Abstract
YKL-40, also called chitinase3-like-1 protein, is an inflammatory biomarker which has been associated with disease severity in inflammatory and malignant diseases, including acute myeloid leukemia (AML), multiple myeloma and lymphomas. The objective of the current study was to assess the prognostic value of pre-transplant recipient and donor plasma YKL-40 concentrations in patients with AML (n=624) or myelodysplastic syndrome (MDS) (n=157) treated with allogeneic hematopoietic cell transplantation (HCT). In recipients, the plasma YKL-40 concentrations were increased when the HCT-comorbidity index was ≥5 (p=0.028). There were no significant associations between plasma YKL-40 concentrations in recipients and any outcome measures. In donors with YKL-40 plasma concentrations above the age adjusted 95th percentile a trend towards increased grade II-IV acute graft versus host disease in recipients was observed (adjusted hazard ratio 1.39 (95% confidence interval 1.00–1.94), P=0.050), with no significant associations with overall survival, treatment-related mortality or relapse. In conclusion, our study shows that YKL-40 does not aid risk stratification of patients undergoing allogeneic HCT, but suggests that YKL-40 may aid donor selection when multiple, otherwise equal, donors are available.
Keywords: YKL-40, Chitinase-3-like-1 protein, inflammatory biomarker, allogeneic hematopoietic cell transplantation, graft versus host disease
INTRODUCTION
YKL-40 (chitinase-3-like-1 protein (CHI3L1)) is an acute phase reactant, which is mainly secreted by cancer cells1, vascular smooth muscle cells2, connective tissue cells3, and immune cells4,5.Increased levels have been associated with poor prognosis in patients with different types of cancer and inflammatory diseases1,3,6–16. YKL-40 plays a role in angiogenesis17,inflammation18 and its synthesis is stimulated by IL-619. Compared to CRP, which is secreted by hepatocytes as response to IL-6, YKL-40 originates from cells directly involved in the disease process and has a different tempero-spatial secretion profile20,21, and may therefore reflect disease activity more accurately.
We have previously investigated YKL-40 in HCT after nonmyeloablative conditioning22. Recipients with pre-transplant YKL-40 plasma concentration above the age adjusted 95th percentile had higher relapse-related mortality and lower progression free (PFS) and overall survival (OS), while recipients transplanted with donors with YKL-40 plasma concentration above the age adjusted 95th percentile had increased probability of experiencing grade 2–4 GVHD.
The objective of the current study was to validate the role of recipient and donor plasma YKL-40 as a prognostic biomarker in patients undergoing unrelated donor HCT for acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS).
Patients and methods
The study cohort consisted of 781 donor/ recipient pairs with AML or MDS undergoing allogeneic hematopoietic cell transplantation with bone marrow or granulocyte colony stimulating factor (G-CSF) mobilized peripheral blood stem cells (PBSC) from 7/8or 8/8 allele (HLA-A, B, C and DRB1) matched unrelated donors. Early stage disease was defined as AML in first complete remission or MDS with refractory anemia with or without ringed sideroblasts. Intermediate stage disease was defined as AML in second or subsequent complete remission or in first relapse. Advanced stage disease was defined as MDS subtype refractory anemia with excess blasts or in transformation, or MDS not otherwise specified. Transplantation demographics are shown in table 1. The median follow-up was 3.2 (range 0.4–5.1) years.
Table 1.
Transplantation demographics
| Variable | |
|---|---|
| Number of patients | 781 |
| Number of centers | 98 |
| Male patient gender | 401 (51) |
| Patient age, median (range), years | 50 (18–78) |
| 18–20 years | 28 (4) |
| 21–30 years | 91 (12) |
| 31–40 years | 105 (13) |
| 41–50 years | 174 (22) |
| 51–60 years | 248 (32) |
| >60 years | 135 (17) |
| Donor age, median (range), years | 31 (18–60) |
| Sex of donor/patient | |
| Female/male | 110 (14) |
| Other combinations | 669 (86) |
| Missing | 2 (<1) |
| Karnofsky prior to transplant ≥ 90 (only evaluable for 750 patients) | 500 (64) |
| CMV serostatus of donor/patient | |
| Negative/negative | 235 (30) |
| Other combinations | 529 (68) |
| Unknown | 17 (2) |
| HLA match (HLA-A, B, C, DRB1) | |
| 7/8 | 175 (22) |
| 8/8 | 606 (78) |
| Disease at transplant | |
| Acute myeloid leukemia | 624 (80) |
| Early | 439 (70) |
| Intermediate | 185 (30) |
| Myelodysplastic syndrome | 157 (20) |
| Early | 79 (50) |
| Advanced | 78 (50) |
| IPSS prior to transplant | |
| Low | 29 (18) |
| Intermediate 1 | 81 (52) |
| Intermediate 2 | 36 (23) |
| Unknown | 11 (7) |
| Graft Type | |
| Bone marrow | 136 (17) |
| Peripheral blood stem cells | 645 (83) |
| Conditioning regimen | |
| Myeloablative | 564 (72) |
| Busulphan based | 402 (71) |
| Total body irradiation based | 162 (29) |
| Reduced intensity | 217 (28) |
| Busulphan based | 126 (58) |
| Melphalan based | 41 (19) |
| Nonmyeloablative | 50 (23) |
| Graft-versus-host disease prophylaxis | |
| Calcinerin inhibitor + methotrexate ± other | 502 (64) |
| Calcinerin inhibitor ± other (no methotrexate) | 266 (34) |
| Other combinations | 13 (2) |
| Transplantation year | |
| 2008 | 159 (20) |
| 2009 | 361 (46) |
| 2010 | 261 (33) |
Values are number of cases with percents in parenthesis, unless otherwise specified.
Transplantations were facilitated through the National Marrow Donor Program (NMDP) and performed between 2008 and 2010. Data collection and analysis was performed under the auspices of the Center for International Blood and Marrow Transplant Research (CIBMTR). Pre-transplant donor and patient research samples were provided by the NMDP/CIBMTR Research Repository.
Observational studies conducted by the CIBMTR are performed in compliance with the privacy rule (HIPAA) as a Public Health Authority and in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review of the Institutional Review Boards (IRB) of the NMDP.
Outcomes
OS was defined as time from HCT to death from any cause. Treatment-related mortality (TRM) was defined as death in continuous remission from primary disease with relapse as a competing risk. Disease-free survival (DFS) was defined as time to death from any cause or relapse. Acute GVHD grades II-IV and III-IV were defined according to the Glucksberg scale23. Primary malignancy relapse was defined using CIBMTR criteria with death as a competing risk24.
YKL-40 plasma analysis
From the 781 included recipient/donor pairs plasma was available from all recipients and 576 donors. Plasma was prepared from EDTA-anticoagulated blood within 8 hours of sampling and stored at −80°C until YKL-40 analysis. YKL-40 concentrations are stable at room temperature in EDTA anticoagulated blood for up to 8 hours25. Plasma concentrations of YKL-40 were determined in duplicates using a commercial sandwich-type enzyme-linked immunosorbent assay (Quidel, Santa Clara, CA). The detection limit was 10 µg/L. The intra-assay coefficients of variations were 5% (at 40 µg/L), 4% (at 104 µg/L), and 4% (at 155 µg/L). The inter-assay coefficient of variation was <6%. Because YKL-40 levels vary with age, patients and donors were divided into subjects with normal plasma YKL-40 concentrations, i.e. equal to or below the age adjusted 95th percentile, and subjects with high YKL-40 concentrations, i.e. above the age adjusted 95th 26.
Statistics
The main variable, donor YKL-40 plasma concentration, was treated as a categorical variable with at or above versus below the age adjusted 95% percentile consistent with previous analyses in the transplant population. Chi-square or Fisher’s exact tests were used to compare frequencies for categorical variables, and ANOVA was used to compare means for continuous variables. Univariate probabilities for OS were calculated using the Kaplan-Meier estimator27. Comparison of survival curves was done using the log-rank test. The cumulative incidences of acute GVHD II-IV and TRM were calculated using a Taylor series linear approximation to estimate the variance28. Multivariate analysis was performed using Cox proportional hazards models. Only recipients and donors for whom complete data were available were included in the multivariate analyses, resulting in the inclusion of 745 recipients for testing the recipient plasma YKL-40 effect and 565 recipients for testing the donor plasma YKL-40 effect. First, we build preliminary multivariate models for OS, DFS, relapse, TRM, acute GVHD II-IV and III-IV. All the clinical variables were tested for affirmation of the proportional hazards assumption. Factors that violated the proportional hazards assumption were adjusted through stratification. Then a stepwise model-building procedure was used to develop models for each outcome with a threshold of 0.05 for both entry and retention in the model. Based on these preliminary models, the main variable was tested by forcing it into the models. Interactions between the main variable and the adjusted covariates were also tested at a significance level of 0.01, and no significant interactions were detected. SAS version9.3 (SAS Institute, Cary, NC) was used for all the analyses.
Results
Associations between plasma YKL-40 concentrations and pre-transplant variables
Table 2 shows associations between recipient mean plasma YKL-40 concentrations and pre-transplant variables, and the proportion of recipients with plasma YKL-40 concentrations above the age adjusted 95th percentile. Plasma was available from 781 recipients and 576 donors. The mean plasma YKL-40 concentration was 61 µg/L (range, 20–864) and correlated significantly with age both among donors (r2 = 0.03, p<0.001) and recipients (r2 = 0.11, p<0.001). No significant associations were observed between Karnofsky score and recipient mean plasma YKL-40 concentration (Table 2). However the proportion of recipients with plasma YKL-40 concentrations above the age adjusted 95th percentile was highest in the subset of recipients that had a Karnofsky score <90 (Karnofsky score < 90, n=72 (29%); Karnofsky ≥90, n=114 (23%); P=0.059). Mean plasma YKL-40 concentrations were similar in recipients with HCT-CI between 0–4, and only increased significantly in recipients with HCT-CI ≥5 (p=0.028). There were no differences in mean plasma YKL-40 concentrations or proportion of recipients with plasma YKL-40 above the age adjusted 95th percentile between recipients with AML or MDS (Table 2). No differences in YKL-40 plasma concentrations or proportion of patients with YKL-40 plasma concentration above the age adjusted 95th percentile were observed according to AML or MDS disease status, cytogenetics or international prognostic scoring system (IPSS) (Table 2).
Table 2.
Median plasma YKL-40 concentrations in 781 recipients and number and proportion of recipients with plasma YKL-40 over the age adjusted 95th percentile
| Variable | Plasma YKL-40 | ||||
|---|---|---|---|---|---|
| n (%) | Mean (range) µg/L |
P | >95th percentile n (%) |
P | |
| Karnofsky | 0.679 | 0.059 | |||
| ≥90 | 500 (64) | 110 (20–4644) | 114 (23) | ||
| <90 | 247 (32) | 117 (20–1152) | 72 (29) | ||
| Missing | 34 (4) | NA | NA | ||
| HCT-CI | 0.130 | 0.198 | |||
| 0 | 265 (34) | 106 (20–1446) | 55 (21) | ||
| 1–2 | 242 (31) | 105 (20–1176) | 63 (26) | ||
| 3–4 | 206 (26) | 106 (20–636) | 56 (27) | ||
| ≥5 | 68 (9) | 171 (20–4644) | 21 (31) | ||
| Disease | 0.911 | 0.471 | |||
| AML | 624 (80) | 111 (20–4644) | 152 (24) | ||
| MDS | 157 (20) | 113 (20–714) | 43 (27) | ||
| AML | |||||
| Disease status | 0.801 | 0.475 | |||
| Early | 439 (70) | 110 (20–4644) | 111 (34) | ||
| Intermediate | 185 (30) | 115 (20–1332) | 41 (28) | ||
| AML | |||||
| Cytogenetics | 0.630 | 0.061 | |||
| Favorable | 44 (7) | 70 (20–248) | 8 (20) | ||
| Intermediate | 431 (70) | 116 (20–4644) | 97 (29) | ||
| Poor | 143 (23) | 109 (20–1176) | 45 (46) | ||
| Missing | 6 (<1%) | NA | NA | ||
| MDS | |||||
| Disease status | 0.341 | 0.593 | |||
| Early | 79 (50) | 105 (20–624) | 20 (25) | ||
| Advanced | 78 (50) | 122 (20–714) | 23 (29) | ||
| Cytogenetics | 0.779 | 0.384 | |||
| Favorable | 55 (35) | 121 (20–714) | 18 (33) | ||
| Intermediate | 35 (22) | 110 (20–301) | 10 (29) | ||
| Poor | 65 (41) | 107 (20–630) | 14 (21) | ||
| Missing | 2 (1) | NA | NA | ||
| IPSS | 0.524 | 0.485 | |||
| Low | 29 (18) | 102 (20–299) | 9 (31) | ||
| Intermediate-1 | 81 (52) | 108 (20–714) | 19 (23) | ||
| Intermediate-2 | 36 (23) | 131 (20–630) | 36 (33) | ||
| Missing | 11 (7) | NA | NA | ||
HCT-CI, hematopoietic cell transplantation comorbidity index; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; IPSS, international prognostic scoring system.
Associations between plasma YKL-40 concentrations and outcome
Although plasma YKL-40 concentrations above the age adjusted 95thpercentile in donors or recipients in general were associated with hazard ratios >1 (Table 3), no significant associations between donor plasma YKL-40 concentrations above the age adjusted 95th percentile and grade III-IV acute GVHD, TRM, relapse, OS or DFS were observed (Table 3). However, a trend towards an association between donor plasma YKL-40 concentrations above the age adjusted 95th percentile and recipient grade II-IV acute GVHD (adjusted hazard ratio (HR) 1.39, 95% confidence interval 1.00–1.94, P=0.050 (Figure 1), was observed. When plasma YKL-40 concentrations were analyzed as a continuous value or dichotomized at the best cutpoint, conclusions were similar to the primary analysis (data not shown). The impact of extremely low plasma YKL-40 concentrations were also analyzed, however no significant associations were observed (data not shown).
Table 3.
Multivariate analyses of the association between recipient and donors with YKL-40 plasma concentrations at or above versus below the age adjusted 95% percentile.
| YKL-40 plasma concentration above the age adjusted 95% percentile |
|||||||
|---|---|---|---|---|---|---|---|
| Recipient | Donor | ||||||
| N | HR (95% CI) | P | N | HR (95% CI) | P | ||
| Overall survivala | 745 | 1.13 (0.89–1.43) | 0.320 | 565 | 1.30 (0.95–1.79) | 0.099 | |
| Disease free survivalb | 745 | 1.07 (0.85–1.35) | 0.573 | 565 | 1.13 (0.83–1.53) | 0.441 | |
| Relapsec | 745 | 1.13 (0.83–1.54) | 0.436 | 565 | 1.25 (0.84–1.86) | 0.277 | |
| Treatment related mortalityd | 745 | 0.98 (0.69–1.38) | 0.894 | 565 | 1.20 (0.75–1.94) | 0.447 | |
| Acute GVHD | |||||||
| Grade II–IVe | 745 | 1.09 (0.84–1.41) | 0.531 | 565 | 1.39 (1.00–1.94) | 0.050 | |
| Grade III–IVe | 745 | 0.87 (0.55–1.37) | 0.551 | 565 | 1.09 (0.62–1.93) | 0.760 | |
Patients who had the donor plasma YKL-40 concentration missing (n=205) were deemed ineligible for testing the donor plasma YKL-40 effect. In addition, 36 (or 11) patients were excluded in multivariate testing for recipient (or donor) plasma because of missing information on either the time-to-event endpoints or covariates that were adjusted in the models.
HR, hazard ratio; CI, confidence interval
: adjusted for patient age, patient BMI, disease status, HLA match, sex match, and conditioning regimen intensity.
: adjusted for patient age, patient BMI, cytogenetics, disease status, sex match, and conditioning regimen intensity.
: adjusted for patient age, cytogenetics, disease status, and conditioning regimen intensity.
: adjusted for patient BMI, disease, CMV match status, HLA match and conditioning regimen intensity.
: adjusted for disease, CMV match status, conditioning regimen and HLA match
Figure 1.
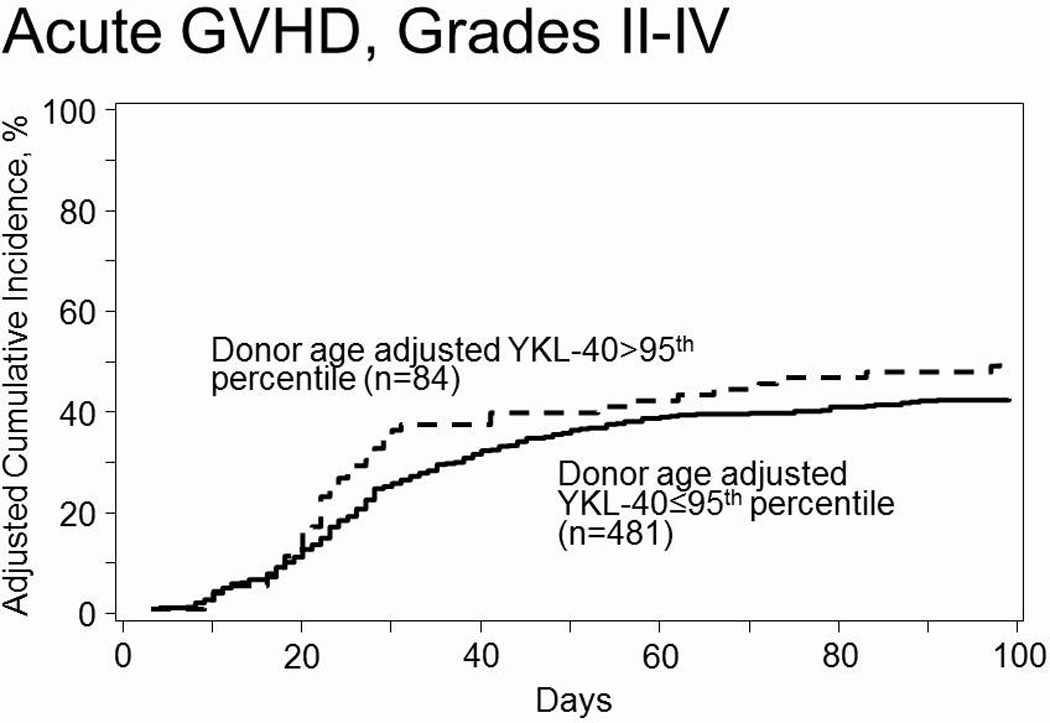
Discussion
In contrast to our previous study22 no significant associations between recipient plasma concentrations of YKL-40 and relapse related outcome measures were observed. The discrepancy can be explained by differences in diagnoses and remission status between patients included in the current study and the previous. Increased serum or plasma concentrations have been associated with disease severity in Hodgkin9 and non-Hodgkin lymphomas29,30 and in our previous study, approximately half of the recipients had a variety of lymphoid diseases in various stages of remission, allowing the association between plasma YKL-40, remission status and relapse to become evident22.
Although YKL-40 has been shown to be secreted by myeloid leukemia cell lines5 a previous study of YKL-40 in AML demonstrated that increased YKL-40 serum concentrations prior to chemotherapy only were associated with lower survival due to infections, and not prognostic factors such as cytogenetics or French-American-British (FAB) classification7. This may reflect that increased YKL-40 concentrations are associated with the degree of inflammation rather than the disease burden in AML. In line with these observations high YKL-40 levels in in the current study were associated with high HCT-CI but not with disease-related variables.
As the current study cohort was relatively homogenous, only consisting of patients with MDS and AML with low disease burden at time of transplant, it was not possible to observe any association between recipient YKL-40 and relapse. It should be noted that the cohort in our previous study only included nonmyeloablative transplants. However, as there is no evidence to support that the function of YKL-40 is dependent on the conditioning regimen, there is no reason to believe that the observed differences in relapse related outcome measures between our studies is related to differences in conditioning regimens.
Overall no statistically significant findings between plasma concentrations of YKL-40 and outcome parameters were observed in the current study. However a signal at the p=0.05 level in line with our previous finding in nonmyeloablative HCT22, was detected. Namely, a trend towards an association between donor plasma YKL-40 concentrations above the age adjusted 95th percentile and increased incidence of grade II-IV acute GVHD.
Although no pathophysiological mechanism linking YKL-40 to GVHD has been described, observations from a knockout model of the murine analog of YKL-40, BRP-39, may offer some explanation31. Knockout of BRP-39 entails defective interleukin (IL) 13 induced inflammatory responses and increased apoptosis of macrophages and CD4 T-cells. In our setting with increased YKL-40 concentrations, an opposite reaction may be hypothesized with increased accumulation of macrophages, dendritic cells, T-cells and enhanced IL-13 mediated inflammation, which previously has been associated with acute GVHD32 thus augmenting inflammatory and acute GVHD.
As plasma samples were only available from 576 of 781 donors, the risk of type I errors was increased. However, as missing samples were randomly distributed throughout the cohort, only overall statistical power was weakened, while bias due to skewing of the cohort was unlikely.
In conclusion, the main findings of the current study show that pre-transplant plasma YKL-40 concentrations do not increase the fidelity of the overall risk assessment of patients with AML or MDS undergoing allogeneic HCT. However, a borderline significant association between the inflammatory state of the donor, at time of donation, and grade II–IV acute GVHD was observed. In current practice, choosing the best donor for a patient is based on fairly blunt clinical criteria such as HLA-match, blood type, age, gender and cytomegalovirus serostatus33–37. Investigation of donor-specific parameters such as inflammatory state, immune responsiveness or other measures of the potential to be a safe and effective donor is another approach to try to improve the success of transplantation. In this context donor YKL-40 plasma concentrations could be relevant in different transplant settings, such as in HLA-identical related donor transplants, where the inflammatory state of the donor could be of more importance due to the lower degree of inherent alloreactivity than in the unrelated donor setting. Plasma YKL-40 could also be of relevance in haploidentical transplants where the inflammatory state of the donor could aid selection between a larger number of potential donors. To establish the role of YKL-40 in donor selection further studies are needed.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from Actinium Pharmaceuticals; Allos Therapeutics, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;GentiumSpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; TarixPharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; University of Minnesota; University of Utah; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
The study was also supported by grants from the Danish Biotechnology Program, the Danish Child Cancer Foundation and the Dagmar Marshall Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
BK designed the study, analyzed and interpreted data and drafted and revised the manuscript and. TW, XZ, SS, MH, and SJL provided patient and donor material, performed statistical analyses, interpreted data and revised the manuscript. JSJ performed plasma YKL-40 measurements, interpreted data and revised the manuscript. KM, LV and PG planned and designed the study, interpreted data and revised the manuscript.
FINANCIAL DISCLOSURES
The authors report no potential conflicts of interest
Reference List
- 1.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan. Med. Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- 2.Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJ. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp. Cell Res. 1999;250:168–173. doi: 10.1006/excr.1999.4511. [DOI] [PubMed] [Google Scholar]
- 3.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J. Biol. Chem. 1993;268:25803–25810. [PubMed] [Google Scholar]
- 4.Volck B, Price PA, Johansen JS, Sorensen O, Benfield TL, Nielsen HJ, et al. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc. Assoc. Am. Physicians. 1998;110:351–360. [PubMed] [Google Scholar]
- 5.Rehli M, Niller HH, Ammon C, Langmann S, Schwarzfischer L, Andreesen R, et al. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J. Biol. Chem. 2003;278:44058–44067. doi: 10.1074/jbc.M306792200. [DOI] [PubMed] [Google Scholar]
- 6.Johansen JS, Schultz NA, Jensen BV. Plasma YKL-40: a potential new cancer biomarker? Future. Oncol. 2009;5:1065–1082. doi: 10.2217/fon.09.66. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann OJ, Johansen JS, Klausen TW, Mylin AK, Kristensen JS, Kjeldsen E, et al. High serum concentration of YKL-40 is associated with short survival in patients with acute myeloid leukemia. Clin. Cancer Res. 2005;11:8644–8652. doi: 10.1158/1078-0432.CCR-05-1317. [DOI] [PubMed] [Google Scholar]
- 8.Mylin AK, Rasmussen T, Johansen JS, Knudsen LM, Norgaard PH, Lenhoff S, et al. Serum YKL-40 concentrations in newly diagnosed multiple myeloma patients and YKL-40 expression in malignant plasma cells. Eur. J. Haematol. 2006;77:416–424. doi: 10.1111/j.0902-4441.2006.t01-1-EJH2879.x. [DOI] [PubMed] [Google Scholar]
- 9.Biggar RJ, Johansen JS, Smedby KE, Rostgaard K, Chang ET, Adami HO, et al. Serum YKL-40 and interleukin 6 levels in Hodgkin lymphoma. Clin. Cancer Res. 2008;14:6974–6978. doi: 10.1158/1078-0432.CCR-08-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawada M, Hachiya Y, Arihiro A, Mizoguchi E. Role of mammalian chitinases in inflammatory conditions. Keio J. Med. 2007;56:21–27. doi: 10.2302/kjm.56.21. [DOI] [PubMed] [Google Scholar]
- 11.Johansen JS, Christoffersen P, Moller S, Price PA, Henriksen JH, Garbarsch C, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. J. Hepatol. 2000;32:911–920. doi: 10.1016/s0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 12.Ostergaard C, Johansen JS, Benfield T, Price PA, Lundgren JD. YKL-40 is elevated in cerebrospinal fluid from patients with purulent meningitis. Clin. Diagn. Lab Immunol. 2002;9:598–604. doi: 10.1128/CDLI.9.3.598-604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knudsen LS, Ostergaard M, Baslund B, Narvestad E, Petersen J, Nielsen HJ, et al. Plasma IL-6, plasma VEGF, and serum YKL-40: relationship with disease activity and radiographic progression in rheumatoid arthritis patients treated with infliximab and methotrexate. Scand. J. Rheumatol. 2006;35:489–491. doi: 10.1080/03009740600904300. [DOI] [PubMed] [Google Scholar]
- 14.Kucur M, Isman FK, Karadag B, Vural VA, Tavsanoglu S. Serum YKL-40 levels in patients with coronary artery disease. Coron. Artery Dis. 2007;18:391–396. doi: 10.1097/MCA.0b013e328241d991. [DOI] [PubMed] [Google Scholar]
- 15.Rathcke CN, Vestergaard H. YKL-40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm. Res. 2006;55:221–227. doi: 10.1007/s00011-006-0076-y. [DOI] [PubMed] [Google Scholar]
- 16.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N. Engl. J. Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 17.Shao R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front Physiol. 2013;4:122. doi: 10.3389/fphys.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CG, Da Silva CA, la Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen AR, Plomgaard P, Krabbe KS, Johansen JS, Pedersen BK. IL-6, but not TNF-alpha, increases plasma YKL-40 in human subjects. Cytokine. 2011;55:152–155. doi: 10.1016/j.cyto.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa KC, Millis AJ. gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp. Cell Res. 2003;287:79–87. doi: 10.1016/s0014-4827(03)00069-7. [DOI] [PubMed] [Google Scholar]
- 21.Kzhyshkowska J, Mamidi S, Gratchev A, Kremmer E, Schmuttermaier C, Krusell L, et al. Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood. 2006;107:3221–3228. doi: 10.1182/blood-2005-07-2843. [DOI] [PubMed] [Google Scholar]
- 22.Morup AM, Kornblit B, Johansen JS, Masmas TN, Madsen HO, Vindelov L, et al. The prognostic value of YKL-40 concentrations in nonmyeloablative conditioning allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2011;17:1299–1307. doi: 10.1016/j.bbmt.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 24.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Hogdall EV, Johansen JS, Kjaer SK, Price PA, Blaakjaer J, Hogdall CK. Stability of YKL-40 concentration in blood samples. Scand. J. Clin. Lab Invest. 2000;60:247–251. doi: 10.1080/00365510050184886. [DOI] [PubMed] [Google Scholar]
- 26.Bojesen SE, Johansen JS, Nordestgaard BG. Plasma YKL-40 levels in healthy subjects from the general population. Clin. Chim. Acta. 2011;412:709–712. doi: 10.1016/j.cca.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 28.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat. Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.El-Galaly TC, Bilgrau AE, Gaarsdal E, Klausen TW, Pedersen LM, Nielsen KR, et al. Circulating tumor necrosis factor-alpha and YKL-40 level is associated with remission status following salvage therapy in relapsed non-Hodgkin lymphoma. Leuk. Lymphoma. 2015:1–3. doi: 10.3109/10428194.2014.1001984. [DOI] [PubMed] [Google Scholar]
- 30.Hottinger AF, Iwamoto FM, Karimi S, Riedel E, Dantis J, Park J, et al. YKL-40 and MMP-9 as serum markers for patients with primary central nervous system lymphoma. Ann. Neurol. 2011;70:163–169. doi: 10.1002/ana.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J. Exp. Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan WJ, Brookes PA, Szydlo RM, Goldman JM, Lechler RI, Ritter MA. IL-13 production by donor T cells is prognostic of acute graft-versus-host disease following unrelated donor stem cell transplantation. Blood. 2004;103:717–724. doi: 10.1182/blood-2003-01-0192. [DOI] [PubMed] [Google Scholar]
- 33.Logan AC, Wang Z, Alimoghaddam K, Wong RM, Lai T, Negrin RS, et al. ABO Mismatch Is Associated with Increased Nonrelapse Mortality after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2015;21:746–754. doi: 10.1016/j.bbmt.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howard CA, Fernandez-Vina MA, Appelbaum FR, Confer DL, Devine SM, Horowitz MM, et al. Recommendations for donor human leukocyte antigen assessment and matching for allogeneic stem cell transplantation: consensus opinion of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Biol. Blood Marrow Transplant. 2015;21:4–7. doi: 10.1016/j.bbmt.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J. Infect. Dis. 2002;185:273–282. doi: 10.1086/338624. [DOI] [PubMed] [Google Scholar]
- 36.Stern M, Passweg JR, Locasciulli A, Socie G, Schrezenmeier H, Bekassy AN, et al. Influence of donor/recipient sex matching on outcome of allogeneic hematopoietic stem cell transplantation for aplastic anemia. Transplantation. 2006;82:218–226. doi: 10.1097/01.tp.0000226156.99206.d1. [DOI] [PubMed] [Google Scholar]
- 37.Confer DL, Abress LK, Navarro W, Madrigal A. Selection of adult unrelated hematopoietic stem cell donors: beyond HLA. Biol. Blood Marrow Transplant. 2010;16:S8–S11. doi: 10.1016/j.bbmt.2009.10.031. [DOI] [PubMed] [Google Scholar]


