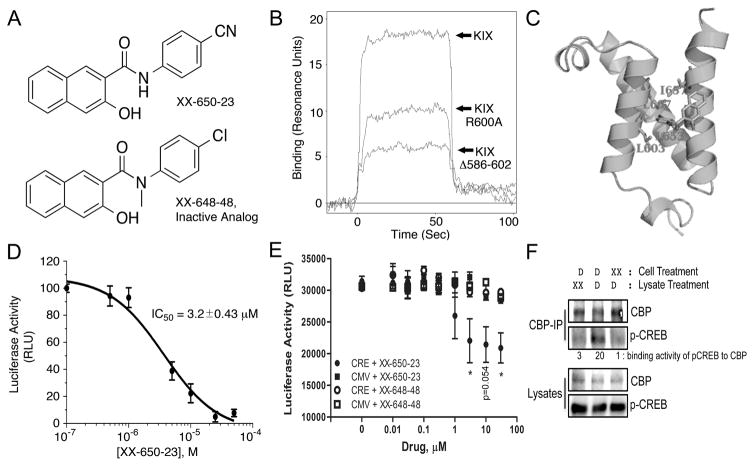
Fig. 1. XX-650-23 Binds to the KIX Domain of CBP and Blocks CREB-Dependent Gene Transcription.
A) The structure of XX-650-23 and its inactive analog XX-648-48. B) The native human KIX domain and two mutant proteins were expressed as a fusion protein with GST and subjected to Biacore analysis to assess XX-650-23 binding characteristics. Mutation of Arginine-600 to Alanine reduced binding of XX-650-23 by ~45% at a concentration of 5μ, while a KIX mutant lacking amino acids 586-602 reduced binding by ~70%. C) Binding model of XX-650-23 to CBP KIX domain. D) Split-Renilla luciferase assays with 293T cells treated with forskolin demonstrated XX-650-23 is a direct inhibitor of CREB-CBP binding, with an IC50 of approximately 3.2 μM. Data are presented as mean ± SD, n=3. E) Two KG-1 cell lines were generated in which luciferase was expressed either under the control of a CREB-driven promoter (CRE) or a CMV-driven promoter (CMV). These two cell lines were each treated with a range of XX-650-23 or XX-648-48 concentrations for 6 hours. Luciferase activity was significantly decreased following XX-650-23 treatment at concentrations of 3, 10 and 30 μM. No statistical difference in luciferase activity was detected following XX-648-48 treatment. Data are presented as mean ± SEM, n=3. *p < 0.05, t-test. F) XX-650-23 inhibits CREB/CBP association. HEK293 cells were transfected with a plasmid expressing CREB. Cells were treated with XX-650-23 (XX, 5 μM, lane 3) or DMSO vehicle (D) for 1 hour. Total lysates of transfected HEK293 cells were immunoprecipitated using anti-CBP antibody. XX-650-23 (XX, 5 μM) was added during anti-CBP Immunoprecipation process (lane 1). Immunoprecipitates (CBP-IP) and total lysates were analyzed by immunoblotting for phospho-CREB (p-CREB) and CBP. Binding activities were calculated by the relative protein amounts of p-CREB bound to CBP normalized against CBP amounts in CBP IP products.