Abstract
The wide use of pyrethroids has resulted in the emergence and spread of resistance in mosquito populations, which represent a major obstacle in the struggle against vector-borne diseases. Resistance to pyrethroids is a complex genetic phenomenon attributed by polygenetic inheritance. We previously have sequenced and analyzed the miRNA profiles of Culex pipiens pallens. MiR-92a was found to be overexpressed in a deltamethrin-resistant (DR) strain. The association of miR-92a with pyrethroid-resistance was investigated by quantitative reverse transcription PCR (qRT-PCR). Expression levels of miR-92a were 2.72-fold higher in the DR strain than in the deltamethrin-susceptible (DS) strain. Bioinformatic analysis suggested that CpCPR4, a mosquito cuticle gene, is the target of miR-92a. Dual luciferase reporter assays further confirmed that CpCPR4 is modulated by miR-92a through binding to a specific target site in the 3′ untranslated region (3′ UTR). Microinjection of the miR-92a inhibitor upregulated CpCPR4 expression levels, leading to an increase in the susceptibility of the DR strain in the Centers for Disease Control and Prevention (CDC) bottle bioassay (a surveillance tool for detecting resistance to insecticides in vector populations). Taken together, our findings indicate that miR-92a regulates pyrethroid-resistance through its interaction with CpCPR4.
Keywords: CpCPR4, Culex pipiens pallens, miR-92a, pyrethroid-resistance
1. Introduction
Mosquitoes transmit many of the world’s most devastating human diseases, such as malaria, dengue fever, yellow fever and West Nile fever, which constitute an enormous public health burden (Gendrin et al. 2015; Hegde et al. 2015; LaDeau et al. 2015). Effective methods for the control of mosquitoes, which are the vector for these diseases, are urgently required. Chemical control is efficient and convenient for large-scale application (Marimuthu et al. 2012). Pyrethroids, which are associated with low toxicity in humans and rapid eradication of insects, are commonly used to control mosquitoes by application to bed nets and indoor spraying (Nauen 2007; White et al. 2014). However, after more than 70 years of chemical pesticide use, resistances to pyrethroids has become wide spread, representing the major obstacle to control the mosquito-borne diseases (Benelli 2015; Siegwart et al. 2015). Resistance to pyrethroids is a complex genetic phenomenon driven by polygenetic inheritance (Zou et al, 2015). Multiple resistance mechanisms have been found in individual mosquito species. In particular, extensive investigation of the metabolic resistance and target-site insensitivity mechanisms has indicated that resistance may arise from improved detoxification or through reduced sensitivity of target proteins (Liu 2015). A role for cuticular changes in conferring resistance has yet to be characterized. Genomic, proteomic, and transcriptomic studies have facilitated the identification of numerous insect cuticle proteins (ICPs) related to resistance (Dittmer et al. 2012; Futahashi et al. 2008). However, the mechanism by which cuticular proteins contribute to resistance remains largely unknown. One class of endogenous small RNAs, known as microRNAs (miRNAs), are the key regulators of gene expression. By base pairing of the miRNA “seed” with complementary sites within the 3′ UTR of mRNA, these molecules function at the post-transcriptional level to play important roles in translational inhibition and mRNA decay (Djuranovic et al. 2012; Drayton et al. 2012; Lucas & Raikhel 2013). These small RNAs have functions in regulation of a wide range of cellular pathways that affect several biological processes such as development, survival, metabolism and the immune system (Asgari 2014). Recently, many studies have indicated the involvement of miRNAs in pyrethroid-resistance (Hong et al. 2014; Lei et al. 2015). Studying miRNA function in the mosquito may provide an improved understanding of pyrethroid-resistance and pave the way toward the utilization of these small molecules as novel control approaches.
In a previous study, miR-92a was found to be expressed at high levels of in a deltamethrin-resistant (DR) strain (Hong et al. 2014), suggesting the involvement of miR-92a might in pyrethroid-resistance. In this study, we conducted a functional characterization of miR-92a in mosquitoes to establish an interaction between miR-92a and its target, the cuticular protein gene, CpCPR4. The results of this study provides evidences that the interactions between miR-92a and CpCPR4, affect pyrethroid-resistance in mosquitoes.
2. MATERIALS AND METHODS
2.1 Mosquito strains
Two Culexpipiens pallens strains were used in this study. The deltamethrin-susceptible (DS) strain was collected from Tang Kou, Shan Dong Province, and then reared in our laboratory without exposure to any insecticides. The DR-strain was selected from the DS strain by exposure to deltamethrin (obtained from Institute of Nanjing General Hospital of Nanjing Military Region) for more than 60 generations. The 50% larval lethal concentrations (LC50) of DS and DR strains were 0.04 mg/L and 7.3 mg/L, respectively. Both strains were maintained on 10% glucose solution at 28–30°C and 70–80% humidity, respectively, with a 16/8 h day-night light cycle.
2.2 Total RNA extraction and real-time quantitative PCR
Total RNA was extracted from 15 adult female mosquitoes collected from each group at three days post-eclosion (PE) using RNAiso Plus reagent (TaKaRa, Dalian, China). The quality of the total RNA was assessed by 1% denaturing agarose gel electrophoresis. cDNA was synthesized from 500 ng total RNA with PrimeScript™ RT Master Mix (Perfect Real-time) (TaKaRa) according to the manufacturer’s protocol. The reaction mixture was incubated at 37°C for 15 min followed by 85°C for 5 s. The reverse transcription primers are shown in Table 1. qRT-PCR was performed on the ABI Prism 7300 HT Sequence Detection system (Applied Biosystem, Foster City, CA, USA) using FastStart® SYBR Green Master (ROX) (Roche, Swiss) according to the manufacturer’s protocol. Reactions were incubated in a 96-well optical plate at 50°C for 2 min and 95°C for 10 min and followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Melting curves were determined immediately after the PCR and the data were analyzed using the 7300 System SDS Software v1.2.1 (Applied Biosystems). The raw threshold cycle (Ct) values of miR-92a and CpCPR4 were normalized against the Ct values of U6 and β-actin, respectively. These values were then used to calculate relative expression levels in the samples using the 2−ΔΔCt method (Livak et al. 2001). The primers used in this experiment are shown in Table 1. The qRT-PCR analysis was performed three times using independent purified RNA samples and P values were calculated by Student’s t-test.
Table 1.
Oligonucleotide primers used in qRT-PCR and PCR
| Name | Sequence |
|---|---|
| MiR-92a SL | 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGATAGGCCG-3′ |
| U6 F | 5′-GCTTCGGCTGGACATATACTAAAAT-3′ |
| U6 R | 5′-GAACGCTTCACGATTTTGCG-3′ |
| MiR-92a F | 5′-ACACTCCAGCTGGGTATTGCACTTGTCCCG-3′ |
| URP | 5′-TGGTGTCGTGGAGTCG-3′ |
| β-actin F | 5′-CGCTTCCTCGTCTACACTGG-3′ |
| β-actin R | 5′-GTGTTGGCGAACAGATCCTT-3′ |
| CpCPR4 qPCR F | 5′TCTTACTCCCTGGTTGAGCCCGA-3′ |
| CpCPR4 qPCR R | 5′-AGTGGCTCACGGTGGACAACAG-3′ |
F: Forward; R: Reverse
2.3 Target gene prediction
To determine the function of miR-92a, we attempted to use the method for target gene prediction described by Allen (Allen et al. 2005). However, the use of computational prediction tools to predict targets for miRNAs proved problematic due to the lack of 3′ UTR sequences in the Cx. quinquefasciatus database. In a previous study, we observed differential expression of cuticular genes in susceptible versus resistant Cx. pipiens pallens strains (Fang et al. 2015). Among these, the 3′ UTR sequence of CpCPR4 was found to contain a complete complementary miR-92a target site of based on transcripts from Cx. quinquefasciatus.
2.4 Vectors construction
The CpCPR4 3′ UTR sequence was amplified by PCR from cDNAs of 3d PE female mosquitoes using the following conditions: denaturation at 95°C for 30 s followed by 35 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s with a final extension at 72°C for 10 min. The following primers were used: forward primer, 5′-CGACGCGTCACCCGTCGCGTCGTTGAGT-3′; reverse primer, 5′-CCAATCATCAGGACATCGTGCAATAAGCTTGGG-3′. For CpCPR4 mutagenesis, the 3′ UTR sequence (GTGCAAT) complementary to the binding site for the miR-92a seed sequence was replaced by ATTCGAC. The wild-type and mutant 3′ UTR sequences of CpCPR4 were both cloned into the pMIR-REPORT™ miRNA Expression Reporter Vector (Ambion, US) using the Hind III and Mlu I sites. The pMIR-CpCPR4-UTR and pMIR-CpCPR4-MUT constructs were validated by sequencing.
2.5 Cell transfection and dual luciferase reporter assays
The HEK-293 cell line (derived from human embryonic kidney) was obtained from the American Type Culture Collection (ATCC) and cultured with the Dulbeccos’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bone serum (FBS) (Gibco, Australia) as recommended by the ATCC. Cells (4×105 cells/well) were seeded into 6-well plates 24 h before transfection. The sequences of the miR-92a mimic and Negative Control 1(NC1) are shown in Table 2, both were designed and procured from GenePharma (GenePharma, Shanghai, China). The cells were divided into four groups: pMIR-CpCPR4-UTR + miR-92a mimic + PGL; pMIR-CpCPR4-UTR + NC1 + PGL; pMIR-CpCPR4-MUT + miR-92a mimic + PGL; pMIR-CpCPR4-MUT + NC1 + PGL. Cells were transfected with 0.4 μg reporter construct and 100 nM miRNA mimics per well using FuGENER® HD Transfection Reagent (Promega, WI, USA). The solutions were mixed gently and incubated at room temperature for 20 min. The cells were lysed after 48 h in 1×Passive Lysis Buffer (Promega), and the activity of the firefly and Renilla luciferases was determined using the dual luciferase reporter assay system (Promega) according to the manufacturer’s instructions.
Table 2.
Sequence of miR-92a mimic, inhibitor and negative control
| Mimic | 5′-UAUUGCACUUGUCCCGGCCUAU-3′ |
| NC1 | 5′-UUCUCCGAACGUGUCACGUTT-3′ |
| Inhibitor | 5′-UCAGGACAUCGUGCAA-3′ |
| NC2 | 5′-CAGUACUUUUGUGUAGUACAA-3′ |
NC: Negative control
2.6 Microinjection of DS-strain female mosquitoes
The sequences of the miR-92a inhibitor and Negative Control 2 (NC2) are shown in Table 2, both were designed and procured from GenePharma. A Nanoject IIAuto-Nanoliter Injecto (Drummond, USA) was used to introduce 69 nL solution (inhibitor, 7.5 μM; NC2, 7.5 μM) into the thorax of cold-anesthetized DS-strain female mosquitoes at 24 h PE. The control group was injected 69 nL water pretreated with diethylpyrocarbonate (DEPC-treated water) at the same time. After microinjection, mosquitoes were immediately placed into small plastic tubes and were allowed to recover at 28–30°C and 70%–80% humidity with 16/8 h day-night light cycle, with access to with 10% glucose solution. After 72 h of recovery, mosquitoes were selected for qRT-PCR and subsequent experiments.
2.7 The Centers for Disease Control and Prevention Centers (CDC) bottle bioassay
The CDC bottle bioassay was used as a surveillance tool for detecting resistance to insecticides in vector populations (Aizoun et al. 2013; William & Adeline 2015). The recommended dosage of deltamethrin did not cause 100% mortality of susceptible mosquitoes in our first bioassay; therefore, we increased the diagnostic dose in this study to 15 mg per bottle (250 mL). Deltamethrin (1 mL at 15 mg/mL) dissolved in acetone was smeared evenly in each bottle; one empty bottle smeared with an equivalent volume of acetone only was used as the control. The treated bottles were placed in a dark and ventilated environment for almost 3 h to ensure that the bottles were completely dry. Approximately 20 female mosquitoes were placed into each bottle 72h after microinjection, and the mortality of the mosquitoes was recorded every 15 min for 2 h.
2.8 Statistical analysis
Data represent the Student’s t test (Fig. 1, Fig. 2, Fig. 3A; all values represent the mean ± SD) and Chi-square analysis (Fig. 3B) of three independent experiments. Any P-value of <0.05 is considered statistically significant.
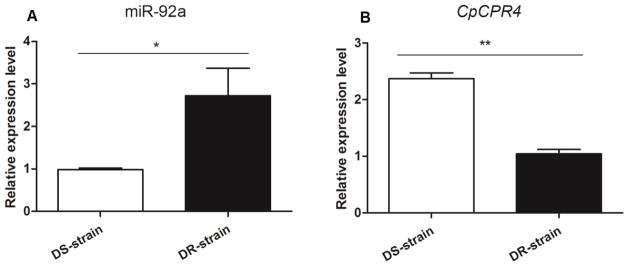
Figure 1.
Expression levels of miR-92a and CpCPR4 in the DS and DR strains of Cx. pipiens pallens (A) MiR-92a. (B) CpCPR4. The expression level of U6/β-actin in the same template is considered as background level or 1, and the expression level of miR-92a/CpCPR4 was shown as the relative value against U6/β-actin expression. Data represent the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared with the DS strain.
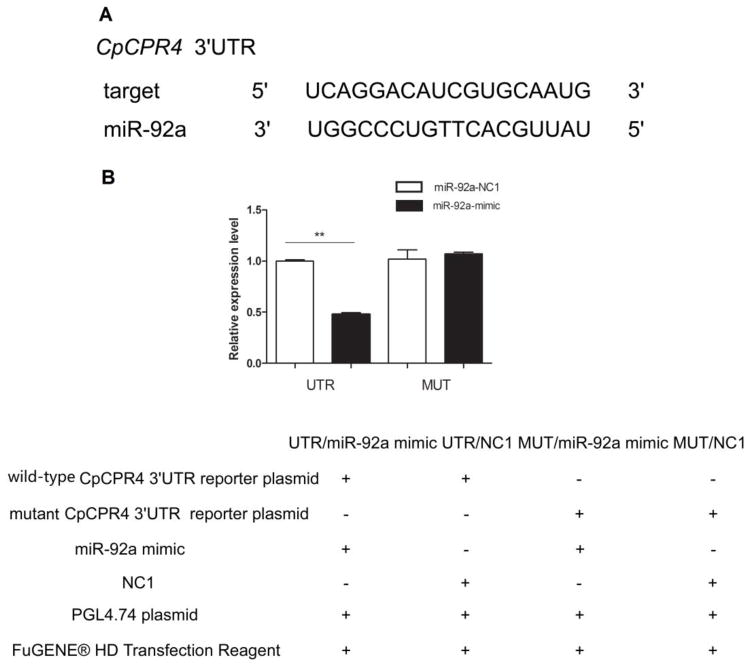
Figure 2.
Interaction between miR-92a and CpCPR4 revealed through a dual luciferase reporter assay (A) Base pairing between the seed sequence and the 3′UTR of CpCPR4. (B) Luciferase activity was decreased to 48% compared with the control after miR-92a overexpression. Data represent the mean ± SD of three independent experiments. **P<0.01.
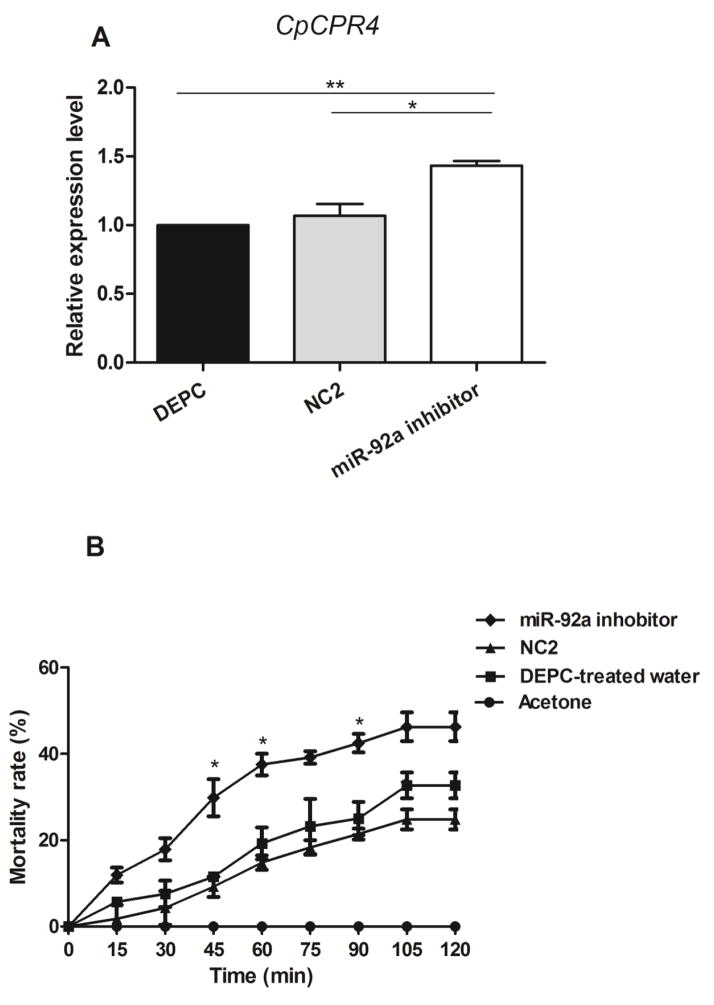
Figure 3.
Microinjection of the miR-92a inhibitor into female adult mosquitoes and the American CDC contact bottle mortality test (A) qRT-PCR analysis of CpCPR4 expression after microinjection. Compared with the NC2 and DEPC-treated water groups, the CpCPR4 target gene was overexpressed by 34.3% and 43.3%, respectively. (B) Mortality of microinjected mosquitoes observed during 2 h of exposure to CDC bottles treated with deltamethrin (15 mg/ml). Compared with NC2 group, the mortality in the miR-92a inhibitor group was significantly increased, especially at 45, 60 and 90 mi. There was no significant difference between the DEPC and NC2 microinjection groups. A: Data represent the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01. B: Data represent the Chi-square analysis of three independent experiments. *P < 0.05.
3. RESULTS
3.1 Differential expression of miR-92a is observed in the DS and DR strains
To explore the role of miRNAs in pyrethroid-resistance in Cx. pipiens pallens, we examined 228 differentially expressed miRNAs in the DS and DR strains (Hong et al. 2014). In this study, we performed a qRT-PCR analysis of the relative of miR-92a expression levels in total RNA samples obtained from female DS and DR strain mosquitoes at 3 days PE. Significantly higher expression in the DR strain indicated that miR-92a plays an important role in events associated with the mechanisms of pyrethroid-resistance in Cx. pipiens pallens (2.37-fold, P < 0.05; Fig. 1A).
3.2 MiR-92a regulates CpCPR4
The functions of miRNAs are mediated through imperfect base-paring to target seed region sites in the 3′ UTR of mRNAs resulting in translational inhibition or mRNA degradation (Lucas & Raikhel 2013). Based on the Cx. Quinquefasciatus sequence, we predicted the miR-92a target gene using miRNA seed regions binding to the 3′ UTR of mRNAs. In a previous study, we observed differential expression of cuticular genes in susceptible versus resistant Cx. pipiens pallens strains (Fang et al. 2015). Among these, qRT-PCR analysis showed that CpCPR4 was up-regulated in the DS-strain (2.72-fold, P < 0.01; Fig. 1B) and the 3′ UTR sequence of CpCPR4 was found to contain a complete complementary miR-92a target site (Fig. 2A). We used a dual fluorescent reporter system to verify that miR-92a regulates CpCPR4 by directly binding to the 3′ UTR of the gene, with a miRNA mimic that functions as an endogenously expressed miRNA. The PGL4.74 plasmid served as an internal control for evaluation of the efficiency of transfection using the FuGENE® HD Transfection Reagent (Mottahedin et al. 2013). Specific-miR-92a targeting resulted in CpCPR4 degradation. As predicted, the miR-92a mimic significantly decreased luciferase activity to approximately 48% in the CpCPR4 UTR PMIR-reporter vector, compared to the negative mimic control (Fig. 2B). This result indicated that miR-92a directly regulates CpCPR4 in vitro. We then generated a reporter construct containing a mutant of the miR-92a binding site to confirm whether miR-92a specifically inhibited CpCPR4 by binding to the seed sequence. In this case, miR-92a overexpression did not affect the CpCPR4 MUT PMIR-reporter vector activity (Fig. 2B). These results confirmed that CpCPR4 was an authentic target of miR-92a that binds specifically at the seed sequence.
3.3 MiR-92a regulates deltamethrin-resistant by negatively modulating CpCPR4 expression in female mosquitoes
To evaluate the authenticity of CpCPR4 as a miR-92a target in vivo, we performed qRT-PCR analysis of the CpCPR4 transcript levels in females injected with the miR-92a inhibitor, NC2 and DEPC-treated water. Compared with the levels detected in the NC2 and DEPC-treated water groups, CpCPR4 was enriched significantly in female mosquitoes following microinjection of the miR-92a inhibitor (34.3% (P < 0.05) and 43.3% (P < 0.01), respectively (Fig. 3A).
In CDC bottle bioassay performed 72 h after microinjection, the mortality of the miR-92a inhibitor group was dramatically higher than that in the DEPC-treated water and NC2 groups, with significant differences detected at 45, 60 and 90 min (P < 0.05; Fig. 3B). Together, these findings suggest that miR-92a-depletion in female mosquito results in an increase in CpCPR4 expression, which could increase the susceptibility of the DR strain mosquitoes to deltamethrin.
4. DISCUSSION
Our results show that depletion of the conserved miRNA, miR-92a, increasing susceptibility to deltamethrin in mosquito. This study represents a significant step toward defining the regulatory roles of miRNAs in pyrethroid-resistance in mosquito. Using microinjection and the CDC bottle bioassay, we have demonstrated that miR-92a functions as a regulator of pyrethroid-resistance events in mosquitoes by negatively modulating CpCPR4 expression.
Next, miRNA target prediction was conducted to identify the physiologically relevant miR-92a target contributing to the miR-92a-depletion phenotypes. We identified CpCPR4 as a direct target of miR-92a both in vitro and in vivo. A dual luciferase reporter assay using a luciferase reporter vector containing Cx. pipiens pallens CpCPR4 3′ UTR cotransfected with the miR-92a mimic resulted in a decrease in Renilla luciferase activity in vitro. In accordance with this, miR-92a-depletion increased the expression of CpCPR4. These results further confirmed CpCPR4 as an authentic miR-92a target in mosquitoes. The small endogenous RNAs named miRNAs (approximately 22 nt) play important regulatory roles (Bartel, 2004). Typically, the miRNA “seed sequence” (nucleotides 2–8 at the 5′ end) binds to complementary seed match sites within the 3′ UTR of mRNAs, resulting in translational inhibition and/or mRNA degradation. In the 1990s, miRNAs were first discovered in Caenorhabditis elegans and shown to be involved in the timed regulation of developmental events (Lucas & Raikhel 2013). To date, thousands of potential miRNAs have been identified in various organisms and played significantly functional roles of gene regulation in animals, plants, fungi and viruses (Vincent & Wegst 2004). In insects, miRNAs appear to be involved in regulation of a wide range of cellular pathways that affect biological processes such as development, the immune system, survival, metabolism and host-pathogen interactions (Asgari 2014). Furthermore, some reports have shown that miRNAs are related to resistance to insecticides. For instance, miR-278-3p is involved in the regulation of the pyrethroid-resistance in mosquitoes by decreasing CYP6AG11 expression (Lei et al. 2015). In this study, we found that miR-92a acts as a regulator of the mosquito cuticle gene, CpCPR4, in pyrethroid-resistance. CpCPR4 is a component of insect cuticle proteins (ICPs), which are structural proteins that form the insect cuticle. Together with chitin, the cuticle serves as a barrier to the external environment (Papandreou et al. 2010). In recent years, the rapid development of advanced biological technologies and transcriptomic and proteomic analyses of sensitive and resistant insects have revealed differential expression of ICPs, such as CPR, CPLC, and CPLCG subfamily members (Awolola et al. 2009; Nkya et al. 2014; Reid et al. 2012; Silva et al. 2012). In Anopheles gambiae, temporal and spatial differences in transcript abundance and protein localization mean that the structural cuticular proteins account for only about 2% of its protein coding genes. Furthermore, the location of CPLCG3/4, one of the components known to be involved in insecticide resistance, in the endocuticle can contribute to the thickness of the cuticle (Vannini et al. 2014). Similarly, in mosquitoes, it is possible that thicker cuticles lead to slower rates of pyrethroid absorption, which is likely to increase the efficiency of metabolic detoxification (Ahmad et al. 2006; Wood et al. 2010). Previous studies have also shown that CpCPR4 expressed differently with other cuticle protein genes in Cx. pipiens pallens. Allen et al. found that CpCPR4 may function together with other cuticular protein genes that contribute to Cx. pipiens pallens pyrethroid-resistance (Allen et al. 2005). Moreover, following the development of pyrethroid-resistance, clarification of the mechanism of cuticle resistance in mosquitoes is increasingly imperative for identification of markers of resistance and the development of novel strategies to control mosquito disease vectors.
In summary, our findings have established a fundamental role for miR-92a and its target gene CpCPR4 in pyrethroid-resistance of mosquitoes. Further investigation of the cuticular protein gene CpCPR4, in insecticide resistance in mosquitoes is warranted to determine its exact function in the regulation of insecticide resistance and in identification of markers of resistance.
Acknowledgments
This work was supported by the National Institutes of Health of US (NIH) [Grant no. 2R01AI075746], the National Natural Science Foundation of China [Grant nos. 81171610, 81101279 and 81301458], the National S & T Major Program [Grant nos. 2012ZX10004-219 and 2012ZX10004-220], and the Natural Science Foundation of Jiangsu Province, China [Grant no. 81101279].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad M, Denholm I, Bromilow RH. Delayed cuticular penetration and enhanced metabolism of deltamethrin in pyrethroid resistant strains of Helicoverpa armigera from China and Pakistan. Pest Manag Sci. 2006;62(9):805–10. doi: 10.1002/ps.1225. [DOI] [PubMed] [Google Scholar]
- Allen E, Xie Z, Gustafson AM, Carrington JC. MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell. 2005;121(2):207–21. doi: 10.1016/j.cell.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Aïzoun N, Ossé R, Azondekon R, Alia R, Oussou O, Gnanguenon V, Aikpon R, Padonou GG, Akogbéto M. Comparison of the standard WHO susceptibility tests and the CDC bottle bioassay for the determination of insecticide susceptibility in malaria vectors and their correlation with biochemical and molecular biology assays in Benin, West Africa. Parasite Vectors. 2013;6:147. doi: 10.1186/1756-3305-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari S. Role of microRNAs in Arbovirus/Vector Interactions. Viruses. 2014;6(9):3514–34. doi: 10.3390/v6093514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awolola TS, Oduola OA, Strode C, Koekemoer LL, Brooke B, Ranson H. Evidence of multiple pyrethroid resistance mechanisms in the malaria vector Anopheles gambiae sensu stricto from Nigeria. Trans R Soc Trop Med Hyg. 2009;103(11):1139–45. doi: 10.1016/j.trstmh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Benelli G. Research in mosquito control: current challenges for a brighter future. Parasitol Res. 2015;114(8):2801–5. doi: 10.1007/s00436-015-4586-9. [DOI] [PubMed] [Google Scholar]
- Dittmer NT, Hiromasa Y, Tomich JM, Lu N, Beeman RW, Kramer KJ, Kanost MR. Proteomic and transcriptomic analyses of rigid and membranous cuticles and epidermis from the elytra and hindwings of the red flour beetle, Tribolium castaneum. J Proteome Res. 2012;11(1):269–78. doi: 10.1021/pr2009803. [DOI] [PubMed] [Google Scholar]
- Djuranovic S, Nahvi A, Green R. MiRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336(6078):237–40. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton RM. The role of microRNA in the response to cisplatin treatment. Biochem Soc Trans. 2012;40(4):821–5. doi: 10.1042/BST20120055. [DOI] [PubMed] [Google Scholar]
- Futahashi R, Okamoto S, Kawasaki H, Zhong YS, Iwanaga M, Mita K, Fujiwara H. Genome-wide identification of cuticular protein genes in the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2008;38(12):1138–46. doi: 10.1016/j.ibmb.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Fang Fujin, Wang Weijie, Zhang Donghui, Lv Y, Zhou D, Ma L, Shen B, Sun Y, Zhu C. The cuticle proteins: a putative role for deltamethrin resistance in Culex pipiens pallens. Parasitol Res. 2015;114(12):4421–9. doi: 10.1007/s00436-015-4683-9. [DOI] [PubMed] [Google Scholar]
- Gendrin M, Rodgers FH, Yerbanga RS, Ouédraogo JB, Basáñez MG, Cohuet A, Christophides GK. Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nat Commun. 2015;6:5921. doi: 10.1038/ncomms6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S, Rasgon JL, Hughes GL. The microbiome modulates arbovirus transmission in mosquitoes. Curr Opin Virol. 2015;15:97–102. doi: 10.1016/j.coviro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Guo Q, Wang W, Hu S, Fang F, Lv Y, Yu J, Zou F, Lei Z, Ma K, Ma L, Zhou D, Sun Y, Zhang D, Shen B, Zhu C. Identification of differentially expressed microRNAs in Culex pipiens and their potential roles in pyrethroid resistance. Insect Biochem Mol Biol. 2014;55C:39–50. doi: 10.1016/j.ibmb.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDeau SL, Allan BF, Leisnham PT, Levy MZ. The ecological foundations of transmission potential and vector-borne disease in urban landscapes. Funct Ecol. 2015;29:889–901. doi: 10.1111/1365-2435.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas K, Raikhel AS. Insect microRNAs: Biogenesis, expression profiling and biological functions. Insect Biochem Mol Biol. 2013;43(1):24–38. doi: 10.1016/j.ibmb.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Liu N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu Rev Entomol. 2015;60:537–59. doi: 10.1146/annurev-ento-010814-020828. [DOI] [PubMed] [Google Scholar]
- Marimuthu G, Rajamohan S, Mohan R, Krishnamoorthy Y. Larvicidal and ovicidal properties of leaf and seed extracts of Delonix elata (L.) Gamble (family: Fabaceae) against malaria (Anopheles stephensi Liston) and dengue (Aedes aegypti Linn.) (Diptera: Culicidae) vector mosquitoes. Parasitol Res. 2012;111(1):65–77. doi: 10.1007/s00436-011-2802-9. [DOI] [PubMed] [Google Scholar]
- Mottahedin A, Paidikondala M, Cholleti H, Baule C. NF-kappa B activation by equine arteritis virus is MyD88 dependent and promotes viral replication. Arch Virol. 2013;158(3):701–5. doi: 10.1007/s00705-012-1515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag Sci. 2007;63(7):628–33. doi: 10.1002/ps.1406. [DOI] [PubMed] [Google Scholar]
- Nkya TE, Poupardin R, Laporte F, Akhouayri I, Mosha F, Magesa S, Kisinza W, David JP. Impact of agriculture on the selection of insecticide resistance in the malaria vector Anopheles gambiae: a multigenerational study in controlled conditions. Parasit Vectors. 2014;7:480. doi: 10.1186/s13071-014-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou NC, Iconomidou VA, Willis JH, Hamodrakas SJ. A possible structural model of members of the CPF family of cuticular proteins implicating binding to components other than chitin. J Insect Physiol. 2010;56(10):1420–6. doi: 10.1016/j.jinsphys.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid WR, Zhang L, Liu F, Liu N. The transcriptome profile of the mosquito Culex quinquefasciatus following permethrin selection. PLoS ONE. 2012;7(10):e47163. doi: 10.1371/journal.pone.0047163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AX, Jander G, Samaniego H, Ramsey JS, Figueroa CC. Insecticide resistance mechanisms in the green peach aphid Myzus persicae (Hemiptera: Aphididae) I: A transcriptomic survey. PLoS ONE. 2012;7(6):e36366. doi: 10.1371/journal.pone.0036366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegwart M, Graillot B, Blachere Lopez C, Besse S, Bardin M, Nicot PC, Lopez-Ferber M. Resistance to bio-insecticides or how to enhance their sustainability: a review. Front Plant Sci. 2015;6:381. doi: 10.3389/fpls.2015.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JF, Wegst UG. Design and mechanical properties of insect cuticle. Arthropod Struct Dev. 2004;33(3):187–99. doi: 10.1016/j.asd.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Vannini L, Reed TW, Willis JH. Temporal and spatial expression of cuticular proteins of Anopheles gambiae implicated in insecticide resistance or differentiation of M/S incipient species. Parasit Vectors. 2014;7:24. doi: 10.1186/1756-3305-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MT, Lwetoijera D, Marshall J, Caron-Lormier G, Bohan DA, Denholm I, Devine GJ. Negative cross resistance mediated by co-treated bed nets: a potential means of restoring pyrethroid-susceptibility to malaria vectors. PLoS One. 2014;9(5):e95640. doi: 10.1371/journal.pone.0095640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood O, Hanrahan S, Coetzee M, Koekemoer L, Brooke B. Cuticle thickening associated with pyrethroid resistance in the major malaria vector Anopheles funestus. Parasit Vectors. 2010;3:67. doi: 10.1186/1756-3305-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William G, Chan Adeline. Guideline for Evaluating Insecticide Resistance in Vectors Using the CDC Bottle Bioassay. 2015 ( http://www.cdc.gov/malaria/resources/pdf/fsp/ir_manual/ir_cdc_bioassay_en.pdf)
- Zou F, Chen C, Zhong D, Shen B, Zhang D, Guo Q, Wang W, Yu J, Lv Y, Lei Z, Ma K, Ma L, Zhu C, Yan G. Identification of QTLs Conferring Resistance to Deltamethrin in Culex pipiens pallens. PLoS One. 2015;10(10):e0140923. doi: 10.1371/journal.pone.0140923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Zhentao, Yuan Lv, Wang Weijie, Guo Q, Zou F, Hu S, Fang F, Tian M, Liu B, Liu X, Ma K, Ma L, Zhou D, Zhang D, Sun Y, Shen B, Zhu C. MiR-278-3p regulates pyrethroid resistance in Culex pipiens pallens. Parasitol Res. 2015;114:699–706. doi: 10.1007/s00436-014-4236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]