Abstract
Objectives
Identification of biologic pathways of symptom clusters is necessary to develop precision therapies for distressing symptoms. This review examined extant literature evaluating relationships between biomarkers and symptom clusters in cancer survivors.
Data Sources
PubMed, CINAHL, Web of Science and Cochrane Library were searched using terms “biological markers” or “biomarkers” and “symptom cluster” or “symptom complex” or “multiple symptoms”.
Results
Biomarkers related to inflammation (e.g., cytokines) were the most studied and showed the most significant relationships with clusters of symptoms.
Conclusion
This review suggest that clustering of symptoms related to cancer or cancer therapy is linked to immune/inflammatory pathways.
Implications for Nursing Practice
Understanding the etiology of symptom clusters may guide future nursing interventions for symptom management.
Keywords: symptom cluster, inflammation, cancer-related symptoms, symptom complex, biological, pathways
Advances in treatment and screening for early detection of cancer have resulted in increased survival for millions of individuals.1 There are over 14 million cancer survivors living in the United States, and this number is expected to reach 19 million by 2024.2 Regardless of where one is in the spectrum of cancer survivorship, symptoms are a major concern for individuals and their caregivers. Symptoms may be associated with the stage of the cancer or adverse effects of treatments. Frequency, severity, and the number of co-occurring symptoms contribute to symptom burden, which negatively affects health-related quality of life of cancer survivors.3
Although individual symptoms may have unique manifestations clinically, it is hypothesized that co-occurring symptoms may share common biologic underpinnings.4,5 The etiology behind clustering of symptoms observed in oncology patients is poorly understood. A recent review identified inflammatory pathways to explain the biology of symptom cluster (pain, fatigue, sleep disturbance, and depression) reported by breast cancer women after therapy.6
Cancer and its treatment are known triggers of acute and persistent inflammatory and immune responses,7–9 as well as influential players in the hypothalamic-pituitary-adrenal (HPA) axis function.10–12 It was reported that cancer treatment directly increases the synthesis and release of cytokines from macrophages,13,14 which bind to receptors in target cells and produce additional inflammatory cytokines and chemokines to mount a systemic inflammatory response to counter the cellular damage inflicted by the cancer therapy.15 This systemic inflammatory response is believed to target inflammatory-responsive neurons in the central nervous system, which produces co-occurrence of symptoms to include fatigue, anorexia, cognitive dysfunction, and other cytokine-induced sickness behavior.16,17
Inflammatory pathways were also suggested to explain the biology of clustering of symptoms in clinical populations other than cancer. Levels of interleukin (IL)-15 and IL-1 receptor agonist were negatively associated with the clustering of neuropsychiatric symptoms including depression, apathy, agitation, and sleep in individuals with Alzheimer’s disease.18 In women with fibromyalgia, an inflammatory transcriptome profile distinctly delineated subjects complaining of fatigue associated with pain from those reporting fatigue associated with catastrophizing.19
The goal of this review is to investigate the biology of clustering of symptoms related to cancer or cancer therapy. Specifically, this review examined studies focusing on relationships of biological markers and the co-occurrence of cancer-related symptoms. Central to the development and implementation of targeted, individualized, symptom management strategies for symptom clusters is to understand the mechanisms undergirding their development and/or persistence.
Methods
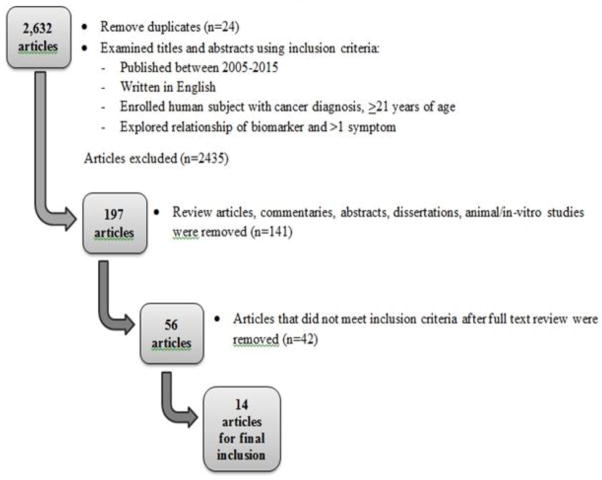
An extensive literature search was performed with the assistance of a medical librarian at the University of Florida using four commonly referenced databases (PubMed, CINAHL, Web of Science, and Cochrane Library). The initial search resulted in 2656 articles using search terms listed in Table 1. After removal of duplicates (n= 24), 2632 articles remained. Articles were included if they were written in English, published between 2005 and 2015 to capture the most recent information, and enrolled only human subjects who were at least 21 years of age. All articles reviewed were studies in which participants had a cancer diagnosis (any type, any stage, receiving any cancer treatment), reported co-occurrence of more than one symptom, and explored the relationship of biological markers and reported symptoms. The 2632 articles were assessed for relevance to the review by visually examining their titles and abstracts using the inclusion/exclusion criteria, and additional 2435 articles were removed, which left 197 articles for consideration. Articles were further excluded if they were reviews, commentaries, editorials, or dissertations, which left 56 articles for full text review. After a thorough review of the full text of the remaining articles, an additional 42 articles were excluded because they did not explore a symptom cluster (n= 21), did not include a biomarker in relation to a symptom cluster (n=19), or were previously unidentified duplicates (n=2). The process for article selection is described in Figure 1.
Table 1.
Summary of Search Terms
| Database | Search Strategy |
|---|---|
| PubMed | (“Biological Markers”[Mesh] OR biomarker*[Text Word] OR “Inflammation”[Mesh] OR inflammatory markers”[Text Word] OR “Epigenesis, Genetic”[Mesh] OR “Genomics”[Mesh] OR “Metabolomics”[Mesh] OR “Proteomics”[Mesh] OR “Mitochondria”[Mesh] OR “Cytokines”[Mesh] OR “Bacteria”[Mesh] OR “Viruses”[Mesh]) AND (“Syndrome”[Mesh] OR “symptom cluster”[Text Word] OR “symptom clusters’[Text Word] OR “symptom complex”[Text Word] OR “multiple symptoms”[Text Word]). Filters: 10 years, Humans, English, Cancer Subset (subset search strategy available here: https://www.nlm.nih.gov/bsd/pubmed_subsets/cancer_strategy.html). |
| Cumulative Index of Nursing and Allied Health Literature | (((MH “Biological Factors”) OR (MH “Immunologic and Biologic Factors”) OR (MH “Biological Markers+”) OR (MH “Inflammation”) OR (MH “Genomics”) OR (MH “Nutrigenomics:) OR (MH “Epigenomics”) OR (MH “Proteomics”) OR (MH “Mitochondria”) OR (MH “Cytokines+”)) OR biomark* OR inflammation OR genomic* OR epigenetic* OR proteomic* OR metabolomic* OR mitochondria* OR cytokine*) AND ((MH “Neoplasms+”) OR cancer OR neoplasm*) AND ((MH “Symptoms”) OR (MH “Syndrome”) OR “symptom cluster*” OR syndrome). Limiters: Published Date: 20050101-2015-1231; Language: English. |
| Web of Science | TOPIC: ((biological marker* OR biomarker* OR inflammation OR epigenetic* OR genomic* OR metabolomic* OR proteomic* OR mitochondria* OR cytokine* OR “inflammatory marker*”)) AND TOPIC: (cancer* OR neoplasm* OR tumor*) AND TOPIC: (“symptom cluster*” OR syndrome OR “multiple symptoms” OR “symptom complex”) AND TOPIC: (human OR participant* OR volunteer* OR patient*) NOT TOPIC: (animal OR mouse OR mice OR rat OR murine). Refined by PUBLICATION YEARS: (2015 OR 2014 OR 2013 OR 2012 OR 2011 OR 2010 OR 2009 OR 2008 OR 2007 OR 2006 OR 2005) AND LANGUAGE: (ENGLISH) AND WEB OF SCIENCE CATEGORIES: ONCOLOGY |
| Cochrane Library | ‘biological marker* OR biomarker* OR inflammation OR epigenetic* OR genomic* OR metabolomic* OR proteomic* OR mitochondria* OR cytokine* OR “inflammatory marker*” and cancer* OR neoplasm* OR tumor* and “symptom cluster*” OR syndrome OR “multiple symptoms” OR “symptom complex”. |
Figure 1.
Process of selecting articles for inclusion in this review
Results
There were a total of 14 articles (see Table 2) that explored biological mechanisms related to clustering of cancer-related symptoms.20–33 Of these 14 studies, 8 were longitudinal20,22,24,26,27, 30, 32,33 and 6 were cross-sectional in design.21,23,25,28,29,31 The majority (12/14, 86%) of articles reviewed were published within the last five years.20–26,28–31,33 In addition, most studies (n=12/14, 86%) were conducted in an outpatient setting with varying sample sizes ranging from N=24 participants25 to N=718 participants.24 The predominant cancer populations explored were women with breast cancer (n=4/14, 29%) and individuals with lung cancer (n=4/14, 29%). Other populations explored were individuals with hematologic cancers (n=3/14, 21%); those with hepatic carcinoma and malignant melanoma (n=2/14, 14%); and one on gastrointestinal cancer, lung cancer, or pancreatic cancer.24
Table 2.
Summary of Reviewed Articles
| Citation | Symptom Cluster Definition | Study Design | Symptoms Examined | Biomarker(s) | Statistics | Findings |
|---|---|---|---|---|---|---|
| Doong, S.H., et al. (20) | Symptoms assumed to be common and occur together were used as a symptom cluster No specific definition provided |
Design: prospective, longitudinal design Setting: outpatient Sample Group: 516 adults with breast cancer Stage: 0–IV Control Group: none reported |
Pain: numeric rating scale Fatigue: Lee Fatigue Scale Sleep Disturbance: General Sleep Disturbance Scale (GSDS) Depression: Center for Epidemiologic Studies-Depression (CES-D) Scale |
SNPs of pro- inflammatory and anti-inflammatory cytokines-yielding 82 SNPs among 15 candidate genes Source: blood; buffy coat (leukocytes and platelets) |
Descriptive statistics and frequency distributions; Latent Class Analysis; Bayesian information criteria; bootstrapped likelihood ratio test; p< .05 | Identified three relatively distinct latent classes of patients (low levels of all 4 symptoms; high levels of all 4 symptoms; low pain & high fatigue); there were significant demographic differences among the three groups; there were significant genotype differences (p= .044) for nine SNPs and two haplotypes spanning seven genes between two groups; rare A allele was associated with being in the all high group |
| Giannousi, Z., et al. (21) | No specific definition provided |
Design: cross-sectional survey design Setting: outpatient Sample Group: 122 adults with non-small cell lung cancer (NSCLC) Stage: IV Control Group: none reported |
Cachexia - Nutritional status: Mini Nutritional Assessment (MNA) Anxiety and Depression: Hospital Anxiety and Depression Scale (HADS) |
C-reactive protein and albumin for the Glasgow Prognostic Scale (GPS) Source: blood |
Descriptive statistics; Spearman rho correlation coefficients; p< .05 | The following correlations were observed: MNA and GPS (r=0.289, p=0.001), MNA and HADS (depression scale) (r=0.275, p=0.002), GPS and HADS (depression scale (r=0.256, p=0.004), and GPS and HADS (anxiety scale) (r=0.194, p=0.033). In univariate analysis, GPS (p=0.002) and MNA (p=0.010) emerged as significant predictors of survival. In multivariate analysis, both MNA (p=0.032) and GPS (p=0.020) retained their importance. |
| Heinze, S., et al. (22) | Symptoms assumed to occur together were used as a symptom cluster No specific definition provided |
Design: prospective, randomized control trial Setting: outpatient Sample Group: 282 adults with primary melanomas Stage: Unspecified Control Group: none reported |
Physical & Psychological symptoms: Symptom Check List 90-Revised Depressive symptoms: Beck Depression Inventory (BDI) |
IFN-α Source: blood |
T-tests for independent samples (normally distributed) and Mann-Whitney U (not normally distributed) used for between-group comparisons. Wilcoxon tests or Friedman tests for within group comparisons. Odds ratios estimated the predictive value of BDI scores with regard to risk of early discontinuation of treatment; p=0.05 | Low pretreatment BDI scores with increased scores in the first 6 months of IFN-alpha. Females had more pronounced changes in BDI scores. 12 month scores were higher than baseline. 5% of patients developed a clinically significant depressive syndrome during the 12 month period. Significant changes in indecisiveness, fatigue, pessimism, dislike of self, irritability, libido, insomnia, suicidal ideation, dissatisfaction, work difficulties, self-accusation, social withdrawal, loss of appetite and loss of weight were revealed in the first year of INF-alpha. Fatigue increased at 3 months and remained elevated up to 1 year. Insomnia increased significantly until 1 year. Mean SCL-90-R scores and each global indices of SCL 90-R increased significantly. SCL 90-R subscales of depression, somatization, interpersonal sensitivity, hostility, and paranoid ideation significantly increased at some point during treatment compared to baseline. Patients with a BDI score > or equal to 5 before treatment had a 3-fold increased risk to discontinue IFN treatment due to side effects. |
| Jaremka, L.M., et al. (23) | Pain, depression and fatigue were assumed to be a cluster No specific definition provided |
Design: prospective, observational, cross-sectional Setting: outpatient Sample Group: 200 women with breast cancer Stage: 0–IIIA Control Group: none reported |
Loneliness: The UCLA scale Pain: RAND SF-36 Depression: CES-D Fatigue: Multidimensional Fatigue Symptom Inventory and RAND SF-36 Sleep: Pittsburg Sleep Quality Index |
Cytomegalovirus and Epstein Barr virus Source: blood; plasma |
Linear regression model; p< .001 | CMV did not differ by loneliness or pain, depression, fatigue cluster; EBV was not related to loneliness or the symptom cluster; loneliness was associated with CMV titers (b = 0.31, t(74) = 2.65, p = .010); CMV was associated with higher symptom scores (b = 0.24, t(74) = 2.63, p = .010) |
| Laird, B.J., et al. (24). | Symptoms assumed to be common were used as a symptom cluster No specific definition provided |
Design: secondary analysis of a double-blind, placebo-controlled, randomized study Setting: none reported Sample Group:718 adults with advanced GI, lung, or pancreatic cancer Stage: Advanced Control Group: none reported |
Pain: EORTC QLQ-30 Depression: EORTC QLQ- 30 Fatigue: EORTC QLQ-30 |
CRP Source: blood; plasma |
Kruskal-Wallis test; p<0.05 | Pain, depression and fatigue clustered in 2–4 times the number of advanced cancer patients with cachexia. Single and pair representation of symptoms is less likely. CRP was not related to the symptom cluster. |
| Lynch Kelly, D., et al. (25). | No specific definition provided |
Design: prospective, cross-sectional, correlational design Setting: outpatient Sample Group: 24 adults who had received AHSCT Stage: Unspecified Control Group: none reported |
Pain: Brief Pain Inventory (BPI) Depression: HADS Fatigue: Brief Fatigue Inventory (BFI) General Symptoms: Lee cGVHD Symptom scale & the Memorial Symptom Assessment Scale (MSAS) |
IL-1β, IL-6, IL-10, TNF, IFN-γ; CRP Source: blood; plasma |
Student’s t tests to compare cytokine and CRP levels between groups (with and without symptoms). Pearson product-moment correlation coefficient for all normally distributed pairwise combinations of variables. Spearman’s rank correlation coefficient was used for skewed data. | Levels of cytokines were higher in individuals with symptoms; IL6 correlated with interferon-gamma, lack of energy and dry mouth; IL1B was significantly positively correlated with TNF, interferon-gamma, IL-6 and IL-10; IL-10 was significantly correlated with difficulty sleeping and INF-gamma; TNF was significantly correlated with interferon-gamma; CRP and the social and family well-being subscale. |
| Lyon, D.E., et al. (26). | Symptom cluster defined as at least three concurrent symptoms |
Design: prospective, 3-group, randomized, double blinded, longitudinal Setting: outpatient Sample Group:35 adults with breast cancer Stage: I–IIIA Control Group: none reported |
Depression & Anxiety: HADS Fatigue: BFI Sleep: GSDS Pain: BPI |
IL-6, TNF-α, IL-1β, CRP Source: blood; plasma |
Random effects regression models; Kruskal-Wallis test; ANOVA; r correlations; p <0.05 | CRP was associated with depression, fatigue and pain at baseline. Depression and TNF-alpha were positively correlated. There was less of an increase in the intervention group, but this was not significantly different. |
| Meyers, C.A., et al. (27). | No specific definition provided |
Design: longitudinal study Setting: not reported Sample Group:54 adults with AML or MDS Stage: Unspecified Control Group: none reported |
Cognitive function: The Digit Span to measure attention span; Digit Symbol to measure graphomotor speed; Hopkins Verbal Learning Test for memory; Controlled Oral Word Association for verbal fluency; Trial Making Test Part A for visual-motor scanning speed; Trial Making Test Part B for executive function; Grooved Pegboard for fine motor dexterity Fatigue: BFI |
IL-1, IL-1RA, IL-6, IL-8, and TNF-α Source: blood; serum |
Spearman correlation; r ≥ .35 considered significant | Levels of IL-6, IL-1RA, and TNF-α were related significantly to fatigue and overall QOL ratings. Higher IL-6 levels also were associated with worse performance on the Trial Making Test Part B for executive function; Higher IL- 8 levels were related to better memory performance; Fine motor control associated with worsen fatigue 1 month after the therapy; Patients who achieved a complete response to have a lower circulating IL-1 levels at baseline (p=0.05) |
| Reyes-Gibby, C.C., Swartz, M.D., et al. (28). | Symptom cluster of pain, depressed mood, and fatigue are assumed to be a cluster Definition of symptom cluster provided as multiple, co-occurring symptoms experienced by cancer patients |
Design: cross-sectional Setting: outpatient Sample Group: 599 adults with NSCLC Stage I–IV Controls: none reported |
Pain: numeric rating scale Depressed mood: 12-Item Short-Form Health Survey Fatigue: 12-Item Short-Form Health Survey |
SNPs from cytokines (IL-1A, IL-1B, IL-2, IL-6, IL-8, IL-12, IL-16, TNF-α, TNF- β, GMCSF, MCP, MIF, IFNγ, IL-1RA, IL-4, IL-4R, IL-10, IL-10RA, IL-10RB, IL-13) prostaglandin and nitric oxide (PTGS2, ENOS,INOS) and intracellular signaling molecules (IKB, PPARA, PPARD, PPARG) Source: blood |
Descriptive statistics of demographic data; Kolmogorov-Smirnov Z test for normality of symptoms (then dichotomized symptoms using NCCN cut-off scores); Associations were measured using Chi-square and Pearson correlation coefficients; Tree-Based Multivariate Analysis; Odds ratios; set alpha was not given nor the strength of r for significance. | Among patients with advanced-stage disease, IL-8-T251A was the most relevant genetic factor for pain (odds ratio [OR]=2.18, 95% confidence interval [CI]=1.34,3.55; P=0.001), depressed mood (OR=0.37, 95% CI=0.14,1.0), and fatigue (OR=2.07, 95% CI=1.16,3.70); Among those with early-stage NSCLC, variants in the IL-10 receptor were relevant for fatigue among women. Specifically, women with genotype Lys_Glu or Glu_Glu in the IL-10 gene had a 0.49 times lower risk of severe fatigue compared with those with genotype Lys_Lys (OR=0.49, 95% CI=0.25, 0.92; P=0.027); Among men with early-stage lung cancer, a marginal significance was observed for IL-1A C-889T, C/T or T/T genotype: these men had a lower risk of severe fatigue compared with those with genotype C/C (OR=0.38, 95% CI=0.13, 1.06) |
| Reyes-Gibby, C.C., Wang, J., et al. (29). | No definition of symptom cluster given |
Design: cross-sectional Setting: outpatient Sample Group: 599 adults with NSCLC Stage: I–IV Controls: none reported |
Pain: numeric rating scale Depressed mood: 12-Item Short-Form Health Survey Fatigue: 12-Item Short-Form Health Survey |
SNPs from cytokines (IL-1A, IL-1B, IL-2, IL-6, IL-8, IL-12, IL-16, TNF-α, TNF- b, GMCSF, MCP, MIF, INFg, IL-1RA, IL-4, IL-4R, IL-10, IL-10RA, IL-10RB, IL-13) prostaglandin and nitric oxide (PTGS2, ENOS, INOS) and intracellular signaling molecules (IKB, PPARA, PPARD, PPARG) Source: blood |
Descriptive statistics of demographic data; Kolmogorov-Smirnov Z test for normality of symptoms (then dichotomized symptoms using NCCN cut-off scores); Hierarchical clustering analysis; Euclidian distance metric; Stochastic search variable selection (Bayesian model)applied to logistic regression | Of the 55 SNPs assessed, an additive effect of mutant alleles in ENOS (−1474 T/A) (Posterior Probability of Inclusion (PPI) = 0.78, OR = 0.54, 95% CI = (0.31, 0.93); IL1B T-31C (PPI = 0.72, OR = 55, 95% CI = (0.31, 0.97)); TNFR2 Met196Arg (PPI = 0.70; OR=1.85;95%CI=(1.03,3.36)); PTGS2 exon 10+837T>C (PPI = 0.69, OR = 0.54, 95%CI = (0.28, 0.99)); and IL10RB Lys47Glu (PPI = 0.68; OR=1.74; 95%CI=(1.04,2.92)) were predictive for symptom clusters of pain, depressed mood and fatigue; IL1B T-31C, PTGS2 exon 10+837T>C, and SNP rs1800783 affect symptom burden |
| Steel, J.L., et al. (30). | Pain, depression and fatigue were assumed to be a symptom cluster Symptom cluster was defined as three or more symptoms occurring together at the same time |
Design: longitudinal Setting: outpatient Sample Group: 206 adults with hepatobiliary carcinoma Stage: Unspecified Control Group: none reported |
Pain: Functional Assessment of Cancer Therapy-Hepatobiliary cancer (FACT-Hep) Scale Depression: FACT-Hep Fatigue: FACT-Hep |
Monocytes, eosinophils and basophils Source: blood |
Two-step hierarchical cluster analysis; Schwarz’s Bayesian Information Criterion; Multivariate latent growth curve and structural equation modeling; Comparative fit index and root mean square error of approximation; Cox regression; Kaplan-Meier survival and p value not reported | High levels of pain, depression and fatigue were associated with elevated eosinophils (p= .05) |
| Thornton, L.M., et al. (31). | Symptoms occur together were assumed to be a symptom cluster No specific definition provided |
Design: cross-sectional observational study Setting: outpatient Sample Group: 104 adults with advanced breast cancer Stage: IV, Recurrence Control Group: none reported |
Pain: BPI Depression: CES-D Fatigue: BFI |
Norepinephrine, epinephrine, ACTH, cortisol Source: blood; plasma |
Correlations; Covariance structure modeling; Alpha was set at .05 | The data confirm the hypothesis model derived from the neuroendocrine-immune models of cancer symptoms. The result of the final model showed elevation of the SNS and HPA axis hormones were associated with increased pain, fatigue and depression (Beta =0.23, p <.05) |
| Wang, X.S., et al. (32). | No specific definition provided |
Design: longitudinal Setting: inpatient Sample Group:30 adults scheduled for AHSCT Stage: Unspecified Control Group: none reported |
General Symptoms: M.D. Anderson Symptom Inventory | IL-1b, IL-6, IL-8, IL-10, IL12p40p70, IL-1ra, IFN-γ, sTNF-R1 Source: blood; serum |
Descriptive statistics; Lowess curves; Mixed effects modeling; p < .05 | An increase in IL-6 from baseline to nadir (8 days post-transplant)significantly predicted symptom severity (p = .0006); IL-6 and sTNF-R1 predicted changes in the component scores of the symptoms inventory (p < .0001) and (p < .05) respectfully |
| Wang, X.S., et al. (33). | No specific definition provided |
Design: longitudinal Setting: outpatient Sample Group: 62 adults with NSCLC Stage: I–IV Control Group: none reported |
General Symptoms: M.D. Anderson Symptom Inventory | IL-6, IL-8, IL-10, IL12p40p70, IL-1ra, TNF-a, sTNF-R1 Source: blood; serum |
Descriptive statistics; Lowess curves; Mixed effects modeling; p < .05 | IL-6 was the only cytokine to demonstrate a significant positive correlation (est, .32; SE, .16; p,.05) over time with the five most severe symptoms (pain, fatigue, disturbed sleep, lack of appetite and sore throat); sTNF-R1 was significantly correlated with an increased component symptom score (est, 1.74; SE, .69; p< .05). Pain and sore throat were significantly positively associated with IL-6 (est, .77; SE, .19, p< .05) and significantly negatively associated with IL-8 (est, .49; SE, .17, p< .05) |
Cluster of Symptoms
The symptom cluster most frequently examined was pain, depression, and fatigue in 64% (n=9/14) of the studies, 5 of which were cross-sectional23,25,28,29,31 and 4 were longitudinal studies.20,24,26,30 Other symptom clusters explored included cognitive symptoms and fatigue (n=1/14, 7%),27 anxiety and depression (2/14, 14%),21,26 and depression, anxiety, fatigue, and sleep disturbance.26
Symptoms were assessed using varied self-report measures. Pain was most often assessed using a multi-item scale (n=7/14, 50%) and less frequently measured using a numeric scale (n=3, 21%) and the Brief Pain Inventory (n=3, 21%). Depressive symptoms were measured using the Hospital Anxiety and Depression Scale (n=3, 21%) or the Center for Epidemiologic Studies Depression Scale (n=3, 21%). Fatigue was most often assessed (n=4, 29%) using the Brief Fatigue Inventory.
Biologic Pathway
The biologic pathway most frequently explored (n=13, 93%) to explain the clustering of cancer-related symptoms was the immune/inflammation pathway. Cytokines were examined in almost half (n=6/14, 43%) of the studies.22,25–27,32,33 C-reactive protein was examined in five (36%) studies.21,24–26,31 Immune markers including viral load and white blood count were examined in two (14%) studies,23,30 and cytokine encoding genes were examined in three (21%) studies.20,28,29
Inflammation
IL-6 and Tumor Necrosis Factor (TNF)- alpha (α) were the most measured cytokines (n=5, 36%).25–27,32,33 In fact, IL-6 was measured in 50% of the longitudinal studies.26,27,32,33 Specific cytokines were observed to correlate with specific symptoms, but not with clusters of symptoms. For example, TNF-α significantly correlated with depression, but not with pain, fatigue, or anxiety.26 IL-6 significantly predicted worsening of symptoms.32 Inconsistencies with these associations were observed among the reviewed studies. For example, TNF-α was significantly associated with fatigue only in one study,27 in another study TNF-α was only significantly correlated to depression,26 and in a third study, no significant associations between with TNF-α levels and symptom scores were found.25 In addition, two studies from the same group of authors reported that they were unable to detect TNF-α levels in their samples.32,33 Inconsistent findings were also reported in the association of C-reactive protein (CRP) with symptom clusters. For example, two studies reported significant correlations between CRP and the clustering of depression, pain, and fatigue,24,26 while another study did not.25 One study used the Glasgow Prognostic Scale, where a numeric value from levels of CRP and albumin was assigned as a biologic marker, and found that this value was significantly correlated with depression and anxiety.21
Immune Markers
Viral load and white blood cells were assessed in two (14%) studies.23,30 High viral load for cytomegalovirus (CMV) was associated with high levels of pain, depression, and fatigue.23 In addition, elevated levels of eosinophils were associated with pain, fatigue, and depression.30
Genetics
There were three (21%) studies that examined genes encoding cytokines as a biological marker of cancer-related symptoms.20,28,29 Homozygous for rare allele in IL-6 rs2069845 increased the odds for participants to have high symptom burden from the clustering of pain, depression, fatigue, and sleep disturbance.20 Two studies were done by the same research group, which investigated the involvement of immune response genes with the clustering of pain, depressed mood, and fatigue in lung cancer patients with different disease stages.28,29 IL-8-T251A was significantly associated with pain, depressed mood, and fatigue, and individuals with IL-8-T251A T/T genotypes were less prone to report severe pain or fatigue, but more prone to have severe depression compared with individuals with A/T and A/A genotypes.29 These significant associations were not observed in participants with early-stage lung cancer.29 In addition, these authors found that mutant alleles in ENOS (−1474 T/A); IL1B T-31C; TNFR2 Met196Arg mutant alleles of PTGS2 exon 10+837T>C; and IL10RBLys Glu predicted the clustering of pain, depression, and fatigue.28
Hormones
Hormones were the least studied. Investigators from only one study reported significant associations with sympathetic nervous system and hypothalamic-pituitary-adrenal axis hormones (epinephrine, norepinephrine, and cortisol) with the clustering of pain, depression, and fatigue.31
Discussion
This review describes the current investigations conducted in humans to explain the biological underpinnings of clustering of cancer-related symptoms. Although our findings suggest that inflammatory, immune, and hormonal markers are associated with the clustering of cancer-related symptoms, several inconsistencies in these associations were observed. These inconsistencies may result from different measurement techniques of biological markers and symptoms. In addition, the inconsistencies with the definition of symptom cluster itself may contribute to our findings.
Recent technological advances have allowed investigators an unprecedented opportunity to measure different blood markers (i.e., cytokines) with more accuracy; however, this has also created significant challenges contributing to inconsistencies observed in the literature.34 For example, inconsistencies in the measurement of cytokines stem from the experimental design including the method of collection, the time of day for collection, the type of specimen obtained (serum vs. plasma), where the specimen is obtained (arterial vs. venous), processing and storage of specimen, as well as the health status of the research participant.34,35 In addition, the technical expertise of the lab personnel and the commercially-available assay used greatly influence the findings.34,36 Most of these important variables (e.g., time of day, technical expertise of the lab personnel often reported as inter-/intra-assay variations) are not mentioned in most of the reviewed articles.
The three clustering symptoms (pain, depression, fatigue) that were most frequently measured in the reviewed articles were assessed using various unidimensional and multidimensional questionnaires. For example, pain was measured by a numeric rating scale in two studies from the same sample28,29 and measured by a health-related quality of life instrument (SF-36) in another study.23 This discordance in measuring a specific symptom in a cluster might influence the association of the symptom cluster with a potential biomarker. Because symptoms are subjective experiences, researchers often rely on patient self-report to measure them.37 It is important to measure the same construct across studies to collectively understand their biological underpinnings.37 As we move into the era of “Big Data,” symptom science researchers must find ways to gather, store, and share information about symptoms to work across studies to make comparable associations among symptoms and symptom clusters to move symptom science forward. The use of common data elements (CDEs) makes certain that a construct is consistently measured across studies both conceptually and operationally.38 It is also recommended that symptoms should be consistently measured across disciplines.39
The clinical relevance of the cytokine-induced clustering of cancer-related symptoms observed from our findings warrants further investigation. To move us forward with our mechanistic investigations, it is very important to address the other limitations we observed while conducting this review. There was no clear definition of symptom cluster. Two of the reviewed articles26,30 used a previously reported definition of symptom cluster of three or more co-occurring symptoms.4 Although several authors of the reviewed articles mentioned “symptom cluster” in the titles, a clear definition of how they conceptually defined symptom cluster was not provided.20,24,28,31 Other terms used to denote co-occurrence of symptoms included, “multiple co-occurring symptoms experienced by cancer patients”.29 Several of the studies did not define symptom cluster, but used various statistical methods to confirm the relationship among symptoms.21–23,25,27,32,33 Having an agreed upon definition of symptom cluster will advance our science investigating the biological basis for clustering of cancer-related symptoms.
Other challenges encountered while reviewing the articles included the clustering of a specific symptom with a related physical condition. For example, fatigue with vomiting or fatigue with weight loss. For consistency, the authors of this review only included articles that explored the relationship of biomarkers with subjective report of co-occurrence of symptoms related to cancer or its therapy. In addition, the biologic samples used in all studies were only from peripheral blood; hence, the interplay of peripheral and central mechanisms causing the clustering of symptoms can only be hypothesized. Further, most of the reviewed articles used small sample sizes,21,25,26,31–33 making it challenging to generalize the findings of this review.
Future investigations should explore the biological basis of clustering of cancer-related symptoms in larger samples. Use of longitudinal studies will greatly inform and validate the relationship of symptom clusters with specific biomarkers. The knowledge gained from this review is critically relevant to nurses and other health care providers. Understanding the biological mechanisms associated with the clustering of cancer-related symptoms will inform the future development and testing of targeted therapeutics and personalized interventions to mitigate cancer-related symptoms and improve the health-related quality of life of cancer survivors. Optimization of targeted therapeutics will more likely result in successful symptom management and may improve adherence to treatments.
This review brings to light an interesting question regarding symptom science research. In this review five studies investigated the associations of pain, depression, and fatigue as a symptom cluster based on assumptions: 1) pain, depression and fatigue are common cancer-related symptoms and 2) they commonly occur together.40,41 Examining these symptoms as a cluster decreases spurious statistical associations by accounting for the co-variance of symptoms. However, at what point is it necessary to examine these symptoms as a cluster and not as isolated symptoms? When investigating a particular symptom, should other symptoms be examined to provide perspective, knowing that oftentimes, symptoms cluster together? There are many symptoms associated with a single illness or with co-morbid conditions. Providing perspective of both clinical and biologic features of symptoms is critical to scientific progression toward alleviating suffering due to symptoms.42
Conclusion
The current state of our understanding of the pathobiology of clustering of symptoms related to cancer and cancer therapy is limited. This review provides initial information that inflammatory and neuroimmune markers are associated with the clustering of common cancer-related symptoms such as pain, fatigue, and depression. Further investigations are warranted to identify and validate potential pharmacodynamics targets. This information will be particularly important to nurses and other health care providers in planning optimal interventions to manage these symptom clusters.
Footnotes
Financial Disclosures:
This research was supported by the Division of Intramural Research, National Institute of Nursing Research, National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Comprehensive Cancer Network. [Accessed August 1, 2016];Clinical practice guidelines in oncology: Non-small cell lung cancer. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated Version 7.2015.
- 2.American Cancer Society. [Accessed July 28, 2016];Cancer treatment and survivorship facts & figures 2014–2015. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-042801.pdf. Updated 2014.
- 3.Shi Q, Smith TG, Michonski JD, Stein KD, Kaw C, Cleeland CS. Symptom burden in cancer survivors 1 year after diagnosis. Cancer. 2011;117(12):2779–2790. doi: 10.1002/cncr.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28(3):465–470. [PubMed] [Google Scholar]
- 5.Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Semin Oncol Nurs. 2007;23(2):99–105. doi: 10.1016/j.soncn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Fagundes C, LeRoy A, Karuga M. Behavioral Symptoms after Breast Cancer Treatment: A biobehavioral approach. J Pers Med. 2015;5(3):280–295. doi: 10.3390/jpm5030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33(suppl 1):S79–84. doi: 10.1007/s10875-012-9847-0. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 9.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine & Growth Factor Reviews. 2011;22(2):83–89. doi: 10.1016/j.cytogfr.2011.02.003. http://dx.doi.org/10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Artherholt SB, Fann JR. Psychosocial care in cancer. Curr Psychiatry Rep. 2012;14(1):23–29. doi: 10.1007/s11920-011-0246-7. [DOI] [PubMed] [Google Scholar]
- 11.Lutgendorf SK, Andersen BL. Biobehavioral approaches to cancer progression and survival: Mechanisms and interventions. Am Psychol. 2015;70(2):186–197. doi: 10.1037/a0035730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol. 2010;28(26):4094–4099. doi: 10.1200/jco.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood LJ, Weymann K. Inflammation and neural signaling: Etiologic mechanisms of the cancer treatment-related symptom cluster. Curr Opin Support Palliat Care. 2013;7(1):54–59. doi: 10.1097/SPC.0b013e32835dabe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauter KA, Wood LJ, Wong J, Iordanov M, Magun BE. Doxorubicin and daunorubicin induce processing and release of interleukin-1beta through activation of the NLRP3 inflammasome. Cancer Biol Ther. 2011;11(12):1008–1016. doi: 10.4161/cbt.11.12.15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adelman JS, Martin LB. Vertebrate sickness behaviors: Adaptive and integrated neuroendocrine immune responses. Integr Comp Biol. 2009;49(3):202–214. doi: 10.1093/icb/icp028. [DOI] [PubMed] [Google Scholar]
- 16.Weymann KB, Wood LJ, Zhu X, Marks DL. A role for orexin in cytotoxic chemotherapy-induced fatigue. Brain Behav Immun. 2013;37:84–94. doi: 10.1016/j.bbi.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossberg AJ, Zhu X, Leinninger GM, et al. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J Neurosci. 2011;31(31):11376–86. doi: 10.1523/JNEUROSCI.2311-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall JR, Wiechmann AR, Johnson LA, et al. Biomarkers of vascular risk, systemic inflammation, and microvascular pathology and neuropsychiatric symptoms in Alzheimer’s disease. J Alzheimers Dis. 2013;35(2):363–371. doi: 10.3233/jad-122359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukkahatai N, Majors B, Reddy S, Walitt B, Saligan LN. Gene expression profiles of fatigued fibromyalgia patients with different categories of pain and catastrophizing: a preliminary report. Nurs Outlook. 2013;61(4):216–224. e212. doi: 10.1016/j.outlook.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doong SH, Dhruva A, Dunn LB, et al. Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biol Res Nurs. 2015;17(3):237–247. doi: 10.1177/1099800414550394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannousi Z, Gioulbasanis I, Pallis AG, et al. Nutritional status, acute phase response and depression in metastatic lung cancer patients: correlations and association prognosis. Supportive Care in Cancer. 2012;20(8):1823–1829. doi: 10.1007/s00520-011-1282-x. [DOI] [PubMed] [Google Scholar]
- 22.Heinze S, Egberts F, Rotzer S, et al. Depressive mood changes and psychiatric symptoms during 12-month low-dose interferon-alpha treatment in patients with malignant melanoma results from the multicenter DeCOG trial. J Immunother. 2010;33(1):106–114. doi: 10.1097/CJI.0b013e3181b8bdb9. [DOI] [PubMed] [Google Scholar]
- 23.Jaremka LM, Fagundes CP, Glaser R, Bennett JM, Malarkey WB, Kiecolt-Glaser JK. Loneliness predicts pain, depression, and fatigue: Understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38(8):1310–1317. doi: 10.1016/j.psyneuen.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laird BJ, Scott AC, Colvin LA, et al. Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manage. 2011;42(1):1–11. doi: 10.1016/j.jpainsymman.2010.10.261. [DOI] [PubMed] [Google Scholar]
- 25.Lynch Kelly D, Lyon DE, Ameringer SA, Elswick RK, Jr, McCarty JM. Symptoms, cytokines, and quality of life in patients diagnosed with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Oncol Nurs Forum. 2015;42(3):365–375. doi: 10.1188/15.onf.265-275. [DOI] [PubMed] [Google Scholar]
- 26.Lyon DE, Schubert C, Taylor AG. Pilot study of cranial stimulation for symptom management in breast cancer. Oncol Nurs Forum. 2010;37(4):476–483. doi: 10.1188/10.onf.476-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104(4):788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 28.Reyes-Gibby CC, Swartz MD, Yu X, et al. Symptom clusters of pain, depressed mood, and fatigue in lung cancer: assessing the role of cytokine genes. Support Care Cancer. 2013;21(11):3117–3125. doi: 10.1007/s00520-013-1885-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyes-Gibby CC, Wang J, Spitz M, Wu X, Yennurajalingam S, Shete S. Genetic variations in interleukin-8 and interleukin-10 are associated with pain, depressed mood, and fatigue in lung cancer patients. J Pain Symptom Manage. 2013;46(2):161–172. doi: 10.1016/j.jpainsymman.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steel JL, Kim KH, Dew MA, et al. Cancer-related symptom clusters, eosinophils, and survival in hepatobiliary cancer: an exploratory study. J Pain Symptom Manage. 2010;39(5):859–871. doi: 10.1016/j.jpainsymman.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol. 2010;29(3):333–337. doi: 10.1037/a0018836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang XS, Shi Q, Williams LA, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer. 2008;113(8):2102–2109. doi: 10.1002/cncr.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24(6):968–974. doi: 10.1016/j.bbi.2010.03.009. http://dx.doi.org/10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou X, Fragala MS, McElhaney JE, Kuchel GA. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care. 2010;13(5):541–547. doi: 10.1097/MCO.0b013e32833cf3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008;63(8):879–884. doi: 10.1093/gerona/63.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong TS. 2013 Special Topics Conference: Peaks and pitfalls in longitudinal studies of symptom outcome data. Nurs Outlook. 2014;62(5):305–312. doi: 10.1016/j.outlook.2014.05.005. http://dx.doi.org/10.1016/j.outlook.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Redeker NS, Anderson R, Bakken S, et al. Advancing Symptom Science Through Use of Common Data Elements. J Nurs Scholarsh. 2015;47(5):379–388. doi: 10.1111/jnu.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henly SJ. The symptom science model: Challenges in dissemination across the investigative sequence. Nurs Res. 2015;64(5):329–330. doi: 10.1097/nnr.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 40.Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 41.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. doi: 10.1200/jco.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cashion AK, Grady PA. The National Institutes of Health/National Institutes of Nursing Research intramural research program and the development of the National Institutes of Health Symptom Science Model. Nurs Outlook. 2015;63(4):484–487. doi: 10.1016/j.outlook.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]