Abstract
Background
Limited evidence from small scale studies, mainly involving end stage renal disease (ESRD) patients, suggests that kidney transplantation may improve cognitive function. We examined changes in cognitive function after a kidney transplant and its association with survival in advanced chronic kidney disease (CKD)/ESRD patients.
Methods
In a prospective study design, cognitive performance of 90 patients (50.6±13.1 yrs, 66.7% males, 27.8% blacks, 76% CKD stage 4–5) was assessed at patients’ homes using established neurocognitive tests.
Results
Among the 90 patients, 44 received a kidney transplant (KTx group) while 46 did not (no-KTx group). After a mean follow-up of ~19 months, there was no significant change in scores for majority of cognitive tests in either group. Older age but not diabetes or renal function status (CKD vs ESRD) were determinants of poor follow-up cognitive performance. Additionally, poor attention/psychomotor speed and executive performance (as measured by Trails A and Stroop respectively) was associated with higher mortality over a mean follow-up of 4.7 years, even after adjustment for age, sex, diabetes, CKD or ESRD status, and kidney transplant status
Conclusion
Overall, cognitive function does not significantly improve after kidney transplant or significantly decline in non-transplanted, advanced CKD/ESRD patients. Poor attention, psychomotor speed and executive performance independent of transplant status was associated with higher mortality over time.
Keywords: kidney transplant, cognitive function, mortality
Introduction
Up to 30–70% of end stage renal disease (ESRD) and 17–30% of advanced chronic kidney disease (CKD) patients are reported to have significant cognitive deficits [1–4]. Cognitive impairment may affect patient’s daily functioning, independence, social adjustments, employment options, medication adherence, medical decision making [5], and is also an independent predictor of mortality [6, 7]. Although there is well established survival benefit of renal transplantation, the evidence of its effect on cognitive function is limited and not entirely consistent.
Previous studies demonstrated advanced CKD/ESRD patients have significant impairments in verbal memory and executive functioning [1, 2, 4, 8]. There also appears to be an association between severity of kidney disease and the degree of cognitive impairment [3, 5, 9]. The etiology of cognitive impairment in this population is multifactorial including high prevalence of cerebrovascular risk factors, cerebrovascular disease itself, and increased exposure to acute changes in metabolic abnormalities/fluid shifts associated with renal replacement therapy (RRT) [10]. Kidney transplantation, perhaps by improving uremic mileu and metabolic abnormalities, may improve cognitive function. However, it is possible that the risk factors contributing to cognitive impairment may not be corrected or reversed by transplant alone. In fact, cognitive function may worsen post-transplant due to effect of immunosuppressive medications.
Early studies demonstrated a beneficial effect of kidney transplantation on cognitive function, specifically verbal memory [11–13]. More recent studies revealed improvement in psychomotor speed, abstract thinking, attention, and executive functioning with improvements sustaining well past initial transplantation [14–16]. While encouraging, these studies remain limited by small sample size, lack of non-transplant comparison group, and lack of generalizability to the United States CKD/ESRD population. Moreover, almost all of these studies were done in patients on HD only, and very limited information is available on outcomes of non-dialysis CKD undergoing renal transplant.
The primary aim of our study was to examine changes in cognitive function after a kidney transplant in a cohort of patients with advanced CKD/ESRD. We compared changes in cognitive function over time in patients who received a kidney transplant with those who did not. We hypothesized that those receiving transplant will have improved cognitive function compared to those without a transplant. Finally, we examined whether baseline cognitive tests were associated with mortality in the transplanted (KTx) and non-transplanted (no-KTx) groups.
Subjects and Methods
Study Participants
This cohort comprises data collected as part of a prospective study that investigated memory, sleep and quality of life in adult, English-speaking patients with advanced CKD (dialysis dependent or Modification of Diet in Renal Disease [MDRD] estimated glomerular filtration rate [eGFR] ≤30 ml/min/1.73m2) or ESRD, defined as being on RRT (either three times per week in-center HD or peritoneal dialysis [PD]) for at least 3 months [17]. We approached patients during routine CKD or dialysis clinic visit, or initial evaluation at a kidney transplant clinic in Western Pennsylvania between March 2004 and December 2008. Exclusion criteria included age (<18 or >90 years), craniofacial abnormalities, active malignancy, treated sleep apnea, active infection, active coronary artery disease, advanced cirrhosis, active alcohol abuse, history of head injury or refractory psychiatric disease. Patients with severe cognitive impairment (advanced dementia, clinically confused, or those unable to provide basic personal information) were also excluded. Baseline cognitive evaluation was performed shortly after enrollment during initial home visit and patients were followed prospectively. All participants signed informed consent and the study was approved by the University of Pittsburgh Institutional Review Board (PRO14010028).
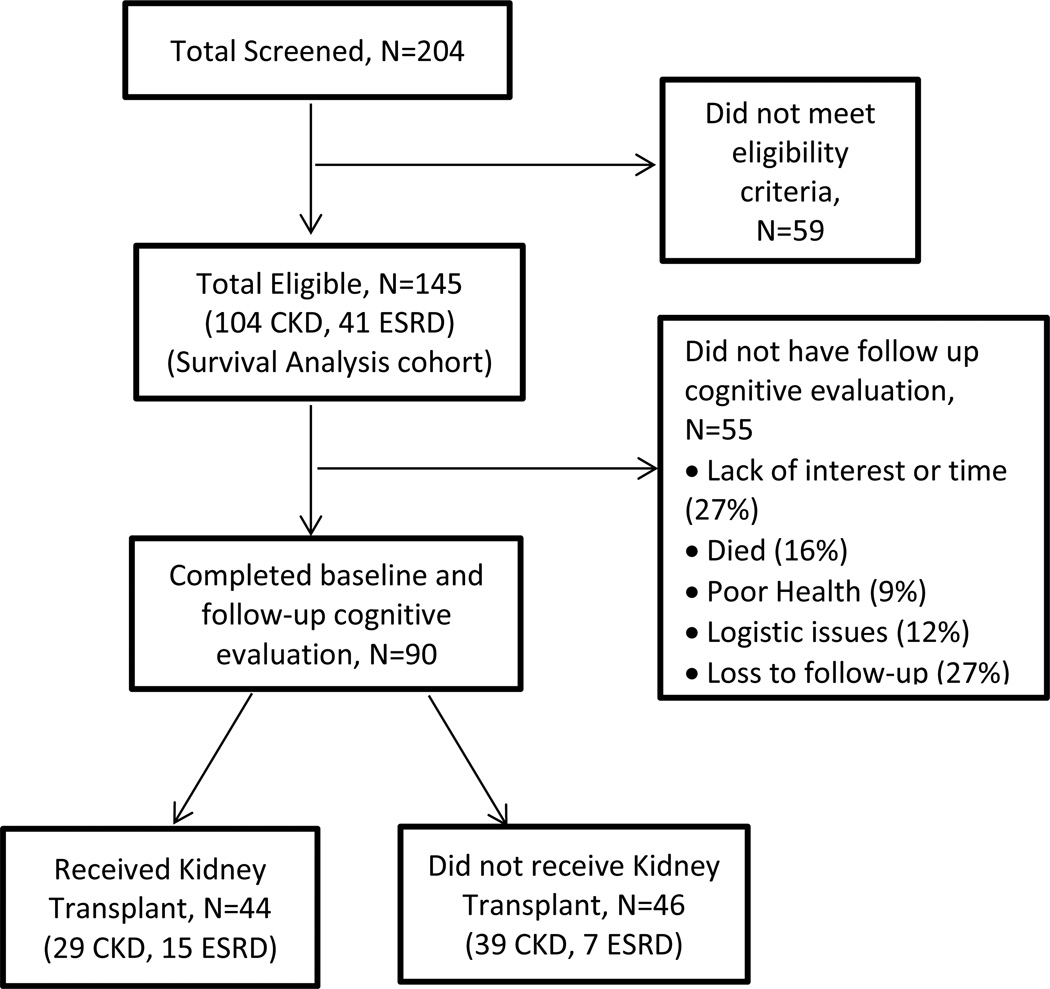
Out of the 145 patients who met the eligibility criteria and consented for this study, 55 failed to complete follow-up cognitive evaluation (Figure 1). Among the 90 participants who completed a baseline and follow-up cognitive evaluation per study protocol, 44 (15 ESRD, 29 CKD) were transplanted during study period (KTx group) and completed follow-up cognitive evaluation at least 6 months post-transplant. We chose this interval to allow for stabilization of renal allograft function and immunosuppressive medication regimen. The remaining 46 patients (7 ESRD, 39 CKD) were not transplanted during study period (No-KTx group) (Figure 1).
Figure 1. Flowchart of patient selection for inclusion in the study cohort.
Data Collection
Baseline data was collected from a standardized health interview and questionnaire that included assessment of previous medical problems, surgeries and relevant medical procedures. Current medications and anthropometric measurements were obtained with self-reporting of age, race and socioeconomic status. Baseline serum laboratory tests were collected from medical record.
Neurocognitive Assessment
Patients were assessed using a battery of well-validated neuro-cognitive tests with high inter- and intra-rater reliability (Table 1). Tests were administered by research assistants trained and supervised by an expert in neuropsychological assessment (C.S.). To ensure a quiet, distraction free environment, assessments were performed at patients’ homes. By avoiding assessment during HD, we minimized the effect of rapid fluid/electrolyte shifts during HD on cognitive performance. The tests were administered in a standardized way using the same sequence of tests and on paper except the WCST, which was administered on a laptop.
Table 1.
Description of Cognitive Tests Used
| Cognitive Test | Brief Description | Measurement Criteria |
|---|---|---|
| Clock Drawing Test (CLOX) 1 and 2 [39, 40] |
Assesses abstract thinking and visual spatial constructive abilities [39, 40]. |
Scored based on accuracy. Higher score is better. |
| Controlled Oral Word Association (COWA) [41] |
Language assessment test for phonetic/semantic fluency components. Individual required to produce words with a designated first letter and a list of animals each within a one minute period [41]. |
Combined score used. Higher score is better. 20 words or less is considered seriously deficient. |
| Digit Span Subtest [42] |
Individual is required to repeat increasingly longer number sequences in either forward/reverse order. A subtest from Wechsler Memory Scale [42]. |
Age-based scaled score was generated and used. |
| Digit-Symbol Substitution Subtest (DSST) [42] |
Test for visual scanning and rapid response where individual substitutes numbers for symbols based on a learned code. A subtest from the Wechsler Memory Scale [42]. |
Score equaled total number correct within the given time frame. |
| Wechsler Abbreviated Scale of Intelligence (IQ) [42] |
Abbreviated intelligence quotient scale assessment where two of four subtests (vocabulary and matrix reasoning) were used to generate age-related IQ score [42]. |
50% percentile for population is 100. Less than 80 is considered borderline. |
| Logical Memory Subtest [42] |
Assessment for verbal memory. Individual is read a story, asked to recall story related details immediately and 30 minutes post. A subtest of Wechsler Memory Scale [42]. |
Age-based standard score was used. |
| Mini Mental State Examination [43, 44] |
Tests orientation, attention, calculation, recall, language, and visual-spatial skills [43, 44]. |
Raw Scores used. |
| Rey-Osterrieth Complex Figure (RCF) Copy Condition [18, 45] |
Assesses visual spatial/constructional abilities. Requires individuals to copy complex figures with immediate spontaneous and delayed (30 minutes later) recall trial [18, 45]. |
Each copy is scored for accurate reproduction and placement of specific elements of figure. Higher score is better. |
| Stroop Test [46] | Assessment of interference in the reaction time of a task. Test requires individual to read the names of colors, name of the color of an image, and name the color of ink of a printed color word [46]. |
Age adjusted color- word raw score reported. Higher score is better. |
| Trail-Making Tests Parts A and B [47] |
Provides an estimate of attention, psychomotor speed, executive functioning, and working memory. Part A requires subjects to connect a series of numbers in sequential order as quickly as possible. Part B requires subjects to connect letters and numbers in sequential order as quickly as possible [47]. |
Measured as time required completing each task. Quicker (lower) scores are better. |
| Wisconsin Card Sorting Test (WCST) [47] |
Assessment for problem solving that requires flexibility in the face of changing schedules of reinforcement. Individual is given a set of stimulus cards, instructed to match the cards but not told how, and then told whether correct or not [47]. Computerized version used. |
Number of completed categories reported. Higher score is better. |
Definition of Exposures
Depression was defined as Patient Health Questionnaire-9 (PHQ-9) score of ≥10, with 5 or more symptoms – including either depressed mood or anhedonia – present for more than half the days over the prior 2 weeks; and/or self-reported treatment with anti-depressants. Diabetes was evaluated by both self-report and/or current use of insulin or hypoglycemic agents. Cardiovascular disease was evaluated by patient self-reporting (of previous myocardial infarction or heart attack, stroke, or previous coronary artery bypass/angioplasty). Patient’s overall health-related quality of life (HRQOL) was assessed using the short form health survey (SF 36).
Statistical Analysis
Categorical and continuous variables were presented using frequencies with percentages and means with standard deviations (or medians with interquartile range for skewed distributions) respectively. Baseline characteristics were compared between KTx and No-KTx group. Baseline, follow-up and changes in neurocognitive measures were also compared between the two groups. Within-group changes in neurocognitive measures from baseline to follow-up were assessed using linear mixed model with random subject intercept. Between-group comparisons used two-sample t-test or Wilcoxon rank sum test and Chi-square or Fisher exact test for continuous and categorical measures respectively. Adjusted analyses of change scores of each neurocognitive measure were conducted using linear models that adjust for age, gender, baseline cognitive score and IQ. IQ score was used as a proxy for education due to limited capture of the education data by the binary “high school graduate” variable. In multivariable linear regression models stratified by transplant group, we examined the association between select follow-up neurocognitive tests (MMSE, logical memory 1 and 2, and trails A and B) and patient characteristics [age, diabetes, renal function status (CKD vs ESRD), and IQ] that may be important determinants of cognitive function after adjusting for the baseline cognitive score. Due to skewness, Trails A and Trails B were log-transformed prior to modeling. Within the KTx group, neurocognitive scores at pre- and at post-transplant were also compared between CKD and RRT using two-sample t-test or Wilcoxon rank sum test.
To examine the relationship between cognitive performance (MMSE, Logical Memory 1 and 2, Trails A and B) and overall survival, we fitted Cox proportional hazards model with and without adjusting for age, gender, diabetes, renal function status and a time-dependent covariate, receipt of kidney transplant. Hazards ratios along with the 95% confidence intervals were reported. National Death Index data until Dec 31st, 2011 was obtained and patients still alive on this date were censored.
For sensitivity analysis, we applied principal component analysis (PCA) of the neurocognitive measures to reduce the dimensionality. We examined the scree plot, eigenvalues, and cumulative proportion of variance explained to determine the number of principal components (PC) to keep. Loadings and contributions of the neurocognitive measures were used to characterize the PCs and to examine agreement with the components of commonly presented latent constructs. PC scores were calculated and analyzed using the same methods applied to the original neurocognitive measures The tests included in PCA analysis were CLOX 1 and 2, COWA, Logical Memory 1 and 2, Trails A and B, Stroop, Digit Span Forward and Backward, DSST, RCF copy and WCST.
All statistical analyses were carried out in R (version 13.2) [18] using the packages dplyr [19] for data manipulation, compareGroups [20] for descriptive tables, ggplot2 [21] for graphics, survival [22] for survival analysis, and lme4 for linear mixed modeling.
Results
Baseline characteristics of the KTx and no-KTx group are shown in Table 2. Patients who received KTx had significantly lower prevalence of diabetes (27.3% vs 53.3%, p=0.02) as compared to the no-KTx group. The KTx group had trends toward more patients on RRT and lower eGFR among non-dialysis dependent CKD (eGFR 16.2±4.9 vs 19.9±8.0, p=0.04) than the no-KTx group. Except for better self-reported physical health (SF-36 PCS) in the KTx group, both groups were similar in terms of baseline demographics, comorbidities (including history of stroke), cognitive reserve (IQ score), mental health including depression, antidepressant or antihistamine use and biochemical status. Among the KTx group, those with non-dialysis dependent CKD were more likely to be Whites, employed, less anemic, and have higher cognitive reserve (IQ score) and lower SF-36 mental component summary score as compared to those with ESRD (Supplementary Table 1). Among the no-KTx group, there were no differences in any demographics, comorbidities or biochemical variables (Supplementary Table 2). At CE#1, the KTx and no-KTx group had similar scores for all cognitive tests except CLOX 1 and 2, for which the KTx group performed slightly better (Table 3).
Table 2.
Baseline Characteristics comparing Transplant vs. Non-Transplant Group
| All (N=90) | KTx group (N=44) |
No-KTx group (N=46) |
p- valueb |
|
|---|---|---|---|---|
| Age (years) | 50.6 (13.1) | 48.0 (13.9) | 53.0 (11.8) | 0.07 |
| Male | 60 (66.7%) | 33 (75.0%) | 27 (58.7%) | 0.16 |
| Race | 0.08 | |||
| White | 63 (70.0%) | 35 (79.5%) | 28 (60.9%) | |
| Black | 25 (27.8%) | 9 (20.5%) | 16 (34.8%) | |
| Others | 2 (2.2%) | 0 (0.0%) | 2 (4.4%) | |
| High School graduate | 83 (92.2%) | 42 (95.5%) | 41 (89.1%) | 0.44 |
| Married | 55 (61.1%) | 29 (65.9%) | 26 (56.5%) | 0.49 |
| Employed | 35 (38.9%) | 20 (45.5%) | 15 (32.6%) | 0.30 |
| Ever Smoker | 47 (52.2%) | 25 (56.8%) | 22 (47.8%) | 0.52 |
| Cardiovascular Disease | 16 (17.8%) | 5 (11.4%) | 11 (23.9%) | 0.20 |
| Diabetes | 36 (40.4%) | 12 (27.3%) | 24 (53.3%) | 0.02 |
| Hypertension | 75 (89.3%) | 39 (90.7%) | 36 (87.8%) | 0.74 |
| Stroke | 5 (5.75%) | 1 (2.38%) | 4 (8.89%) | 0.36 |
| Depression | 8 (8.99%) | 2 (4.55%) | 6 (13.3%) | 0.27 |
| Renal function status | 0.07 | |||
| CKD | 68 (75.6%) | 29 (65.9%) | 39 (84.8%) | |
| HD | 20 (22.2%) | 14 (31.8%) | 6 (13.0%) | |
| PD | 2 (2.22%) | 1 (2.27%) | 1 (2.17%) | |
| Dialysis Vintage (months)e | 9.0 [4.5;19.0] | 5.0 [3.5;11.5] | 15.0 [10.5;40.2] |
0.07 |
| Cognitive Reserve (IQ score) |
101 (15.6) | 103 (16.2) | 99.4 (14.9) | 0.33 |
| Anti-depressant use | 15 (16.7%) | 9 (20.5%) | 6 (13.0%) | 0.51 |
| Anti-histamine use | 2 (2.2%) | 1 (2.3%) | 1 (2.2%) | 1.00 |
| SF36 Physical Component Summary (PCS) score |
41.4 (6.6) | 43.0 (6.0) | 40.0 (6.8) | 0.04 |
| SF 36 Mental Component Summary (MCS) score |
44.5 (7.5) | 44.8 (6.8) | 44.2 (8.2) | 0.73 |
| Systolic Blood Pressured | 147 (23.2) | 147 (19.5) | 146 (26.7) | 0.79 |
| Diastolic Blood Pressured | 83.6 (13.6) | 85.7 (13.1) | 81.4 (13.9) | 0.14 |
| Serum creatinine (mg/dL) | 5.0 (2.3) | 5.5 (2.4) | 4.4 (2.0) | 0.04 |
| eGFR (MDRD; ml/min/1.73m2)f |
18.2 (7.0) | 16.2 (4.9) | 19.9 (8.0) | 0.04 |
| Hemoglobin (g/dL) | 11.8 (1.4) | 11.7 (1.2) | 11.9 (1.5) | 0.56 |
| Albumin (g/dL) | 3.8 (0.6) | 3.9 (0.5) | 3.8 (0.6) | 0.24 |
| Phosphorus (mg/dL) | 4.8 (1.4) | 4.9 (1.4) | 4.7 (1.5) | 0.69 |
| Bicarbonate (mEq/L) | 23.5 (3.3) | 22.9 (3.0) | 24.1 (3.5) | 0.12 |
| Parathyroid Hormone (pg/ml) |
176 [108; 310] |
227 [124; 263] |
156 [104; 320] |
0.68 |
| Time from 1st to 2nd cognitive evaluation (months) |
19.1 (9.0) | 17.7 (8.8) | 20.4 (9.0) | 0.15 |
| Time from 1st cognitive evaluation to KTx (months) |
9.3 (7.7) | |||
| Time from KTx to 2nd cognitive evaluation (months) |
- | 8.3 (5.2) | - | - |
Data are presented as percentage n(%), mean(sd) or median[Q1;Q3] where Q1 is the 25th percentile and Q3 is the 75th percentile.
P-values use analysis of variance (ANOVA) or Kruskal-Wallis for continuous variables and Fisher exact or Chi-square for categorical variables.
Blood Pressure was measured during home visit at the time of cognitive evaluation
Dialysis vintage missing for 1 patient
For non-dialysis dependent CKD only
Table 3.
Baseline Cognitive Scores (CE#1) comparing KTx versus no-KTx group
| Neurocognitive Test |
All (N=90) | KTx group (N=44) |
No-KTx group(N=46) |
p- value |
|---|---|---|---|---|
| MMSE | 27.7 (2.1) | 28.0 (2.0) | 27.4 (2.1) | 0.20 |
| CLOX 1 | 12.9 (2.2) | 13.5 (2.1) | 12.4 (2.2) | 0.02 |
| CLOX 2 | 14.5 (0.9) | 14.7 (0.5) | 14.3 (1.1) | 0.03 |
| COWA total | 35.3 (13.0) | 36.2 (12.9) | 34.5 (13.2) | 0.53 |
| Digit Span Forward |
10.1 (2.3) | 10.2 (2.5) | 9.9 (2.0) | 0.46 |
| Digit Span Backward |
6.8 (2.5) | 7.2 (2.5) | 6.4 (2.4) | 0.15 |
| DSST | 49.0 (14.5) | 51.3 (16.1) | 46.7 (12.5) | 0.14 |
| Logical Memory 1 | 8.0 (3.1) | 8.3 (2.8) | 7.8 (3.3) | 0.45 |
| Logical Memory 2 | 8.9 (2.7) | 9.1 (2.7) | 8.7 (2.8) | 0.49 |
| RCF copy | 31.6 (5.0) | 32.2 (4.2) | 31.1 (5.7) | 0.29 |
| RCF delayed recall |
16.1 (7.2) | 17.0 (7.8) | 15.1 (6.5) | 0.22 |
| Stroop | 41.5 (10.0) | 42.2 (11.7) | 40.9 (8.3) | 0.55 |
| Trails A time (sec) | 31.5 [26.0;39.8] |
29.0 [26.5;39.0] |
33.0 [26.0;41.8] |
0.18 |
| Trails B time (sec) | 79.0 [54.0;105] |
69.0 [53.0;95.8] |
87.0 [59.0;116] | 0.13 |
| WCST | 2.2 (1.6) | 2.4 (1.8) | 1.9 (1.4) | 0.15 |
Data are presented as percentage n(%), mean(sd) or median[Q1;Q3] where Q1 is the 25th percentile and Q3 is the 75th percentile.
Legend:
MMSE – Mini Mental Status Exam
CLOX 1 and 2 – Clock Drawing Test
COWA – Controlled Oral Word Association
DSST – Digit Symbol Substitution Subtest
RCF - Rey-Osterrieth Complex Figure (RCF) Copy Condition Test
WCST – Wisconsin Card Sorting Test
In our KTx group, 78% of patients were on a single immunosuppressant, most commonly tacrolimus (95% patients) with an average daily dose of 8.1 mg. Cellcept was used in 22%, rapamune in 4.9%, and a combination of one of these and steroids in 2.4% of the patients. The average daily doses of cellcept, rapamune and steroids were 984 mg, 4 mg and 30 mg respectively.
Longitudinal Change in cognitive Function
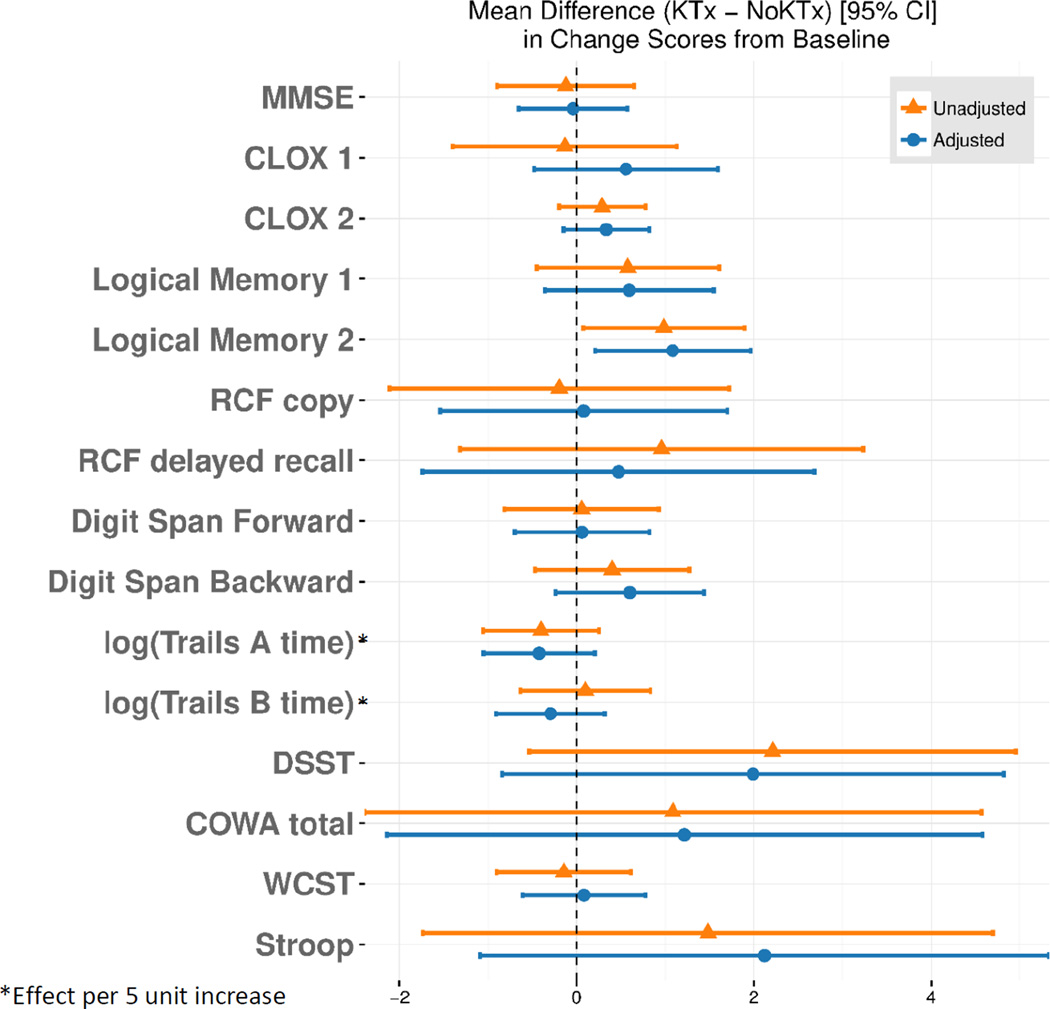
The time interval between the first (CE#1) and the final (CE #2) cognitive evaluation for the entire cohort was 19.1±9.0 months and was comparable between the KTx and no-KTx groups (Table 1). For KTx group, the mean time interval from kidney transplant to CE #2 was 8.3±5.2 months. Table 4 shows the longitudinal changes in cognitive scores for both the groups. In the KTx group, there was no significant change in cognitive scores for all tests except slight improvement in RCF delayed recall testing (19.1±8.4 vs 17.0±7.8, p=0.02) compared to pre-transplant score. We also tested whether the patients’ post-transplant scores were different based on patients’ CKD or ERSD status at baseline and found no significant difference (Supplementary Table 3). Similarly, in the no-KTx group, there was no change in cognitive scores over 20.4±9.0 months period. Figure 2 displays unadjusted and adjusted [for age, sex, baseline IQ (as proxy for education) and baseline cognitive score] mean difference of longitudinal changes in cognitive scores between KTx and No-KTx groups. Overall, there was no significant difference in the change of cognitive function over time in the two groups except for greater improvement in Logical Memory 2 within KTx group [adjusted: 1.08 (0.20–1.96); p=0.02].
Table 4.
Raw Score Comparison for Cognitive Tests Pre vs Post Kidney Transplant in the KTx and no-KTx group
| Neurocognitive Test |
KTx group | No-KTx group | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre KTx score b |
Post KTx score b |
Difference (Post-Pre) |
P- valuea |
Baseline scorec |
Follow-up scorec |
Difference (Follow up- Baseline) |
P- valuea |
|
| MMSE | 28.0 (2.0) | 28.1 (2.1) | 0.1 (1.7) | 0.60 | 27.4 (2.1) | 27.7 (1.9) | 0.3 (2.0) | 0.39 |
| IQ | 102.6 (16.2) | 103.9 (16.0) | 1.3 (8.1) | 0.30 | 99.4 (14.9) | 99.7 (17.8) | 0.3 (10.0) | 0.86 |
| CLOX 1 | 13.5 (2.1) | 13.1 (1.9) | −0.4 (2.9) | 0.34 | 12.4 (2.2) | 12.1 (3.0) | −0.3 (3.2) | 0.59 |
| CLOX 2 | 14.7 (0.5) | 14.5 (0.8) | −0.3 (0.9) | 0.06 | 14.3 (1.1) | 13.8 (1.6) | −0.5 (1.4) | 0.01 |
| COWA total | 36.2 (12.9) | 37.7 (12.4) | 1.5 (9.6) | 0.32 | 34.5 (13.3) | 34.9 (12.9) | 0.4 (7.1) | 0.73 |
| Digit Span Forward |
10.3 (2.5) | 10.6 (2.4) | 0.3 (2.5) | 0.41 | 9.9 (2.0) | 10.2 (1.9) | 0.3 (1.6) | 0.28 |
| Digit Span Backward |
7.2 (2.5) | 7.3 (2.7) | 0.2 (2.4) | 0.61 | 6.4 (2.4) | 6.2 (2.6) | −0.2 (1.9) | 0.43 |
| DSST | 51.3 (16.1) | 52.1 (17.7) | 0.9 (6.2) | 0.36 | 46.7 (12.5) | 45.4(13.4) | −1.4 (7.0) | 0.20 |
| Logical Memory 1 |
8.3 (2.8) | 8.6 (3.4) | 0.3 (2.7) | 0.50 | 7.8 (3.3) | 7.5 (2.9) | −0.3 (2.3) | 0.38 |
| Logical Memory 2 |
9.1 (2.7) | 9.7 (2.9) | 0.6 (2.3) | 0.09 | 8.7 (2.8) | 8.4 (2.7) | −0.4 (2.1) | 0.23 |
| RCF copy | 32.2 (4.2) | 32.6 (3.9) | 0.3 (4.3) | 0.60 | 31.1 (5.7) | 31.3 (5.3) | 0.5 (4.7) | 0.58 |
| RCF delayed recall |
17.0 (7.8) | 19.1 (8.4) | 2.1 (5.7) | 0.02 | 15.1 (6.5) | 16.6 (8.3) | 1.2 (5.3) | 0.13 |
| Stroop | 42.2 (11.7) | 43.3 (11.9) | 1.8 (7.2) | 0.14 | 40.9 (8.3) | 41.3 (9.0) | 0.3 (7.9) | 0.77 |
| Trails A time (sec) |
29.0 [26.5;39.0] |
27.5 [20.8;35.3] |
−1.5 [− 7.3;1.5] |
0.16 | 33.0 [26.0;41.8] |
34.0 [26.0;43.5] |
0.0 [− 4.8;6.8] |
0.43 |
| Trails B time (sec) |
69.0 [53.0;95.8] |
61.0 [43.8;99.5] |
−2.5 [− 13.3;9.3] |
0.84 | 87.0 [59.0;116.0] |
78.0 [57.3;123.3] |
−4.0 [−25. 0;8.0] |
0.60 |
| WCST | 2.4 (1.8) | 2.6 (1.6) | 0.3 (2.0) | 0.49 | 1.9 (1.4) | 2.3 (1.7) | 0.4 (1.5) | 0.10 |
Data are presented as mean (sd) where sd is standard deviation or median [Q1;Q3] where Q1 is the 25th percentile and Q3 is the 75th percentile.
P-value obtained from unadjusted random intercept model
In KTx group, for baseline (pre KTx score) - Stroop and WCST missing for 2 subjects. For post KTx score, CLOX-1 and CLOX-2 scores missing for 1 subject and WCST missing for 3 subjects
In no-KTx group, for baseline scores - Trails B missing for 1 subject, WCST missing for 2 subjects, Stroop missing for 1 subject. For follow-up scores, RCF copy missing for 6 subjects, RCF delayed recall missing for 1 subject, WCST missing for 2 subjects, Stroop missing for 3 subjects
- MMSE – Mini Mental Status Exam
- CLOX 1 and 2 – Clock Drawing Test
- COWA – Controlled Oral Word Association
- DSST – Digit Symbol Substitution Subtest
- RCF - Rey-Osterrieth Complex Figure (RCF) Copy Condition Test
- WCST – Wisconsin Card Sorting Test
Figure 2. Mean Difference in the Longitudinal Change in Cognitive Scores (Post-Pre) in KTx vs. No-KTx group.
$ Adjusted for age, sex and baseline cognitive score
- MMSE – Mini Mental Status Exam
- CLOX 1 and 2 – Clock Drawing Test
- COWA – Controlled Oral Word Association
- DSST – Digit Symbol Substitution Subtest
- RCF - Rey-Osterrieth Complex Figure (RCF) Copy Condition Test
- WCST – Wisconsin Card Sorting Test
Determinants of Cognitive Function
We conducted multivariable analysis of association of CE#2 with select patient characteristics that may be important determinants of cognition [age, baseline IQ (as proxy for education), diabetes, renal function status (CKD vs ESRD)] after adjusting for baseline score and stratified by transplant status. For both the KTx and no-KTx group, lower IQ was associated with lower MMSE score but did not associate with Trails A or B or Logical Memory 1 or 2. Within no-KTx group, increasing age was associated with poorer performance on MMSE, Trails A, Logical Memory 1 and 2. Diabetes and renal function status were not significant determinants of cognitive function for either group. (Supplementary Fig 1)
Association of Cognitive Function with Survival
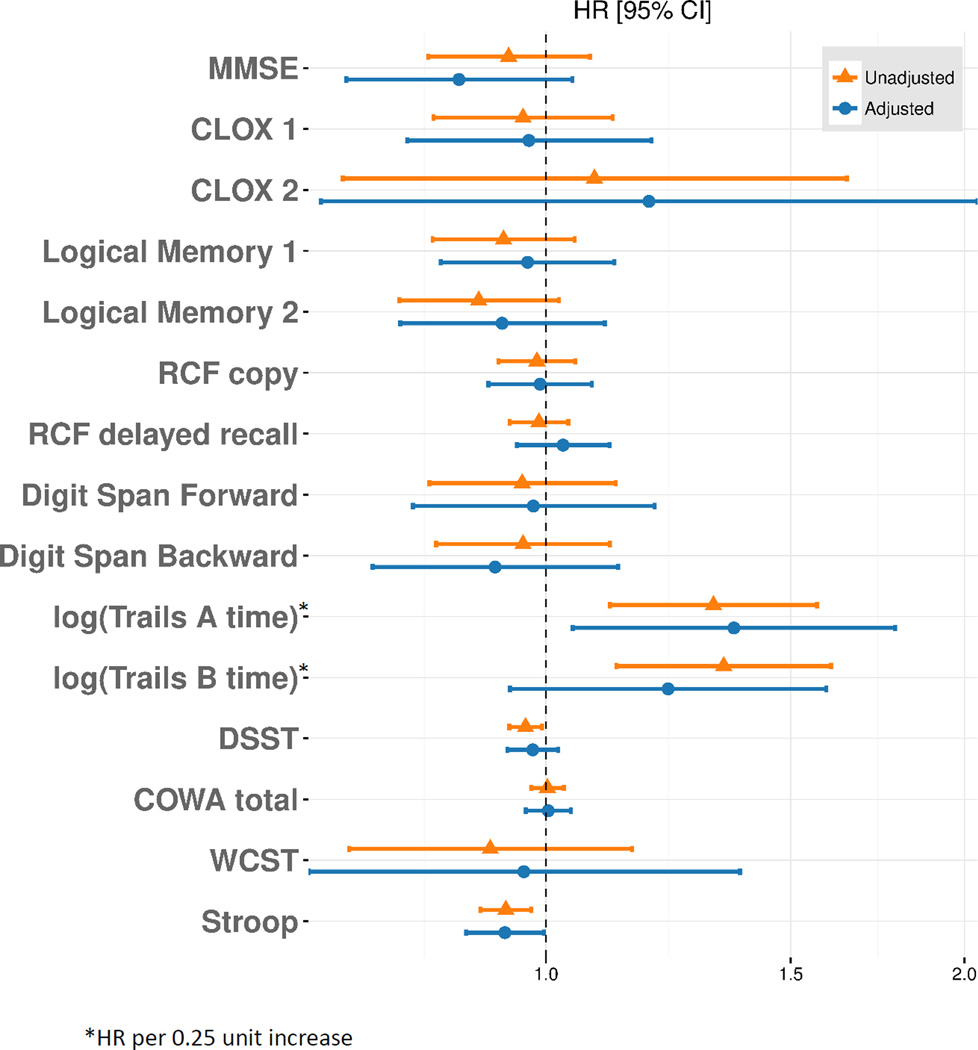
For survival analysis, our cohort included all subjects who had completed a baseline cognitive evaluation (n=145). There were 35 deaths over a mean follow-up period of 4.7±1.8 years. Association of baseline cognitive scores with mortality was assessed adjusting for transplant status as a time-dependent variable. In unadjusted analysis, poor performance on Trails A, Trails B, DSST and Stroop test were associated with higher mortality [unadjusted HR: 3.03 (1.53, 6.02); p=0.002 for Trails A; 3.25 (1.60, 6.61); p=0.002 for Trails B, 0.97 (0.94, 0.99); p=0.016 for DSST; 0.94 (0.90, 0.98); p= 0.002 for Stroop] (Figure 3). However, after adjustment for age, sex, diabetes, CKD or ESRD status, and kidney transplant status, only poor performance on Trails A and Stroop were significantly associated with mortality [adjusted HR: 3.47 (1.19, 10.1); p=0.02 and 0.93 (0.88, 1.00); p = 0.04 respectively]. There was no association of baseline MMSE, CLOX 1 or 2, Logical Memory 1 or 2, RCF copy, RCF delayed recall, Digit Span Forward or Backward with mortality.
Figure 3. Association of baseline cognitive function with survival.
Sensitivity Analysis using Principal Component Analysis
We employed PCA to derive a composite score of cognition. The number of PCs to keep, however, were not consistent among the different criteria. The scree plot showed that 1 component is sufficient, but only 36% of the variance would be accounted for. The eigenvalue-greater-than-one criteria would keep 5 components, which would account for about 70% of the variance. To account for at least 80% of the variability, we would have to keep 7 components. These findings suggest that PCA was not very effective for this dataset. Nevertheless, we chose to keep 5 components. Based on the neurocognitive loadings and contributions, these components represent (1) executive functioning, (2) logical memory, (3) digit span, (4) RCF, and (5) CLOX. There was no significant difference between the KTx and no-KTx group’s baseline or follow-up cognitive functioning. In the KTx group, the only significant change was a slight improvement in CLOX [post-pre Tx 0.3 (1.0); p=0.04]. For survival analysis using PCA, we found that worse performance on Principal Component Executive functioning was associated with mortality in unadjusted analysis [HR: 0.82(0.68, 0.99); p = 0.04], however this became insignificant when adjusted for age, sex, diabetes, CKD vs ESRD status and KTx status
Selection Bias Analysis
To rule out selection bias, we compared the characteristics of the study cohort who had 2 cognitive evaluations (n=90) with patients who completed only baseline cognitive evaluation (n=55) and subsequently dropped out. The groups were similar in terms of demographics, comorbidities and renal function status, with the only significant difference being dialysis vintage duration [median 9.0 (4.5; 19.0) months for study cohort vs 45.0 (11.0; 61.0) months, p=0.01 for the drop-outs] (Supplementary Table 4). There was no significant difference in any of the baseline cognitive scores among the 2 groups (Supplementary Table 5).
Discussion
Our prospective study demonstrated that kidney transplantation was not associated with significant improvement in cognitive function among patients with advanced CKD/ESRD. In patients with advanced CKD/ESRD who were not transplanted, cognitive function remained stable over a mean follow-up of about 20 months. Multivariable stratified analysis demonstrated that diabetes and renal function status (CKD vs ESRD) were not associated with changes in cognitive performance over time, but lower age was associated with better performance on certain tests. We also found that poor attention/psychomotor speed and executive performance (as measured by Trails A and Stroop respectively) were significantly associated with higher mortality over a mean follow-up of 4.7 years, even after adjustment for age, sex, diabetes, CKD or ESRD status, and kidney transplant status.
Our study adds to existing literature by examining effect of transplant on cognitive function in a representative US CKD/ESRD population. While most studies have evaluated the effect of transplant in HD patients only, we extend these findings to non-dialysis dependent CKD patients, which comprised 76% of our cohort. A unique strength of our study was that both baseline and follow-up cognitive assessments were done in patients’ homes, away from distractions and acute effect of HD, as opposed to during HD or in a research office in prior studies, which by itself may have introduced bias [13–16]. There is emerging evidence that, in addition to the uremic mileu, HD procedure itself poses significant circulatory stress and causes irreversible ischemic brain injury in this already vascularly diseased population [23]. The longer dialysis vintage in our cohort compared to most other studies (median 5 yrs compared to mean 2.9±2.5 yrs in Gelb et al., 2.6±2.7 yrs in Griva et al., and 3.6±3.6 yrs in Harciarek et al.) may have contributed to lack of improvement in cognitive performance in our study [13, 14, 24]. Another possible explanation may be the patient characteristics of our cohort, which was slightly older than most other studies and may have chronic aging related changes in cerebrovascular structure/function that are not reversed by transplantation. We did find that older age was strongly associated with worse cognitive performance. Our patients also had a higher prevalence of diabetes, which is a risk factor for microvascular changes, although we did not see any differences in our stratified analysis, which was underpowered. Although the KTx group had more ESRD patients (34%) than the no-KTx group (15 %), we do not feel that this affected our results. It may be argued that cognitive function may be directly correlated with the severity of kidney disease. However, the baseline cognitive scores for the KTx and no-KTx groups were similar for all cognitive tests except CLOX 1 and 2, for which the KTx had slightly better scores. Moreover, there was no significant difference in post-transplant scores for any of the tests based on patients’ pre-transplant renal function status or association of CE#2 with renal function status in multivariable analysis.
The high prevalence of traditional vascular risk factors such as hypertension and diabetes in CKD/ESRD patients, may contribute to the high prevalence of clinical and subclinical cerebrovascular disease leading to abnormalities such as cerebral atrophy, silent brain infarcts and white matter hyper intensities [25–29]. Studies indicate cognitively impaired ESRD patients have more frequent and severe multiple infarct dementia, vascular dementia, and cerebrovascular lesions even in the absence of clinical stroke [30–33]. Such findings suggest that cerebrovascular disease is the primary cause of cognitive impairment in this population [23], thus not likely to be corrected or reversed by kidney transplant.
An interesting finding in our study was stable cognitive function in non-transplanted advanced CKD/ESRD patients over time; contrary to some studies suggesting decline in cognition in ESRD patients over a period of 2 years [15]. It may be that cognitive function is better preserved over time in non-dialysis CKD patients, which was the majority of our cohort. Additionally, the mean baseline IQ our cohort was 101±15.6 (near average per normative data) which may have contributed to better cognitive preservation or test performance over time. Alternatively, we might have selected a group of patients who are more cognitively intact, although we did not find any evidence of selection bias when comparing to patients who completed only baseline evaluation.
To our knowledge, none of the prior studies assessing cognitive function and mortality in CKD accounted for transplant status [27]. We found that poor baseline cognitive function, especially attention, psychomotor speed and executive functioning was independently associated with higher mortality. Despite reaching statistical significance, our results should be interpreted cautiously given the limited sample size and multiple testing in our study. Nevertheless, our findings are consistent with and add to prior literature, and may have important clinical implications in identifying high-risk patients [7].
Our study has several areas of strength. Ours is the largest study evaluating the effect of kidney transplant using a prospective study design. Moreover, the inclusion of a CKD/ESRD control group of patients who did not receive kidney transplant allows for better comparison between the transplant/non-transplanted groups and assessment of longitudinal changes in the non-transplanted group over time. Additionally, unlike most prior studies, we administered in-home cognitive tests both times, thus minimizing the effect of rapid fluid/electrolyte shifts and hemodynamic changes during HD on cognitive function as well providing a consistent testing environment across the two tests. Lastly, we accounted for depression and medications that may impair cognition, both of which may be important confounders in this patient population [34].
Our study has some limitations. We did not have longer term cognitive follow-up to evaluate the effect of transplant on cognition. However, the mean time from kidney transplant to follow-up evaluation in our study was 8.5±5.3 months, which should have allowed adequate time for stabilization of renal function and immunosuppressive drug levels post-transplant. Moreover, prior studies have demonstrated changes in cognitive function over a similar follow-up time interval [13, 14, 35]. Thus, longer follow-up would not be expected to change our results. Secondly, although a large number of patients who initially enrolled in the study failed to complete follow-up testing, we did not find any evidence of selection bias in terms of demographics, comorbidities, renal function status or baseline cognitive function. However, it is possible that there may be differences in other variables that were not captured in our study, thus limiting the generalizability of our findings Thirdly, the transplant allocation was non-random, and one could posit that those transplanted had higher baseline and follow-up cognitive performance and may be more likely to have a ceiling effect. However, we did not find any significant differences in baseline cognitive scores or follow-up scores for any of the measured domains in our cohort. Fourth, although we did not adjust for the effect of immunosuppressive medications on cognitive performance, a contemporary lower-immunosuppression protocol was the approach at our transplant center during the time period of this study. Lastly, because of the small number of deaths in our cohort, especially in the KTx group, we were not able to adjust for variables that may affect patient survival.
In conclusion, in a prospective study of CKD/ESRD patients receiving a kidney transplant compared with those who did not receive a transplant, we observed no significant improvement in cognitive function post- transplant. Moreover, we did not find any significant decline in cognitive function over time in those CKD/ESRD patients who were not transplanted. We did find poor cognitive function, especially attention, psychomotor speed and executive functioning, was independently associated with increased mortality.. More studies are needed to investigate determinants of cognitive function in this population and long term effects on cognitive function post kidney transplant.
Supplementary Material
Acknowledgments
We would like to thank Mary Fletcher, BS for help with all data management.
Sources of Support: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P30-DK-079307, R01DK077785 (Unruh) and American Heart Association grant 11FTF7520014 (Jhamb). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Sarnak MJ, Tighiouart H, Scott TM, et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology. 2013;80:471–480. doi: 10.1212/WNL.0b013e31827f0f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 3.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52:1863–1869. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 4.Seidel UK, Gronewold J, Volsek M, et al. The prevalence, severity, and association with HbA1c and fibrinogen of cognitive impairment in chronic kidney disease. Kidney Int. 2014;85:693–702. doi: 10.1038/ki.2013.366. [DOI] [PubMed] [Google Scholar]
- 5.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis. 1997;30:41–49. doi: 10.1016/s0272-6386(97)90563-1. [DOI] [PubMed] [Google Scholar]
- 6.Griva K, Stygall J, Hankins M, et al. Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis. 2010;56:693–703. doi: 10.1053/j.ajkd.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Drew DA, Weiner DE, Tighiouart H, et al. Cognitive function and all-cause mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2015;65:303–311. doi: 10.1053/j.ajkd.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurella Tamura M, Larive B, Unruh ML, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network trials. Clin J Am Soc Nephrol. 2010;5:1429–1438. doi: 10.2215/CJN.01090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornton WL, Shapiro RJ, Deria S, et al. Differential impact of age on verbal memory and executive functioning in chronic kidney disease. J Int Neuropsychol Soc. 2007;13:344–353. doi: 10.1017/S1355617707070361. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan AV, Kiernan MC. Neurological complications of chronic kidney disease. Nat Rev Neurol. 2009;5:542–551. doi: 10.1038/nrneurol.2009.138. [DOI] [PubMed] [Google Scholar]
- 11.Kramer L, Madl C, Stockenhuber F, et al. Beneficial effect of renal transplantation on cognitive brain function. Kidney Int. 1996;49:833–838. doi: 10.1038/ki.1996.115. [DOI] [PubMed] [Google Scholar]
- 12.Griva K, Hansraj S, Thompson D, et al. Neuropsychological performance after kidney transplantation: a comparison between transplant types and in relation to dialysis and normative data. Nephrol Dial Transplant. 2004;19:1866–1874. doi: 10.1093/ndt/gfh141. [DOI] [PubMed] [Google Scholar]
- 13.Griva K, Thompson D, Jayasena D, et al. Cognitive functioning pre- to post-kidney transplantation--a prospective study. Nephrol Dial Transplant. 2006;21:3275–3282. doi: 10.1093/ndt/gfl385. [DOI] [PubMed] [Google Scholar]
- 14.Harciarek M, Biedunkiewicz B, Lichodziejewska-Niemierko M, et al. Cognitive performance before and after kidney transplantation: a prospective controlled study of adequately dialyzed patients with end-stage renal disease. J Int Neuropsychol Soc. 2009;15:684–694. doi: 10.1017/S1355617709990221. [DOI] [PubMed] [Google Scholar]
- 15.Harciarek M, Biedunkiewicz B, Lichodziejewska-Niemierko M, et al. Continuous cognitive improvement 1 year following successful kidney transplant. Kidney Int. 2011;79:1353–1360. doi: 10.1038/ki.2011.40. [DOI] [PubMed] [Google Scholar]
- 16.Radic J, Ljutic D, Radic M, et al. Kidney transplantation improves cognitive and psychomotor functions in adult hemodialysis patients. Am J Nephrol. 2011;34:399–406. doi: 10.1159/000330849. [DOI] [PubMed] [Google Scholar]
- 17.Unruh ML, Sanders MH, Redline S, et al. Sleep apnea in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the Sleep Heart Health Study. J Am Soc Nephrol. 2006;17:3503–3509. doi: 10.1681/ASN.2006060659. [DOI] [PubMed] [Google Scholar]
- 18.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. URL http://www.R-project.org/ [Google Scholar]
- 19.Wickham H, Francois R. dplyr: A Grammar of Data Manipulation. R package version 0.4.1. 2015 Retrieved from http://CRAN.R-project.org/package=dplyr. [Google Scholar]
- 20.Subirana I, Sanz H, Villa J. Building Bivariate Tables: The compareGroups Package for R. Journal of Statistical Software. 2014;57(12):1–16. URL http://www.jstatsoft.org/v57/i12/ [Google Scholar]
- 21.Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2009. [Google Scholar]
- 22.Therneau T. A Package for Survival Analysis in S. version 2.38. 2015 <URL: http://CRAN.R-project.org/package=survival>. [Google Scholar]
- 23.McIntyre CW, Goldsmith DJ. Ischemic brain injury in hemodialysis patients: which is more dangerous, hypertension or intradialytic hypotension? Kidney Int. 2015;87:1109–1115. doi: 10.1038/ki.2015.62. [DOI] [PubMed] [Google Scholar]
- 24.Gelb S, Shapiro RJ, Hill A, Thornton WL. Cognitive outcome following kidney transplantation. Nephrol Dial Transplant. 2008;23:1032–1038. doi: 10.1093/ndt/gfm659. [DOI] [PubMed] [Google Scholar]
- 25.Kurella Tamura M, Xie D, Yaffe K, et al. Vascular risk factors and cognitive impairment in chronic kidney disease: the Chronic Renal Insufficiency Cohort (CRIC) study. Clin J Am Soc Nephrol. 2011;6:248–256. doi: 10.2215/CJN.02660310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seliger SL, Sarnak MJ. Subclinical vascular disease of the brain in dialysis patients. Am J Kidney Dis. 2007;50:8–10. doi: 10.1053/j.ajkd.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Weiner DE, Scott TM, Giang LM, et al. Cardiovascular disease and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis. 2011;58:773–781. doi: 10.1053/j.ajkd.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drew DA, Bhadelia R, Tighiouart H, et al. Anatomic brain disease in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 2013;61:271–278. doi: 10.1053/j.ajkd.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madero M, Sarnak MJ. Does hemodialysis hurt the brain? Semin Dial. 2011;24:266–268. doi: 10.1111/j.1525-139X.2011.00857.x. [DOI] [PubMed] [Google Scholar]
- 30.Lass P, Buscombe JR, Harber M, et al. Cognitive impairment in patients with renal failure is associated with multiple-infarct dementia. Clin Nucl Med. 1999;24:561–565. doi: 10.1097/00003072-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Morosanu AI, Alexa ID, Badescu M, Ilie AC. Correlation between cognitive impairment and cardiovascular risk factors in dialysis vs. non-dialysis elderly patients. Rev Med Chir Soc Med Nat Iasi. 2011;115:1057–1061. [PubMed] [Google Scholar]
- 32.Naganuma T, Uchida J, Tsuchida K, et al. Silent cerebral infarction predicts vascular events in hemodialysis patients. Kidney Int. 2005;67:2434–2439. doi: 10.1111/j.1523-1755.2005.00351.x. [DOI] [PubMed] [Google Scholar]
- 33.Nakatani T, Naganuma T, Uchida J, et al. Silent cerebral infarction in hemodialysis patients. Am J Nephrol. 2003;23:86–90. doi: 10.1159/000068034. [DOI] [PubMed] [Google Scholar]
- 34.Agganis BT, Weiner DE, Giang LM, et al. Depression and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis. 2010;56:704–712. doi: 10.1053/j.ajkd.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaya Y, Ozturkeri OA, Benli US, Colak T. Evaluation of the cognitive functions in patients with chronic renal failure before and after renal transplantation. Acta Neurol Belg. 2013;113:147–155. doi: 10.1007/s13760-012-0139-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.