-199715070-00001. [DOI] [PubMed] [Google Scholar]
- 47.Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: Preeclampsia and beyond. Circulation Research. 2013;113:78–87. doi: 10.1161/CIRCRESAHA.113.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quitterer U, Lother H, Abdalla S. At1 receptor heterodimers and angiotensin ii responsiveness in preeclampsia. Seminars in Nephrology. 2004;24:115–119. doi: 10.1016/j.semnephrol.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 49.AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased at(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin ii responsiveness. Nature Medicine. 2001;7:1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]