Abstract
Previous studies showed that P2 receptors are involved in neutrophil migration via stimulation of chemokine release and by facilitating chemoattractant gradient sensing. Here, we have investigated whether these receptors are involved in LPS-induced neutrophil transendothelial migration (TEM) using a Boyden chamber where neutrophils migrated through a layer of lipopolysaccharide (LPS)-stimulated human umbilical vein endothelial cells (HUVECs). In line with a role of P2 receptors, neutrophil TEM was inhibited by the P2 receptor antagonists suramin and reactive blue 2 (RB-2) acting on the basolateral, but not luminal, HUVECs’ P2 receptors. HUVECs express P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11. The involvement of P2Y4 was unlikely as this receptor is insensitive to suramin while P2Y1, P2Y6 and P2Y11 were excluded with available selective antagonists, leaving P2Y2 as the only candidate. Indeed, the P2Y2 knockdown in HUVECs inhibited neutrophil TEM compared to control HUVECs transfected with scrambled siRNA. Moreover, UTP, a P2Y2 ligand, markedly potentiated LPS-induced TEM. Interestingly, IL-8 and ICAM-1 had a modest effect on neutrophil TEM in this 3 h assay which was significantly diminished by the inhibition of Rho kinase in HUVECs with Y27632. In summary, endothelial P2Y2 receptors control the early LPS-induced neutrophil TEM in vitro via Rho kinase activation.
Keywords: Extracellular nucleotides, Rho kinase, Cell trafficking, Inflammation, Boyden chamber
1. Introduction
Transendothelial migration (TEM) is a critical step in neutrophil recruitment to sites of infection and/or inflammation. TEM is a step-wise process that involves complex neutrophil–endothelium interactions leading to neutrophil rolling, firm adhesion, and ultimately exit from blood vessels (Wagner and Roth, 2000; Eltzschig et al., 2006). These steps are regulated by many factors including inducible adhesion molecules and chemokines produced by these cell types (Wagner and Roth, 2000; Becker et al., 2001; Yang et al., 2005). Other studies have shown that neutrophil migration is also controlled by Rho kinase as its pharmacological inhibition markedly reduces neutrophil TEM in vivo and in vitro (Tasaka et al., 2005; Saito et al., 1998). LPS, a cell wall component of Gram-negative bacteria recognized by cells through Toll like receptor 4 (TLR4), is a potent activator of neutrophil TEM (Wagner and Roth, 2000; Lu et al., 2008). Endothelial cells stimulated with LPS exhibit an increased expression of ICAM-1 and activation of Rho kinase (Basit et al., 2006; Essler et al., 2000) which have been shown to play a critical role in LPS-induced neutrophil recruitment in the lungs (Becker et al., 2001; Basit et al., 2006; Tasaka et al., 2005). In addition, LPS can also stimulate either endothelial cells or neutrophils to secrete interleukin 8 (IL-8), a chemokine that has a major role in leukocyte TEM.
Extracellular nucleotides such as ATP, ADP, UTP and UDP serve as danger signals that are rapidly released by cells during inflammatory responses (Bours et al., 2006; Yegutkin, 2008). These molecules act via the activation of P2 receptors that include the ion-channel P2X receptors (P2X1–7) and G-protein-coupled P2Y receptors (P2Y1,2,4,6,11–14). All P2X receptors as well as P2Y2 and P2Y11 are activated by ATP. P2Y1, P2Y12 and P2Y13 are activated by ADP, P2Y4 by UTP, P2Y6 by UDP, and P2Y14 by UDP-glucose (Bours et al., 2006). Distinct P2Y receptors have been implicated in LPS-induced inflammation. For example, we have previously demonstrated that in human monocytes, LPS-induced IL-8 release is mediated via activation of P2Y6 receptors (Warny et al., 2001; Kukulski et al., 2007). As endothelial cells such as human umbilical vein endothelial cells (HUVECs) express various P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11) (Wang et al., 2002) and release large quantities of IL-8 in response to LPS (Beck et al., 1999; Kukulski et al., 2007), we hypothesized that these receptors might trigger neutrophil TEM via IL-8 release. We indeed found that endothelial nucleotide receptors are instrumental for LPS-induced neutrophil TEM in vitro, but interestingly, this migration was regulated mainly by endothelial Rho kinase and not by IL-8 which was not secreted in significant amounts during the course of the TEM assay performed in this work (3 h).
2. Materials and methods
2.1. Materials
LPS from E. coli O111:B4, potato apyrase grade VII, nucleotides (ATP, ADP, UTP, UDP, ATPγS and β-NAD), pyridoxal-phosphate-6-azophenyl-2′, 4′-disulfonate (PPADS), suramin, nucleotides (ATP, UTP, ADP and UDP) and fish oil were purchased from Sigma (St. Louis, MO). MRS2500, MRS2578, NF157 and Y27632 were obtained from Tocris Bioscience (Bristol, UK). Reactive blue 2 (RB-2) was bought from ICN Biochemicals Inc. (Aurora, OH). IL-8 and ICAM-1 neutralizing antibodies (nIL-8 and nICAM-1 ab) MAB208 and AF720, respectively, were from R&D Systems (Minneapolis, MN). CellTracker™ Green CMFDA (5-chloromethylfluorescein diacetate) was obtained from Invitrogen (Burlington, On, Canada). Goat serum was purchased from Wisent (St.-Bruno, Canada) and bovine serum albumin (BSA) as well as Tween-20 from VWR (West Chester, PA).
The stock of LPS (5 mg/ml) was prepared in an endotoxin-free saline (Sigma). Before stimulation, LPS was sonicated for 10 min in a water bath sonicator and diluted in RPMI-5% FBS medium. P2 receptor antagonists (suramin, RB-2, PPADS, MRS2500 and NF157) were prepared at 10 mM in endotoxin-free water from Sigma, fil-trated and used for TEM assays. MRS2578 (10 mM) was prepared in 100% DMSO, filtrated and diluted with RPMI-5% FBS medium to 10 μM for neutrophil TEM assays. Appropriate controls containing 0.01% DMSO were also performed.
2.2. Isolation of human blood neutrophils
Human neutrophils were isolated as described (Boyum, 1968), with some modifications. Briefly, venous blood of healthy volunteers was collected on isocitrate anticoagulant solution, centrifuged (250 × g, 10 min, 24 °C), and the resulting platelet-rich plasma discarded. Leukocytes were obtained following erythrocyte sedimentation in 2% Dextran T-500 and centrifuged (525 × g, 20 min, 24 °C) through a 10-ml Ficoll–Paque cushion (Wisent). The neutrophil-enriched pellet was subjected to a 15-s hypotonic lysis to remove the remaining erythrocytes and centrifuged (1000 × g, 5 min, 24 °C). The purified neutrophils were re-suspended in RPMI 1640 medium containing 5% FBS (RPMI 1640-5% FBS) and used for TEM assays. The purity of neutrophils obtained using this protocol is >95% (Chakravarti et al., 2009).
2.3. HUVEC culture and stimulation
HUVECs (Cambrex Bio Science, Walkersville, MD) were cultured in a complete EGM Bulletkit® medium (Cambrex Bio Science) and used at passage 3–5. Each experiment was performed with HUVECs of at least two independent donors from distinct passages.
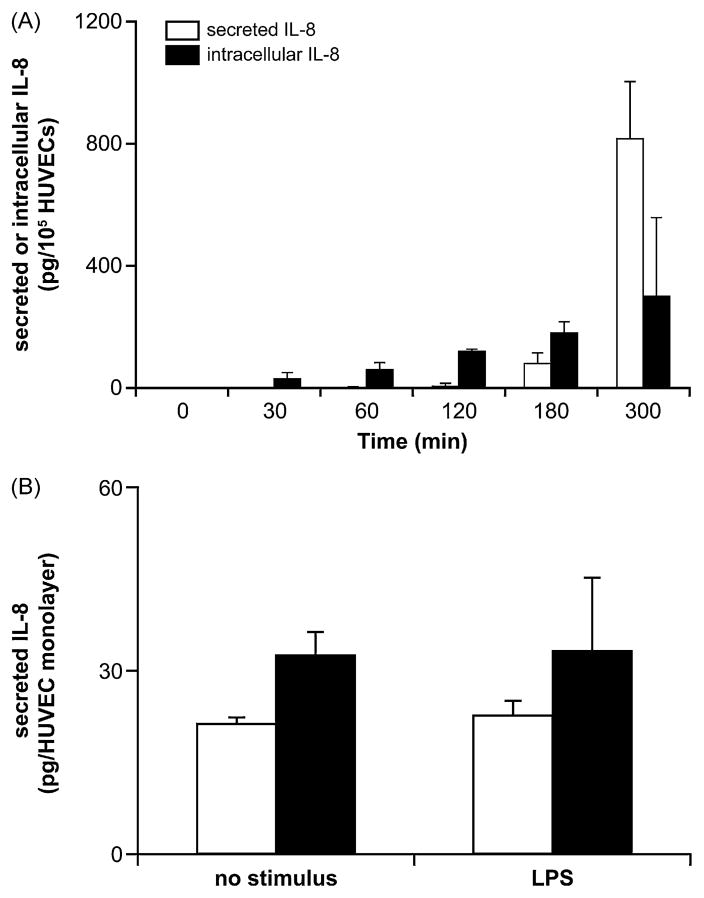
For LPS stimulation, the cells were seeded at 105/well in a 24-well plate and grown for 24 h. Next, these cells were stimulated for up to 5 h with 0.1 μg/ml LPS at 37 °C in a humid atmosphere containing 5% CO2. After the stimulation, the media of HUVECs (supernatants) were collected and centrifuged (1000 × g, 10 min, 4 °C) to remove the detached cells. The adherent HUVECs were washed with PBS, lysed with 1% Triton X-100/PBS for 2 h at 4 °C and the resulted lysates centrifuged (1000 × g, 10 min, 4 °C) to remove non-dissolved material. These samples were analyzed by ELISA for IL-8 as described below.
2.4. Neutrophil TEM assay
Neutrophil TEM was carried out in a Boyden chamber system as described (Issekutz et al., 1995), with some modifications. Briefly, cell culture inserts (3 μm pore size) were used to form dual compartments (chambers) in a Falcon™ 24-well culture plate (Becton Dickinson, Franklin Lakes, NJ). The polyethylene membrane filters (6.4 mm diameter) of the inserts were coated successively with 1% (w/v) gelatine (overnight; Sigma), 0.006% (v/v) stabilized human fibronectin (2 h; Biomedical Technologies, Stoughton, MA) and 15 × 104 HUVECs (2 days for confluence). Freshly isolated human neutrophils (106 cells in 0.2 ml of RPMI 1640–5% FBS) were loaded to the upper chamber and their migration was initiated with LPS (100 ng/ml) added to the medium of the bottom chamber. Where indicated, P2 receptor antagonists were added to the media of the bottom or upper chambers 15 min before the addition of neutrophils and LPS. Basal neutrophil migration observed in the absence of LPS was less than 20% of that induced with LPS and was subtracted from the data presented in the figures. In the indicated assays, the monolayers of HUVECs were preincubated for 30 min with the antibodies neutralizing IL-8 or ICAM-1, or irrelevant mouse IgG1 antibody as a control (10 μg/ml; added to both upper and bottom chambers), the Rho kinase inhibitor Y27632 (10 μM; added to the upper chamber), or sialic acid (50 mM; added to the upper chamber). Neutrophil migration was carried out for 3 h at 37 °C and 5% CO2. The migrated neutrophils were collected from the bottom chambers and counted with a hemocytometer. Depending on the experiment, from 50 to 80% of the neutrophils loaded to the upper chambers migrated to the bottom chambers as a result of HUVECs stimulation with LPS added to the bottom chambers. These numbers are expressed in the Figures as 100%.
To exclude the effect of LPS and/or inhibitors on neutrophils and therefore specifically address the role of endothelial P2 receptors in neutrophil TEM, the above assays were also performed as follows: HUVEC monolayers were pre-stimulated with LPS added to the upper or bottom chamber (to address the role of luminal and basolateral P2 receptors, respectively) in the presence or absence of the P2 receptor antagonists for 1 h, the media of the bottom and upper chambers discarded, the inserts transferred to a new 24-well plate and HUVEC monolayers washed twice with PBS. Neutrophils were then loaded to the upper chamber and allowed to migrate for 2 h. The same setup was also used to investigate whether 100 μM exogenous nucleotides (ATP, UTP, ADP and UDP; added to the bottom chamber) would increase neutrophil TEM due to suboptimal LPS concentration (1 ng/ml; in the bottom chamber).
2.5. P2Y2 knockdown
To knockdown the P2Y2 receptor in HUVECs, these cells were transfected with a specific validated anti-P2Y2 siRNA (NM 002564, Ambion) using siPORT™ NeoFX™ transfection agent (Ambion) according to the manufacturer’s protocol with some optimization. Control cells were transfected with an irrelevant scrambled siRNA (Ambion). Briefly, freshly trypsinized HUVECs (2 × 105 in 0.2 ml of OPTI-MEM® medium) were mixed 1:1 with the transfection complexes containing 5 μM siRNA and 8 μl of the NeoFX™, and loaded on the precoated cell culture inserts. The transfection was carried out for 18 h and then HUVECs were cultured for 2 more days as it has previously reported that P2Y2 expression in PC12 cells was significantly knocked down 3 days after transfection (Arthur et al., 2005). P2Y2 knockdown in HUVECs was verified by RT-PCR as described below.
2.6. RNA extraction and reverse transcriptase (RT)-PCR
Total HUVECs’ RNA was isolated 66 h after transfection with ice-cold lysis buffer and the complementary cDNA was prepared using “from cell to cDNA” kit (Ambion) according to the two-step protocol supplied by the manufacturer. The amplifications were performed in Peltier thermal cycler (Bio-Rad). Samples of 2.5 μl of cDNA were amplified with a Taq DNA Polymerase (New England BioLabs Ltd., On, Canada) in 25 μl of a reaction mixture containing 10 pmol primer mixture for the P2Y2 or 5 pmol primer mixture for the gene of reference (actin, Ambion) using ThermoPol buffer (New England Biolabs) and 0.2 mM dNTP. The following primers were designed based on the 5′ and 3′ ends of published human P2Y2 sequence: forward 5′-GCT-ACA-GGT-GCC-GCT-TCA-ACG-AGG-ACT-TC-3′ and reverse 5′-GGC-AGG-CCA-GCA-CCA-ACA-CCC-ACA-C-3′, that give an amplified fragment of 429 bp. The amplification was started by 2 min incubation at 94 °C followed by 35 cycles of 45 s denaturation at 94 °C, 45 s annealing at 66 °C, 45 s primer extension at 72 °C and ended by a 7-min incubation at 72 °C. A similar program was used for the amplification of the gene of reference with 25 cycles and the annealing temperature of 60 °C.
2.7. IL-8 secretion by HUVEC monolayers
To quantify IL-8 secreted by LPS-treated HUVEC monolayers in the Boyden chamber, these cells were stimulated for 3 h with LPS (100 ng/ml) added to the bottom chamber. To quantify cell-bound IL-8, HUVECs were treated for 1 h with a mixture of heparinases I, II and III (2 U/ml of each enzyme; Sigma) to digest heparan sulphate residues binding IL-8 at the surface of these cells. These heparinase treatments were performed in different experiments either before or after LPS stimulation. The media of the upper and bottom chamber were collected and their IL-8 content measured by Flexia enzyme-linked immunosorbent assay kit (ELISA, Medicorp, Montréal, Canada), following the manufacturer’s instructions. Human recombinant IL-8 was used as a standard.
2.8. Laser scanning cytometry (LSC) for quantification of the cell-bound IL-8
HUVECs (5 × 104/cover slip) were stimulated for 3 h with LPS (100 ng/ml) and the CellTracker™ Green (20 μM) was added 30 min before the end of stimulation to visualize the cells for LSC analysis. After the stimulation, HUVECs were fixed for 20 min with 4% paraformaldehyde in PBS, washed with PBS (3 × 5 min), and incubated for 45 min in the blocking solution containing 7% goat serum, 0.5% BSA, 0.2% Tween-20, 0.1% fish oil and 0.05% NaN3. The cells were next incubated for 1 h with a mouse anti-IL-8 monoclonal antibody MAB208 diluted in blocking solution (25 μg/ml), washed with PBS (3 × 5 min) and incubated with Alexa594-conjugated goat anti-mouse IgG antibody (Invitrogen) diluted in blocking solution (1 μg/ml). HUVECs were then washed with PBS (3 × 5 min) and incubated for 10 min with 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen) diluted in PBS (1 μM) to visualize the nuclei. After final wash (3 × 5 min), the cover slips were mounted in Mowiol medium containing DABCO antifade reagent and kept at −80 °C until analyzed. The quantification of the cell-bound IL-8 was done using a Laser Scanning Cytometer system (CompuCyte Corporation, Cambridge, MA).
2.9. Flow cytometry for ICAM-1 expression on HUVECs
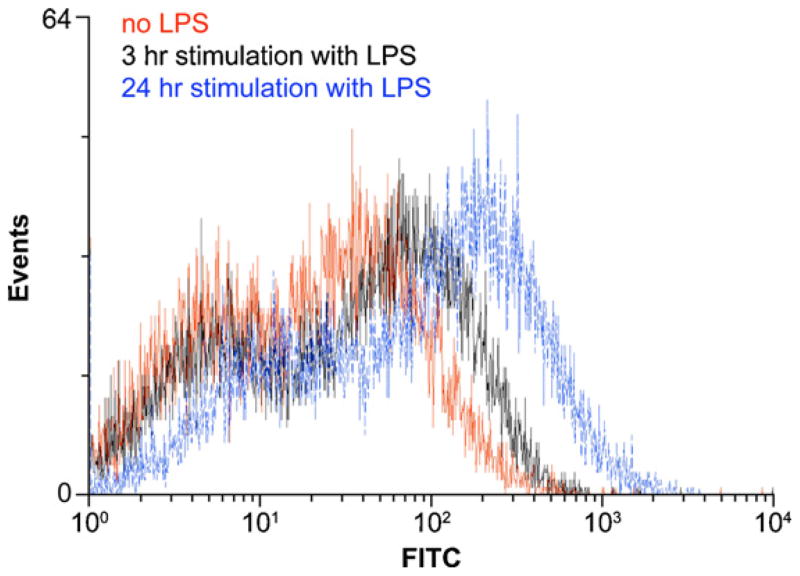
HUVECs (105 cell/well in a 24-well plate) were stimulated for 3 and 24 h with LPS (100 ng/ml), washed twice with PBS, detached by a 15-min incubation with 15 mM sodium citrate and 135 mM KCl at 37°C. The detached cells were centrifuged (500 × g, 5 min, 4 °C), re-suspended in PBS containing 1% FBS and 0.1% NaN3 (PBS/FBS/NaN3), incubated with the mouse anti-ICAM-1 antibody AF720 (25 μg/ml) for 1 h at 4 °C and washed twice with PBS/FBS/NaN3 solution. The HUVECs were next incubated with the secondary anti-mouse FITC-conjugated antibody (1 μg/ml) for 30 min at 4 °C, washed twice with PBS/FBS/NaN3 solution and analyzed by flow cytometry.
2.10. Statistical analysis
Student’s t-test was performed using Excel software (Microsoft® Office OneNote™ 2003).
3. Results
3.1. P2 receptor(s) mediate LPS-induced neutrophil TEM in vitro
LPS-induced neutrophil TEM across HUVEC monolayers was investigated using a modified Boyden chamber assay (see Material and methods). Preliminary experiments showed that 0.1 μg/ml LPS added to the bottom chamber caused a transmigration of ~50–80% of neutrophils loaded in the upper chamber (106 cells) within 3 h of stimulation. This time point was used for all subsequent TEM assays.
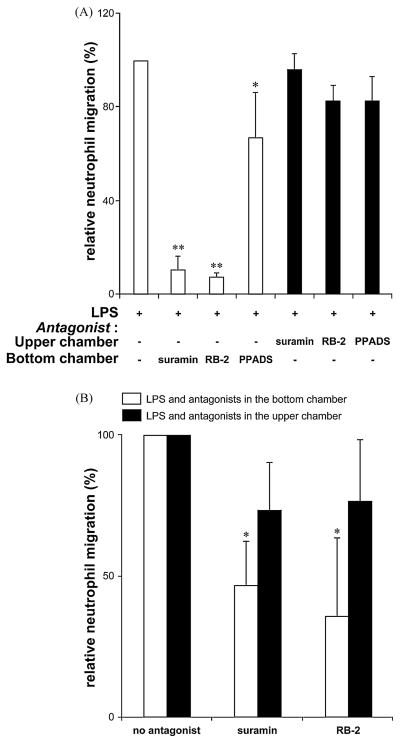
To test whether P2 receptors expressed on HUVECs and/or neutrophils are involved in LPS-induced TEM, the migration of human neutrophils across HUVEC monolayers stimulated with LPS was performed either in the presence or absence of the general P2 receptor antagonists, suramin, RB-2 and PPADS (all at 100 μM; added to HUVECs 15 min before LPS stimulation). As seen in Fig. 1A, suramin and RB-2 present in the media of the bottom chambers (open bars) completely inhibited LPS-induced neutrophil TEM while PPADS had a weaker effect. In contrast, the presence of these inhibitors in the media of the upper chambers (solid bars) in addition to their preincubation with neutrophils (for 15 min) only slightly decreased neutrophil TEM (Fig. 1A). These data suggested that LPS-induced neutrophil TEM was inhibited by blocking P2 receptor(s) present on HUVECs or, less likely, on the neutrophils already migrated to the bottom chambers. To exclude the effect of suramin and RB-2 on neutrophil P2 receptors, the TEM assays were modified as follows: the HUVEC monolayers were pre-stimulated with LPS alone or in combination with either suramin or RB-2 for 1 h (all added to the bottom chamber), the media of the upper and bottom chambers removed, the HUVEC monolayers washed and transferred to a new 24-well plate. Then fresh neutrophils were added for a 2-h migration, and as for the assay above, suramin and RB-2 also markedly decreased their migration across HUVECs (Fig. 1B; open bars). These results confirmed that LPS-induced neutrophil TEM involves the activation of HUVECs’ basolateral P2 receptors and that P2 receptors on neutrophils do not appear to have a role in this model.
Fig. 1.
Endothelial P2 receptors control LPS-induced neutrophil TEM. Panel A: HUVEC monolayers were preincubated for 15 min with the indicated P2 receptor antagonists (100 μM) added either to the bottom (open bars; to block basolateral P2 receptors) or the upper chamber (solid bars; to block luminal P2 receptors). In the latter case, the antagonists were also added to neutrophils and preincubated for 15 min. The neutrophil TEM was initiated with LPS (100 ng/ml) added to the bottom chamber and carried out for 3 h. These data represent the mean + S.D. of at least three assays with neutrophils from independent donors. The assays were carried out in duplicate or triplicate. *P < 0.03 vs. LPS alone, **P < 0.001 vs. LPS alone. Suramin and RB-2 added to the bottom chamber suppressed LPS-induced neutrophil TEM (open bars) while these antagonists were ineffective when added to the upper chamber (solid bars). Panel B: The HUVEC monolayers were pre-stimulated for 1 h with LPS (100 ng/ml) in the presence or absence of suramin or RB-2 (100 μM), all added together either to the bottom or to the upper chamber (open and solid bars, respectively). To exclude the effect of LPS and the antagonists on neutrophils, these cells were loaded for migration once the media of the bottom and upper chambers had been removed and the HUVEC monolayers washed. The migration was carried out for 2 h. These data represent the mean + S.D. of at least three assays with neutrophils from different donors. The assays were carried out in duplicate or triplicate. *P < 0.007 vs. LPS.
All previous TEM assays were performed with LPS in the bottom chamber. We next tested whether the addition of LPS to the upper chamber would also trigger neutrophil TEM and whether this process would involve P2 receptors. To avoid neutrophil stimulation with LPS, HUVEC monolayers were pre-stimulated with LPS added to the upper chamber for 1 h, the media of the upper and bottom chambers removed, the HUVEC monolayers washed and transferred to a new 24-well plate. Then fresh neutrophils were added for a 2-h migration. Note that HUVEC pre-stimulation was performed either in the presence or absence of suramin and RB-2 in the upper chamber. As seen in Fig. 1B (solid bars), LPS added to the upper chamber triggered neutrophil TEM which was comparable to that observed with LPS in the bottom chamber, however this migration was not inhibited by P2 antagonists. Based on these data, it was concluded that the luminal HUVECs’ P2 receptors are not involved in LPS-induced neutrophil TEM and therefore all subsequent TEM assays were performed with LPS and antagonists in the bottom chamber.
3.2. Endothelial P2Y2 receptor regulates LPS-induced neutrophil TEM
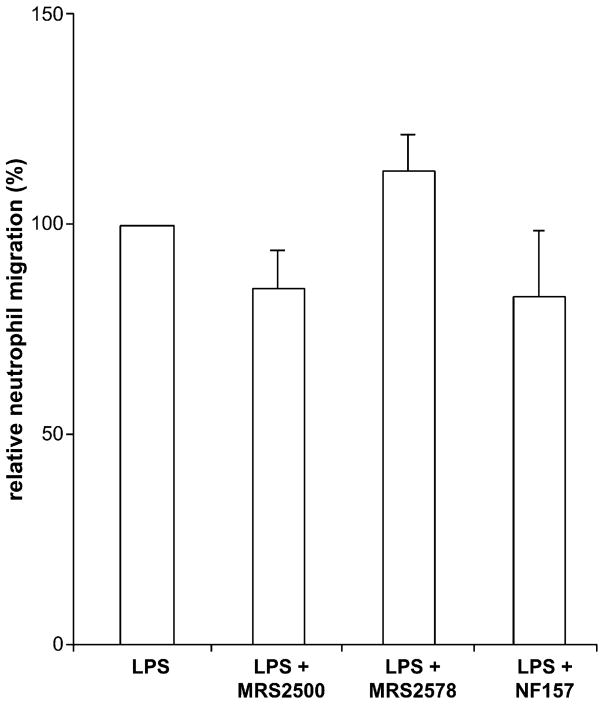
Suramin and RB-2 are potent inhibitors of all P2Y subtypes expressed in HUVECs (P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11) with the exception of P2Y4 that is insensitive to suramin (Brunschweiger and Muller, 2006). To further define the P2Y receptor involved, the selective antagonists of P2Y1, P2Y6 and P2Y11 (MRS2500, MRS2578 and NF157, respectively) were tested on LPS-induced neutrophil TEM. The efficacy of these molecules at their respective target receptor has previously been validated in multiple works. To ascertain the efficacy of the compounds that we used, we have performed positive controls. For example, MRS2500 inhibited, as expected, ADP-induced and P2Y1-mediated mouse endothelial cell vasodilation (Kauffenstein et al., in press), MRS2578 inhibited P2Y6-mediated IL-8 secretion from human monocytes (Kukulski et al., 2007; Ben Yebdri et al., 2009) while NF157 inhibited P2Y11-mediated neutrophil chemotaxis and apoptosis (Moreschi et al., 2006; Vaughan et al., 2007). All these antagonists had only minor effects on neutrophil TEM that were not statistically different from the control (Fig. 2). Note that for the selective inhibition of P2Y11 we used 1 μM NF157 as higher concentrations of this antagonist affect other P2Y subtypes including P2Y2 (Ullmann et al., 2005). In agreement, 100 μM NF157 inhibited neutrophil migration by 70% (data not shown). Taken together, these data suggested that the P2Y1, P2Y4, P2Y6 and P2Y11 receptors were not involved in LPS-induced neutrophil TEM.
Fig. 2.
The P2Y1, P2Y6 and P2Y11 receptors are not involved in LPS-induced neutrophil TEM. HUVEC monolayers were preincubated for 15 min with MRS2500 (10 μM; a P2Y1 antagonist), MRS2578 (10 μM; a P2Y6 antagonist) or NF157 (1 μM; a P2Y11 antagonist) added to the bottom chamber. The neutrophil TEM was initiated by the addition of LPS (100 ng/ml) to the bottom chamber and carried out for 3 h. These data represent the mean + S.D. of at least three assays with neutrophils and HUVECs of three independent donors. The assays were carried out in triplicate.
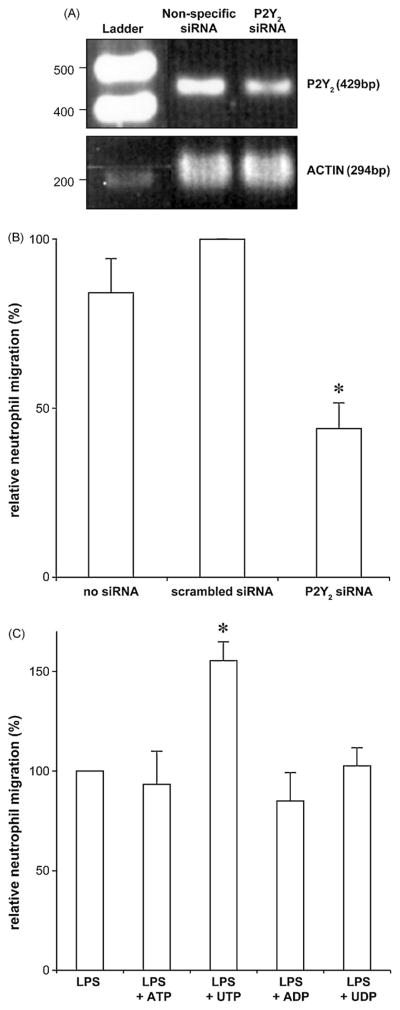
No selective P2Y2 antagonist is available and therefore the role of this receptor in neutrophil TEM was addressed using a gene silencing technique with a validated anti-P2Y2 siRNA. Density analysis of the cDNA bands for P2RY2 revealed >50% decrease of the P2Y2 transcript in HUVECs treated with anti-P2Y2 siRNA compared to the cells treated with scrambled siRNA (scanned density of 323 vs. 690; Fig. 3A). P2Y2 protein expression knockdown was not assessed as to our knowledge there are no specific antibodies against this receptor. The P2Y2 knockdown in HUVECs significantly decreased neutrophil TEM compared to the control cells which were either untransfected or transfected with scrambled siRNA (~50% decrease; Fig. 3B). These data demonstrate that LPS-induced neutrophil TEM was mediated by the endothelial P2Y2 receptor.
Fig. 3.
Endothelial P2Y2 receptors control LPS-induced neutrophil TEM. Panels A and B: HUVEC monolayers (2 × 105 cells/insert) were transfected with a validated anti-P2Y2 or with a control siRNA, as described in Material and Methods. The knockdown efficiency was verified by RT-PCR (panel A), as described in Material and Methods. The neutrophil TEM was initiated by the addition of LPS (100 ng/ml) to the bottom chamber and carried out for 3 h (panel B). These data represent the mean + S.D. of at least three experiments carried out in triplicate with neutrophils from different donors. Upon LPS stimulation, P2Y2 -depletion in HUVECs resulted in a diminished neutrophil migration compared to the HUVECs transfected with scrambled siRNA (*P < 0.01). Panel C: The HUVEC monolayers were pre-stimulated for 1 h with a suboptimal concentration of LPS (1 ng/ml) in the presence or absence of either 100 μM ATP, UTP, ADP or UDP, also added to the bottom chamber. To exclude an effect of LPS and nucleotides on neutrophils, these cells were loaded for migration once the media of the bottom and upper chambers had been removed and the HUVEC monolayers washed. The migration was carried out for the following 2 h. These data represent the mean + S.D. of at least three assays with neutrophils from different donors. The assays were carried out in duplicate or triplicate. UTP, an agonist of P2Y2, but not the other nucleotides tested, potentiated LPS-induced neutrophil TEM (*P < 0.01 vs. LPS alone).
As ATP and UTP are natural ligands of P2Y2, the role of this receptor in LPS-induced TEM was further addressed by testing whether these two nucleotides (100 μM) would potentiate neutrophil migration due to a suboptimal concentration of LPS (1 ng/ml). Note that this low concentration of LPS induced only a weak migration of neutrophils (~20–30% of the maximum migration obtained with 100 ng/ml) that could therefore be further increased. ADP and UDP which are not the agonists of P2Y2 were used as negative controls. Note that none of the nucleotides induced neutrophil migration per se in this model ((Kukulski et al., 2007) and data not shown). As seen in Fig. 3C, UTP, but not other nucleotides tested, potentiated LPS-induced neutrophil migration. The lack of the effect of ATP was probably due to its hydrolysis to adenosine which is a potent inhibitor of neutrophil migration in the model used as this nucleotide also potentiated neutrophil TEM but only in the presence of adenosine deaminase (data not shown).
3.3. Rho kinase activation is required for LPS-induced neutrophil TEM
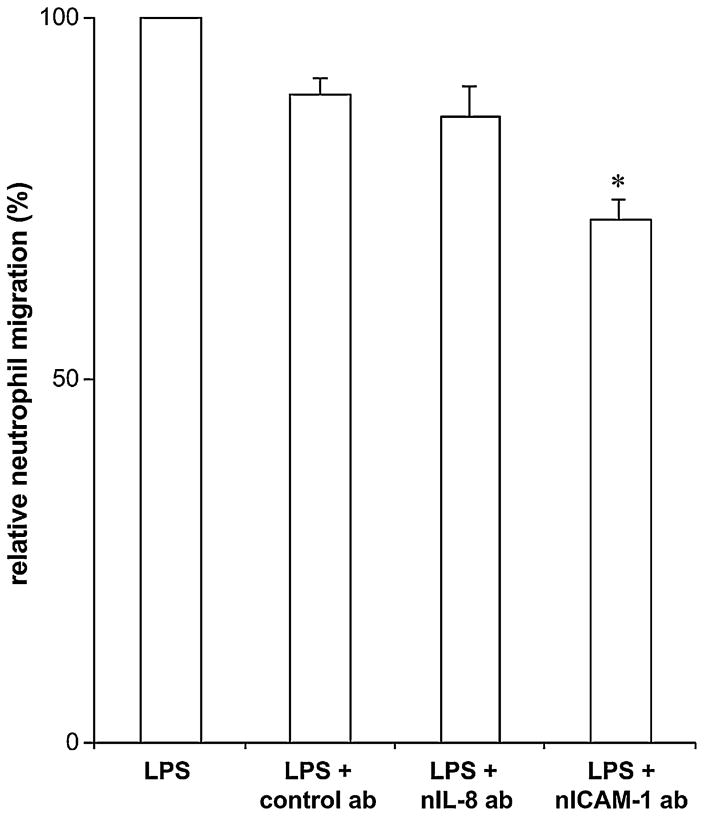
We next investigated the mechanism(s) responsible for LPS-induced TEM. LPS-treated HUVECs secrete significant amounts of IL-8 that could trigger neutrophil recruitment. However, in the TEM assays performed here, IL-8 neutralizing antibodies added to both the upper and bottom chambers had no effect on LPS-induced neutrophil migration (Fig. 4). In control experiments, these antibodies completely inhibited IL-8-induced neutrophil migration (Kukulski et al., 2007) and in contrasts to LPS-induced neutrophil TEM, IL-8 neutralizing antibodies inhibited by ~80% neutrophil TEM due to poly(I:C) (data not shown). Note however, that to take effect, this migration required at least 4 h of HUVEC stimulation with poly(I:C) which correlates with the kinetics of HUVECs’ IL-8 secretion for this stimulus (F.K. and J.S. unpublished observation). In keeping with the data obtained with IL-8 neutralizing antibodies, LPS required more than 3 h to induce a significant IL-8 production and secretion from HUVECs (Fig. 5A). This time course of IL-8 secretion was also confirmed by IL-8 ELISA of the media collected from the bottom and upper chambers 3 h after LPS stimulation, where both untreated and LPS-treated HUVEC monolayers produced low and comparable amounts of this chemokine (Fig. 5B). It has been recently proposed that endothelial cells not only secrete but also bind IL-8 via extracellular heparan sulphate residues (cell-bound IL-8) and subsequently present it to neutrophils, thus increasing their migration (Middleton et al., 2002). To verify this possibility, we treated HUVECs with a mixture of heparinases I, II and III to remove heparan sulphate residues from their surface either before or after stimulation with LPS. This treatment did not increase IL-8 in the media of these cells compared to the untreated cells (data not shown). We also performed a laser scanning cytometry of HUVECs incubated with anti-IL-8 antibodies to quantify cell-bound IL-8 which showed no differences in fluorescence corresponding to cell-bound IL-8 on HUVECs stimulated with LPS for 3 h compared to unstimulated HUVECs (data not shown). These data demonstrated that neither soluble nor cell-bound IL-8 was responsible for LPS-induced TEM in this work.
Fig. 4.
Anti-IL-8 and ICAM-1 antibodies have a modest effect on the initial LPS-induced neutrophil TEM. The antibodies were added to the HUVEC monolayers (to the bottom and upper chambers) and then neutrophil TEM was initiated by the addition of LPS (100 ng/ml) to the bottom chamber and carried out for 3 h. These data represent the mean + S.D. of at least three assays with neutrophils from different donors. The assays were carried out in triplicate. nIL-8 ab: IL-8 neutralizing antibody; nICAM-1 ab: ICAM-1 neutralizing antibody; *P < 0.006 vs. LPS + control ab.
Fig. 5.
Time scale of IL-8 secretion by HUVECs stimulated with LPS. Panel A: HUVECs (seeded at 105/well in a 24-well plate) were stimulated with LPS (100 ng/ml) and both the supernatants and cells were collected at the indicated time points up to 5 h. The collected HUVECs were lysed with 1% Triton X-100/PBS for 2 h at 4 °C. The resulted samples (supernatants and cell lysates) were analyzed by ELISA for IL-8 as described below. These data represent the mean + S.D. of at least three experiments in triplicate. Panel B: HUVEC monolayers of Boyden chamber were stimulated for 3 h with LPS (100 ng/ml) added to the bottom chamber in the absence of neutrophils. The media of the bottom and upper chambers (open and solid bars, respectively) were collected and analyzed for IL-8 by ELISA. These data represent the mean + S.D. of at least three experiments in triplicate. As expected from panel A, at this time point, LPS did not yet induce the secretion of IL-8 when compared to unstimulated control.
LPS-induced neutrophil TEM can also be activated by ICAM-1 (Becker et al., 2001; Basit et al., 2006). However, the anti-ICAM-1 blocking antibody only slightly decreased LPS-induced neutrophil migration by ~15% (Fig. 4). In agreement with these data, a low increase in ICAM-1 expression was seen in HUVECs stimulated with LPS for 3 h compared to unstimulated HUVECs, as determined by flow cytometry (Fig. 6). However, a longer stimulation time (24 h; Fig. 6) resulted in further increase in ICAM-1 expression suggesting that this protein plays a more significant role at later stages of neutrophil migration, as previously reported (Basit et al., 2006). In addition, LPS-induced neutrophil TEM did not involve the upregulation of E-selectin expression as the incubation of HUVECs with 50 mM sialic acid did not affect neutrophil migration (data not shown).
Fig. 6.
Time scale of ICAM-1 expression by HUVECs stimulated with LPS. HUVECs (seeded at 105/well in a 6-well plate) were stimulated with LPS (100 ng/ml) for 3 and 24 h. The detached cells were collected, incubated successively with the mouse anti-ICAM-1 antibody AF720 and the secondary anti-mouse FITC-conjugated antibody, and analyzed by FACS. At 3 h post-stimulation there is a modest increase in cell surface expression of ICAM-1 which is much stronger after 24 h. A representative experiment out of two, with cells from different donors, is shown.
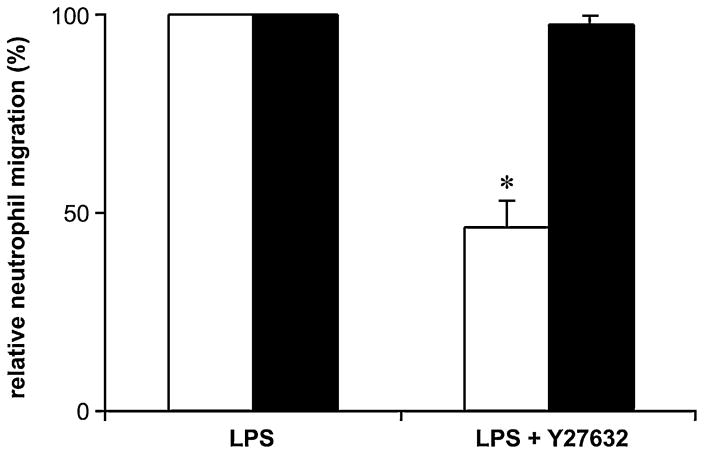
Neutrophil TEM can be regulated by Rho kinase that induces cytoskeleton reorganization in endothelial cells (Tasaka et al., 2005; Essler et al., 2000; Saito et al., 1998). The involvement of Rho kinase in LPS-induced neutrophil TEM was determined with the specific Rho kinase inhibitor Y27632. The pre-treatment of HUVEC monolayers with this molecule (10 μM) diminished neutrophil migration by 50% (Fig. 7, open bars). Importantly, the pre-treatment of HUVEC with Y27632 (10 μM) in combination with suramin or RB-2 (100 μM) only slightly increased the inhibition of neutrophil migration (~10% increase compared to Y27632 alone; data not shown) suggesting that all these molecules block the same cellular response. To determine whether Rho kinase expressed in neutrophils was also involved in this phenomenon, these cells were also preincubated with Y27632 (10 and 100 μM) for 30 min before migration. As this treatment did not affect neutrophil migration (solid bars; Fig. 7, not shown for 100 μM Y27632), it was concluded that LPS-induced neutrophil TEM was regulated by endothelial Rho kinase. Noteworthy, Y27632 efficiently inhibited neutrophil migration toward IL-8 in a transwell chemotaxis assay without HUVEC monolayers (100% inhibition at 100 μM; data not shown) which was performed as a positive control.
Fig. 7.
Endothelial Rho kinase is involved in LPS-induced neutrophil TEM. HUVEC monolayers (open bars) or neutrophils (solid bars) were preincubated for 15 min with Y-27632 (10 μM) and washed. Fresh neutrophils were then loaded onto the Y-27632-treated HUVECs (open bars) whereas Y-27632-treated neutrophils were loaded onto untreated HUVECs (solid bars). The TEM was initiated with LPS (100 ng/ml) added to the bottom chamber and carried out for 3 h. These data represent the mean + S.D. of three experiments in triplicate with neutrophils from different donors. *P < 0.001 vs. LPS.
Altogether, these data indicate that LPS-induced endothelial Rho kinase activation is required for neutrophil TEM. The minor effect of IL-8 and ICAM-1 on this process correlates with the kinetics of production/expression of these proteins that takes several hours in HUVECs. The weak inhibition of TEM with ICAM-1 neutralizing antibodies suggests however that the slight increased expression of this protein may somehow facilitate neutrophil transmigration, for example by increasing their adhesion to HUVECs.
4. Discussion
This work demonstrates that endothelial P2Y2 receptor regulates LPS-induced neutrophil TEM in vitro. Indeed, neutrophil migration across LPS-stimulated HUVEC monolayers was inhibited by two non-specific P2Y2 antagonists, suramin and RB-2 as well as by specific P2Y2 knockdown in HUVECs with a validated siRNA. Moreover, this migration was potentiated by UTP, a P2Y2 ligand. The recent works by Dr. Junger’s group and us showed that the P2Y2 expressed in neutrophils is also important for neutrophil migration in vitro (Chen et al., 2006; Kukulski et al., 2009). However, these works investigated neutrophil migration using a trans-well chemotaxis model where neutrophils migrated through an uncoated synthetic membrane without an endothelial cell layer which contrasts with the model used here. Interestingly, P2Y2 knockout mice showed reduced neutrophil migration that has been proposed to be due to P2Y2 deficiency in neutrophils (Inoue et al., 2008). In the light of the data presented here, it cannot be excluded that the impaired neutrophil migration in these mice could also (or rather) be a result of the P2Y2 deficiency in endothelial cells. In further support of this view, multiple P2Y receptors, including P2Y2, have been suggested to mediate nucleotide-induced increase of HUVECs’ permeability for FITC-labeled dextran (Tanaka et al., 2003).
There are other reports demonstrating an important role of either non-endothelial or endothelial P2Y2 receptor in immune cell migration in vitro via stimulation of mechanisms distinct from Rho kinase activation described in this work. Our group has recently demonstrated that the P2Y2 (and P2Y6) receptor may promote the migration of neutrophils by mediating IL-8 secretion induced by TLR2 activation in human monocytic THP-1 cells (Ben Yebdri et al., 2009). P2Y2 has also been implicated in the recruitment of monocytes/macrophages and lymphocytes by inducing vascular cell adhesion molecule-1 (VCAM-1) expression in human endothelial cells and in a submandibular gland cell line, respectively (Seye et al., 2003; Baker et al., 2008). In addition, P2Y2 may also promote monocyte migration by activating MCP-1 release from murine macrophages (Stokes and Surprenant, 2007).
The data presented here suggest that LPS-induced neutrophil TEM involves P2Y2-dependent Rho kinase activation. In keeping with these data, P2Y2 has previously been shown to be coupled to Rho kinase activation in vascular myocytes and 1321N1 astrocy-toma cells (Sauzeau et al., 2000; Liao et al., 2007). The involvement of the endothelial Rho kinase in LPS-induced neutrophil migration in our model was confirmed with Y27632, a selective inhibitor of this enzyme. In line with these data, this molecule has previously been shown to attenuate neutrophil migration in endotoxin-induced acute lung injury and also neutrophil TEM in vitro with leukotriene B4 (LTB4) as a migration stimulus (Tasaka et al., 2005; Saito et al., 1998; Mong and Wang, 2009). Neutrophil Rho kinase also plays an important role in neutrophil migration, for instance during chemotaxis (Junger, 2008). However, in the TEM model presented here, the inhibition of the neutrophil Rho kinase with Y27632 did not affect the migration of these cells. This suggests that the neutrophil motility response controlled by Rho kinase is not necessary for TEM in the model used in this work, and the passage of these cells through endothelium may be driven mainly by the latter cells. Indeed, in contrast to neutrophil chemoattractants such as IL-8, fMLP and C5a which are all known to activate Rho kinase/motility response in these cells, LPS used in these assays as a migration inducer is not a neutrophil chemoattractant and therefore was not expected to have this effect.
IL-8 has been shown to play an important role in LPS-induced neutrophil recruitment but had limited effect in our model. This is in agreement with the fact that HUVECs do not pre-store IL-8 (unless they have been previously stimulated) and require a long stimulation (~5 h) for physiologically important IL-8 synthesis and release (Fig. 5A). On the other hand, IL-8 secreted by HUVECs could also bind to extracellular heparan sulphate residues of these cells (cell-bound IL-8) and therefore be undetectable by ELISA. This cell-bound IL-8 plays an important role in neutrophil extravasation (Middleton et al., 2002). However, no LPS-induction of cell-bound IL-8 was detected in HUVECs by laser scanning microscopy analysis. In agreement with these results, the treatment of LPS-stimulated HUVECs with heparinases to digest heparan sulphate residues did not increase IL-8 level in their media. Altogether with the results obtained using the neutralizing IL-8 antibody, these data suggest that IL-8 is not involved in initial LPS-induced neutrophil TEM studied by this work but may certainly come into play at a later stage when it is produced and secreted in significant amounts.
There is compelling evidence that ICAM-1 plays important role in LPS-induced neutrophil migration (Wagner and Roth, 2000; Becker et al., 2001; Basit et al., 2006). Vascular endothelium expresses low levels of ICAM-1 that is upregulated by inflammatory stimuli such as LPS and TNF-alpha (Yan et al., 2002). However, several hours of stimulation are required to induce a significant upregulation of ICAM-1 expression. For example, it takes ~12 h for a significant increase of ICAM-1 expression in the lungs challenged with LPS while 24 h stimulation was needed for a 2-fold increase of ICAM-1 expression in ATPγS-stimulated human dermal microvascular endothelial cells-1 (HMEC-1) (Yan et al., 2002; Seiffert et al., 2006). In our TEM assays carried out for 3 h, only low increase of ICAM-1 expression was detected which could explain the ~15% decrease of neutrophil TEM by ICAM-1 blocking antibodies. Therefore, as for IL-8, it appears that ICAM-1 becomes physiologically important at later stages of LPS-induced neutrophil migration but is not responsible for the large TEM observed in our model.
Taken together, the above data suggest that LPS-induced neutrophil TEM is mediated by the activation of Rho kinase in HUVECs, and not via the upregulation of the production/expression of IL-8, ICAM-1 and/or E-selectin that do have an effect, but later on. The lack of the major effect of all these molecules may be specific to the TEM models with HUVECs as the distinct types of endothelial cells may exhibit a different kinetics of IL-8 and ICAM-1 production in response to LPS. On the other hand, our results are in agreement with previously reported in vivo data showing for example that ICAM-1 expression in the lung endothelium increases after ~12 h from LPS-challenge (Basit et al., 2006). It is also conceivable that the model used in this work may reflect the situation that takes place in capillaries, where neutrophils are in close contact with endothelial cells, and thus only the change in endothelium permeability and basal expression of IL-8 and ICAM-1 may be sufficient for their extravasation. In contrast, the latter proteins could be expected to play a more important role in large vessels where endothelial cells have to attract and subsequently capture circulating neutrophils before their actual egress (Middleton et al., 2002).
Collectively, our data show a novel role of endothelial P2Y2 receptor in inflammatory neutrophil migration that may be crucial in early responses to Gram-negative bacteria. These data are in line with previous reports demonstrating other functions of P2Y2 in immune responses and inflammation, making this receptor a potential target for new therapies.
Acknowledgments
The authors thank Mrs. J.-C. Lévesque for the LSC analysis and Dr. M. Dufour for the flow cytometry analysis. This work was supported by grants to J.S. from the Canadian Institutes of Health Research (CIHR) and from The Arthritis Society (TAS). F.K. and F.B. were recipients of a fellowship from the CIHR/Wyeth Pharmaceuticals, F.B.Y. of a scholarship from Fonds de la Recherche sur l’Arthrite et les Maladies Rhumatismales (FRAMR) and J.S. of a new investigator award from the CIHR and of a Junior 2 scholarship from the FRSQ.
References
- Arthur DB, Akassoglou K, Insel PA. P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci USA. 2005;102:19138–19143. doi: 10.1073/pnas.0505913102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker OJ, Camden JM, Rome DE, Seye CI, Weisman GA. P2Y2 nucleotide receptor activation up-regulates vascular cell adhesion molecule-1 expression and enhances lymphocyte adherence to a human submandibular gland cell line. Mol Immunol. 2008;45:65–75. doi: 10.1016/j.molimm.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basit A, Reutershan J, Morris MA, Solga M, Rose CE, Jr, Ley K. ICAM-1 and LFA-1 play critical roles in LPS-induced neutrophil recruitment into the alveolar space. Am J Physiol Lung Cell Mol Physiol. 2006;291:L200–L207. doi: 10.1152/ajplung.00346.2005. [DOI] [PubMed] [Google Scholar]
- Beck GC, Yard BA, Breedijk AJ, Van Ackern K, Van Der Woude FJ. Release of CXC-chemokines by human lung microvascular endothelial cells (LMVEC) compared with macrovascular umbilical vein endothelial cells. Clin Exp Immunol. 1999;118:298–303. doi: 10.1046/j.1365-2249.1999.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MD, Garman K, Whitcup SM, Planck SR, Rosenbaum JT. Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2001;42:2563–2566. [PubMed] [Google Scholar]
- Ben Yebdri F, Kukulski F, Tremblay A, Sévigny J. Concomitant activation of P2Y2 and P2Y6 receptors on monocytes is required for TLR1/2-induced neutrophil migration by regulating IL-8 secretion. Eur J Immunol. 2009;39:2885–2894. doi: 10.1002/eji.200939347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Brunschweiger A, Muller CE. P2 receptors activated by uracil nucleotides—an update. Curr Med Chem. 2006;13:289–312. doi: 10.2174/092986706775476052. [DOI] [PubMed] [Google Scholar]
- Chakravarti A, Raquil MA, Tessier P, Poubelle PE. Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood. 2009;114:1633–1644. doi: 10.1182/blood-2008-09-178301. [DOI] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- Essler M, Staddon JM, Weber PC, Aepfelbacher M. Cyclic AMP blocks bacterial lipopolysaccharide-induced myosin light chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling. J Immunol. 2000;164:6543–6549. doi: 10.4049/jimmunol.164.12.6543. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Chen Y, Hirsh MI, Yip L, Junger WG. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock. 2008;30:173–177. doi: 10.1097/shk.0b013e318160dad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz AC, Chuluyan HE, Lopes N. CD11/CD18-independent transendothelial migration of human polymorphonuclear leukocytes and monocytes: involvement of distinct and unique mechanisms. J Leukoc Biol. 1995;57:553–561. doi: 10.1002/jlb.57.4.553. [DOI] [PubMed] [Google Scholar]
- Junger WG. Purinergic regulation of neutrophil chemotaxis. Cell Mol Life Sci. 2008;65:2528–2540. doi: 10.1007/s00018-008-8095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffenstein G, Fürstenau CR, D’Orléans-Juste P, Sévigny J. NTPDase1 distinctly regulates P2Y1 and P2Y2 receptor-dependent vasorelaxation. Br J Pharmacol. doi: 10.1111/j.1476-5381.2009.00566.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukulski F, Ben Yebdri F, Lecka J, Kauffenstein G, Lévesque SA, Martín-Satué M, Sévigny J. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine. 2009;46:166–170. doi: 10.1016/j.cyto.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukulski F, Ben Yebdri F, Lefebvre J, Warny M, Tessier PA, Sévigny J. Extracellular nucleotides mediate LPS-induced neutrophil migration in vitro and in vivo. J Leukoc Biol. 2007;81:1269–1275. doi: 10.1189/jlb.1206758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor requires interaction with alpha v integrins to access and activate G12. J Cell Sci. 2007;120:1654–1662. doi: 10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leuko-cyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- Mong PY, Wang Q. Activation of Rho kinase isoforms in lung endothelial cells during inflammation. J Immunol. 2009;182:2385–2394. doi: 10.4049/jimmunol.0802811. [DOI] [PubMed] [Google Scholar]
- Moreschi I, Bruzzone S, Nicholas RA, Fruscione F, Sturla L, Benvenuto F, Usai C, Meis S, Kassack MU, Zocchi E, De Flora A. Extracellular NAD+ is an agonist of the human P2Y11 purinergic receptor in human granulocytes. J Biol Chem. 2006;281:31419–31429. doi: 10.1074/jbc.M606625200. [DOI] [PubMed] [Google Scholar]
- Saito H, Minamiya Y, Kitamura M, Saito S, Enomoto K, Terada K, Ogawa J. Endothelial myosin light chain kinase regulates neutrophil migration across human umbilical vein endothelial cell monolayer. J Immunol. 1998;161:1533–1540. [PubMed] [Google Scholar]
- Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Vaillant N, Gadeau AP, Desgranges C, Scalbert E, Chardin P, Pacaud P, Loirand G. P2Y1, P2Y2, P2Y4, and P2Y6 receptors are coupled to Rho and Rho kinase activation in vascular myocytes. Am J Physiol – Heart Circ Physiol. 2000;278:H1751–H1761. doi: 10.1152/ajpheart.2000.278.6.H1751. [DOI] [PubMed] [Google Scholar]
- Seiffert K, Ding W, Wagner JA, Granstein RD. ATPgammaS enhances the production of inflammatory mediators by a human dermal endothelial cell line via purinergic receptor signaling. J Invest Dermatol. 2006;126:1017–1027. doi: 10.1038/sj.jid.5700135. [DOI] [PubMed] [Google Scholar]
- Seye CI, Yu N, Jain R, Kong Q, Minor T, Newton J, Erb L, Gonzalez FA, Weisman GA. The P2Y2 nucleotide receptor mediates UTP-induced vascular cell adhesion molecule-1 expression in coronary artery endothelial cells. J Biol Chem. 2003;278:24960–24965. doi: 10.1074/jbc.M301439200. [DOI] [PubMed] [Google Scholar]
- Stokes L, Surprenant A. Purinergic P2Y2 receptors induce increased MCP-1/CCL2 synthesis and release from rat alveolar and peritoneal macrophages. J Immunol. 2007;179:6016–6023. doi: 10.4049/jimmunol.179.9.6016. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Kawasaki K, Nejime N, Kubota Y, Takahashi K, Hashimoto M, Kunitomo M, Shinozuka K. P2Y receptor-mediated enhancement of permeation requires Ca2+ signalling in vascular endothelial cells. Clin Exp Pharmacol Physiol. 2003;30:649–652. doi: 10.1046/j.1440-1681.2003.03893.x. [DOI] [PubMed] [Google Scholar]
- Tasaka S, Koh H, Yamada W, Shimizu M, Ogawa Y, Hasegawa N, Yamaguchi K, Ishii Y, Richer SE, Doerschuk CM, Ishizaka A. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol. 2005;32:504–510. doi: 10.1165/rcmb.2004-0009OC. [DOI] [PubMed] [Google Scholar]
- Ullmann H, Meis S, Hongwiset D, Marzian C, Wiese M, Nickel P, Communi D, Boeynaems JM, Wolf C, Hausmann R, Schmalzing G, Kassack MU. Synthesis and structure-activity relationships of suramin-derived P2Y11 receptor antagonists with nanomolar potency. J Med Chem. 2005;48:7040–7048. doi: 10.1021/jm050301p. [DOI] [PubMed] [Google Scholar]
- Vaughan KR, Stokes L, Prince LR, Marriott HM, Meis S, Kassack MU, Bingle CD, Sabroe I, Surprenant A, Whyte MK. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J Immunol. 2007;179:8544–8553. doi: 10.4049/jimmunol.179.12.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52:349–374. [PubMed] [Google Scholar]
- Wang L, Karlsson L, Moses S, Hultgardh-Nilsson A, Andersson M, Borna C, Gudbjartsson T, Jern S, Erlinge D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- Warny M, Aboudola S, Robson SC, Sévigny J, Communi D, Soltoff SP, Kelly CP. P2Y6 nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J Biol Chem. 2001;276:26051–26056. doi: 10.1074/jbc.M102568200. [DOI] [PubMed] [Google Scholar]
- Yan W, Zhao K, Jiang Y, Huang Q, Wang J, Kan W, Wang S. Role of p38 MAPK in ICAM-1 expression of vascular endothelial cells induced by lipopolysaccharide. Shock. 2002;17:433–438. doi: 10.1097/00024382-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]