Abstract
Portable electronic devices and wireless communication systems enable a broad range of applications such as environmental and food safety monitoring, personalized medicine and healthcare management. Particularly, hybrid smartphone and microfluidic devices provide an integrated solution for the new generation of mobile sensing applications. Such mobile sensing based on microfluidic devices (broadly defined) and smartphones (MS2) offers a mobile laboratory for performing a wide range of bio-chemical detection and analysis functions such as water and food quality analysis, routine health tests and disease diagnosis. MS2 offers significant advantages over traditional platforms in terms of test speed and control, low cost, mobility, ease-of-operation and data management. These improvements put MS2 in a promising position in the fields of interdisciplinary basic and applied research. In particular, MS2 enables applications to remote infield testing, homecare, and healthcare in low-resource areas. The marriage of smartphones and microfluidic devices offers a powerful on-chip operating platform to enable various bio-chemical tests, remote sensing, data analysis and management in a mobile fashion. The implications of such integration are beyond telecommunication and microfluidic-related research and technology development. In this review, we will first provide the general background of microfluidic-based sensing, smartphone-based sensing, and their integration. Then, we will focus on several key application areas of MS2 by systematically reviewing the important literature in each area. We will conclude by discussing our perspectives on the opportunities, issues and future directions of this emerging novel field.
Keywords: Smartphone, Microfluidic Device, Mobile Sensing, Environment and Food Safety, Point-of-Care (PoC), Health Management, Personalized Medicine
1 Introduction
With the rapid emergence of materialization and the improvement of living standards in modern civilization, people become increasingly concerned about their personal health status and are more aware of various influencing factors. Most of the current health monitoring services are still based on professional technologies operated by highly skilled professionals, which are time-consuming, costly and labor-intensive. Consequently, health monitoring and the accompanying data management are mainly limited to resource-rich areas, resulting in high burden in costs. Therefore, there is a growing trend to develop portable sensing devices that can be used outside professional facilities (e.g. in-field testing, homecare test, and testing in low-resource areas) and extend the capabilities of current health-related monitoring patterns while reducing costs and operation requirements1. To this end, lab-on-a-chip (LoC) devices are drawing increasing attention in many areas for diagnostic applications, such as environmental monitoring2, health management3, and medical diagnosis4. A successful LoC device must meet the requirements of low cost, high sensitivity and specificity, and userfriendliness. In view of these requirements, microfluidic devices are important enabling technologies for the emerging field of LoC-based point-of-care (PoC) applications5.
Microfluidic devices can precisely manipulate microliter volume of fluidic samples in micrometer length scale of channels and reaction chambers6, 7. These devices enable various on-chip chemical and biological assays with reduced reagent and sample consumption, well-controlled microenvironments, high-throughput experimentation, batch processing, and controlled sample handling and reaction8. Microfluidic devices have been broadly applied as useful experimental tools in chemistry, biology, physics, engineering and biomedical sciences9, 10, 11. Early microfluidic devices were fabricated from silicon, glass and polydimethylsiloxane (PDMS)12, 13. Recently, both thermoplastics14 and cheap paper15 were adopted as novel fabrication materials (Fig. 1). These microchips are usually operated by guiding the transport and reaction of fluidic samples based on capillary forces. Some of them have been used for routine diagnostic monitoring through colorimetric sensing (e.g. the well-known pregnancy rapid diagnostic test strip (RDTs) based on lateral flow immunochromatography). On one hand, the evolution of fabrication materials technologically enabled scientists to develop low-cost diagnostic tools using microfluidic devices with built-in analysis capabilities16, 17, 18. On the other hand, adoption of current microfluidic diagnostic devices for routine tests faces the challenge of inertia for using traditional assays in the biomedical field and the limitation of existing microfluidic devices to obtain reliable quantitative test results19, 20. Furthermore, the complex external instruments that are required for operating microfluidic devices and labbased tests present a major bottleneck for practical implementation of microfluidic diagnostic methods.
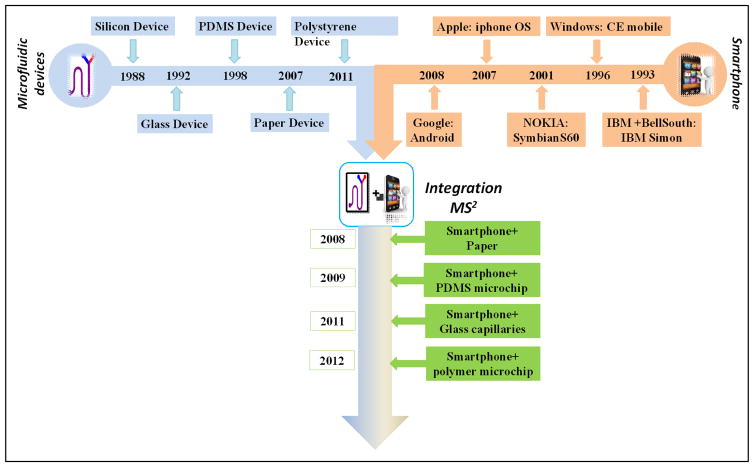
Fig. 1. Historical development of microfluidic device, smartphone and MS2.
The first smartphone was developed by IBM more than 20 years ago (Fig. 1). The early generation of smartphones was limited in terms of the performance of processors and sensors and short unplugged battery operation time. The new generation of smartphones significantly improved their performance and functionalities in these aspects through enabling technologies such as back-illuminated sensors, highperformance processors, and access to on-board power. Thus, the new generation of smartphones and their functions provide a new mobile platform to implement microfluidic diagnostic devices (Fig. 1). The rapid development of smartphones with various embedded sensors enabled applications in health-related monitoring. For example, smartphone cameras and audio jacks have been widely used to obtain health-related images and audio signals such as heart rate, blood oxygen saturation, and spirometry data21. In addition to smartphones’ built-in sensors, external sensors (e.g., circuit and probe) have also been integrated into the smartphones for more sophisticated detection (e.g. electrochemical (EC) reaction, body temperature, and ultrasound imaging)22–24. In this direction, smartphone-based diagnosis also aims to enable easier and more efficient testing in resource-limited settings, which coincides with the important goal of microfluidic diagnostic devices. The common features of smartphones and microfluidic devices with regard to miniaturization, ease-of-operation and portability suggest the high potential for their effective integration toward enabling a wide range of new mobile diagnostic applications. Such mobile sensing based on microfluidic devices (broadly defined) and smartphones (abbreviated as MS2) can be generalized as a unique class of applications.
As one of the earliest development in this area, in 2008, Martinez and colleagues from Harvard University integrated a smartphone with paper-based microfluidic chips to detect the concentrations of glucose and proteins in artificial urine25. In 2009, Lu and colleagues demonstrated a low-cost, portable immunoassay system, which integrated a PDMS microfluidic device and a smartphone26. Integration with the smartphone platform not only allows microfluidic systems to perform convenient and fast diagnosis testing but also permits long distance transmission and management of the test data from the microfluidic systems. If remote diagnosis and decision-making are required, the smartphone can collect test data and communicate remotely to an expert for feedback in real time. The key concept of MS2 is adding new diagnostic functions to the pre-existing mobile communication equipment owned by the users instead of creating new test systems. Such a model makes it possible to perform highly specialized diagnostic tests by end-users on a globally accepted platform27, 28.
In the past few years, the new generation of smartphones created opportunities for the development of portable quantitative bio-chemical analysis tools integrated with microfluidic devices29. Recent critical reviews described the development of smartphone-based PoC applications in medicine, imaging, cellular and bio-molecular analysis30–39. However, to our best knowledge, a comprehensive review focused on the integration of smartphones and microfluidic devices for mobile sensing applications is not yet available. To fill this gap, we provide a timely review of the latest development of MS2 based on the relevant literature over the past few years, which covers the application areas in environmental and food safety testing (e.g. water and food pollution detection), routine health testing and management (e.g. cholesterol and glucose detection) and biomedical diagnosis (e.g. bacteria, pathogen, gene and nucleic acid detection) (Table 1). In addition, this review highlights the applications of MS2 in telemedicine and commercialization. Lastly, we present a brief discussion on the current limitations, challenges and future directions of MS2.
Table 1.
A systematic summary of MS2 applications.
| Detection Target | Test Principle | Microfluidic Devices | Comments | Ref. | |
|---|---|---|---|---|---|
| Environment and food safety monitoring | E. coli | Fluorescence | Glass capillary | LOD: 5–10 CFU /mL | 42 |
| E. coli | Mie scattering | Paper device | LOD: 100 CFU/mL | 47 | |
| E. coli | EC | Polymer chip | LOD: 100 nM | 62 | |
| B. anthracis Sterne spores | Direct image sensing | PDMS chip | detect 50 to 5000 B. anthracis Sterne spores in 3 to 5 hours | 52 | |
| BDE-47 | ELISA | PDMS chip | Testing range: 10−3–104 μg/L | 48 | |
|
| |||||
| Cu,Ni,Cd,Cr | Colorimetric | Paper device | LOD: 0.29, 0,33, 0.35, 0.19 ppm | 56 | |
| Arsenic | EC | Polymer chips | LOD: 1 ppb | 57 | |
|
| |||||
| Nitrite | Colorimetric | Paper device | LOD: 0.52 mg/L | 29 | |
| Nitrate | EC | Test strip | LOD: 0.2 ppm | 61 | |
|
| |||||
| Routine health tests | Glucose | Colorimetric | Paper device | LOD: 0.μmM | 70 |
| pH. | Colorimetric | Test strip | Monitoring in sweat and saliva | 74 | |
| Cholesterol | Colorimetric | Test strip | 1.8% accuracy (140 to 400 mg dL−1) | 72 | |
| Vitamin D | Colorimetric | Test strip | Precision 10 nM. | 73 | |
| Cholesterol, Bile Acid | BL-CL | Test strip | Cholesterol LOD: 20 mg dL−1 Bile Acid LOD: 5 μmol L−1 |
76 | |
|
| |||||
| Biomedical Diagnosis | E. coli | LAMP | Polymer chip | Finish testing in 30 min | 89 |
| Kaposi’s Sarcoma | PCR | PDMS chip | Power consumption is 80 mW | 84 | |
| Salmonella Typhimurium | Fluorescent | Paper device | Cellulose paper: LOD:103 CFU mL−1 Nitrocellulose paper: LOD:104 CFU mL−1 |
90 | |
|
| |||||
| Loa loa | Direct image sensing | Glass capillary | 94% specificity and 100% sensitivity | 91 | |
| HRP-2 | Mie scattering | PDMS | LOD: 1 pg mL−1 in 10% blood3 | 92 | |
| HIV, syphilis | ELISA | PDMS | Sensitivity: 92 to 100% Specificity : 79 to 100% |
94 | |
| TSH | Mie scattering | Test strip | LOD: 0.31 mUI L−1 | 93 | |
| PfHRP2 | EC | PDMS | LOD: 16 ng mL−1 | 60 | |
| Malaria, Tuberculosis, HIV | Colorimetric | Test strip | Fast test result evaluation | 104 | |
2 MS2 for Environment and Food Safety Monitoring
2.1. Bacteria and Inorganic Pollutants Detection
Improper handling of food or water sources increases the chances of contamination. Bacterial or inorganic infections can even lead to death. Therefore, effective food and water contamination detection methods are in high demand. Traditionally, contamination in food and water was detected by microscopy-based methods. Other examples of commonly used methods for detecting bacterial concentration included nitrite assays and RDTs. However, conventional bright-field and fluorescence microscopes were large and expensive. In order to address these limitations, optical modification of smartphones has been implemented to develop compact and cost-effective microscopy modalities. In early 2009, Breslauer and colleagues developed a microscope attachment for a smartphone (CellScope) that was capable of both bright-field and fluorescence imaging for global health applications40. In addition, Smith and colleagues reported a smartphone-based microscope fabricated via integration of a commercial smartphone having a 1 mm ball lens with a 350× objective microscope and a visible-light fluorescence spectrometer for biomedical applications41. These smartphonebased imaging modalities opened up opportunities for developing new portable detection methods for food and water contamination.
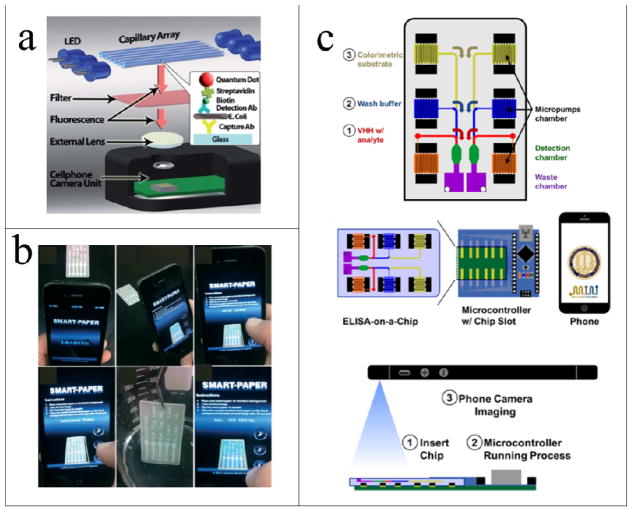
Zhu and colleagues used antibody-functionalized glass capillaries with quantum dots for detection of Escherichia coli (E. coli) contamination in water and food samples42 (Fig. 2a). The smartphone, glass capillaries, filter and external lens were assembled into a light plastic case. The glass capillaries pretreated with antibodies against E. coli served as the microfluidic channels. This system offered a more compact, lightweight and cost-effective solution for specific detection of E. coli compared with traditional methods such as flow cytometry, polymerase chain reaction (PCR), enzyme-linked immunosorbent assay (ELISA) and mass spectroscopy. In addition, the high surface-to-volume ratio of the glass capillaries improved the limit of detection (LOD) to 5–10 cfu mL−1, which was significantly lower than the commercial 18 minute test strip for bacteria detection (LOD is 103 cfu mL−1). The fluorescently conjugated antibodies binding to E. coli were excited by battery-powered light-emitting diodes (LEDs) and the fluorescence images were captured using the smartphone camera, which was positioned at the bottom of these glass capillaries. The signal-to-noise ratio increased due to the high photostability of the quantum dots compared to traditional organic dyes43. The glass capillaries can be replaced with parallel glass substrates to achieve cost-effective and wide-field fluorescence imaging with <10 μm resolution44. In addition, the same group developed an optofluidic fluorescence imaging platform integrated with a PDMS microchip and a smartphone to detect white blood cells and Giardia lamblia from drinking water45.
Fig. 2. MS2 for environmental and food safety monitoring.
(a) Schematic illustration of E. coli detection with a cell phone using a quantum dotbased sandwich assay42. (b) Smartphone application for Salmonella detection from a multi-channel microfluidic device47. (c) An integrated mobile PoC system including a camera phone, an Arduino microcontroller on copper electrodes with a microfluidic chip slot, and a microfluidic ELISAon-a-chip48. The figures are adapted from ref. 42, 47 and 48 with permission from the Royal Society of Chemistry for (a and b) and AIP Publishing LLC for (c), respectively.
To reduce the cost of optical devices, Park and colleagues developed a paper-based device to detect Salmonella typhimurium in water by Mie scattering46, 47 (Fig. 2b). The device was preloaded with antibody-conjugated polystyrene microbeads, which recognized the target pathogen. This, in turn, triggered the immunoagglutination of the microbeads and the resulting intensity of light scattering was correlated with the target pathogen concentration. Paper microfluidic devices not only are more cost-effective and easier to use, but also have competitive advantages in terms of mass manufacturing over conventional photo- or soft-lithographic techniques. In addition, paper devices with cellulose fibers have the advantage of filtering out contaminants such as dust/soil particles and algae in field samples that might cause interference. In this method, the smartphone position and detection angle were optimized and only the ambient light source was used for image acquisition. In addition, the LOD was at the single-cell level and the total assay time was shortened to 90 s.
In a recent study, Chen and colleagues reported a smartphone-based ELISA assay for detecting 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), an environmental contaminant found in food supply48 (Fig. 2c). The microfluidic chip contained three layers (i.e. a top sample injection layer with the sample chambers, a middle network layer with microfluidic channels, and a bottom electrode layer made by mixing carbon black with PDMS). Carbon– PDMS electrodes controlled using an Arduino controller (powered by the USB interface from the smartphone) have been incorporated to generate electrolytic reactions for microfluidic manipulations (i.e. produce gaseous bubbles with volume displacement to drive liquid movement and control experiment operation without external pressure equipment). The smartphone captured the colorimetric signal in the test zone as the quantitative readout49. In this study, the detection range was 10−3–104 μg L−1, which was comparable to that of standard ELISA. The platform was not limited only to BDE-47 detection, but can also be applied to detection of some antigens. In addition to application of the electrolytic principle to generate gas for microfluidic manipulations, both the on-chip pneumatic system and modular digital microfluidic system have also been successfully integrated with a smartphone as a high-level controller and post processing station for immunoassay and droplet actuation without human intervention50, 51.
The smartphone-based colorimetric sensing significantly reduces the cost compared to traditional methods with specialized imaging instruments such as digital color detectors, photodetectors, spectrophotometers, charge-coupled devices (CCDs), and complementary metal oxide semiconductors (CMOS). To further reduce the complexity of the setup, Hutchison and colleagues developed a simple microfluidic incubation device (MID) combined with a smartphone-based microscope for rapid, sensitive and reagent-free detection of viable B. anthracis spores52. In this work, the PDMS-based microfluidic incubation chamber consisted of a coarse filter, an incubation chamber, a fine filter, and a waste chamber. The sample was injected into the inlet, and the large debris and particulates in the sample were removed by the coarse filter before the filtered sample entered the incubation chamber. After incubation, the sample was aspirated into the waste chamber and vegetative bacterial filaments were trapped by the fine filter for optical monitoring. The images were captured using a smartphone. In this platform, no external light source was needed for illumination. In comparison with existing traditional culture plating techniques, such as immunoassays and PCR-based methods, this new approach reduced the assay time and was highly sensitive, specific, and cost-effective.
2.2. Heavy Metal Pollution Detection
Heavy metals in water pollution include arsenic, cadmium, iron, cobalt, chromium, copper, manganese, mercury, molybdenum, nickel, lead, selenium, vanadium and zinc. Heavy metals can enter water via various natural processes. For example, rain or flowing water can leach heavy metals out of geological formations. Heavy metals are bio-accumulative, are toxic at high concentrations, and cause various health problems such as cancers. To detect heavy metal pollution, portable, qualitative and quantitative analytical assays are critically required. Commercial colorimetric test kits such as the ‘Heavy Metals Water Test Kit’ can be used only for qualitative detection. The AAS method for metal ion detection allows quantitative testing but suffers from complicated operation and relatively long test times. MS2 offers a new approach to enable quantitative, user-friendly and faster heavy metal detection.
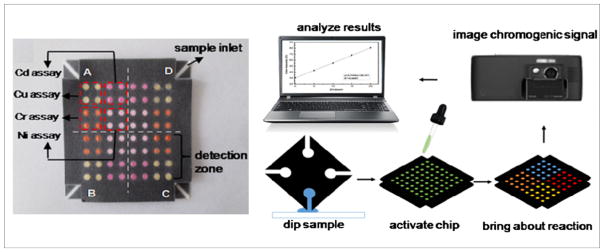
Among the different MS2 devices, three-dimensional (3D) paper-based microfluidic devices have shown attractive features. Compared with 3D polymeric systems53, 3D paper systems significantly reduce fabrication complexity and cost. The combination of smartphones and 3D paper devices further enables quantitative testing in resource-limited settings54, 55. One of such examples was a paper-based 3D multi-layer microfluidic chip for colorimetric sensing of four heavy metal ions developed by Wang and colleagues (Fig. 3)56. The 3D paper chip was designed with multiple detection zones. Driven by capillary force, four different samples from a water sample were introduced into the 3D chip independently without crossing. The samples reacted with the specific metalselective chromogenic reagents within minutes and the colorimetric images, which indicated the concentration of heavy metal ions, were captured and analyzed using the smartphone. The LODs for Cu, Ni, Cd, and Cr were 0.29, 0.33, 0.35, and 0.19 ppm, respectively, which were lower than that obtained by flame atomic absorption spectroscopy (FAAS) after digestion with 1 : 1 nitric acid according to the Environmental Protection Agency (EPA) Method 200.7.
Fig. 3. MS2 application for heavy metal detection.
Schematic illustration of a metal assay based on a 3D paper microfluidic device and a cell phone. Samples are added to the paper device for metal chromogenic reaction in the detection zone. The chromogenic signals are imaged using a camera cell phone and analyzed through a personal computer with image processing and analysis software56. The figure is adapted from ref. 56 with permission from Springer.
In contrast to optical detection methods, the electrochemical (EC) protocol provides more stable and quantifiable signals, which are insensitive to environmental optical variations. In a recent development, the USB protocol replaced the traditional camera-based MS2 for capturing images or fluorescence signals. One representative example for USB-based MS2 was the implementation of the EC sensing mode. Unyoung and colleagues demonstrated an EC sensor combined with the microfluidic platform, smartphone and EC analyzer to detect arsenic as the main groundwater contaminant57. The microfluidic platform comprised an ink-based three-electrode system used for loading water samples. The EC analyzer ran a voltammetry test on the sample to determine the arsenic concentration. The smartphone communicated with the EC analyzer through a USB cable and the data was processed and shown on the user interface. The LOD for heavy metal arsenic was 1 ppb. Such an MS2-based EC detection method offered advantages in terms of portability, low cost and userfriendliness, which permitted its effective use in remote or resource-limited areas.
2.3. Nitrate, Nitrite and pH Test
Nitrate and nitrite are associated with septic waste and agricultural processes. Therefore, they are indicators of environmental pollution such as bacterial contamination, which are commonly assessed by nitrite assays and RDTs58. Due to its high solubility in water, nitrate is one of the most common contaminants in groundwater in rural and suburban areas. Nitrite, as a reduced form of nitrate, is known as a major cause of food poisoning in human. Therefore, monitoring nitrate and nitrite in drinking water and food is in high demand. According to the World Health Organization (WHO), the maximal admissible concentration of nitrite is 3 mg L−1. In addition, the pH of water is also known as one of the common indicators for safe drinking, swimming, and other water-related industries59. As one example, bacterial contamination can be indicated by acidic pH.
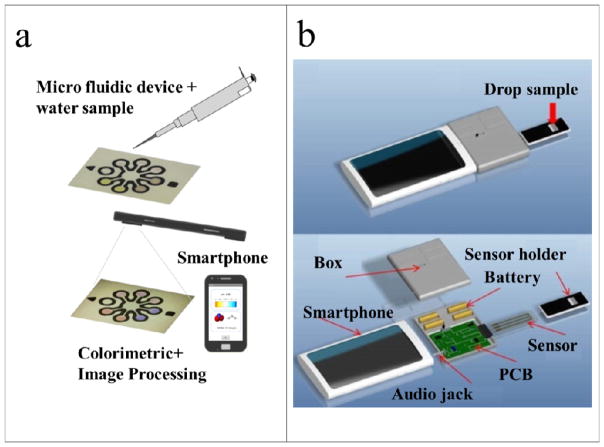
Lopez-Ruiz and colleagues developed a paper-based colorimetric sensor for simultaneous determination of nitrite and pH in water samples (Fig. 4a)29. The paper chip was designed with four detection areas for testing the pH, two detection areas for testing the nitrite concentration, and a reference detection area. For the pH test, the four areas were separated into two regions, wherein two different pH indicators (i.e. phenol red and chlorophenol red) were preloaded. For the nitrite test, the color change was based on the Griess reaction. An Android application for smartphone-based image acquisition and processing was developed in order to test the performance of the system; nine solutions with different concentrations of nitrite at different pH values were examined. In addition, the authors tested the system in a second smartphone and obtained similar results that demonstrated the applicability of the method to different smartphones with high reproducibility. This method achieved a resolution of 0.04 units and an accuracy of 0.09 units for the pH test, which were significantly better than those obtained with the commercial pH test paper; for the nitrite test, it achieved a resolution of 0.51% at 4.0 mg L−1, a linear range from 4.0 mg L−1 to 85 mg L−1 (wider than that of the commercial ‘Nitrate and Nitrite Test Strips’), and an LOD of 0.52 mg L−1.
Fig. 4. MS2 applications for nitrite, nitrate and pH detection.
(a) Schematic illustration of a smartphone-based application for measurement of nitrite concentration and pH in combination with a low-cost paper-based microfluidic device29. (b) A mobile EC sensing system for nitrate detection. It includes a miniaturized EC sensor and a mobile phone-based control platform61. The figures are adapted from ref. 29 and 61 with permission from the American Chemical Society for (a) and Elsevier for (b), respectively
A micro-USB interface can be used as the communication interface for rapid EC detection57. However, reliable test results on different operating systems such as Android and iOS is difficult to achieve when a USB protocol alone is used. Therefore, a USB host-enabled accessory needs to be integrated into the MS2 systems to make it compatible with smartphones running on USB client mode-only versions of Android60. In order to address this limitation, audio jacks have been introduced into MS2 for EC sensing. For example, Wang and colleagues demonstrated an EC sensing system (Fig. 4b), which included a miniaturized EC sensor, a smartphone, a circuit board for controlling the EC reaction, and a paper microfluidic chip for quantitative measurement of nitrate concentration from only a few microliters of a field liquid sample61. The audio jack in the smartphone was used to connect the electrical input and output signals between the smartphone and the circuit. The LOD of the nitrate test was 0.2 ppm and the test time was within 1 min. A similar concept was applied by Unyoung and colleagues to test Arsenic57 and E. coli62 in field water.
3 Routine Health Tests Using MS2
Many routine health tests detect different health indicators in human fluids. Among them, salivary pH is known as an important factor for preventing enamel decalcification63, and sweat pH indicates the risk of dehydration during physical activity64. In addition, serum cholesterol monitoring helps prevent heart disease65, 66, and serum Vitamin D is associated with diabetes and cardiovascular diseases67, 68, 69. These routine tests can be performed using self-diagnostic tools. However, existing self-diagnostic tools are relatively expensive and usually require specialized equipment. MS2 offers a promising new solution for routine health tests inside and outside the clinics.
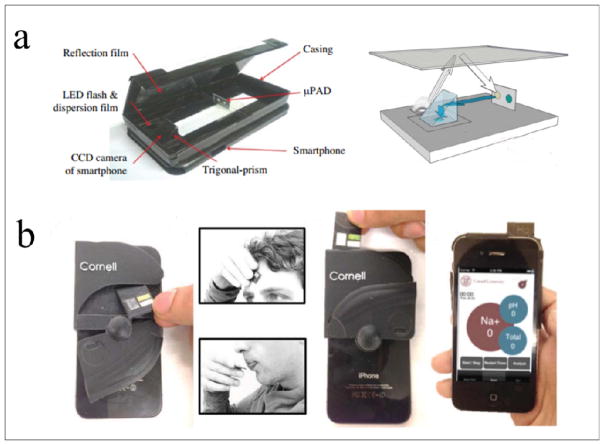
Chun and colleagues developed a paper-based glucose sensing system, which used a smartphone as the signal reader combined with other accessories including a case, a hard board, a reflection film, and a prism (Fig. 5a)70. The paper device consisted of two layers: the top layer had a loading zone and a detection zone and the bottom layer contained microfluidic channels. A blood sample was added to the loading zone and reached the detection zone driven by capillary force. In the detection zone, the reaction between glucose oxidase (GOx) and horseradish peroxidase triggered a bluecolored signal as the signal readout. An opaque case was used to house the smartphone which blocks light from the environment, and a flashlight was used as the light source. The performance of this system was validated by testing various concentrations of glucose. The LOD value was approximately 0.3 mM, which was higher than that for the commercial test strip (in the order of 3M)71. However, the cutoff glucose concentration in serum for assessment of diabetes is generally ~7 mM. Therefore, this developed system allowed effective glucose detection in blood for diabetes assessment.
Fig. 5. MS2 applications for routine health tests.
(a) Schematic illustration of a smartphone-based optical biosensor for glucose monitoring in combination with a low-cost paper-based microfluidic device70. (b) Smartphone-based health sweat and saliva biomarker monitoring on the test strip74. The figures are adapted from ref. 70 and 74 with permission from Springer for (a) and the Royal Society of Chemistry for (b), respectively.
Oncescu and colleagues demonstrated the rapid quantification of cholesterol levels from whole blood based on colorimetric changes in portable test strips72. Generally, test strips containing dry reagents are disposable and provide a visual readout of the results by changing colors or forming lines at specific locations (e.g. the famous pregnancy test strips). Directly reading the results of the test strips by naked eyes can be inaccurate. This issue can be addressed by smartphonebased imaging and analysis methods. The current test strip was designed to separate plasma from whole blood based on a series of filter papers and the separated plasma was directed to the reaction pad. A mini-3D printed supporter was used to block the ambient light and the smartphone flash was used for illumination. The test accuracy for cholesterol levels was within 1.8% from 140 mg dL−1 to 400 mg dL−1. The detection results were compared to those of the CardioChek PA system and the maximum difference of the test results between the two systems was less than 5.5%. The same group also tested the vitamin D levels in blood and pH values in sweat and saliva. In these studies, the developed MS2 offered an integrated design that permits non-invasive real-time analysis using disposable test strips and colorimetric signal detection on a mobile platform (Fig. 5b) 73, 74. For testing of pH values in sweat and saliva, the accuracy was within 0.2 pH unit. For testing of vitamin D levels, the MS2 can measure physiological levels of vitamin D and the test results were comparable with those for commercial ELISA kits.
Coupled biochemiluminescence (BL–CL) enzymatic reactions can also be used as a method for biosensing with increased sensitivity and LOD compared to conventional colorimetric substrates75. Roda and colleagues reported the use of a smartphone for BL–CL detection coupled with a 3D-printed microfluidic accessory to detect bile acids and cholesterol in blood and oral fluids76. Two specific assays were tested as the proof-of-principle: a bioluminescence (BL) assay for total bile acid detection and a chemiluminescence (CL) assay for total cholesterol detection. In this work, the sample cartridge contained a blood filter pad, which was housed in a holder and connected to a reaction chamber through a microfluidic channel. The reaction chamber consisted of a nitrocellulose disk for supporting the specific enzymes. In addition, a separate 15 μL reservoir for BL–CL reagents was connected to the reaction chamber via another microfluidic channel. The mini-cartridge was made by 3D printing for fast and easy fabrication. During the experiment, 15 μL of blood was loaded into the cartridge from the inlet and directed toward the reaction chamber. The cartridge was then inserted into an adaptor, which was attached to the smartphone, and the luminescence signal was captured using the smartphone camera. The advantage of this system was that the whole assay can be performed within only 3 min. The LOD was 20 mg dL−1 and 0.5 μmol L−1 for analyses of total cholesterol and total bile acid in serum, respectively.
Although CL increases the detection sensitivity compared to conventional colorimetric methods, the reaction timing and level are difficult to control77, 78. In this regard, electrochemiluminescence (ECL) sensing presents a possible solution because the CL reaction can be initiated and controlled by the application of an EC potential79, 80. Delaney and colleagues reported a portable ECL sensing platform using a smartphone and paper microfluidic sensors, which can control both the EC excitation and photonic detection using a smartphone application81, 82. Altogether, MS2 provides a new approach for routine health tests based on various sensing methods. Further developments are needed to better control the reaction sensitivity, stability, reproducibility, and reliability.
4 MS2 for Biomedical Diagnostic Applications
Traditional methods for protein or genetic biomarker diagnosis such as PCR or ELISA are expensive, time-consuming, laborious, and often require specialized facilities. Microscopybased clinical diagnostic methods for bloodborne pathogens (e.g. malaria) are impractical in some developing regions or in the field83. MS2 opens up new approaches for easy biomedical diagnostics.
4.1 Genetic Analysis
Jiang and colleagues developed a solar-powered quantitative PCR system, which leveraged natural energy sources84. The microfluidic chip was integrated with the solar heating configuration to achieve the required thermal cycling. The test sample was transported through three ring-shaped temperature zones in a repeated sequence. This continuous flow PCR test presented several attractive features over traditional PCR85 for PoC applications including high reaction speed, low cross-contamination, and high throughput. On-chip thermocouples were operated using a microcontroller connected to the smartphone for measuring and displaying the temperature of each zone. The power consumption was reduced to 80 mW. This system was used to test nucleic acids in human skin biopsies within 30 minutes. In another relevant study, Angus and colleagues developed a novel smartphone-based PCR device for quantitative detection of E. coli in a droplet using a similar heating control strategy with three printed circuit board (PCB) heaters for thermal cycling86. The fluorescence signal was detected using a smartphone-based microscope and the PCR test was completed within only 15 min.
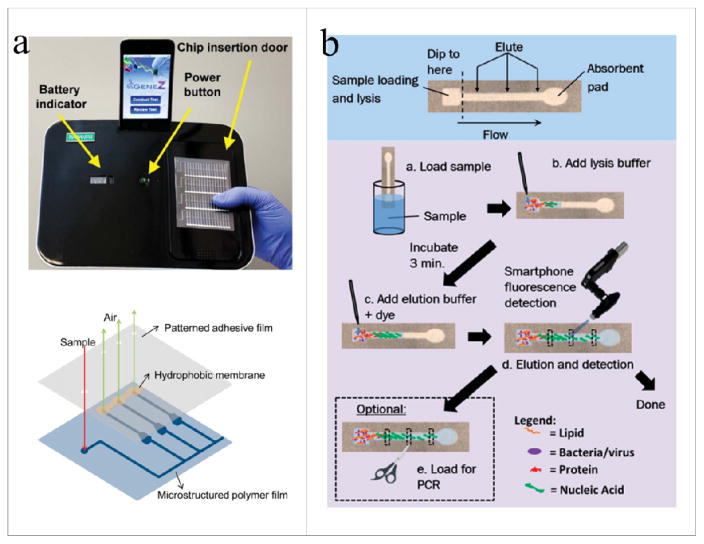
While the competitive advantage of solar-based or PCB-based PCR tests was recognized, the thermal cycling accuracy and the system complexity remain to be challenges for these mobile systems. To this end, Liu and colleagues developed a portable genetic testing platform utilizing loop-mediated isothermal amplification (LAMP) technology87, 88 without the requirement of thermal cycling for nucleic acid amplification (Gene-Z-system)(Fig. 6a)89. The Gene-Z system consisted of a polymer microfluidic chip with arrays of reaction wells, which were pre-loaded with the PCR reagents for bacteria detection. This fully integrated microfluidic system permitted rapid PCR with reduced reagent consumption. The microfluidic chip was heated by means of an aluminum heater to obtain the desired temperature and the embedded shellstructured reaction wells prevented the crossing of optical signals between the reaction wells. Sixty-four individually addressable green LEDs and 64 polymer optical fibers were embedded and epoxied onto the bottom of the aluminum heater for collecting the emitted fluorescence signal. The fluorescence signal was then converted to the electrical voltage reading through a photodiode connected to a microcontroller. The smartphone was used to control the heater and LEDs, collect and analyze the data from the microcontroller, and finally transmit the data by Wi-Fi. This prototype system can simultaneously detect two different genes from two different pathogens within 8.6 minutes and the minimal copy number that could be detected was 13 copies per reaction well.
Fig. 6. MS2 applications for genetic tests.
(a) The Gene-Z prototype system with a disposable chip for E. coli detection89. (b) A smartphone with a mini-fluorescence microscope to identify pathogenic nucleic acids from field and clinical samples using paper microfluidic chips90. The figures are adapted from ref. 89 and 90 with permission from the Royal Society of Chemistry.
Fronczek and colleagues developed a one-step direct detection method to detect the genomic nucleic acid (DNA) of Salmonella typhimurium without amplification using a smartphone and a paper microfluidic chip (Fig. 6b)90. Salmonella Typhimurium in water was spiked to 10% poultry packaging liquid, which was subsequently loaded into the paper chip. After 3 minutes of incubation, the buffer was added for lysis and elution. Finally, two microliters of Qubit fluorescent intercalating dye were loaded to 3 pre-determined locations along the paper channel. A mini-fluorescence microscope was attached to a smartphone for measuring the fluorescence reflectance from the paper chip. The fluorescence microscope included two bandpass filters, two 10× objective lenses, a dichroic mirror, and a blue LED. This developed system permitted fast testing within 5 minutes. The LOD was 103 CFU mL−1 and 104 CFU mL−1 using cellulose paper and nitrocellulose paper, respectively.
4.2 Other MS2 for Pathogen Detection
Direct bloodborne pathogen detection using a smartphonebased microscope offers a new diagnostic approach. D’Ambrosio and colleagues developed a smartphone-based microscope combined with a thin glass capillary to detect Loa loa microfilariae (mf) in blood91. This system directly measured the “wriggling” motion of individual mf instead of molecular biomarkers or morphology. A single field-of-view (FOV) was used to quantify the number of moving mf within 2.59 μL of whole blood. Additional FOVs were captured along the length of the capillary by driving the capillary using a servo motor and this method can quantify five FOVs in less than 2 min. The quantitative results were highly correlated with manual thick smear counts (94% specificity and 100% sensitivity). This system could also be used for screening and quantification of other bloodborne infectious agents such as trypanosomes and other species of filariae.
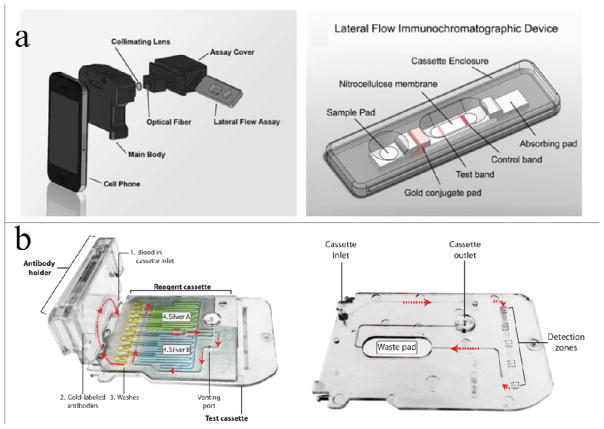
Stemple and colleagues demonstrated a handheld optofluidic device utilizing a microbead immunoagglutination assay combined with Mie scattering for detecting histidine-rich protein 2 (HRP-2) as a model malaria antigen, which was expressed exclusively by Plasmodium falciparum (P. falciparum) in blood92. The reaction between HRP-2 and antibody-conjugated microbeads took place in a PDMS-based microchip. A smartphone was used as the imaging device in this assay. Specifically, the smartphone LED was used as the only light source, which was guided to the reaction channel through a series of mirrors and lens. The scattering light intensity from immunoagglutination between HRP-2 and the antibody-conjugated microbeads was captured using the smartphone camera. The assay time was approximately 10 minutes. The LOD was 1 pg mL−1 in 10% blood. The linear range was from 1 pg mL−1 to 10 ng mL−1. The same group used the Rayleigh/Mie scattering detection protocol, which was integrated with an RDT and a smartphone to quantitatively monitor thyroid-stimulating hormone (TSH) in human serum93. The RDT-based tests required no sample pretreatment and can be operated by end-users without professional facilities and skills, thereby offering a practical approach for PoC diagnosis (Fig. 7a). In addition to the Mie scattering test module, EC-based bio-sensing was also used for rapid quantification of HRP-2 in human serum60. This EC-based test system consisted of a disposable microfluidic chip for bio-sensing, a smartphone for user operation, and an embedded circuit for signal processing and transmission. This system can provide quantifiable measurements with an LOD of 16 ng mL−1.
Fig. 7. MS2 applications for pathogen detection.
(a) A handheld smartphone-based optofluidic device for quantitative measurement of TSH based on Mie scattering and lateral flow assays93. (b) A benchtop ELISA instrument based on a disposable microfluidic cassette and a smartphone for simultaneous pathogen detection in blood94. The figures are adapted from ref. 93 and 94 with permission from Elsevier for (a) and the American Association for the Advancement of Science for (b), respectively.
Early diagnosis and treatment of HIV, syphilis and other sexually transmitted diseases in pregnant women can reduce the health risk to both mothers and newborns. Laksanasopin and colleagues developed a portable ELISA instrument by integrating a disposable microfluidic cassette with a smartphone (Fig. 7b)94. The handheld microfluidic cassette was designed with multiple detection zones for detecting HIV, syphilis and the control sample. Gold nanoparticles and silver ions were used for signal amplification. Each detection zone was pre-loaded with specific capturing antibodies against the target antigens. A negative pressure was manually activated on the microfluidic cassette to drive the ELISA assay for target antigen detection in blood samples95. Optical density (OD) absorbance measurement was used as the test readout by connecting photodetectors and LEDs to a microcontroller. The smartphone powered the microcontroller through the audio jack interface and collected OD data. The assay required only 2 μL of finger-prick blood and the diagnostic result was obtained within 15 minutes. This triplex test was comparable with the gold standard of laboratory-based HIV ELISA tests. It had a sensitivity of 92–100% and a specificity of 79–100%, which met the clinical test requirements.
4.3 Commercial Application for Mobile Diagnostics
Recently, new companies were established based on MS2 technologies. As an example, Holomic LLC developed an innovative handheld tool for alcohol and drug abuse testing. The RDT was inserted into a smartphone for lateral flow immunochromatographic assay96. In addition, the same company developed several test platforms for environmental monitoring and routine health management such as Allergen Tester97, Mercury Analyzer98 and Urine Analyzer99. Another company developed a commercial mobile diagnostic reader based on mobile image radiometry that converted the analog visual signal from RDTs to a digital signal100. The test data was stored in the mobile assay’s secure cloud. The reader operated with a standardized rapid immunoassay strip and a smartphone for detecting cocaine and benzoylecgonine. The mobile application was compatible with iOS, Android, and Windows. This reader can provide quantitative test results with an enhanced sensitivity of 1 ppb.
4.4 MS2 for Diagnostic Data Management and Remote Monitoring
MS2 has a clear advantage to enable remote data management101, 102, 103. This allows the users to store the test information in a smartphone, which can be easily edited, printed, e-mailed or wirelessly uploaded to a secure cloud server for remote access. Furthermore, the global positioning system (GPS) in the smartphone allows MS2 applications for epidemiological studies specific to geographical locations58. Such remote data management is particularly useful for low-resource areas.
Toward this direction, Mudanyali and colleagues developed microfluidics-based lateral flow immunochromatographic testing strips to monitor the presence of target analytes including malaria, tuberculosis (TB) and HIV104. The diagnostic data and related geography information can be transmitted to a central server for epidemiological research through a custom smartphone application. Moreover, dynamic mapping of the test results can be viewed and shared using a web browser. In case the internet connection is not available, the test results can be stored in the memory card and automatically transferred to the server upon the availability of internet connection. Similarly, Unyoung and colleagues demonstrated a smartphone-interfaced microfluidic EC biosensor for continuous real-time detection of waterborne pathogens or arsenic57, 62. Most recently, Sicard and colleagues used a smartphone as a portable image acquisition and processing tool for colorimetric quantification and monitoring of organophosphate pesticides in field water105. The smartphone was used with two paper-based analytical chips (one for testing the contaminated water sample and the other for testing clean water as a control group). Both the testing results and the geographical locations were simply uploaded to a centralized web server, which was accessible through Google Maps.
5 Conclusions and Future Perspectives
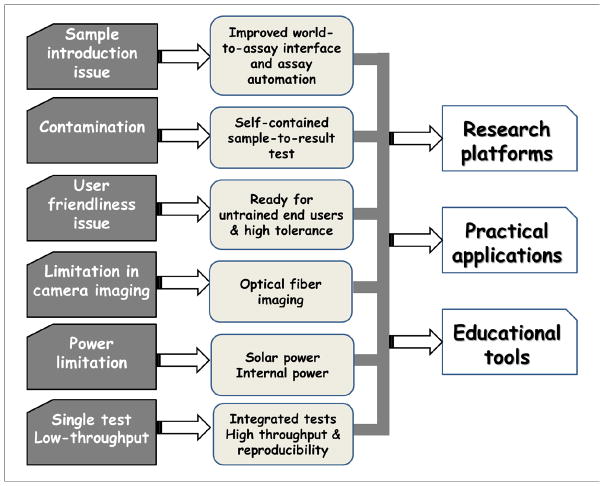
Mobile sensing based on MS2 is a very recent and fast developing field. Evidently, most of the literature reports reviewed here were published from 2011 to 2015. These studies demonstrated the unique features of MS2 in various areas of applications covering environmental and food safety monitoring, routine health monitoring, biomedical disease diagnosis and health data management. We believe that MS2 is a valuable and promising area that has high scientific and commercial potential. This powerful technology will inevitably continue to develop at high speed. The successful development of this hybrid platform will have a profound impact on the quality of life of the citizens in modern society. On one hand, there are unlimited opportunities for MS2 to embrace. On the other hand, this emerging field faces many challenges that require further development. Here, we pinpoint some common issues of the current MS2 systems and highlight future directions based on our perspective (Fig. 8).
Fig. 8. Summary of common issues of current MS2 systems and future perspectives on MS2 development.
We identified sample introduction and contamination, operator safety, limitations in power, imaging, throughput and user-friendliness as the main common issues of the current MS2 systems. We envision future MS2 systems to have improved world-to-assay interface, assay automation and self-containment, incorporation of new power sources and optical fiber imaging, and integration of different tests and operation with high throughput, tolerance and reproducibility, which can be used even by untrained end-users. We believe that future MS2 technologies will serve as a powerful experimental platform for scientific research, and will enable a wide range of practical diagnostic applications in environmental monitoring, food safety and healthcare. In addition, we believe that MS2 will revolutionarily create new methodologies for science education.
The introduction of the test sample in current MS2 systems requires additional interface apparatus besides the microfluidic device and the smartphone. In addition, conventional pipettors, tips, and pumps are often used to deliver samples. Automated sample manipulation requires specialized laboratory tools and skills. Therefore, for future development of MS2, the sample-to-test interface (or more generally world-to-assay interface) needs to be improved. Ideally, the interface should be built into the microfluidic device and can directly interact with sample-acquiring supplies such as finger sticks or swabs. 3D-printed microfluidic devices and their associated accessories have great potential to enable such an improved sample-to-test interface. In addition, self-powered microfluidic devices or microfluidic devices with simple and easy-to-operate on-chip valves and pumps should be developed to enable improved fluidic transport and assay automation in MS2. Moreover, advanced diagnostic assays often require complicated external control equipment, which is ill-suited for MS2 applications. One such example is nucleic acid-based diagnostic tests that require precise temperature control, which is challenging to implement in a simple microfluidic device at low cost. To this end, further development of smartphone-compatible microfluidic devices with a simple integrated heating component such as embedded carbon wires is required.
Current MS2 systems generally require safety supplies to protect the operator and to reduce the chances of sample contamination, which often limits MS2 applications to research labs. Outside the lab, test operation complexity, reproducibility, and reliability become significant issues. Future development of MS2 should eliminate direct exposure of the operators to the samples by incorporating an on-chip sample-to-test interface and develop the MS2-based tests in a self-contained manner without requiring operator intervention beyond initial sample acquisition and final test device disposal. Some sterilization strategies such as the use of cellulose fibers and other filters have been developed in MS2 tests to reduce contaminants in the test sample. Further development of sterilization methods for different types of MS2 is critically required. Again, improvement and innovation in world-to-assay interface and assay automation will play important roles in advancing the development of MS2 technology. In addition, optical fibers can be used as an innovative data acquisition method for the imagebased MS2 applications, which currently use optical cameras and suffer from low test throughput, low tolerance to environmental factors, limited test accuracy and the requirement of post-test image analysis.
In many low-resource areas, the wireless network suffers from poor connectivity and signal quality. In addition, in these areas, it can be inconvenient to locate reliable electrical power sources to charge the smartphone. Thus, to ensure reliable MS2 applications, the smartphone must support asynchronous data transmission so that the collected data can be stored locally in the device until a network connection or sufficient bandwidth becomes available for data transmission. In this context, MS2 applications will also benefit from further development of battery optimization technology and alternative power sources such as solar power106.
Current MS2 systems employ specialized microfluidic devices for different applications. It will be highly beneficial to develop integrated MS2 applications for detection of environmental pollutants, food contaminants and health indicators on a single microfluidic platform. For medical diagnosis applications, the MS2 should be able to effectively integrate sample acquisition and processing, diagnostic testing, data analysis and management. Such a platform will improve the speed, accuracy and versatility for diagnostic tests.
We believe that future MS2 technologies will serve as a powerful experimental platform for scientific research, and will enable a wide range of practical diagnostic applications in environmental monitoring, food safety and healthcare. Further efforts to improve the user-friendliness and suitability of testing outside the labs for MS2 applications will enable the practical use of MS2 technologies by untrained end-users to easily obtain reliable test results. In addition, we believe that MS2 will revolutionarily create new methodologies for science education. Foldscope is one such inspiring example that allows kids to watch biological samples on the microscopic scale using an extremely cheap paper-based microscope and take pictures with their own smartphones107. Further incorporation of microfluidic devices to science education in combination with smartphones is not far from becoming a reality. Toward this direction, MS2 is anticipated to become a common science educational tool at all levels of schools and institutions in the near future108.
Acknowledgments
The development of this paper is supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Institutes of Health Research (CIHR). We thank Dr. Ying Wei Xia for helpful comments on the manuscript.
List of Abbreviations
- 3D
three-dimensional
- BL-CL
biochemiluminescence
- BL
bioluminescence
- BDE-47
2,2′,4,4′-tetrabromodiphenyl ether
- CL
chemiluminescence
- ELISA
enzyme-linked immunosorbent assay
- ECL
electrochemiluminescence
- E. coli
Escherichia coli
- EPA
Environmental Protection Agency
- EC
electrochemical
- FOV
field of view
- FAAS
flame atomic absorption spectroscopy
- GPS
global positioning system
- GOx
glucose oxidase
- HRP-2
histidine-rich protein 2
- LoC
lab-on-chip
- LEDs
light-emitting diodes
- LAMP
loop-mediated isothermal amplification
- LOD
limit of detection
- Mf
microfilariae
- MID
microfluidic incubation device
- MS2
mobile sensing based on microfluidic devices and smartphone
- PoC
point-of-care
- PDMS
polydimethylsiloxane
- PCR
polymerase chain reaction
- PCB
printed circuit board
- P. falciparum
Plasmodium falciparum
- RDTs
rapid diagnostic test strip
- TB
tuberculosis
- TSH
thyroid stimulating hormone
- WTO
World Health Organization
References
- 1.Solanas A, Patsakis C, Conti M, Vlachos I, Ramos V, Falcone F, Postolache O, Perez-Martinez P, Pietro R, Perrea D, Martinez-Balleste A. IEEE Commun Mag. 2014;52:74–81. [Google Scholar]
- 2.Miró M, Hansen EH. Anal Chim Acta. 2007;600:46–57. doi: 10.1016/j.aca.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 3.Dell N, Francis I, Sheppard H, Simbi R, Borriello G. Proceedings of the 16th international conference on Human-computer interaction with mobile devices & services ACM; 2014; pp. 33–42. [Google Scholar]
- 4.Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Nature. 2006;442:412–418. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- 5.Mabey D, Peeling RW, Ustianowski A, Perkins MD. Nat Rev Microbiol. 2004;2:231–240. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]
- 6.Wu JD, Wu X, Lin F. Lab Chip. 2013;13:2484–2499. doi: 10.1039/c3lc50415h. [DOI] [PubMed] [Google Scholar]
- 7.Reyes DR, Iossifidis D, Auroux PA, Manz A. Anal chem. 2002;74:2623–2636. doi: 10.1021/ac0202435. [DOI] [PubMed] [Google Scholar]
- 8.Mao X, Huang TJ. Lab Chip. 2012;12:1412–1416. doi: 10.1039/c2lc90022j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Tasoglu S, Chen PZ, Chen M, Akbas R, Wach S, Ozdemir CI, Gurkan UA, Giguel FF, Kuritzkes DR, Demirci U. Sci Rep-Uk. 2014;4:3796. doi: 10.1038/srep03796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee WG, Kim YG, Chung BG, Demirci U, Khademhosseini A. Adv Drug Deliver Rev. 2010;62:449–457. doi: 10.1016/j.addr.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu J, Wang S, Wang L, Li F, Pingguan-Murphy B, Lu TJ, Xu F. Biosens Bioelectron. 2014;54:585–597. doi: 10.1016/j.bios.2013.10.075. [DOI] [PubMed] [Google Scholar]
- 12.van Lintel HTG, van De Pol FCM, Bouwstra S. Sensor Actuat. 1988;15:153–167. [Google Scholar]
- 13.Harrison DJ, Manz A, Fan Z, Luedi H, Widmer HM. Anal Chem. 1992;64:1926–1932. [Google Scholar]
- 14.Berthier E, Young EWK, Beebe D. Lab Chip. 2012;12:1224–1237. doi: 10.1039/c2lc20982a. [DOI] [PubMed] [Google Scholar]
- 15.Martinez AW, Phillips ST, Butte MJ, Whitesides GM. Angew Chem Int Edit. 2007;46:1318–1320. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sia SK, Whitesides GM. Electrophoresis. 2003;24:3563–3576. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- 17.Janasek D, Franzke J, Manz A. Nature. 2006;442:374–380. doi: 10.1038/nature05059. [DOI] [PubMed] [Google Scholar]
- 18.Henares TG, Mizutani F, Hisamoto H. Anal Chim Acta. 2008;611:17–30. doi: 10.1016/j.aca.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen NT, Lam YC, Ho SS, Low CLN. Biomicrofluidics. 2008;2:034101. doi: 10.1063/1.2959099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ungerbock B, Charwat V, Ertl P, Mayr T. Lab Chip. 2013;13:1593–1601. doi: 10.1039/c3lc41315b. [DOI] [PubMed] [Google Scholar]
- 21.Gallegos D, Long KD, Yu H, Clark PP, Lin Y, George S, Nath P, Cunningham BT. Lab Chip. 2013;13:2124–2132. doi: 10.1039/c3lc40991k. [DOI] [PubMed] [Google Scholar]
- 22.Khandoker AH, Black J, Palaniswami M. Electrical and Computer Engineering (ICECE), 2010 International Conference on. IEEE; 2010; pp. 634–637. [Google Scholar]
- 23.Xu X, Akay A, Wei H, Wang S, Pingguan-Murphy B, Erlandsson B-E, Li X, Lee W, Hu J, Wang L, Xu F. P IEEE. 2015;103:236–247. [Google Scholar]
- 24.Wojtczak J, Bonadonna P. Am J Emerg Med. 2013;31:573–577. doi: 10.1016/j.ajem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Martinez AW, Phillips ST, Carrilho E, Thomas SW, Sindi H, Whitesides GM. Anal Chem. 2008;80:3699–3707. doi: 10.1021/ac800112r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y, Shi W, Qin J, Lin B. Electrophoresis. 2009;30:579–582. doi: 10.1002/elps.200800586. [DOI] [PubMed] [Google Scholar]
- 27.Vashist S, Schneider E, Luong J. Diagnostics. 2014;4:104–128. doi: 10.3390/diagnostics4030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erickson D, O’Dell D, Jiang L, Oncescu V, Gumus A, Lee S, Mancuso M, Mehta S. Lab Chip. 2014;14:3159–3164. doi: 10.1039/c4lc00142g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Ruiz N, Curto VF, Erenas MM, Benito-Lopez F, Diamond D, Palma AJ, Capitan-Vallvey LF. Anal Chem. 2014;86:9554–9562. doi: 10.1021/ac5019205. [DOI] [PubMed] [Google Scholar]
- 30.Steinhubl SR, Muse ED, Topol EJ. Sci Transl Med. 2015;7:283rv3. doi: 10.1126/scitranslmed.aaa3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huckle D. Expert Rev Mol Diagn. 2015;15:815–827. doi: 10.1586/14737159.2015.1033405. [DOI] [PubMed] [Google Scholar]
- 32.Zhu H, Isikman SO, Mudanyali O, Greenbaum A, Ozcan A. Lab Chip. 2013;13:51–67. doi: 10.1039/c2lc40864c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coskun AF, Ozcan A. Curr Opin Biotech. 2014;25:8–16. doi: 10.1016/j.copbio.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vashist S, Mudanyali O, Schneider EM, Zengerle R, Ozcan A. Anal Bioanal Chem. 2014;406:3263–3277. doi: 10.1007/s00216-013-7473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Lin TY, Lillehoj P. Ann Biomed Eng. 2014;42:2205–2217. doi: 10.1007/s10439-014-1055-z. [DOI] [PubMed] [Google Scholar]
- 36.Gravenhorst F, Muaremi A, Bardram J, Grünerbl A, Mayora O, Wurzer G, Frost M, Osmani V, Arnrich B, Lukowicz P, Tröster G. Pers Ubiquit Comput. 2015;19:335–353. [Google Scholar]
- 37.Azzazy HME, Elbehery AHA. Clin Chim Acta. 2015;438:186–194. doi: 10.1016/j.cca.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 38.Vashist SK, Luppa PB, Yeo LY, Ozcan A, Luong JHT. Trends Bio Technol. 2015;33:692–705. doi: 10.1016/j.tibtech.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Zhang D, Liu Q. Biosens Bioelectron. 2016;75:273–284. doi: 10.1016/j.bios.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 40.Breslauer DN, Maamari RN, Switz NA, Lam WA, Fletcher DA. PLoS ONE. 2009;4:e6320. doi: 10.1371/journal.pone.0006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith ZJ, Chu K, Espenson AR, Rahimzadeh M, Gryshuk A, Molinaro M, Dwyre DM, Lane S, Matthews D, Wachsmann-Hogiu S. PLoS ONE. 2011;6:e17150. doi: 10.1371/journal.pone.0017150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu H, Sikora U, Ozcan A. Analyst. 2012;137:2541–2544. doi: 10.1039/c2an35071h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanvicens N, Pascual N, Fernández-Argüelles M, Adrián J, Costa-Fernández J, Sánchez-Baeza F, Sanz-Medel A, Marco MP. Anal Bioanal Chem. 2011;399:2755–2762. doi: 10.1007/s00216-010-4624-5. [DOI] [PubMed] [Google Scholar]
- 44.Zhu H, Yaglidere O, Su TW, Tseng D, Ozcan A. Lab Chip. 2011;11:315–322. doi: 10.1039/c0lc00358a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu H, Mavandadi S, Coskun AF, Yaglidere O, Ozcan A. Anal Chem. 2011;83:6641–6647. doi: 10.1021/ac201587a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park TS, Yoon JY. IEEE Sens J. 2015;15:1902–1907. [Google Scholar]
- 47.Park TS, Li W, McCracken KE, Yoon JY. Lab Chip. 2013;13:4832–4840. doi: 10.1039/c3lc50976a. [DOI] [PubMed] [Google Scholar]
- 48.Chen A, Wang R, Bever CRS, Xing S, Hammock BD, Pan T. Biomicrofluidics. 2014;8:064101. doi: 10.1063/1.4901348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee DS, Jeon BG, Ihm C, Park JK, Jung MY. Lab Chip. 2011;11:120–126. doi: 10.1039/c0lc00209g. [DOI] [PubMed] [Google Scholar]
- 50.Li B, Li L, Guan A, Dong Q, Ruan K, Hu R, Li Z. Lab Chip. 2014;14:4085–4092. doi: 10.1039/c4lc00227j. [DOI] [PubMed] [Google Scholar]
- 51.Yafia M, Ahmadi A, Hoorfar M, Najjaran H. Micromachines. 2015;6:1289–1305. [Google Scholar]
- 52.Hutchison JR, Erikson RL, Sheen AM, Ozanich RM, Kelly RT. Analyst. 2015;140:6269–6276. doi: 10.1039/c5an01304f. [DOI] [PubMed] [Google Scholar]
- 53.Luo Y, Zare RN. Lab Chip. 2008;8:1688–1694. doi: 10.1039/b807751g. [DOI] [PubMed] [Google Scholar]
- 54.Lewis GG, DiTucci MJ, Baker MS, Phillips ST. Lab Chip. 2012;12:2630–2633. doi: 10.1039/c2lc40331e. [DOI] [PubMed] [Google Scholar]
- 55.Martinez AW, Phillips ST, Whitesides GM. P Natl Acad Sci. 2008;105:19606–19611. doi: 10.1073/pnas.0810903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, Li YJ, Wei JF, Xu JR, Wang YH, Zheng GX. Anal Bioanal Chem. 2014;406:2799–2807. doi: 10.1007/s00216-014-7715-x. [DOI] [PubMed] [Google Scholar]
- 57.Unyoung K, Demaree B, van der Giessen J, Reynolds M, Perricone K, Seubert J, Elahi Z, Gandhi S, Krishnan S, Figueira S. e-Health Networking, Applications & Services (Healthcom). 2013 IEEE 15th International Conference on. IEEE; 2013; pp. 575–579. [Google Scholar]
- 58.Usydus Z, Szlinder-Richert J, Polak-Juszczak L, Komar K, Adamczyk M, Malesa-Ciecwierz M, Ruczynska W. Chemosphere. 2009;74:1420–1428. doi: 10.1016/j.chemosphere.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 59.Shadbolt C, Ross T, McMeekin TA. Lett Appl Microbiol. 2001;32:99–102. doi: 10.1046/j.1472-765x.2001.00862.x. [DOI] [PubMed] [Google Scholar]
- 60.Lillehoj PB, Huang MC, Truong N, Ho CM. Lab Chip. 2013;13:2950–2955. doi: 10.1039/c3lc50306b. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Gartia MR, Jiang J, Chang TW, Qian J, Liu Y, Liu X, Liu GL. Sensors and Actuators B. 2015;209:677–685. [Google Scholar]
- 62.Unyoung K, Ghanbari S, Ravikumar A, Seubert J, Figueira S. Translational Engineering in Health and Medicine, IEEE Journal of. 2013;1:3700207–3700207. doi: 10.1109/JTEHM.2013.2281819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.West NX, Hughes JA, Addy M. J Oral Rehabil. 2001;28:860–864. doi: 10.1046/j.1365-2842.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- 64.Morgan RM, Patterson MJ, Nimmo MA. Acta Physiol Scand. 2004;182:37–43. doi: 10.1111/j.1365-201X.2004.01305.x. [DOI] [PubMed] [Google Scholar]
- 65.Murray CJL, Lopez AD. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 66.Malhotra BD, Chaubey A. Sensors and Actuators B. 2003;91:117–127. [Google Scholar]
- 67.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, Lappé DL, Muhlestein JB. Am J Cardiol. 2010;106:963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 68.Holick MF. Am J Clin Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 69.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chun H, Park Y, Han Y, Jang Y, Yoon H. BioChip J. 2014;8:218–226. [Google Scholar]
- 71.Cha KH, Jensen GC, Balijepalli AS, Cohan BE, Meyerhoff ME. Anal Chem. 2014;86:1902–1908. doi: 10.1021/ac4040168. [DOI] [PubMed] [Google Scholar]
- 72.Oncescu V, Mancuso M, Erickson D. Lab Chip. 2014;14:759–763. doi: 10.1039/c3lc51194d. [DOI] [PubMed] [Google Scholar]
- 73.Lee S, Oncescu V, Mancuso M, Mehta S, Erickson D. Lab Chip. 2014;14:1437–1442. doi: 10.1039/c3lc51375k. [DOI] [PubMed] [Google Scholar]
- 74.Oncescu V, O’Dell D, Erickson D. Lab Chip. 2013;13:3232–3238. doi: 10.1039/c3lc50431j. [DOI] [PubMed] [Google Scholar]
- 75.Roda A, Pasini P, Mirasoli M, Michelini E, Guardigli M. Trends Biotechnol. 2004;22:295–303. doi: 10.1016/j.tibtech.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 76.Roda A, Michelini E, Cevenini L, Calabria D, Calabretta MM, Simoni P. Anal Chem. 2014;86:7299–7304. doi: 10.1021/ac502137s. [DOI] [PubMed] [Google Scholar]
- 77.Yu J, Wang S, Ge L, Ge S. Biosens Bioelectron. 2011;26:3284–3289. doi: 10.1016/j.bios.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 78.Wang S, Ge L, Song X, Yu J, Ge S, Huang J, Zeng F. Biosens Bioelectron. 2012;31:212–218. doi: 10.1016/j.bios.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 79.Xu Y, Lv Z, Xia Y, Han Y, Lou B, Wang E. Anal Bioanal Chem. 2013;405:3549–3558. doi: 10.1007/s00216-012-6510-9. [DOI] [PubMed] [Google Scholar]
- 80.Shi CG, Shan X, Pan ZQ, Xu JJ, Lu C, Bao N, Gu HY. Anal Chem. 2012;84:3033–3038. doi: 10.1021/ac2033968. [DOI] [PubMed] [Google Scholar]
- 81.Delaney JL, Doeven EH, Harsant AJ, Hogan CF. Anal Chem Acta. 2013;790:56–60. doi: 10.1016/j.aca.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 82.Delaney JL, Hogan CF, Tian J, Shen W. Anal Chem. 2011;83:1300–1306. doi: 10.1021/ac102392t. [DOI] [PubMed] [Google Scholar]
- 83.Moody AH, Chiodini PL. Clin Lab Haem. 2000;22:189–201. doi: 10.1046/j.1365-2257.2000.00318.x. [DOI] [PubMed] [Google Scholar]
- 84.Jiang L, Mancuso M, Lu Z, Akar G, Cesarman E, Erickson D. Sci Rep-Uk. 2014;4:4137. doi: 10.1038/srep04137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maltezos G, Johnston M, Taganov K, Srichantaratsamee C, Gorman J, Baltimore D, Chantratita W, Scherer A. Appl Phys Lett. 2010;97:264101. doi: 10.1063/1.3530452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Angus SV, Cho S, Harshman DK, Song JY, Yoon JY. Biosens Bioelectron. 2015;74:360–368. doi: 10.1016/j.bios.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu C, Mauk M, Bau H. Microfluid Nanofluid. 2011;11:209–220. doi: 10.1007/s10404-011-0788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu C, Geva E, Mauk M, Qiu X, Abrams WR, Malamud D, Curtis K, Owen SM, Bau HH. Analyst. 2011;136:2069–2076. doi: 10.1039/c1an00007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stedtfeld RD, Tourlousse DM, Seyrig G, Stedtfeld TM, Kronlein M, Price S, Ahmad F, Gulari E, Tiedje JM, Hashsham SA. Lab Chip. 2012;12:1454–1462. doi: 10.1039/c2lc21226a. [DOI] [PubMed] [Google Scholar]
- 90.Fronczek CF, Park TS, Harshman DK, Nicolini AM, Yoon JY. RSC Adv. 2014;4:11103–11110. [Google Scholar]
- 91.D’Ambrosio MV, Bakalar M, Bennuru S, Reber C, Skandarajah A, Nilsson L, Switz N, Kamgno J, Pion S, Boussinesq M, Nutman TB, Fletcher DA. Sci Transl Med. 2015;7:286re4. doi: 10.1126/scitranslmed.aaa3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stemple CC, Angus SV, Park TS, Yoon JY. Jala-J Lab Autom. 2014;19:35–41. doi: 10.1177/2211068213498241. [DOI] [PubMed] [Google Scholar]
- 93.You DJ, Park TS, Yoon JY. Biosens Bioelectron. 2013;40:180–185. doi: 10.1016/j.bios.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 94.Laksanasopin T, Guo TW, Nayak S, Sridhara AA, Xie S, Olowookere OO, Cadinu P, Meng F, Chee NH, Kim J, Chin CD, Munyazesa E, Mugwaneza P, Rai AJ, Mugisha V, Castro AR, Steinmiller D, Linder V, Justman JE, Nsanzimana S, Sia SK. Sci Transl Med. 2015;7:273re1. doi: 10.1126/scitranslmed.aaa0056. [DOI] [PubMed] [Google Scholar]
- 95.Lin YH, Chen YJ, Lai CS, Chen YT, Chen CL, Yu JS, Chang YS. Biomicrofluidics. 2013;7:024103. doi: 10.1063/1.4794974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holomic Substance Test Assistant, Inc. [accessed on 13 January 2016];Substance Abuse Testing. Available online http://www.holomic.com/content/rapid-test-solution/hsta-sat/
- 97.Coskun AF, Wong J, Khodadadi D, Nagi R, Tey A, Ozcan A. Lab Chip. 2013;13:636–640. doi: 10.1039/c2lc41152k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wei Q, Nagi R, Sadeghi K, Feng S, Yan E, Ki SJ, Caire R, Tseng D, Ozcan A. ACS Nano. 2014;8:1121–1129. doi: 10.1021/nn406571t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coskun AF, Nagi R, Sadeghi K, Phillips S, Ozcan A. Lab Chip. 2013;13:4231–4238. doi: 10.1039/c3lc50785h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mobile Assay, Inc. [accessed on 13 January 2016];Home Page. Available online: http://mobileassay.com/
- 101.Berndt RD, Takenga MC, Kuehn S, Preik P, Dubbermann D, Juenger M. Telemed J E-Health. 2012;18:668–673. doi: 10.1089/tmj.2011.0273. [DOI] [PubMed] [Google Scholar]
- 102.van der Heijden M, Lucas PJF, Lijnse B, Heijdra YF, Schermer TRJ. J Biomed Inform. 2013;46:458–469. doi: 10.1016/j.jbi.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Matthews J, Kulkarni R, Gerla M, Massey T. Mobile Netw Appl. 2012;17:178–191. [Google Scholar]
- 104.Mudanyali O, Dimitrov S, Sikora U, Padmanabhan S, Navruz I, Ozcan A. Lab Chip. 2012;12:2678–2686. doi: 10.1039/c2lc40235a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sicard C, Glen C, Aubie B, Wallace D, Jahanshahi-Anbuhi S, Pennings K, Daigger GT, Pelton R, Brennan JD, Filipe CDM. Water Res. 2015;70:360–369. doi: 10.1016/j.watres.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 106.Alshurafa N, Eastwood JA, Nyamathi S, Liu JJ, Wenyao X, Ghasemzadeh H, Pourhomayoun M, Sarrafzadeh M. IEEE T Inf Technol B. 2015;19:57–63. doi: 10.1109/JBHI.2014.2329712. [DOI] [PubMed] [Google Scholar]
- 107.Cybulski JS, Clements J, Prakash M. PLoS ONE. 2014;9:e98781. doi: 10.1371/journal.pone.0098781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koesdjojo MT, Pengpumkiat S, Wu Y, Boonloed A, Huynh D, Remcho TP, Remcho VT. J Chem Educ. 2015;92:737–741. [Google Scholar]