Abstract
Objectives
To determine cost-effectiveness of three community-based acute HIV infection (AHI) testing algorithms compared to HIV antibody testing alone by focusing on the potential of averting new infections occurring within a one-year time horizon among men who have sex with men (MSM).
Methods
Data sources for model parameters included actual cost and prevalence data derived from a community-based AHI screening program in San Diego, and published studies. Main outcome measure was costs per infection averted (IA). The lower end of the cost range of discounted lifetime costs of an HIV infection (i.e. $236,948) was used for defining cost-effectiveness.
Results
The most sensitive algorithm for AHI detection, which was based on HIV nucleic acid amplification testing, was estimated to prevent between 5 and 45 transmissions, with simulated costs per infection averted between $965 and $141,256 when compared to HIV antibody testing alone.
Conclusion
AHI testing was cost-effective in preventing new HIV infections among at risk MSM in San Diego, and also among other MSM populations with similar HIV prevalence but lower proportions of AHI diagnoses. These results indicate that community based AHI testing among MSM in the United States can pay for itself over the long run.
Keywords: HIV transmission, Acute HIV, MSM, testing, cost analysis, NAT, infection averted
Introduction
HIV antibody testing remains the most widely used approach to diagnose HIV infection in community-based settings in the United States [1]. HIV antibody tests, however, fail to detect acute HIV infection (AHI), which is the earliest stage of HIV disease and lasts until the body develops antibodies against HIV[2]. AHI is associated with transient levels of extremely high titer viremia [3] resulting in a high level of infectiousness that serves as a major driver of HIV transmission in the United States and other resource rich countries [4–6]. As many as half of HIV transmissions occur from persons with AHI [7], which makes detection of AHI critical to HIV prevention strategies [4–6, 8]. While guidelines support early initiation of antiretroviral therapy (ART) for the prevention of HIV transmission (i.e. treatment as prevention) [9, 10], AHI diagnosis may reduce transmission risk even in the absence of other interventions, as evidence suggests that individuals generally reduce their risk behavior after being diagnosed with HIV [5, 11].
Although detection of AHI offers opportunities to reduce infectivity (primarily ART and risk reduction) to reduce subsequent transmission risk, screening for AHI is not widely performed in community-based settings. Commercially available point-of-care (POC) assays for AHI have limited sensitivity, while non-POC assays require follow-up for results and are generally more costly to perform. By comparing four community based testing strategies, we have recently shown that costs for detection of one case of AHI may be below US $20,000 in at risk men who have sex with men (MSM) [12] [13]. Calculation of cost-effectiveness per transmission prevented (i.e. infection averted [IA]) is more complicated, but has two major advantages: i.) cost thresholds are easier to define, as there are comprehensive estimates of lifetime treatment- and healthcare costs per HIV infection [14, 15], and ii.) the measure is more complete in terms of costs to the healthcare system. Consistent with federal efforts to reduce the costs of healthcare through the deployment of effective prevention measures, calculation of costs per IA will allow us to determine if testing can pay for itself over the long run.
The objective of this study was to determine cost-effectiveness of three community-based AHI testing algorithms compared to HIV antibody testing alone by focusing on the potential of averting new infections.
Material and Methods
This one-year cost analysis compared community based HIV testing strategies based upon the cost per IA in 2014 US dollars. Cost analyses were conducted using an established HIV testing program perspective. The study evaluated four community based HIV testing strategies [12], including three that detect AHI (EarlyTest [i.e. routine HIV nucleic-acid-amplification testing in all antibody negative persons], Architect, and Determine [both based on HIV p24 antigen detection]), and one that relies on HIV antibody testing alone. The model was built on our recent cost-model that compared these four algorithms with regard to costs per AHI diagnosis in 2014 US dollars [12], which was based on published risk data and HIV observed in MSM undergoing community-based AHI screening in San Diego between 2006 and 2014 [16–20]. Detailed description of the algorithms and methods can be found elsewhere [12], and is summarized in the supplementary appendix (SI Appendix, SI Appendix Table S1, SI Appendix Figure S1).
Cost per infection (i.e. transmission) averted
Estimations of the potential impact of missed AHI diagnoses on subsequent spread of HIV were conducted by combining published transmission risk estimations with data on risk and testing behavior observed in MSM diagnosed with AHI between April 2008 and July 2014 with the “Early Test”, a community-based, confidential AHI screening program in San Diego, California [16]. To assess the frequency of testing in those diagnosed with AHI we calculated the time period between the last negative test and the day they tested positive by NAT and assumed that it would have taken those individuals exactly the same time period to test again. We also assumed that the risk behavior reported by those with AHI for the last 12 months before diagnosis [i.e. condomless insertive anal intercourse (CIAI) and number of male partners] would reflect the ongoing risk behavior in the absence of an HIV diagnosis. In addition, we focused only on direct transmission occurring from individuals with missed AHI diagnoses. Finally, we assumed that those diagnosed with AHI would not transmit HIV during the first year after diagnosis (immediate ART is routinely provided to “Early Test” participants diagnosed with AHI, in addition studies have shown that transmission risk behavior may decrease significantly in the months after HIV diagnosis [5]). Using these assumptions, we calculated estimated numbers of transmissions from undiagnosed (i.e., missed) acute HIV diagnoses. Incremental cost effectiveness ratios (ICERs) were calculated by comparing two different testing algorithms, with the numerator representing the difference in annual cost of the two algorithms and the denominator representing the difference in IA. Numbers of IA by each of the AHI were calculated by two different approaches: a) per-contact transmission risk and b) per-partner transmission risk.
Cost thresholds
Discounted lifetime costs of an HIV infection have recently been updated [i.e between $229,800 and $338,400 depending on the time point of diagnosis and ART initiation [15]]. As those costs were calculated in 2012 US dollars, the thresholds were updated to 2014 US dollars by adding the cumulative rate of inflation (ie, 3.1%), resulting in an updated cost range of $236,948 and $348,927. We conservatively used the lower end of this updated cost range (i.e. $236,948) for defining cost-effectiveness.
Per-contact transmission risk
Focusing on per-contact transmission risk is important as numbers of sexual contacts do not correlate with the number of partners. In previous studies the number of sexual contacts was markedly higher and condomless anal sex was more frequent with the main partner versus casual partners (80.7 annual contacts with main partner vs. 4.0 with casual partners) [21][22]. However, number of contacts was not routinely assessed in our cohort of MSM and we therefore used estimates from a comparable study cohort [23, 24]. In an Australian study that followed more than 1,000 MSM over 4 years, a mean of 41 annual condomless insertive anal intercourse (CIAI) episodes were reported in those reporting any CIAI, however, actual numbers of contacts varied widely [23, 24]. We used these estimates and assumed that in our setting every MSM who reported CIAI for the prior 12 months, would have 41 CIAI episodes (95% confidence interval [CI] 10–70 episodes) per year.
In recent analyses on per-contact HIV transmission risk, condomless receptive anal intercourse (CRAI) with an HIV-positive partner (either acute or chronic) carried the greatest risk of HIV acquisition, with an estimated 1.38% (95%CI 1.02%–1.86%) risk of seroconversion (more than 10 times higher than the risk of acquiring HIV infection during CIAI) [23, 25, 26]. Therefore we focused our transmission risk model on CIAI episodes by the transmitting HIV-positive partners, and assumed a 1.38% (95% CI 1.02%–1.86%) risk per act of transmitting the disease, although this may be an underestimation for those with AHI, where transmission risk is greatest during the initial weeks and months [27, 28].
We combined those two estimations (i.e. 41 CIAI episodes per year [23, 24] and 1.38% risk of transmission per episode [23, 25, 26]), with risk behavior and testing data from individuals diagnosed with AHI by the “Early Test” between 2008 and 2014, and calculated a mean one-year risk of transmitting HIV. Data derived from “Early Test” included: i. proportion who reported CIAI and ii. median time period between the last negative test and the day they tested positive (proportion of a year) with a maximum of 1 year. We calculated a mean one-year risk of HIV transmission per contact (β1) using the following equation:
Considering:
t, Time between last negative and first positive HIV test, in days, range 1 — 365.
c, Proportion who reported CIAI (range 0–1)
To calculate the risk of transmission for the proportion of individuals with missed AHI diagnoses in the different algorithms, we multiplied the respective number of missed AHI diagnoses by the mean risk calculated using the formula above.
Per-partner transmission risk
In a second approach, we assessed the risk of transmission by focusing on number of unique sexual partners. Again, we chose a conservative approach focusing on CIAI only. In a recent meta-analysis of studies evaluating HIV transmission risk through anal intercourse, per-partner HIV transmission probability was 39.9% in MSM (95%CI 22.5–57.%) [29]. The remaining variables were derived from individuals diagnosed with AHI by the “Early Test”. As we did not have number of CIAI partners we assumed that all individuals with AHI that reported insertive anal intercourse (IAI) had IAI with every partner they reported. We calculated the proportion of partners with whom CIAI was performed by using the reported frequency of condom use during IAI episodes. As the frequency of condom use was reported as a percentage range (100% of the time, 50%–99%, 1%–49% or 0%) we chose the median of the percentage range if necessary [i.e. 75% for “condom use in 1% – 49% of IAI episodes” and 25% for “condom use in 50% – 100%”]. We calculated the mean one-year risk of HIV transmission per partner (β2) using the following equation:
Considering:
Estimated per partner risk = 0.399 [29]
n, mean reported number of male partners
μ, mean reported condom use during IAI (range 0–1)
t, Time between last negative and first positive HIV test, in days, range 1 – 365.
c, Proportion who reported CIAI (range 0–1)
Again, the result was multiplied with the respective number of missed AHI diagnoses to calculate transmission risks for the different algorithms.
Sensitivity analyses
We assessed the effect of a number of alternate plausible assumptions and employed a probabilistic sensitivity analysis (PSA) to examine the impact of cost parameter uncertainty. We performed PSA for two different proportions of AHI (0.24 and 0.10 of all HIV diagnoses). While AHI cases represented 24% of all newly diagnosed HIV cases among MSM in the San Diego Primary Infection Resource Consortium (SD PIRC), a lower proportion of 10%, may be more appropriate for settings where clients undergo screening less frequently [30, 31]. For PSA we assigned uniformly distributed 95% CI to applicable cost items, test performance, and loss to follow up in algorithms that do not provide POC positive results for AHI, as described previously [12]. In addition, we assigned uniformly distributed 95% CI to all variables of per-contact and per-partner risk calculation. To determine the frequency at which each algorithm was cost-effective at the given threshold, we conducted Monte Carlo simulations to obtain 1000 samples from all distributions, and used these samples to calculate means and 95% CIs for ICERs per IA, by using the 2.9% HIV prevalence rate, and AHI proportions of 24% and 10%. The model and statistical analyses were performed using Excel 2010 (Microsoft, Seattle, WA, USA) and SPSS 21 (SPSS Inc., Chicago, IL, USA).
Results
Base Model
The base model of costs per IA utilized data from 93 MSM diagnosed with AHI (Fiebig stage I–II) in the Early Test program between April 2008 and July 2014 in conjunction with previously published data and transmission risks. One-year transmission risks per AHI case missed were calculated with per-contact and per-partner analyses, input variables and results are depicted in Table 1. Estimated total annual costs associated with each of the four algorithms are displayed in Table 2.
Table 1.
Input variables for calculation of per-contact and per-partner transmission risk, and one year transmission risk per acute HIV infection missed using per contact and per partner calculation.
| Base model (mean, 95%CI) | Probabilistic Sensitivity Analysis (mean, 95%CI) | |
|---|---|---|
| Variables from 93 MSM diagnosed with AHI with the “Early Test” Program between mid-2008 and mid-2014 | ||
| Proportion who reported recent CIAI | 0.871 (0.804–0.938) | - |
| Time factor (i.e. testing frequency in proportions of one year)* | 0.537 (0.465–0.609) | - |
| Number Partners | 24 (10–37) | - |
| Proportion of condom use in IAI partners | 0.506 (0.438–0.573) | - |
| Variables from literature | ||
| Number of CIAI contacts (23,24) | 41 (10–70) | - |
| Risk per CIAI (26) | 0.0138 (0.0102–0.0186) | - |
| Risk per Partner (29) | 0.399 (0.225–0.574) | - |
| Mean one-year risk of transmitting HIV per missed AHI diagnosis: Per-contact Analysis | 0.2646 | 0.2701 (95%CI 0.0208–0.5193) |
| Mean one-year risk of transmitting HIV per missed AHI diagnosis: Per-partner Analysis | 2.2664 | 2.1762 (95%CI 0.3029–4.0496) |
17% of MSM diagnosed with AHI reported that they have never been tested before. Among those with previous test results, the most recent HIV test before diagnosis was in median 128 days ago (IQR 65–240 days; 14% reported that their most recent test was more than a year ago).
Abbreviations: AHI, acute HIV infection; CI, confidence interval; CIAI, condomless insertive anal intercourse; IAI, insertive anal intercourse; MSM, men who have sex with men.
Table 2.
Number of Diagnoses, total Costs and Cost Differences of the four Testing Algorithms in the San Diego Men who have Sex with Men Model. [Modified from previously published supplementary materials (12)]
| Annual Costs of the Testing Algorithms Base Model | Algorithms | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Actual costs of Early Test | Estimated costs of Architect | Estimated costs of Determine | Estimated costs of Rapid Ab alone | |||||
|
| ||||||||
| BASE MODEL | N | USD | N* | USD | N* | USD | N* | USD |
| Acute HIV (i.e. seronegative) | 19.95 | 1,815.65 | 15.96 | 1,405.12 | 10.5 | 1,394.40 | - | - |
| Acute HIV, but MSM lost to follow up and not informed about diagnosis | 1.05 | 68.48 | 0.84 | 52.29 | - | - | - | - |
| Early or chronic HIV (i.e. seropositive) | 66 | 3,686.10 | 66 | 3,686.10 | 66 | 5,025.24 | 66 | 4,372.50 |
| True Negative Test Result | 2913 | 206,764.74 | 2913 | 198,142.26 | 2913 | 116,053.92 | 2913 | 86,486.97 |
| False Negative Test Result | - | - | 4.2 | 285.68 | 10.5 | 418.32 | 21 | 623,49 |
|
| ||||||||
| TOTAL Costs per Year | 3000 | 212,334.97 | 3000 | 203,571.45 | 3000 | 122,891,88 | 3000 | 91,482.96 |
|
| ||||||||
| Cost Difference vs. Rapid Antibody alone | 120,852.01 | 112,088.49 | 31,408.92 | - | ||||
| Cost Difference vs. Determine | 89,443.09 | 80,659.57 | - | - | ||||
| Cost Difference vs. Architect | 8,763.53 | - | - | - | ||||
Abbreviations: MSM, men who have sex with men
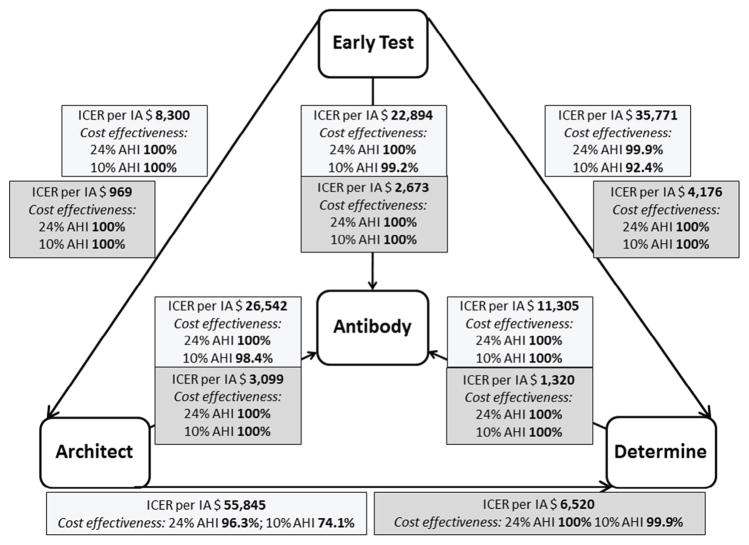
ICERs per IA are displayed in Figure 1. Focusing on per-contact transmission risk, the mean calculated one-year HIV transmission risk for individuals with missed AHI diagnosis was 26.46%. Using these estimations, 5.28 infections were averted within a year using the Early Test algorithm when compared to the Ab alone algorithm (4.22 IA by Architect algorithm when compared to Ab alone, and 2.78 by Determine). The cost per IA by the Early Test algorithm when compared to the Ab alone algorithm was $22,894 (Architect vs. Ab alone was $26,542, and Determine vs. Ab alone $11,305). As these were significantly lower than the lower threshold of the discounted lifetime costs of an HIV infection (i.e. $236,948), all three algorithms that detect AHI were deemed cost-effective compared to the Ab alone algorithm. Numbers and incremental cost effectiveness ratios (ICERs) per IA for per-contact risk comparisons of all four algorithms and for HIV prevalence of 2.9% and AHI proportions of 24% and 10% are depicted in Table 3.
Figure 1.
Incremental cost effectiveness ratios (ICERs) per infection (i.e. transmission) averted for comparing each of the four algorithms in the San Diego men who have sex with men model (i.e. base model), and results of probabilistic sensitivity analyses (percentage of cost-effectiveness in Monte Carlo simulations displayed for 24% AHI proportion and 10% AHI proportion). Light grey boxes represent results of per-contact analysis, dark grey boxes represent results of per-partner analysis.
Table 3.
Per-contact Analysis: Costs per Infection (i.e. Transmission) averted (i.e. incremental cost effectiveness ratio) for comparisons of all four algorithms. Base costs and results of probabilistic sensitivity analyses are displayed.
| Costs per Infection (i.e. Transmission) Averted (i.e. ICER) | HIV prevalence/AHI proportion (%) | Algorithms | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| PER-CONTACT ANALYSIS | Early Test | Architect | Determine | Rapid Ab alone | |||||
|
| |||||||||
| ICER per IA vs. Rapid Ab alone | N* | USD# | N* | USD# | N* | USD# | N* | USD# | |
| ICER per IA (Base costs) | 0.029/24% | 5.28 | 22,893.95 | 4.22 | 26,542.29 | 2.78 | 11,305.10 | - | - |
| ICER per IA, probabilistic sensitivity analysis | 0.029/24% | - | - | - | - | - | - | - | - |
| 95%CI of mean | - | 29,045–31,402 | 33,799–36,552 | 14,326–15,487 | |||||
| Range (% above $236,948, if applicable) | - | 8,779–141,256 | 9,655–179,714 | 3,932–69,804 | |||||
| ICER per IA, probabilistic sensitivity analysis | 0.029/10% | - | - | - | - | - | - | - | - |
| 95%CI | - | 69,590–75,239 | 80,999–87,596 | 33,934–36,685 | |||||
| Range (% above $236,948, if applicable) | - | 21,012–338,095 (0.8%) | 23,113–430,429 (1.6%) | 9,280–165,917 | |||||
|
| |||||||||
| ICER per IA vs. Determine | |||||||||
|
| |||||||||
| ICER per IA (Base costs) | 0.029/24% | 2.50 | 35,770.52 | 1.44 | 55,844.67 | - | - | - | - |
| ICER per IA, probabilistic sensitivity analysis | 0.029/24% | - | - | - | - | - | - | - | - |
| 95%CI of mean | - | 46,211–50,164 | 77,540–85,560 | ||||||
| Range (% above $236,948, if applicable) | - | 13,267–246,923 (0.1%) | 17,356–601,868 (3.7%) | ||||||
| ICER per IA, probabilistic sensitivity analysis | 0.029/10% | - | - | - | - | - | - | - | - |
| 95%CI of mean | - | 111,167–120,682 | 186,692–206,018 | ||||||
| Range (% above $236,948, if applicable) | - | 31,873–593,844 (7.6%) | 41,711–1,449,498 (25.9%) | ||||||
|
| |||||||||
| ICER per IA vs. Architect | |||||||||
|
| |||||||||
| ICER per IA (Base costs) | 0.029/24% | 1.06 | 8,300.71 | - | - | - | - | - | - |
| ICER per IA, probabilistic sensitivity analysis | 0.029/24% | - | - | - | - | - | - | - | - |
| 95%CI of mean | - | 10,865–11,836 | |||||||
| Range (% above $236,948, if applicable) | - | 2,885–53,197 | |||||||
| ICER per IA, probabilistic sensitivity analysis | 0.029/10% | - | - | - | - | - | - | - | - |
| 95%CI of mean | - | 25,964–28,287 | |||||||
| Range (% above $236,948, if applicable) | - | 6,851–127,425 | |||||||
N corresponds to the total number of HIV transmissions averted when compared to alternative algorithm.
USD corresponds to costs (in 2014 USD) per single HIV transmission averted when compared to alternative algorithm.
Abbreviations: 95%CI, 95% confidence interval; IA, infection (i.e. transmission) averted; ICER, incremental cost effectiveness ratio.
Using per-partner transmission risk calculations, the estimated number of HIV transmissions over one year was 2.2664 per undiagnosed AHI diagnosis. Using these estimations, 45 infections were averted by using the Early Test algorithm when compared to Ab alone, with costs per IA as low as $2,673. Numbers and ICERs per IA for per partner-risk comparisons are depicted in Table 4.
Table 4.
Per-partner Analysis: Costs per Infection (i.e. Transmission) averted (i.e. incremental cost effectiveness ratio) for comparisons of all four algorithms. Base costs and results of probabilistic sensitivity analyses are displayed.
| Costs per Infection (i.e. Transmission) Averted (i.e. ICER) | HIV prevalence/AHI proportion (%) | Algorithms | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| PER-PARTNER ANALYSIS | Early Test | Architect | Determine | Rapid Ab alone | |||||
|
| |||||||||
| ICER per IA vs. Rapid Ab alone | N* | USD# | N* | USD# | N* | USD# | N* | USD# | |
| ICER per IA (Base costs) | 0.029/24% | 45.21 | 2,672.85 | 36.17 | 3,098.79 | 23.90 | 1,319.86 | - | - |
| ICER per IA, probabilistic sensitivity analysis | 0.029/24% | - | - | - | - | - | - | - | - |
| 95%CI of mean | - | 3,330–3,540 | 3,832–3,501 | 1,643–1,748 | |||||
| Range (% above $236,948, if applicable) | - | 965–10,944 | 1,199–13,863 | 484–6,207 | |||||
| ICER per IA, probabilistic sensitivity analysis | 0.029/10% | - | - | - | - | - | - | - | - |
| 95%CI | - | 7,979–8,482 | 9,300–9,897 | 3,892–4,140 | |||||
| Range (% above $236,948, if applicable) | - | 2,315–26,228 | 2,876–33,237 | 1,144–14,762 | |||||
|
| |||||||||
| ICER per IA vs. Determine | |||||||||
|
| |||||||||
| ICER per IA (Base costs) | 0.029/24% | 21.42 | 4,176.17 | 12.37 | 6,519.81 | - | - | - | - |
| ICER per IA, probabilistic sensitivity analysis | 0.029/24% | - | - | - | - | - | - | - | - |
| 95%CI of mean | - | 5,294–5,647 | 8,911–9,802 | ||||||
| Range (% above $236,948, if applicable) | - | 1,451–19,733 | 1,921–108,022 | ||||||
| ICER per IA, probabilistic sensitivity analysis | 0.029/10% | - | - | - | - | - | - | - | - |
| 95%CI of mean | - | 12,735–13,585 | 21,455–23,602 | ||||||
| Range (% above $236,948, if applicable) | - | 3,494–47,504 | 4,617–260,681 (0.1%) | ||||||
|
| |||||||||
| ICER per IA vs. Architect | |||||||||
|
| |||||||||
| ICER per IA (Base costs) | 0.029/24% | 9.04 | 969.10 | - | - | - | - | - | - |
| ICER per IA, probabilistic sensitivity analysis | 0.029/24% | - | - | - | - | - | - | - | - |
| 95%CI of mean | - | 1,238–1,325 | |||||||
| Range (% above $236,948, if applicable) | - | 282–4,428 | |||||||
| ICER per IA, probabilistic sensitivity analysis | 0.029/10% | - | - | - | - | - | - | - | - |
| 95%CI of mean | - | 2,959–3,168 | |||||||
| Range (% above $236,948, if applicable) | - | 675–10,620 | |||||||
N corresponds to the total number of HIV transmission averted when compared to alternative algorithm.
USD corresponds to costs (in 2014 USD) per single HIV transmission averted when compared to alternative algorithm.
Abbreviations: 95%CI, 95% confidence interval; IA, infection (i.e. transmission) averted; ICER, incremental cost effectiveness ratio.
Probabilistic Sensitivity Analysis
Results of the probabilistic sensitivity analyses for a 2.9% HIV prevalence with AHI proportions of 24% and 10%, including 95% CI and ranges are depicted in Table 3 (per-contact transmission risk analysis), Table 4 (per-partner transmission risk analysis) and Figure 1. We found that testing for AHI in the per-contact analysis was cost-effective (i.e. ICERs below $236,948 per IA) vs. Ab testing alone. Specifically, the Early Test was cost-effective 100% of the time (with 24% proportion of AHI) and 99.2% (when assuming a 10% proportion of AHI) vs. Ab alone, Architect was cost-effective 100% (24% AHI) and 98.4% (10% AHI) of the time vs. Ab alone, and Determine was always cost-effective vs Ab alone. When comparing the three AHI algorithms, Early Test was cost-effective 100% of the time vs. Architect, 99.9% (24% AHI) and 92.4% (10% AHI) of the time vs. Determine, while Architect was cost-effective 96.3% (24% AHI) and 74.1% (10% AHI) of the time vs. Determine. In all six comparisons of the per-partner analysis those algorithms that were more sensitive for AHI diagnoses (i.e. detect more AHI) were cost-effective ≥ 99.9% of the time vs. those algorithms that were less sensitive for AHI (i.e. detect less AHI; Figure 1).
Discussion
We compared four different community based HIV testing strategies to estimate cost per infection (i.e. transmission) averted, and found that all three algorithms that detect AHI were cost-effective (i.e. one IA costs less than the lifetime medical costs of one HIV infection), when compared to testing relying on Ab testing alone. Among the algorithms that detect AHI, the Early Test algorithm was cost-effective vs. both other algorithms, with Architect being the second best alternative. Cost-effectiveness was established not only among at risk MSM in San Diego, but also among other MSM populations with similar HIV prevalence but lower proportions of AHI diagnoses. By accounting for parameter uncertainty, sensitivity analysis showed that cost-effectiveness of algorithms that detect AHI vs. Ab testing alone is likely to hold over a wide range of parameter values.
While this study indicates that AHI testing is cost-effective among MSM undergoing community-based screening in San Diego and other similar MSM populations in the United States, a previous cost analysis conducted in 2008 found that pooled NAT screening for AHI following negative third-generation antibody or rapid tests was not cost-effective for unselected municipal sexually transmitted diseases clinics and testing and counseling populations [32, 33]. Interestingly, that study used a very low AHI rate of 0.02% for determining cost-effectiveness [32]. Assuming a 10% proportion of AHI, this would relate to a per-test HIV positivity rate of 0.2% (the national HIV prevalence rate is 0.6%). In contrast, the AHI rate of 0.7% evaluated for determining cost-effectiveness in this study is not only more than 10 times higher than the AHI rate used in that previous study, but also in line with AHI rates reported previously for high risk individuals and MSM [30, 31]. While differing study populations may be the main explanation for differing findings [34], other factors such as lower costs for AHI tests in 2014 when compared to 2008 may provide additional explanation.
Costs per IA by algorithms that detect AHI vs. Ab testing alone stayed below $30,000 in the base model, and costs per IA by the Early Test (i.e. the most sensitive algorithm for AHI, but also the most expensive algorithm) vs. the other two algorithms that detect AHI were below $40,000. Costs per IA were therefore markedly below most recently published estimated medical costs saved by avoiding one single HIV infection [i.e updated costs in 2014 US dollars between $236,948 and $348,927 depending on the time point of diagnosis and ART initiation [15]], and also markedly below prior estimations of these costs [14]. Among MSM, community based HIV testing with algorithms that detect AHI was therefore clearly cost effective. Our results further indicate that the most sensitive and most expensive AHI testing algorithm - based on NAT testing - was cost-effective versus the two other (less sensitive and less expensive) AHI testing algorithms. Probabilistic sensitivity analyses indicated, however, that the latter finding may be more uncertain in other MSM settings with lower AHI proportions.
Our study has several limitations. First, calculations were based on 3,000 tests per year with 2.9% HIV prevalence and proportions of 24% and 10% AHI cases among all newly diagnosed HIV cases. The magnitude of effects will vary in other settings with differing numbers of annual tests/proportion of AHI diagnoses. Second, calculations of per-contact and per-partner transmission risk, which formed the basis for calculations of costs per IA, were – at least in part – based on assumptions and utilization of previously published data from other MSM cohorts. Although we did our best to prevent overestimation of transmission risk by choosing conservative approaches, we can’t rule out that real world transmission risks may have been lower. When AHI algorithms were compared to the Ab alone algorithm, however, cost-effectiveness per IA was met by quite a margin (costs per IA below $30,000 for all comparisons of the base model, costs might have been more than eight times higher and still below the cost-effectiveness threshold). We therefore argue that it is unlikely that differing approaches would have changed our outcome. Further, this model was based on the assumption that HIV transmission will drop to zero for the year after AHI diagnosis. While it has been shown previously that risk behavior decreases after diagnosis [5, 11], and early ART may substantially decrease HIV transmission [9, 10], the risk for HIV transmission after AHI diagnosis may still differ in other settings/populations. Our model also focused only on direct transmission occurring from individuals with missed AHI diagnoses and did not account for increased transmission risk during the AHI phase, which may have led to an underestimation of IA. Costs of very early ART were not included in the model, as early ART independent of infection stage (or CD4 cell count) is the standard of care in the United States [9, 10]. Further, the number of annual CIAI contacts in the per contact analysis (i.e. n=41) was derived from a cohort study in Australian MSM and it remains unclear if this number is a realistic estimate also for MSM in the United States. Finally, this analysis was built on a previous cost model, and additional limitations published previously for that model [12] may apply to the current model.
In conclusion, our analysis showed that AHI screening was cost-effective in preventing new HIV infections not only among at risk MSM in San Diego, but also among other MSM populations with similar HIV prevalence (i.e. 2.9%) and lower proportions of AHI diagnoses. The results indicate that community based AHI testing among MSM in the United States can pay for itself over the long run. When comparing the three algorithms that detect AHI, those algorithms that were more sensitive for AHI were cost effective despite higher costs.
Supplementary Material
Highlights.
Cost-effectiveness of community-based acute HIV infection (AHI) testing was evaluated.
The analysis focused on men who have sex with men in San Diego, United States.
Data sources included actual cost and prevalence data from San Diego, and published studies.
Community-based AHI testing screening was cost-effective in preventing new HIV infections.
Also in other MSM populations AHI testing may be preferable over HIV antibody testing alone.
Community based AHI testing among MSM in the United States may pay for itself over the long run.
Acknowledgments
Funding
This work was supported by funds from the following: the Max Kade Foundation, New York (Max Kade Postdoctoral Research grant), Interdisciplicinary Research Fellowship in NeuroAIDS (R25-MH081482), Developmental grant from the UC San Diego Center for AIDS Research (NIAID 5 P30 AI036214), TMARC pilot study (P50DA026306), and grants from the National Institutes of Health (AI043638, AI074621, AI106039, MH100974, and AI108351). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest
Dr. Hoenigl served on the speakers’ bureau of Merck. Dr. Little reported grant funding from Gilead Sciences, Inc. All other authors no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Facente SN, Pilcher CD, Hartogensis WE, Klausner JD, Philip SS, Louie B, et al. Performance of risk-based criteria for targeting acute HIV screening in San Francisco. PLoS One. 2011;6:e21813. doi: 10.1371/journal.pone.0021813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilcher CD, Fiscus SA, Nguyen TQ, Foust E, Wolf L, Williams D, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med. 2005;352:1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 3.Oxenius A, Price DA, Easterbrook PJ, O’Callaghan CA, Kelleher AD, Whelan JA, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci U S A. 2000;97:3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanna AS, Goodreau SM, Gorbach PM, Daar E, Little SJ. Modeling the impact of post-diagnosis behavior change on HIV prevalence in Southern California men who have sex with men (MSM) AIDS Behav. 2014;18:1523–1531. doi: 10.1007/s10461-013-0646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianella S, von Wyl V, Fischer M, Niederoest B, Battegay M, Bernasconi E, et al. Effect of early antiretroviral therapy during primary HIV-1 infection on cell-associated HIV-1 DNA and plasma HIV-1 RNA. Antivir Ther. 2011;16:535–545. doi: 10.3851/IMP1776. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Gay CL, Busch MP, Hecht FM. The detection of acute HIV infection. J Infect Dis. 2010;202(Suppl 2):S270–7. doi: 10.1086/655651. [DOI] [PubMed] [Google Scholar]
- 9.Ambrosioni J, Nicolas D, Sued O, Aguero F, Manzardo C, Miro JM. Update on antiretroviral treatment during primary HIV infection. Expert Rev Anti Infect Ther. 2014;12:793–807. doi: 10.1586/14787210.2014.913981. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MS, Smith MK, Muessig KE, Hallett TB, Powers KA, Kashuba AD. Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: where do we go from here? Lancet. 2013;382:1515–1524. doi: 10.1016/S0140-6736(13)61998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox J, White PJ, Macdonald N, Weber J, McClure M, Fidler S, et al. Reductions in HIV transmission risk behaviour following diagnosis of primary HIV infection: a cohort of high-risk men who have sex with men. HIV Med. 2009;10:432–438. doi: 10.1111/j.1468-1293.2009.00708.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoenigl M, Graff-Zivin J, Little SJ. Costs per Diagnosis of Acute HIV Infection in Community-based Screening Strategies: A Comparative Analysis of Four Screening Algorithms. Clin Infect Dis. 2016;62:501–511. doi: 10.1093/cid/civ912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farnham PG, Sansom SL, Hutchinson AB. How much should we pay for a new HIV diagnosis? A mathematical model of HIV screening in US clinical settings. Med Decis Making. 2012;32:459–469. doi: 10.1177/0272989X11431609. [DOI] [PubMed] [Google Scholar]
- 14.Farnham PG, Gopalappa C, Sansom SL, Hutchinson AB, Brooks JT, Weidle PJ, et al. Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr. 2013;64:183–189. doi: 10.1097/QAI.0b013e3182973966. [DOI] [PubMed] [Google Scholar]
- 15.Schackman BR, Fleishman JA, Su AE, Berkowitz BK, Moore RD, Walensky RP, et al. The Lifetime Medical Cost Savings From Preventing HIV in the United States. Med Care. 2015;53:293–301. doi: 10.1097/MLR.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoenigl M, Chaillon A, Moore DJ, Morris SR, Smith DM, Little SJ. Clear Links Between Starting Methamphetamine and Increasing Sexual Risk Behavior: A Cohort Study Among Men Who Have Sex With Men. J Acquir Immune Defic Syndr. 2016;71:551–7. doi: 10.1097/QAI.0000000000000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoenigl M, Chaillon A, Morris SR, Little SJ. HIV Infection R ates and Risk Behavior among Young Men undergoing community-based Testing in San Diego. Sci Rep. 2016;6:25927. doi: 10.1038/srep25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoenigl M, Green N, Camacho M, Gianella S, Mehta SR, Smth DM, et al. Signs or Symptoms of Acute HIV Infection in a Cohort Undergoing Community-Based Screening. Emerg Infect Dis. 2016;22:532–4. doi: 10.3201/eid2203.151607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoenigl M, Anderson CM, Green N, Mehta SR, Smith DM, Little SJ. Repeat HIV-testing is associated with an increase in behavioral risk among men who have sex with men: a cohort study. BMC Med. 2015;13 doi: 10.1186/s12916-015-0458-5. 218-015-0458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoenigl M, Weibel N, Mehta SR, Anderson CM, Jenks J, Green N, et al. Development and Validation of the San Diego Early Test (SDET) Score to Predict Acute and Early HIV Infection Risk in Men who have Sex with Men. Clin Infect Dis. 2015;61:468–75. doi: 10.1093/cid/civ335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan PS, Salazar L, Buchbinder S, Sanchez TH. Estimating the proportion of HIV transmissions from main sex partners among men who have sex with men in five US cities. AIDS. 2009;23:1153–1162. doi: 10.1097/QAD.0b013e32832baa34. [DOI] [PubMed] [Google Scholar]
- 22.Healthy Living Project Team. Effects of a behavioral intervention to reduce risk of transmission among people living with HIV: the healthy living project randomized controlled study. J Acquir Immune Defic Syndr. 2007;44:213–221. doi: 10.1097/QAI.0b013e31802c0cae. [DOI] [PubMed] [Google Scholar]
- 23.Jin F, Jansson J, Law M, Prestage GP, Zablotska I, Imrie JC, et al. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS. 2010;24:907–913. doi: 10.1097/QAD.0b013e3283372d90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin F, Prestage GP, Mao L, Poynten IM, Templeton DJ, Grulich AE, et al. “Any Condomless Anal Intercourse” is No Longer an Accurate Measure of HIV Sexual risk Behavior in Gay and Other Men Who have Sex with Men. Front Immunol. 2015;6:86. doi: 10.3389/fimmu.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott HM, Vittinghoff E, Irvin R, Sachdev D, Liu A, Gurwith M, et al. Age, race/ethnicity, and behavioral risk factors associated with per contact risk of HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2014;65:115–121. doi: 10.1097/QAI.0b013e3182a98bae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509–1519. doi: 10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero-Severson EO, Alam SJ, Volz E, Koopman J. Acute-stage transmission of HIV: effect of volatile contact rates. Epidemiology. 2013;24:516–521. doi: 10.1097/EDE.0b013e318294802e. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Zhong L, Romero-Severson E, Alam SJ, Henry CJ, Volz EM, et al. Episodic HIV Risk Behavior Can Greatly Amplify HIV Prevalence and the Fraction of Transmissions from Acute HIV Infection. Stat Commun Infect Dis. 2012;4:1041. doi: 10.1515/1948-4690.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39:1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel P, Klausner JD, Bacon OM, Liska S, Taylor M, Gonzalez A, et al. Detection of acute HIV infections in high-risk patients in California. J Acquir Immune Defic Syndr. 2006;42:75–79. doi: 10.1097/01.qai.0000218363.21088.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stekler J, Swenson PD, Wood RW, Handsfield HH, Golden MR. Targeted screening for primary HIV infection through pooled HIV-RNA testing in men who have sex with men. AIDS. 2005;19:1323–1325. doi: 10.1097/01.aids.0000180105.73264.81. [DOI] [PubMed] [Google Scholar]
- 32.Hutchinson AB, Patel P, Sansom SL, Farnham PG, Sullivan TJ, Bennett B, et al. Cost-effectiveness of pooled nucleic acid amplification testing for acute HIV infection after third-generation HIV antibody screening and rapid testing in the United States: a comparison of three public health settings. PLoS Med. 2010;7:e1000342. doi: 10.1371/journal.pmed.1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel P, Mackellar D, Simmons P, Uniyal A, Gallagher K, Bennett B, et al. Detecting acute human immunodeficiency virus infection using 3 different screening immunoassays and nucleic acid amplification testing for human immunodeficiency virus RNA, 2006–2008. Arch Intern Med. 2010;170:66–74. doi: 10.1001/archinternmed.2009.445. [DOI] [PubMed] [Google Scholar]
- 34.Fitzpatrick F, Kminski G, Jones L, Drudy E, Crean M. Use of a fourth generation HIV assay for routine screening – The first year’s experience. Journal of Infection. 2006;53:415–6. doi: 10.1016/j.jinf.2006.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.