Abstract
Background
Decreased kidney function and greater albuminuria are associated with increased incidence and extent of coronary artery calcium (CAC). We investigated whether the associations between kidney function and urine protein-to-creatinine ratio (Uprcr) with CAC differ by HIV serostatus.
Methods
Using data from the Multicenter AIDS Cohort Study (MACS), a prospective multicenter U.S. study of men who have sex with men, we conducted a cross-sectional study comprised of 592 HIV-infected (HIV+) and 378 uninfected (HIV−) men who underwent non-contrast computerized tomography to measure CAC. Logistic and linear regression models were used to determine whether HIV infection modified associations of estimated glomerular filtration rate (eGFR) and Uprcr with the presence and extent of CAC, adjusting for age, race and cardiovascular risk factors.
Results
Every 10-unit decrease in eGFR below 90 ml/min/1.73m2 was significantly associated with 1.3-fold (95% CI: 1.06, 1.51) higher odds of CAC presence and was similar by HIV serostatus (p-interaction=0.37). Greater Uprcr was associated with more extensive CAC, with a change in log CAC score of 0.32 [95% CI: 0.10 – 0.55] per 1% increase in Uprcr. There was a strong trend for effect modification by HIV serostatus for this association (HIV−: 0.75 [0.26, 1.25]; HIV+ 0.22 [−0.03, 0.47], p-interaction=0.06).
Conclusion
Greater CAC burden is apparent among persons with early kidney disease, regardless of HIV serostatus. Increased Uprcr is associated with a greater extent of CAC with a trend for differences by HIV serostatus; a clearer proteinuria/CAC extent relationship was apparent among HIV− persons.
BACKGROUND
Coronary artery calcium (CAC) represents evidence of atherosclerotic plaque and predicts risk for both myocardial infarction and death from cardiovascular disease.i Decreased kidney function has been associated with both incidence and extent of CAC.ii,iii In fact, chronic kidney disease (CKD) is an independent predictor of cardiovascular events. Emerging evidence in the general population suggests that persons with pre-dialysis CKD are more likely to die from cardiovascular disease (CVD) than from kidney disease.iv Additionally, albuminuria, an indicator of endothelial dysfunction, has been shown to be associated both with incident CAC in a large population-based cohort studyv and an independent predictor of cardiovascular mortality across diverse, human immunodeficiency virus (HIV)- uninfected populations.vi
HIV infection is associated with increased risk of acute myocardial infarctionvii and CAC progression.viii CKD is an important complication of HIV infection and occurs in up to 30% of infected patients.ix Using data from Multicenter AIDS Cohort Study (MACS) participants, we sought to investigate whether associations exist between kidney function and urine protein-to-creatinine ratio (Uprcr) with CAC, and whether these associations differ by HIV serostatus.
METHODS
Population
The Multicenter AIDS Cohort Study (MACS) is an ongoing prospective cohort study of the natural and treated history of HIV-1-infected men who have sex with men (MSM). From 1984 – 2003, 6,972 MSM were enrolled at four sites: in Baltimore, Chicago, Pittsburgh and Los Angeles.x The cohort includes both HIV –infected (HIV+) and –uninfected (HIV−) men who attend semiannual study visits that include standardized interviews, physical examinations, and blood and urine collection for laboratory testing and storage in local and central repositories.
Eligibility for the MACS cardiovascular ancillary study included being an active MACS participant, age 40–70 years, weight <300 lbs, and no prior history of cardiac surgery or percutaneous coronary intervention. All participants underwent non-contrast cardiac CT scanning for CAC scoring. The study was approved by the Institutional Review Boards of all participating sites. All participants signed informed consent.
CT Scanning and Analysis Procedures
Aspects of the non-contrast cardiac CT scanning procedures have been previously described.xi The CT scanning equipment included a 64-slice multi-detector CT at 3 centers and a 320-row multi-detector CT at a fourth center. Images were transferred to the core CT reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA) and were analyzed by trained, experienced readers who were blinded to participant characteristics and HIV serostatus. Calcification was quantified as previously described by Agatston et al.xii
Clinical Parameters
During semi-annual MACS visits, data were collected regarding coronary artery disease (CAD) risk factors and HIV clinical parameters by history, physical examination and blood tests. Glomerular filtration rate (eGFR, in ml/min/1.73 m2) was estimated using the CKD-Epi equation,xiii and proteinuria was estimated using a urine protein-to-creatinine ratio (Uprcr) measured from a spot urine sample. For the eGFR and Uprcr, we used the average value from all visits within 1.5 years prior to the CT scan.
For the covariates, we used the value available from the visit closest to the CT scan, generally within 6 months. Race/ethnicity was based on self-report. Glucose, insulin, total and high-density lipoprotein (HDL) cholesterol, and triglycerides were measured from fasting blood samples. For 15 men who did not provide fasting blood samples, total cholesterol and HDL were measured from non-fasting samples. Low density lipoprotein cholesterol (LDL) was calculated using the Friedewald equation or measured directly in persons with triglycerides > 400 mg/dL or with non-fasting samples. Hypertension was defined as systolic blood pressure (BP) ≥140 mm Hg, diastolic BP ≥90 mm Hg, or self-reported use of anti-hypertensive medication among men with a history of hypertension. Diabetes mellitus was defined as a fasting serum glucose ≥ 126 mg/dL or use of medications to treat diabetes among men with a history of diabetes. Measures of HIV disease activity included standardized testing of plasma HIV RNA levelsxiv (Roche ultrasensitive assay with 50 copies/mL limit of detection)xv, CD4+ T-lymphocyte cell counts (CD4)xvi, and history of an AIDS-defining malignancy or opportunistic infection confirmed with medical record review. Also considered was current use of the HIV antiretrovirals abacavir or tenofovir disoproxil fumarate (tenofovir), as well as duration of highly active anti-retroviral therapy (HAART) use based on self-reported information.
Statistical Analyses
The distributions of demographic and clinical factors by HIV serostatus were compared using the Wilcoxon rank-sum test for continuous variables and the Pearson chi-square test for categorical variables.
Multiple logistic and linear regressions were used to evaluate associations of eGFR and Uprcr with the (1) presence of CAC defined as a score above 0 and; (2) extent of CAC among men with a score above 0, respectively. The calcification score was natural-log transformed to normalize its distribution in analyses evaluating the extent of plaque. The effects of the exposure variables were described using piecewise linear splines for eGFR < 90 and ≥ 90 ml/min/1.73m2 (eGFR≥ 90 ml/min/1.73m2 signifies normal kidney function)xvii, and the natural logarithm of Uprcr. To investigate whether these effects were modified by HIV infection, interaction terms between the exposure variables and HIV serostatus were assessed. Non-significant interaction terms were subsequently dropped from the model to obtain the overall effect of exposure variables that correspond to the dropped interaction effects.
All models were initially adjusted for age and race, and then further adjusted for CVD risk factors (body mass index, systolic BP, use of hypertension medications, use of diabetes medications, fasting glucose, total and HDL cholesterol, use of lipid lowering medications and pack-years of tobacco smoking).
Multiple imputation was performed by HIV serostatus on missing CVD risk factor data for the analyses. The imputation models included the outcome, all predictors and auxiliary variables (tobacco use and post CT-scan use of lipid medications) that were strongly correlated with predictors which had the highest % of missing data. HIV-related factors (use of tenofovir or abacavir, history of AIDS, detectable plasma HIV RNA level, duration of HAART, CD4 nadir and current CD4) were also included in the imputation model for HIV+ men. Ten imputed data sets were produced for each HIV+ and HIV− participant using a Markov Chain Monte Carlo (MCMC) method assuming multivariate normality. Values for the following numbers of men were missing and imputed for multiple regression analyses- hypertension medications (6), body mass index (9), diabetes medications (10), smoking pack-years (29), lipid medications (22), total and HDL cholesterol (22), systolic blood pressure (6) and fasting glucose (21).
All significance tests were at the 0.05 level and all analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
The 592 HIV+ and 378 HIV− men significantly differed by age, race, and risk factors for CVD (Table 1). The mean eGFR was similar among HIV− (88±14 [IQR: 78.9, 98.1] ml/min/1.73m2) and HIV+ (88±18 [IQR: 75.6, 102.0] ml/min/1.73m2) men (p=0.37). Median Uprcr was higher among HIV+ men compared with HIV− men (105.7 [IQR: 76.7, 158.3] versus 67.5 [IQR: 55.0, 87.0]; p<0.0001). CAC prevalence was also similar between the 2 groups; 52.4% of HIV+ men had CAC compared with 51.8% of HIV− men (p=0.88).
Table 1.
Characteristics of Study Population
| HIV Seropositive | HIV Seronegative | p-value* | |
|---|---|---|---|
| N | 592 | 378 | |
| Age (years) | 52.5 ± 6.6 | 55.2 ± 7.4 | <0.0001 |
| Race | <0.0001 | ||
| Caucasian (%) | 52.2 | 67.5 | |
| African-American (%) | 34.5 | 24.3 | |
| Hispanic/Other (%) | 13.3 | 8.2 | |
| Hypertension (%) | 48.8 | 43.0 | 0.08 |
| Systolic blood pressure (mm Hg) | 126.7 ± 15.1 | 128.0 ± 14.7 | 0.23 |
| Hypertension medications (%) | 36.0 | 30.7 | 0.09 |
| Diabetes (%) | 12.9 | 9.0 | 0.07 |
| Diabetes medications (%) | 8.6 | 6.9 | 0.36 |
| Tobacco use | 0.0054 | ||
| Never smoker (%) | 25.4 | 25.3 | |
| Current smoker (%) | 30.6 | 21.8 | |
| Former smoker (%) | 44.0 | 52.9 | |
| Smoking pack-years** | 5.9 (0–22.9) | 1.5 (0–21.9) | 0.02 |
| Body Mass Index (kg/m2) | 26.1±4.5 | 27.4±4.8 | <0.0001 |
| Glucose (mg/dL) | 103.3 ± 27.2 | 101.0 ± 32.3 | 0.01 |
| Total Cholesterol (mg/dL) | 186.7±42.7 | 192.5±36.8 | 0.01 |
| LDL Cholesterol (mg/dL) | 106.5±44.2 | 114.4±32.7 | <0.0001 |
| HDL Cholesterol (mg/dL) | 48.4±16.1 | 53.3±15.4 | <0.0001 |
| Triglycerides (mg/dL) | 176.2±202.1 | 123.6±71.7 | <0.0001 |
| Lipid lowering medications (%) | 34.7 | 29.8 | 0.12 |
| Serum Creatinine (mg/dL) | 1.07±0.41 | 1.01±0.19 | 0.07 |
| GFR (CKD-Epi) (ml/min/1.73 m2) | 88±18 | 88±14 | 0.37 |
| Upcr (mg/g creatinine)** | 105.7(76.7–158.3) | 67.5 (55.0–87.0) | <0.0001 |
| Coronary Artery Calcium Present: Agatston Score > 0 (%) | 52.4 | 51.8 | 0.88 |
| Coronary Artery Calcium Score** among those with calcium present (HIV+: 310, HIV−: 196) | 68 (21–191) | 79 (22–242) | 0.36 |
| HIV clinical factors | N=592 | ||
| Current HIV RNA undetectable, < 50 copies/mL (%) | 81.9 | ||
| Current HIV RNA (copies/mL)**# | 723 (139–16400) | ||
| Current CD4+ T-cell count (cells/mm3)** | 599 (424–766) | ||
| CD4+ T-cell count nadir (cells/mm3)** | 284 (172–400) | ||
| HAART experienced (%) | 95.8 | ||
| Duration of HAART (years)** | 9.4 (6.3–12.4) | ||
| Abacavir (%) | 18.8 | ||
| Tenofovir (%) | 68.0 | ||
| History of AIDS (%) | 14.2 |
Data are reported as mean ± standard error or percentage.
Unadjusted p-value comparing seropositive and seronegative values.
Median (interquartile range: 25%–75%) for non-normally distributed variables.
Among 107 HIV+ men with detectable current HIV RNA (>50 copies/mL) levels.
Figure 1 compares the coefficient estimates adjusted for age and race between HIV− and HIV+ men. Associations of eGFR and Uprcr with prevalence and extent of CAC are in the same direction for both groups, with the exception of that between eGFR above 90 ml/min/1.73m2 and CAC extent. However, these associations are generally stronger among HIV− men compared to HIV+ men. This trend persists and estimates were practically the same after adjusting further for CVD risk factors (Tables 2 and 3). Overall, adjusting for demographic and CAD risk factors, each 10-unit decrease in eGFR below 90 ml/min/1.73m2 was significantly associated with 1.3-fold (95% CI: 1.06, 1.51) greater odds of CAC prevalence (Agatston score greater than 0). Odds ratio estimates among HIV+ and HIV− men were 1.21 (95% CI: 0.99, 1.49) and 1.45 (95% CI: 1.04, 2.01), respectively, and were not significantly different (p=0.37).
Figure 1. Association of coronary artery calcium with proteinuria and eGFR levels, by HIV serostatus, among men in the MACS.
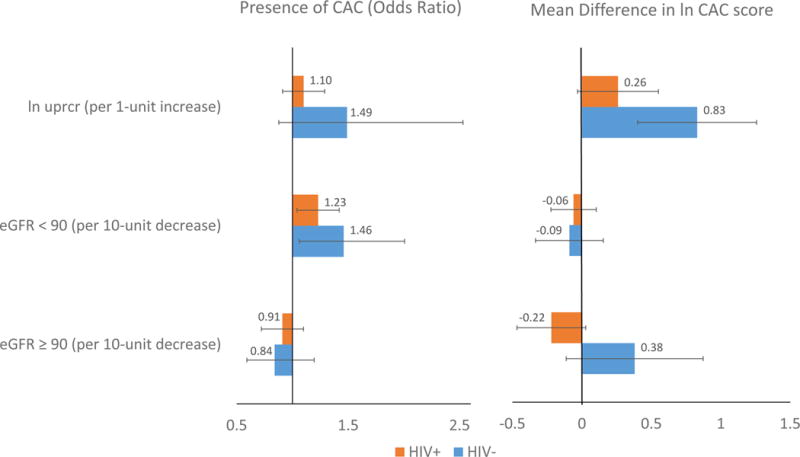
The left panel shows the prevalence and 95% confidence interval of CAC>0 associated with a one unit increase in Uprcr, a ten unit decrease in eGFR among participants with eGFR<90, and a ten unit decrease in eGFR among participants with eGFR >=90 respectively, adjusting for age and race. The right panel shows the extent of CAC (Agatston score) associated with a one unit increase in Uprcr, a ten unit decrease in eGFR among participants with eGFR<90, and a ten unit decrease in eGFR among participants with eGFR >=90 respectively, adjusting for age and race. Abbreviations: eGFR, estimated glomerular filtration rate; MACS, Multicenter AIDS Cohort Study; CAC, coronary artery calcium; Uprcr, urine protein-to-creatinine ratio
Table 2.
Associations between kidney function and presence of coronary artery calcium by HIV infection status. Shown are the results from the fully adjusted multiple logistic regression model* (N=970).
| Exposure | HIV− (N=598) | HIV+ (N=372) | HIV− vs. HIV+ |
|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | P-valuea | |
| eGFR ≥ 90b (per 10-unit decrease) | 0.86 (0.60, 1.25) | 0.89 (0.70, 1.14) | 0.87 |
| eGFR < 90b (per 10-unit decrease) | 1.45 (1.04, 2.01) | 1.21 (0.99, 1.49) | 0.37 |
| Log Uprcr (per 1-unit increase) | 1.37 (0.79, 2.39) | 1.06 (0.81, 1.38) | 0.40 |
Adjusted for age, race and CAD risk factors (body mass index, systolic BP, use of hypertension medications, use of diabetes medications, fasting glucose, total and HDL cholesterol, use of lipid lowering medications and pack-years of tobacco smoking).
p-interaction
ml/min/1.73m2
Table 3.
Associations between kidney function and extent of coronary artery calcium by HIV infection status among those with coronary calcification. Shown are the results from the fully adjusted multiple linear regression model* (N=506).
| Exposure | HIV− (N=310) | HIV+ (N=196) | HIV− vs. HIV+ |
|---|---|---|---|
| Mean Differenceb (95% CI) | Mean Differenceb (95% CI) | P-valuea | |
| eGFR ≥ 90c (per 10-unit decrease) | 0.44 (0.02, 0.86) | −0.22 (−0.51, 0.07) | 0.01 |
| eGFR < 90c (per 10-unit decrease) | −0.08 (−0.32, 0.16) | −0.06 (−0.23, 0.10) | 0.92 |
| LN Uprcr (per 1-unit increase) | 0.75 (0.26, 1.25) | 0.22 (−0.03, 0.47) | 0.06 |
Adjusted for age, race and CAD risk factors (body mass index, systolic BP, use of hypertension medications, use of diabetes medications, fasting glucose, total and HDL cholesterol, use of lipid lowering medications and pack-years of tobacco smoking).
p-interaction
Mean difference in Log CAC score per change in exposure variable
ml/min/1.73m2
Bold indicates significant at 0.05 level
Among men with CAC present and eGFR above 90 ml/min/1.73m2, there was an inverse association between decreasing eGFR and greater extent of CAC in HIV− men [change in log CAC score per 10-unit decrease in eGFR, 0.44 (95% CI: 0.02, 0.86)], but no association in HIV+ men [−0.22 (95% CI: −0.51, 0.07)]. (p=0.01 for interaction by HIV serostatus).
Uprcr was not significantly associated with the prevalence of CAC. However, among men with CAC present, higher Uprcr values were associated with greater CAC extent with a 0.32 (95% CI: 0.10, 0.55) change in log CAC score per 1% increase in Uprcr. There was a strong trend for modification by HIV serostatus for this association. Among HIV− men there was a 0.75 (95% CI: 0.26, 1.25) change in log CAC score per 1% increase in Uprcr. Conversely, among HIV+ men, each 1% higher Uprcr was associated with a 0.22 (95% CI: −0.03, 0.47) change in log CAC score. The interaction by HIV serostatus, however, did not quite reach statistical significance (p=0.06).
DISCUSSION
Decreasing eGFR and CAC
In the MACS, we found that among HIV+ and HIV− men with eGFRs below 90 ml/min/1.73m2, the prevalence of CAC increased with lower eGFRs. To our knowledge, this is the largest population studied to date (n=970) in which increased CAC prevalence has been demonstrated in association with lower GFRs, even among persons with mild kidney dysfunction (i.e. eGFRs between 60 and 90 ml/min/1.73m2), and confirms the previous findings of Roy et al (544 patients)ii, Russo et al (140 patients)xviii, and Piers et al (83 patients)xix in smaller populations.
Our study prominently included many HIV+ persons, a group known to be at increased risk for both CVD and kidney disease. The inclusion of both HIV+ and HIV− men allowed us to investigate how HIV infection modifies the relationship between kidney disease and CAC presence and extent; we believe that ours is the first study to evaluate for such HIV serostatus-based differences.
Recent published data from the MACS suggest that HIV infection predisposes preferentially to non-calcified, rather than calcified, plaque,xx and accordingly we found no significant HIV serostatus-based differences in the associations between eGFR and CAC prevalence. HIV infection itself may increase the risk for cardiovascular disease through adverse effects from chronic inflammation, immune activation, and use of specific antiretroviral treatments that may increase risk for CVD by causing pro-atherogenic dyslipidemia, insulin resistance, and body habitus changes that are linked to coronary atherogenesisxxi. CKD on the other hand may lead to increased CAD by virtue of its pathogenic role in hypertensionxxii, dyslipidemiaxxiii, systemic inflammationxxiv,xxv, as well as altered calcium and phosphorous homeostasisxxvi. Our observation that impaired kidney function is a potential contributor to the increased prevalence of CAC among HIV+ persons, distinct from HIV itself, is novel.
Uprcr and CAC
Albuminuria a sign of endothelial dysfunction, is a known predictor of and marker for both CVD and CKD.xxvii Proteinuria, which includes albuminuria as well as other urinary proteins, was significantly associated with greater CAC extent in those with CAC present among our total study population. A prior study by Jassal et alxxviii undertaken in a smaller population of community-dwelling older adults (421 participants) did not demonstrate a correlation between albuminuria or eGFR and CAC severity or progression, but our study affirms a larger study (by Park et alxxix) involving 1318 asymptomatic, nonhypertensive, nondiabetic adults that showed a correlation between microalbuminuria and increased CAC.
Among HIV− individuals, proteinuria generally reflects albuminuria from endothelial dysfunctionxxx, while among HIV+ infected persons there are additional causes of proteinuria, include the pathognomonic HIV− associated nephropathy (HIVAN) caused by HIV itself as well as renal tubular dysfunction that may occur from exposure to certain antiretroviral medicationsxxxi. In subgroup analyses, we found a significant association between proteinuria and CAC extent among HIV− men, but not HIV+ men. While our differential findings by HIV serostatus did not quite reach statistical significance, which may be explained by lack of statistical power, they suggest the possibility that the clearer association between proteinuria and CAC extent among HIV− men reflects greater uniformity in the pathogenesis of proteinuria in this patient group- a group for whom the causes of proteinuria (largely albuminuria) like diabetes and hypertension are clearly associated with CVD. In contrast, causes of proteinuria among HIV+ persons are more diverse and include tubular proteinuria associated with specific antiretroviral medications use or other causes that are not clearly associated with cardiovascular disease, as shown by Sarnak et al.xxxii
Study Limitations
Roy et alii and Budoff et aliii previously showed that lower GFR among persons with CKD is associated with increased CAC extent. We also found significantly increased CAC extent with decreased eGFR among HIV− men without CKD. However, distinct from findings of these previous studies, in the current study we did not observe a greater extent of CAC in association with decreased GFR in men with CKD or in HIV+ men with eGFR≥ 90 ml/min/1.73m2.
These findings may reflect the fact that in the current study we studied a somewhat younger population with earlier stages of kidney dysfunction and an overall lower prevalence of CAC- factors that limited our ability to demonstrate an association between greater CAC extent and lower GFR. Among participants with preserved kidney function, perhaps there were not sufficient numbers of HIV+ persons to demonstrate increased CAC severity associated with decreased eGFR.
Proteinuria was not independently associated with increased CAC prevalence in our study population, in contrast to previously reported findings from a much larger patient population (6814 patients, Defilippis et alv). However, our population may not have been sufficiently large to demonstrate this correlation.
Since our study is comprised only of males, the generalizability of the findings to other risk groups may be limited. Furthermore, our data are based upon subclinical coronary disease, so our findings require correlation with clinical cardiovascular outcomes over time.
Conclusion
In the MACS, lower eGFR (below 90 ml/min/1.73m2) was significantly associated with increased CAC prevalence. Furthermore, proteinuria (increased Uprcr) was significantly associated with greater CAC extent; there were borderline significant differences by HIV serostatus for this observation with a clearer proteinuria/CAC extent relationship apparent for HIV− persons. The clinical significance of these findings requires further prospective study to demonstrate correlations between CKD, proteinuria, HIV infection and cardiovascular clinical events.
Acknowledgments
The authors thank Sabina Haberlen.
DISCLOSURE OF FUNDING
The MACS CVD2 study is funded by the National Heart, Lung and Blood Institute (NHLBI), R01 HL095129 (Post), with additional support from UL1 TR 001079 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers (Principal Investigators) at: Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1 TR 001079 (JHU CTSA) and UL1-TR000124 (Harbor-UCLA CTSA). Website located at http://www.statepi.jhsph.edu/macs/macs.html. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest to report.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- i.Budoff MJ, Gul KM. Expert review on coronary calcium. Vasc Health Risk Manag. 2008;4(2):315–24. doi: 10.2147/vhrm.s1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ii.Roy SK, Cespedes A, Li D, Choi TY, Budoff MJ. Mild and moderate pre-dialysis chronic kidney disease is associated with increased coronary artery calcium. Vasc Health Risk Manag. 2011;7:719–24. doi: 10.2147/VHRM.S24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- iii.Budoff MJ, Rader DJ, Reilly MP, Mohler ER, 3rd, Lash J, Yang W, Rosen L, Glenn M, Teal V, Feldman HI, CRIC Study Investigators Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) study. Am J Kidney Dis. 2011;58(4):519–26. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- iv.Schiffrin EL, Lipman ML, Mann JFE. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- v.DeFilippis AP, Kramer HJ, Katz R, Wong ND, Bertoni AG, Carr J, Budoff MJ, Blumenthal RS, Nasir K. Association between coronary artery calcification progression and microalbuminuria. JACC Cardiovasc Imaging. 2010;3(6):595–604. doi: 10.1016/j.jcmg.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vi.Chronic Kidney Disease Prognosis Consortium. Matsushita K, Van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vii.Freiberg MS, Chung-Chou HC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Goetz MB, Leaf D, Oursler KA, Rimland D, Barradas MR, Brown S, Gilbert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- viii.Volpe GE, Tang AM, Polak JF, Mangili A, Skinner SC, Wanke CA. Progression of carotid intima-media thickness and coronary artery calcium over 6 years in an HIV-infected cohort. J Acquir Immune Defic Syndr. 2013;64(1):51–7. doi: 10.1097/QAI.0b013e31829ed726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ix.Winston JA. HIV and CKD epidemiology. Adv Chronic Kidney Dis. 2010;17(1):19–25. doi: 10.1053/j.ackd.2009.08.006. [DOI] [PubMed] [Google Scholar]
- x.Kaslow RA, Ostrow DG, Detels R, et al. The multicenter AIDS cohort study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- xi.Hacioglu Y, Gupta M, Choi T-Y, George RT, Deible CR, Jacobson L, et al. Use of Cardiac CT Angiography Imaging in an Epidemiology Study - the Methodology of the Multicenter AIDS Cohort Study Cardiovascular Disease Substudy. Anadolu Kardiyol Derg. 2013;13(3):207–214. doi: 10.5152/akd.2013.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xii.Agatston AS, Janowitz WR, Hildner FJ, Zusmer MR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- xiii.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xiv.Schneider MF, Margolick JB, Jacobson LP, Reddy S, Martinez-Maza O, Munoz A. Improved estimation of the distribution of plasmaHIV-1 RNA in men receiving effective antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;59(4):389–92. doi: 10.1097/QAI.0b013e318246bfce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xv.Erali M, Hillyard DR. Evaluation of the ultrasensitive Roche Amplicor HIV-1 monitor assay for quantitation of human immunodeficiency virus type 1 RNA. J Clin Microbiol. 1999;37(3):792–5. doi: 10.1128/jcm.37.3.792-795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xvi.Hultin LE, Menendez FA, Hultin PM, Jamieson BD, O’Gorman MRG, Borowski L, Matud JL, Denny TN, Margolick JB. Assessing immunophenotyping performance: proficiency-validation for adopting improved flow cytometry methods. Cytometry Part B (Clinical Cytometry) 2007;72B:249–55. doi: 10.1002/cyto.b.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xvii.National Kidney Foundation. Glomerular Filtration Rate. http://www.kidney.org/atoz/content/gfr.cfm.
- xviii.Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE. Coronary artery calcification in patient with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44(6):1024–30. doi: 10.1053/j.ajkd.2004.07.022. [DOI] [PubMed] [Google Scholar]
- xix.Piers LH, Touw HR, Gansevoort R, Franssen CF, Oudkerk M, Zijlstra F, Tio RA. Relation of aortic valve and coronary artery calcium in patients with chronic kidney disease to the stage and etiology of the renal disease. Am J Cardiol. 2009;103(10):1473–7. doi: 10.1016/j.amjcard.2009.01.396. [DOI] [PubMed] [Google Scholar]
- xx.Post WS, Budoff M, Kingsley L, Palella FJ, Witt MD, Li X, George RT, Brown TT, Jacobson LP. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160(7):458–67. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxi.American Heart Association. HIV and Cardiovascular Disease (Heart Disease) http://www.heart.org/HEARTORG/Conditions/More/HIVandYourHeart/HIV-and-Cardiovascular-Disease-Heart-Disease_UCM_315419_Article.jsp.
- xxii.Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet. 2000;356:147–152. doi: 10.1016/S0140-6736(00)02456-9. [DOI] [PubMed] [Google Scholar]
- xxiii.Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290:F262–272. doi: 10.1152/ajprenal.00099.2005. [DOI] [PubMed] [Google Scholar]
- xxiv.Blake JK, Ridker PM. C-reactive protein and other inflammatory risk factors in acute coronary syndromes. J Am Coll Cardiol. 2003;42(6):1142–1143. doi: 10.1016/s0735-1097(02)02953-4. [DOI] [PubMed] [Google Scholar]
- xxv.Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- xxvi.Hage FD, Venkataraman R, Zoghbi GJ, Perry GJ, DeMattos AM, Iskandrian AE. The scope of coronary heart disease in patients with chronic kidney disease. J Am Coll Cardiol. 2009;53(23):2129–2140. doi: 10.1016/j.jacc.2009.02.047. [DOI] [PubMed] [Google Scholar]
- xxvii.Albuminuria. National Kidney Foundation. https://www.kidney.org/atoz/content/albuminuria.
- xxviii.Jassal SK, Chonchol M, Laughlin GA, Cummins KM, Smits G, Kramer CK, Ix JH, Barret-Connor E. Kidney function and progression of coronary artery calcium in community-dwelling older adults (from the Rancho Bernardo study) Am J Cardiol. 2012;110(10):1425–33. doi: 10.1016/j.amjcard.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxix.Park HE, Heo NJ, Kim M, Choi S. Significance of microabluminuria in relation to subclinical coronary atherosclerosis in asymptomatic nonhypertensive, nondiabetic subjects. J Korean Med Sci. 2013;28(3):409–14. doi: 10.3346/jkms.2013.28.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxx.Weir MR. Microalbuminuria and cardiovascular disease. CJASN. 2007;2(3):581–90. doi: 10.2215/CJN.03190906. [DOI] [PubMed] [Google Scholar]
- xxxi.Del Palacio M, RomeroS, Casado JL. Proximal tubular renal dysfunction or damage in HIV-infected patients. AIDS Rev. 2012;14(3):179–87. [PubMed] [Google Scholar]
- xxxii.Sarnak MJ, Katz R, Newman A, Harris T, Peralta CA, Devarajan P, Bennett MR, Fried L, Ix JH, Satterfield S, Simonsick EM, Parikh CR, Shlipak MG, for the Health ABC Study Association of urinary injury biomarkers with mortality and cardiovascular events. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013070713. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]


