Abstract
In this work, we show that P2 nucleotide receptors control lipopolysaccharide (LPS)-induced neutrophil migration in the mouse air pouch model. Neutrophil infiltration in LPS-treated air pouches was reduced by the intravenous (iv) administration of the non-selective P2 receptor antagonist PPADS but not by suramin and RB-2. In addition, the iv administration of a P2 receptor ligand, UTP, enhanced LPS-induced neutrophil migration. In contrast, the iv injection of UDP had no effect on neutrophil migration. These data suggest that LPS-induced neutrophil migration in the air pouch could involve P2Y4 receptor which is antagonized by PPADS, activated by UTP, but not UDP, and insensitive to suramin. The inhibition of neutrophil migration by PPADS correlated with a diminished secretion of chemokines macrophage inflammatory protein-2 (MIP-2) and keratinocyte-derived chemokine (KC) in the air pouch exudates. As determined in vitro, PPADS did not affect MIP-2 and KC release from air pouch resident cells nor from accumulated neutrophils. MIP-2 and KC production in the LPS-treated air pouches correlated with an early neutrophil migration (1 h after LPS injection), and both of these effects were significantly reduced in mice administered with PPADS. Altogether, these data suggest that P2Y4 receptor expressed in circulating leukocytes and/or endothelium controls LPS-induced acute neutrophil recruitment in mouse air pouch.
Keywords: Extracellular nucleotides, PAMPs, Inflammation, Chemokines, Early neutrophil migration
1. Introduction
Lipopolysaccharide (LPS) is a cell wall component of Gram-negative bacteria and potent activator of innate immunity (Freudenberg et al., 2008). LPS and other bacterial cell wall components such as peptidoglycans and flagellin are pathogen associated molecular patterns (PAMPs) (Basu and Fenton, 2004). PAMPs are recognized by host via Toll-like receptors (TLR1-9) of which TLR4 is specific for LPS (Heumann and Roger, 2002). Typically, the exposure of host tissues to LPS initiates the recruitment of neutrophils that rapidly infiltrate the affected site in order to neutralize/eliminate the invading pathogens. Neutrophil migration is regulated by various soluble inflammatory mediators released by the infected tissue such as the chemokine interleukin 8 (IL-8) in human (Wagner and Roth, 2000). In rodents, neutrophil recruitment is activated mainly by the chemokines MIP-2 and KC (Bozic et al., 1995; Kopydlowski et al., 1999; Li et al., 2004; McColl and Clark-Lewis, 1999; Schramm et al., 2000; Shanley et al., 1995).
Extracellular nucleotides are signaling molecules that act via the activation of specific P2 receptors including the ionotropic P2X receptors (P2X1–7) and metabotropic P2Y receptors (P2Y1,2,4,6,11–14). All P2X and P2Y11 receptors are activated by ATP, P2Y2 by ATP and UTP, P2Y1, P2Y12 and P2Y13 by ADP, P2Y4 by UTP, P2Y6 by UDP and P2Y14 by UDP-glucose (Bours et al., 2006). Growing evidence shows that extracellular nucleotides play key roles in inflammatory neutrophil migration. ATP enhances neutrophil adhesion and extravasation (Bours et al., 2006; Dawicki et al., 1995), and also chemotaxis via P2Y2 receptor (Chen et al., 2006; Junger, 2008; Kukulski et al., 2009). In keeping with these data, mice deficient in ATP-dependent P2Y2 receptor exhibit an impaired neutrophil recruitment in sepsis (Inoue et al., 2008). Nucleotides can also induce neutrophil migration via activation of IL-8 secretion by human monocytes (Ben Yebdri et al., 2009; Kukulski et al., 2007).
The murine air pouch model of synovial cavity is widely used to study the basic mechanisms of arthritis and acute inflammatory responses (Alam, 2003; Colville-Nash and Lawrence, 2003; Elliott et al., 2009; Willoughby et al., 1986). Using this model, we show here that the P2 receptor(s) expressed in circulating leukocytes and/or endothelium may play a key role in LPS-induced acute neutrophil migration.
2. Materials and methods
2.1. Materials
LPS from E. coli O111:B4 (TLR4 agonist), potato apyrase grade VII, nucleotides (UTP and UDP), pyridoxal-phosphate-6-azophenyl-2′, 4′-disulfonate (PPADS) and suramin were purchased from Sigma (St. Louis, MO). Reactive blue 2 (RB-2) was from ICN Biochemicals Inc. (Aurora, OH). Pam3CSK4 (TLR2 agonist) and flagellin from S. typhimurium (TLR5 agonist) were obtained from InvivoGen (San Diego, CA). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Gibco (Grand Island, NY) and trypsin–EDTA from Invitrogen (Burlington, On, Canada).
LPS and apyrase were reconstituted in endotoxin-free saline (Sigma) while Pam3CSK4 and flagellin in endotoxin-free water supplied by the manufacturer. Before use, LPS was sonicated for 10 min in a water bath sonicator and diluted in sterile PBS (without Ca2+ and Mg2+; Gibco). The stock solutions of the P2 receptor antagonists (PPADS, suramin and RB-2; 10 mM) and nucleotides (UTP and UDP; 10 mM) were prepared in endotoxin-free water (Sigma), filtrated (0.22 μm membrane, Millipore Corporation, Billerine, MA) and diluted in sterile PBS.
2.2. Murine air pouch
The experimental protocol was approved by the animal protection committee of Université Laval (Québec, QC, Canada). Air pouches were pre-formed on the dorsum of female CD-1 mice (8–12 week old, Charles River) as before (Kukulski et al., 2007). The tail vein injection (iv) of P2 receptor antagonists (PPADS, suramin or RB-2; 200 μM in 0.2 ml) or apyrase (6 U in 0.2 ml) was performed ~2–3 min prior to the induction of inflammation by subcutaneous (sc) injection of LPS, Pam3CSK4, flagellin (10 ng in 1 ml) or mouse recombinant MIP-2 (2 ng in 1 ml) in the air pouch, as indicated. The control mice were injected iv with PBS (0.2 ml) and sc with PAMPs. In some experiments, the mice were injected iv with nucleotides (UTP or UDP; 20 μM in 0.2 ml) or PBS (0.2 ml; control mice) ~2–3 min before the sc injection of a low (suboptimal) dose of LPS (2 ng in 1 ml). Assuming that mice used in these experiments had ~4 ml of blood, the iv injection doses of antagonists and nucleotides were in the range sufficient to inhibit/activate P2 receptors (Abbracchio et al., 2006; Muller, 2002; North and Surprenant, 2000). Some mice were administered sc with LPS alone or with PPADS (10 ng and 10 μM, respectively, in 1 ml of sterile PBS).
The mice were sacrificed by CO2 poisoning 1 or 4 h after LPS injection. The air pouch exudates were collected with a 2 ml wash (PBS-5 mM EDTA) and centrifuged (500 × g, 10 min, 4 °C). The cells present in the pellet were counted with a hemacytometer and analyzed by a Diff quick staining of the cytospins and/or by flow cytometry (showing that neutrophils represented >90% of the migrated cells). The supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA), as described below.
2.3. MIP-2 and KC release in vitro
The air pouch lining (resident) cells were obtained from 8- to 12-week-old female CD-1 mice by air pouch trypsinization with 0.05% trypsin containing 0.5 mM EDTA in DMEM (adjusted to 37 °C before injection; 3 ml/pouch). Each air pouch was trypsinized twice for 5 min and gave ~0.3 × 106 cells.
Peritoneal macrophages were collected from the peritoneal cavities of 8–12-week-old female CD-1 mice with a 7 ml PBS wash 4 days after the intraperitoneal (ip) injection of 4% brewer thioglycolate (1 ml; Difco Laboratories, Detroit, MI).
For stimulation, the air pouch resident cells and peritoneal macrophages were seeded in a 24-well plate (0.15 × 106 and 1 × 106 cells/well, respectively), cultured in DMEM–10% FBS overnight and then incubated for 1 h with LPS (10 ng/ml) in the presence or absence of PPADS (10 μM; added to the cells 15 min before LPS). The media of these cells were centrifuged (500 × g, 5 min, 4 °C) to remove detached cells and analyzed by ELISA, as described below.
Air pouch leukocytes (106/0.5 ml of DMEM–10% FBS in a 5 ml sterile tube) collected 4 h after sc LPS injection (see Section 2.2) were stimulated in vitro with LPS (100 ng/ml) in the presence or absence of PPADS (10 μM) for 1 h, and centrifuged (500 × g, 10 min, 4°C). The supernatants were analyzed by ELISA.
2.4. Enzyme-linked immunosorbent assay (ELISA) for chemokines
MIP-2 and KC were quantified by ELISA (R&D Systems, Minneapolis, MN), following the manufacturer’s instructions.
2.5. Statistical analysis
Student’s t-test was performed using Excel software (Microsoft® Office OneNoteTM 2003).
3 Results
3.1. PPADS inhibits LPS-induced neutrophil migration in the air pouch
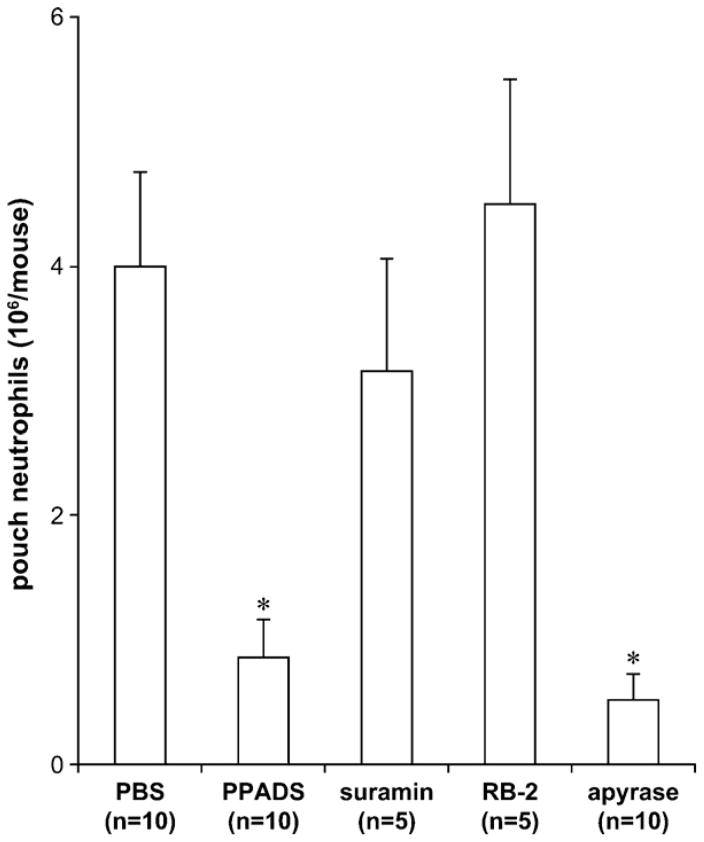
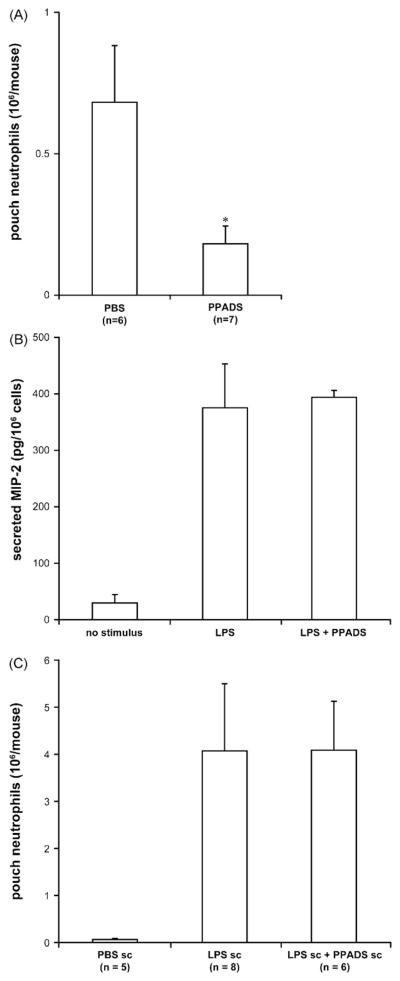
There is growing evidence that extracellular nucleotides act as danger signals which are rapidly released at sites of inflammation and/or infection as a result of TLR activation (Di Virgilio, 2005; Piccini et al., 2008; Trautmann, 2009). Here, using the in vivo murine air pouch model, we investigated whether these molecules would regulate an acute inflammatory response due to LPS. The administration of LPS in the air pouch activates rapid cytokine/chemokine production and massive neutrophil infiltration. The closed environment of the air pouch allows to quantify with precision both these effects directly at the site of inflammation. To specifically address the role of P2 receptors expressed in circulating leukocytes and/or endothelium, their antagonists PPADS, suramin and RB-2, as well as the nucleotide scavenger apyrase, were injected intravenously (tail vein) prior to the induction of neutrophil migration by LPS injection in the air pouches. PPADS and apyrase, but not suramin and RB-2, significantly diminished neutrophil migration (*P < 0.005 vs. PBS, n = 10; Fig. 1) suggesting that PPADS-sensitive and suramin- and RB2-insensitive P2 receptor(s) are important for LPS-induced neutrophil migration in the air pouch.
Fig. 1.
PPADS and apyrase inhibit LPS-induced neutrophil migration in the mouse air pouch. The mice were injected iv with PBS (0.2 ml), P2 receptor antagonists (PPADS, suramin or RB-2; 200 μM in 0.2 ml) or apyrase (6 U in 0.2 ml) followed by sc injection of LPS (10 ng in 1 ml) in the air pouch. The accumulated cells were collected from the air pouches 4 h later and counted. The mean + SEM of at least five mice per group is shown. PPADS and apyrase abrogated neutrophil migration in LPS-treated mouse air pouches (*P < 0.005 vs. PBS). Suramin and RB-2 had no effect on this response.
3.2. UTP potentiates LPS-induced neutrophil migration in the air pouch
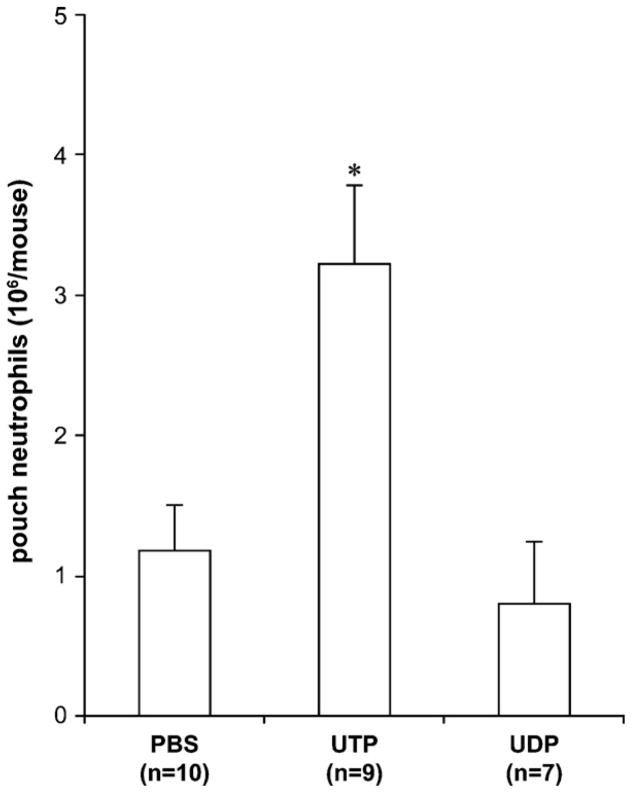
To further investigate the role of P2 receptors in LPS-induced neutrophil migration in the air pouch, we tested whether the iv injection of UTP and UDP would increase LPS-induced neutrophil accumulation in the air pouches. These nucleotides are natural agonists of P2Y2 (UTP) and P2Y6 (in mouse activated by either UDP or UTP; Bar et al., 2008; Kauffenstein et al., in press) receptors that have been shown to control neutrophil migration in vivo and in vitro (Ben Yebdri et al., 2009; Chen et al., 2006; Inoue et al., 2008; Kukulski et al., 2007). UTP is also a ligand of P2Y4 receptor which is highly sensitive to PPADS. We did not perform the iv injections of adenine nucleotides (ATP and ADP) because these molecules are metabolized to adenosine that is generally anti-inflammatory. Moreover, ADP is a potent pro-thrombotic agent that can affect various biological processes in the blood (note that ADP could also be generated from ATP injection by blood ectonucleotidases).
We first determined a suboptimal dose of LPS (2 ng/ml) that by itself elicited a weak neutrophil migration (~1/4 of the migration obtained with 10 ng/ml LPS), allowing an additional potential increased migration by the iv injection of nucleotides. The iv administration of UTP, but not UDP, significantly increased neutrophil migration due to this suboptimal dose of LPS (Fig. 2). Taken together, P2Y4 might be a dominant nucleotide receptor involved in LPS-induced neutrophil migration in the air pouch as, in contrast to P2Y6, it is not activated by UDP, and in contrast to both P2Y2 and P2Y6, it is potently inhibited by PPADS and insensitive to suramin (Abbracchio et al., 2006; Muller, 2002).
Fig. 2.
UTP potentiates LPS-induced neutrophil migration in the air pouch. The mice were injected iv with nucleotides (UTP or UDP; 20 μM in 0.2 ml) or PBS (0.2 ml) prior to sc administration of a suboptimal dose of LPS (2 ng in 1 ml) in the air pouch. The accumulated cells were collected from the air pouches 4 h later and counted. The mean + SEM of at least seven animals per group is shown. UTP, but not UDP, increased LPS-induced neutrophil migration in the air pouch (*P = 0.008 vs. PBS).
3.3. PPADS diminishes MIP-2 and KC production in the air pouch exudates
Mouse chemokines MIP-2 and KC are responsible for neutrophil recruitment in inflammatory air pouches and other rodent models of acute inflammation (Kopydlowski et al., 1999; McColl and Clark-Lewis, 1999; Schramm et al., 2000). Therefore, we investigated whether the inhibition of LPS-induced neutrophil migration in the air pouch by PPADS and apyrase could be related to diminished production of these chemokines in air pouch exudates and blood. Note that MIP-2 and KC production in exudates peaks at ~1 h after LPS injection and then wanes to undetectable level at 4 h after LPS injection (Vandal et al., 2003 and data not shown). Exudates of mice treated iv with PPADS had significantly lower amounts of MIP-2 and KC compared to the exudates of control mice injected iv with PBS, as measured 1 h after LPS injection (Fig. 3A). The exudates of mice injected iv with apyrase had slightly lower levels of MIP-2 and KC compared to the exudates of control mice, however, these differences were not statistically significant (Fig. 3A). No MIP-2 and low amounts of KC were detected in the sera of the mice injected iv with either PPADS or PBS (Fig. 3B). In comparison, the sera of mice injected with apyrase had a high level of both chemokines (Fig. 3B).
Fig. 3.
PPADS diminishes LPS-induced MIP-2 and KC release in the air pouch exudates. The mice were injected iv with PBS (0.2 ml), PPADS (200 μM in 0.2 ml) or apyrase (6 U in 0.2 ml) followed by sc injection of LPS (10 ng in 1 ml) in the air pouch. The air pouch exudates (Panel A) or blood (Panel B) were collected 1 h after LPS injection. The cell-free exudates or sera were prepared as described in Section 2 and analyzed for MIP-2 (open bars) and KC (filled bars) by ELISA. The mean + SEM of 7 or 8 animals per group is shown. *P = 0.05 vs. PBS, **P = 0.003 vs. PBS. PPADS, but not apyrase, diminished significantly MIP-2 and KC production in the air pouch (Panel A).
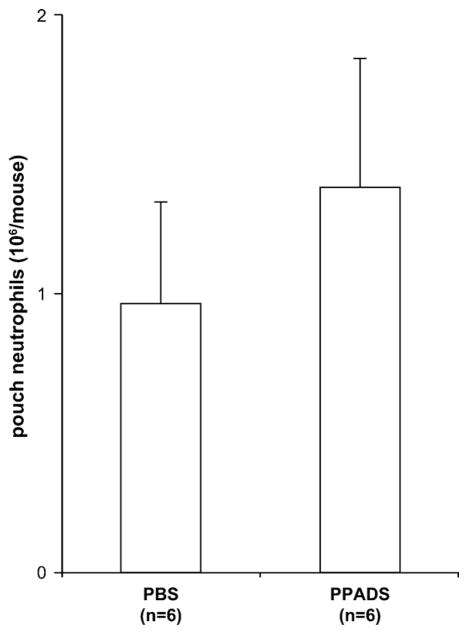
The above results and the fact the iv administration of PPADS did not block neutrophil migration in the air pouches injected with MIP-2 (Fig. 4) suggest that the decrease of LPS-induced neutrophil recruitment by PPADS could be due to inhibition of MIP-2 and KC secretion in the air pouch exudates. As apyrase did not affect MIP-2 and KC production in the air pouch exudates (Fig. 3), its effect on neutrophil migration was not further investigated in this work.
Fig. 4.
PPADS does not inhibit MIP-2-induced neutrophil migration in the air pouch. The mice were injected iv with PBS or PPADS (200 μM in 0.2 ml) prior to sc administration of the mouse recombinant MIP-2 (2 ng in 1 ml). The accumulated cells were collected from the air pouches 4 h later and counted. The mean + SEM of six mice per group is shown.
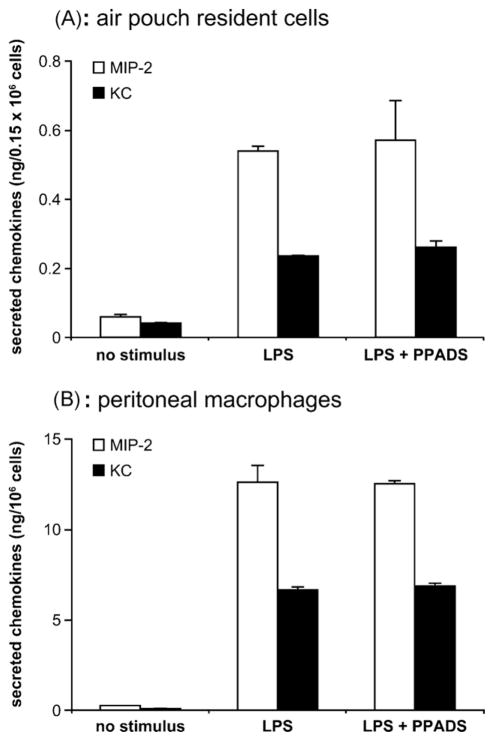
3.4. PPADS has no effect on LPS-induced MIP-2 and KC release by air pouch resident cells and peritoneal macrophages
We next aimed to determine how PPADS inhibits chemokine production in the air pouch. Air pouch lining cells (mainly fibroblasts ~50–90% and macrophages) are assumed to have a predominant role in MIP-2 and KC production in this model (Schramm et al., 2000; Tessier et al., 1997). However, Fig. 5 shows that PPADS had no effect on MIP-2 and KC release from the air pouch resident cells (Panel A) as well as from mouse peritoneal macrophages (Panel B). Noteworthy, in contrast to the air pouch exudates that had more KC than MIP-2 (Fig. 3A), both the air pouch resident cells and peritoneal macrophages stimulated with LPS in vitro produced significantly more MIP-2 than KC (Fig. 5A and B).
Fig. 5.
PPADS does not inhibit LPS-induced MIP-2 and KC release by air pouch resident cells and peritoneal macrophages.
Air pouch resident cells (Panel A) and peritoneal macrophages (Panel B) were pre-incubated with 100 μM PPADS for 15 min before the addition of LPS (10 ng/ml). The media of these cells were collected 1 h after LPS addition, centrifuged and the resulted supernatants analyzed for MIP-2 (open bars) and KC (filled bars) by ELISA. Data are expressed as the mean + SD of three different assays.
These results suggest that the inhibition of MIP-2 and KC production in LPS-treated air pouches is not due to a direct effect of PPADS on resident cells.
3.5. PPADS inhibits early neutrophil migration in the air pouch
Current data suggest that circulating neutrophils possess physiologically significant amounts of MIP-2 produced during their maturation in bone marrow (Komatsu et al., 2006; Matzer et al., 2001). Thus, once migrated in the air pouch, these cells could contribute to MIP-2 release. However, the kinetics of MIP-2 production and neutrophil migration in the air pouch are different. MIP-2 level is maximal at ~1 h after LPS injection that is 2–3 h before the peak of neutrophil infiltration (Vandal et al., 2003). Interestingly, our experiments showed that LPS causes early neutrophil migration as a significant number of neutrophils can be found in the air pouch at 1 h after LPS injection compared to the control pouches treated with PBS (6.0 ± 0.5 × 105 vs. 0.68 ± 0.50 × 105, respectively; *P = 0.03; n = 8). Importantly, this early neutrophil migration, which synchronizes with MIP-2 production in the air pouch, was inhibited in mice treated iv with PPADS (Fig. 6A; *P = 0.05; n = 7).
Fig. 6.
PPADS attenuates the early neutrophil migration in the air pouch. (Panel A) The mice were injected iv with PBS or PPADS (200 μM in 0.2 ml) prior to sc injection of LPS (10 ng in 1 ml) in the air pouch. The accumulated cells were collected from the air pouches 1 h later and counted. The mean + SEM of at least six mice per group is shown. *P = 0.05 vs. PBS. (Panel B) The neutrophils accumulated in the air pouches of 3–5 mice were collected 4 h after LPS injection, pooled and stimulated (106 cells/0.5 ml) with LPS (100 ng/ml) alone or with PPADS (100 μM) in vitro. The supernatants of these cells were analyzed by ELISA for MIP-2. Data of two independent experiments with the neutrophils pooled from different mice are shown. The LPS-stimulated air pouch neutrophils secreted MIP-2 and PPADS did not inhibit this response. (Panel C) The mice were administered sc with LPS alone or in combination with PPADS (10 ng and 10 μM, respectively, in 1 ml of sterile PBS). The accumulated cells were collected from the air pouches 4 h later and counted. The mean + SEM of at least six mice per group is shown. The sc injection of PPADS did not inhibit LPS-induced neutrophil migration in the air pouch.
Using neutrophils collected from the air pouch 4 h after LPS injection, we next demonstrated that these cells can indeed contribute to chemokine production as they release significant amounts of MIP-2, but not KC, due to LPS stimulation in vitro (Fig. 6B; not shown for KC). A high MIP-2 production was also obtained when migrated neutrophils were stimulated with LPS in the air pouch (second sc injection of LPS was performed 4 h after the first one at time 0; data not shown). The production of MIP-2 by air pouch neutrophils was not inhibited by PPADS (Fig. 6B). In agreement, the sc administration of LPS with PPADS did not affect neutrophil migration in the air pouch (Fig. 6C) nor MIP-2 and KC release (data not shown).
Taken together, these data suggest that PPADS decreases the production of MIP-2 and KC in LPS-treated air pouches by inhibiting early neutrophil migration.
3.6. PPADS does not prevent neutrophil migration activated by TLR1/2 and TLR5-agonists
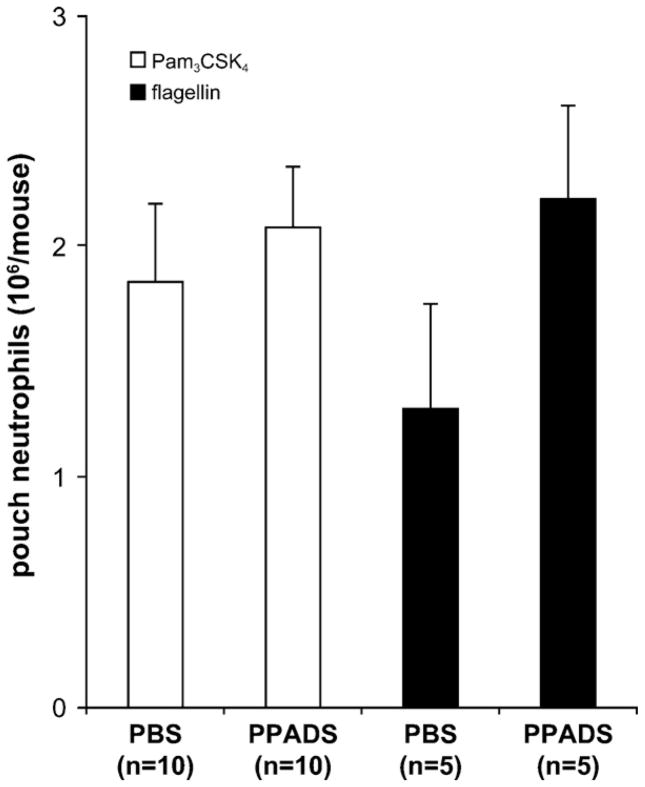
We next investigated whether the iv administration of PPADS would also diminish neutrophil migration due to other bacterial PAMPs, Pam3CSK4 and flagellin that activate TLR1/2 and TLR5, respectively. Both PAMPs induced neutrophil recruitment in the air pouches (Fig. 7) but less potently than LPS. As these migrations were not significantly affected by PPADS (Fig. 7), it can be concluded that PPADS inhibits specifically LPS-induced neutrophil migration.
Fig. 7.
PPADS does not prevent neutrophil migration due to TLR2- and TLR5-agonists. The mice were injected iv with PBS or PPADS prior to sc administration of Pam3 CSK4 (TLR2 agonist; open bars) or flagellin (TLR5 agonist; filled bars; both agonist were injected at 10 ng in 1 ml). The accumulated cells were collected from the air pouches 4 h later and counted. The mean + SEM of at least five mice per group is shown.
4. Discussion
This work demonstrates that P2 receptors regulate LPS-induced neutrophil migration in the murine air pouch model. The iv administration of the P2 receptor antagonist PPADS reduced LPS-induced neutrophil recruitment in this model which was associated with a decreased production of MIP-2 and KC in the air pouch exudates. These chemokines have previously been shown to play a key role in neutrophil migration in the air pouch model due to various inflammatory stimuli such as TNF-α, IL-1β and LPS (McColl and Clark-Lewis, 1999). The production of MIP-2 seems central for the role of extracellular nucleotides in LPS-induced neutrophil migration as PPADS did not affect neutrophil migration due to sc injection of this chemokine. We next showed that there was an early migration of neutrophils in LPS-treated air pouches which was most probably responsible for MIP-2 production. Indeed, PPADS markedly decreased either the number of migrated neutrophils or the amount of MIP-2 produced at 1 h after LPS injection, a time point at which the production of this chemokine in the air pouch is maximal. In keeping with these results, and also with previous reports showing that there are circulating neutrophils loaded with MIP-2 (Komatsu et al., 2006; Matzer et al., 2001), we demonstrated that LPS-stimulated air pouch neutrophils produced physiologically important amounts of this chemokine in vitro (Fig. 6B). As neutrophil MIP-2 production was not affected by PPADS, a potential explanation for this observation would be that extracellular nucleotides control the extravasation of early migrating neutrophils and that the reduced MIP-2 production in the pouches would have been the direct extension of this effect. In further support for the role of early neutrophil migration in chemokine production and in contrast to previous reports proposing a major role for resident cells in this process (Kopydlowski et al., 1999; Mercer-Jones et al., 1999; Schramm et al., 2000; Tessier et al., 1997), PPADS did not inhibit MIP-2 and KC production by air pouch resident cells in vitro and in vivo. Thus, this work puts forward a potential novel role of early migrated neutrophils in the development of the acute inflammatory response in the air pouch. However, we cannot exclude that PPADS affected neutrophil migration over the entire course of their recruitment in the air pouch. Both mechanisms were probably involved.
The experiments reported here suggest that LPS-induced neutrophil migration in the mouse air pouch is controlled by P2Y4 receptor expressed in blood cells and/or endothelium. Indeed, neutrophil migration was inhibited by PPADS, a potent antagonist of this receptor, whereas suramin, largely ineffective at P2Y4, did not affect this response. Moreover, LPS-induced neutrophil migration was potentiated by UTP, a P2Y4 ligand but not by UDP, an agonist of P2Y6 (Fig. 2). However, RB-2 is also a potent antagonist of P2Y4 in vitro (Suarez-Huerta et al., 2001) but did not inhibit neutrophil migration in the air pouch. It is therefore possible that PPADS and UTP acted on distinct P2 receptors. PPADS can inhibit various P2 receptors including P2X (North and Surprenant, 2000) whereas UTP can activate P2Y2, P2Y4, and also P2Y6 in mice (Abbracchio et al., 2006; Bar et al., 2008; Suarez-Huerta et al., 2001). Noteworthy, PPADS does not inhibit neutrophil migration due to Pam3CSK4 and flagellin (Fig. 7) suggesting that these migrations are controlled by PPADS-insensitive P2 receptors (e.g. P2Y2 (Chen et al., 2006; Inoue et al., 2008; Kukulski et al., 2009)) or by other mechanism(s). The iv administration of apyrase also potently inhibited LPS-induced neutrophil migration but in contrast to PPADS, it had no effect on MIP-2 and KC production in the air pouch. A similar effect of apyrase was observed when this enzyme was injected with LPS in the air pouch. These data suggest that apyrase diminishes neutrophil migration in the air pouch via inhibition of a mechanism independent of MIP-2 and KC production which remains to be elucidated (Kukulski et al., 2007 and unpublished data).
In summary, the data presented here suggest that P2 receptor(s), most likely P2Y4, expressed in blood and/or endothelial cells plays a key role in LPS-induced neutrophil migration in the murine air pouch model.
Acknowledgments
The authors thank Mrs. F. Létourneau for her technical assistance with iv injections and Dr. M. Dufour for the flow cytometry analyses. This work was supported by grants to J.S. from the Canadian Institutes of Health Research (CIHR) and from The Arthritis Society (TAS) of Canada. F.K. and F.B. were recipients of a fellowship from the CIHR/Wyeth Pharmaceuticals, F.BY. of a scholarship from Fonds de la Recherche sur l’Arthrite et les Maladies Rhumatismales (FRAMR), S.A.L. of a scholarship from the Fonds de la Recherche en Santé du Québec (FRSQ), M. M-S. of a fellowship from the Spanish Ministry of Education and Science (MEC, José Castillejo Program), and J.S. of a new investigator award from the CIHR and of a Junior 2 scholarship from the FRSQ.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam CA. Quantitative analysis of angiogenesis using the murine chronic granulomatous air pouch. Methods Mol Biol. 2003;225:191–197. doi: 10.1385/1-59259-374-7:191. [DOI] [PubMed] [Google Scholar]
- Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, Bult H, Robaye B. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol. 2008;74:777–784. doi: 10.1124/mol.108.046904. [DOI] [PubMed] [Google Scholar]
- Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L887–L892. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- Ben Yebdri F, Kukulski F, Tremblay A, Sévigny J. Concomitant activation of P2Y2 and P2Y6 receptors on monocytes is required for TLR1/2-induced neutrophil migration by regulating IL-8 secretion. Eur J Immunol. 2009;39(10):2885–2894. doi: 10.1002/eji.200939347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Bozic CR, Kolakowski LF, Jr, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Colville-Nash P, Lawrence T. Air-pouch models of inflammation and modifications for the study of granuloma-mediated cartilage degradation. Methods Mol Biol. 2003;225:181–189. doi: 10.1385/1-59259-374-7:181. [DOI] [PubMed] [Google Scholar]
- Dawicki DD, McGowan-Jordan J, Bullard S, Pond S, Rounds S. Extracellular nucleotides stimulate leukocyte adherence to cultured pulmonary artery endothelial cells. Am J Physiol. 1995;268:L666–L673. doi: 10.1152/ajplung.1995.268.4.L666. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. Purinergic mechanism in the immune system: a signal of danger for dendritic cells. Purinergic Signal. 2005;1:205–209. doi: 10.1007/s11302-005-6312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg MA, Tchaptchet S, Keck S, Fejer G, Huber M, Schutze N, Beutler B, Galanos C. Lipopolysaccharide sensing an important factor in the innate immune response to Gram-negative bacterial infections: benefits and hazards of LPS hypersensitivity. Immunobiology. 2008;213:193–203. doi: 10.1016/j.imbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta. 2002;323:59–72. doi: 10.1016/s0009-8981(02)00180-8. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Chen Y, Hirsh MI, Yip L, Junger WG. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock. 2008;30:173–177. doi: 10.1097/shk.0b013e318160dad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger WG. Purinergic regulation of neutrophil chemotaxis. Cell Mol Life Sci. 2008;65:2528–2540. doi: 10.1007/s00018-008-8095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D’Orleans-Juste P, Marceau F, Thorin E, Sévigny J. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. doi: 10.1093/cvr/cvp265. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu J, Koyama H, Maeda N, Aratani Y. Earlier onset of neutrophil-mediated inflammation in the ultraviolet-exposed skin of mice deficient in myeloperoxidase and NADPH oxidase. Inflamm Res. 2006;55:200–206. doi: 10.1007/s00011-006-0071-3. [DOI] [PubMed] [Google Scholar]
- Kopydlowski KM, Salkowski CA, Cody MJ, van Rooijen N, Major J, Hamilton TA, Vogel SN. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol. 1999;163:1537–1544. [PubMed] [Google Scholar]
- Kukulski F, Ben Yebdri F, Lecka J, Kauffenstein G, Lévesque SA, Martín-Satué M, Sévigny J. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine. 2009;46:166–170. doi: 10.1016/j.cyto.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukulski F, Ben Yebdri F, Lefebvre J, Warny M, Tessier PA, Sévigny J. Extracellular nucleotides mediate LPS-induced neutrophil migration in vitro and in vivo. J Leukoc Biol. 2007;81:1269–1275. doi: 10.1189/jlb.1206758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Klintman D, Liu Q, Sato T, Jeppsson B, Thorlacius H. Critical role of CXC chemokines in endotoxemic liver injury in mice. J Leukoc Biol. 2004;75:443–452. doi: 10.1189/jlb.0603297. [DOI] [PubMed] [Google Scholar]
- Matzer SP, Baumann T, Lukacs NW, Rollinghoff M, Beuscher HU. Constitutive expression of macrophage-inflammatory protein 2 (MIP-2) mRNA in bone marrow gives rise to peripheral neutrophils with preformed MIP-2 protein. J Immunol. 2001;167:4635–4643. doi: 10.4049/jimmunol.167.8.4635. [DOI] [PubMed] [Google Scholar]
- McColl SR, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol. 1999;163:2829–2835. [PubMed] [Google Scholar]
- Mercer-Jones MA, Shrotri MS, Heinzelmann M, Peyton JC, Cheadle WG. Regulation of early peritoneal neutrophil migration by macrophage inflammatory protein-2 and mast cells in experimental peritonitis. J Leukoc Biol. 1999;65:249–255. doi: 10.1002/jlb.65.2.249. [DOI] [PubMed] [Google Scholar]
- Muller CE. P2-pyrimidinergic receptors and their ligands. Curr Pharm Des. 2002;8:2353–2369. doi: 10.2174/1381612023392937. [DOI] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm R, Liu Q, Thorlacius H. Expression and function of MIP-2 are reduced by dexamethasone treatment in vivo. Br J Pharmacol. 2000;131:328–334. doi: 10.1038/sj.bjp.0703543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley TP, Schmal H, Friedl HP, Jones ML, Ward PA. Role of macrophage inflammatory protein-1 alpha (MIP-1 alpha) in acute lung injury in rats. J Immunol. 1995;154:4793–4802. [PubMed] [Google Scholar]
- Suarez-Huerta N, Pouillon V, Boeynaems J, Robaye B. Molecular cloning and characterization of the mouse P2Y4 nucleotide receptor. Eur J Pharmacol. 2001;416:197–202. doi: 10.1016/s0014-2999(01)00875-5. [DOI] [PubMed] [Google Scholar]
- Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal. 2009;2:pe6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- Vandal K, Rouleau P, Boivin A, Ryckman C, Talbot M, Tessier PA. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. J Immunol. 2003;171:2602–2609. doi: 10.4049/jimmunol.171.5.2602. [DOI] [PubMed] [Google Scholar]
- Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52:349–374. [PubMed] [Google Scholar]
- Willoughby DA, Sedgwick AD, Giroud JP, Al-Duaij AY, de Brito F. The use of the air pouch to study experimental synovitis and cartilage breakdown. Biomed Pharmacother. 1986;40:45–49. [PubMed] [Google Scholar]