Summary
Background
Retrospective evidence indicates that disease progression after first-line chemotherapy for metastatic non-small cell lung cancer (NSCLC) occurs most often at sites of disease known to exist at baseline. However, the potential benefit of aggressive local consolidative therapy (LCT) on progression-free survival (PFS) for patients with oligometastatic NSCLC is unknown.
Methods
We conducted a multicenter randomized study (NCT01725165; currently ongoing but not recruiting participants) to assess the effect of LCT on progression-free survival ((PFS). Eligible patients hadwere (1) histologic confirmation of (2) stage IV NSCLC, (3) ≤3 disease sites after systemic therapy, and (4) no disease progression before randomization. Front line therapy was ≥4 cycles of platinum doublet therapy or ≥3 months of inhibitors of epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) for patients with EGFR mutations or ALK rearrangements. Patients were randomized to either LCT ([chemo]radiation or resection of all lesions) +/− maintenance therapy versus maintenance therapy/observation only. Maintenance therapy was recommended based on a list of approved regimens, and observation was defined as close surveillance without cytotoxic therapy. Randomization was not masked and was balanced dynamically on five factors: number of metastases, response to initial therapy, central nervous system metastases, intrathoracic nodal status, and EGFR/ALK status. The primary endpoint was PFS, powered to detect an increase from 4 months to 7 months (hazard ratio [HR}=0.57) using intent-to-treat analysis. The plan was to study 94 randomized patients, with an interim analysis at 44 events. PFS, overall survival (OS), and time to develop a new lesion were compared between arms with log-rank tests.
Results
The study was terminated early after treatment of 49 patients (25 LCT, 24 control), when at a median follow-up time for PFS of 18.7 months, the median PFS time in the LCT group was 11.9 months (90% confidence interval [CI] 5.72 ,20.90) versus 3.9 months (90% CI 2.30, 6.64) in the maintenance group (HR=0.35, 90% CI 0.18,0.66, log rank p=0.005). Toxicity was similar between groups, with no grade 4–5 events. Grade 3 or higher adverse events in the maintenance therapy arm were fatigue (n=1) and anemia (n=1). In the LCT arm, Grade 3 events were: esophagitis (n=2), anemia (n=1), pneumothorax (n=1), and abdominal pain (n=1). Overall survival data are immature, with only 14 deaths recorded.
Interpretation
LCT +/− maintenance therapy for patients with ≤3 metastases from NSCLC that did not progress after initial systemic therapy improved PFS relative to maintenance therapy alone. These findings imply that aggressive local therapy should be further explored in phase III trials as a standard treatment option in this clinical scenario.
INTRODUCTION
Retrospective analyses of patterns of failure after first-line systemic therapy for metastatic non-small cell lung cancer (NSCLC) suggest that most progression events, either within or outside the central nervous system (CNS), occur only at sites of disease known to exist at baseline, rather than in new sites.(1) Consequently, for patients with limited numbers of metastases, ablation of those metastases may be advantageous in terms of cytoreduction or removal of dominant disease sites that may seed other sites in the future. Stage IV disease that is limited to only a small number of sites (“oligometastatic” disease) may reflect a more indolent phenotype that could benefit from local ablative therapy (e.g., surgery or radiation) for consolidation, as suggested by some preclinical and translational analyses.(2, 3) Several retrospective and small prospective trials have suggested that local therapy may be beneficial for patients with stage IV NSCLC presenting with limited metastases, (4–7) including a propensity-matched analysis demonstrating a survival benefit for patients receiving aggressive treatment.(8) However, such an advantage has yet to be shown in well controlled, randomized studies.
To address this gap, we conducted a multi-institutional, randomized, phase II study (NCT01725165) to evaluate progression-free survival (PFS) after aggressive local consolidative therapy (LCT) versus maintenance therapy or observation for patients with stage IV NSCLC with ≤3 metastases remaining after front line systemic therapy. As secondary and exploratory aims, we assessed: (1) safety and the incidence of high-grade toxicity, (2) overall survival (OS), (3) patterns of failure, and (4) time to development of disease at new metastatic sites, and (5) predictors of PFS.
PATIENTS AND METHODS
Study Design and Participants
The trial took place at three institutions: (1) The University of Texas MD Anderson Cancer Center (Houston, TX), (2) London Health Sciences Center (London, ON), and (3) The University of Colorado (Aurora, CO). This study was approved by the institutional review board at all participating sites, and each patient was required to provide written approved consent before enrollment. Recruited patients met the following criteria: (1) diagnosis of pathologically confirmed NSCLC, (2) stage IV disease according to the 7th edition of the American Joint Committee on Cancer staging system, (3) ≤3 metastases, not including the primary tumor (as defined below), (4) Eastern Cooperative Oncology Group (ECOG) performance status ≤2, (5) ≥18 years of age, and (6) receipt of standard front line systemic therapy, defined as (a) ≥4 cycles of platinum doublet chemotherapy, (b) erlotinib or another approved first line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor for ≥3 months if the patient was known to harbor an EGFR mutation, or (c) crizotinib for ≥3 months if the patient was known to have an anaplastic lymphoma kinase (ALK) rearrangement. Presuming that the patient met the above conditions, no exclusion criteria existed for prior therapy, other than that bevacizumab was not permitted within 2 weeks of the initiation of the radiation therapy course.
Laboratory values that were recommended to assess for adequate hematologic function included the following: absolute neutrophil count (ANC) ≥ 1,500/mm3, platelet count ≥ 100,000/mm3, WBC ≥ 3,000/ mm3, and hemoglobin ≥ 9 g/dL within 3 weeks of study entry. We also recommended adequate hepatic function as defined by a total bilirubin level ≤ 1.5 X the upper limit of normal (ULN), serum bilirubin ≤ 1.5× ULN in the setting of known Gilbert’s disease, and alkaline phosphatase, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 2.5 X the upper limit of normal or ≤ 5.0 × ULN if liver metastases were present. Patients with malignant pleural effusion or significant third-space fluid that could not be controlled by drainage were excluded. Patients who had a history of uncontrolled angina, arrhythmias, or congestive heart failure also were excluded. Patients who experienced a complete response to chemotherapy, with no lesions amenable to ablation (including the primary site), were also not eligible for randomization. Notably, while it was anticipated that the vast majority of patients would present with synchronous oligometastases, patients with metachronous metastases were not expressly excluded from the study.
Each lesion was counted separately and contributed to the total number of metastatic lesions, with the following three caveats. First, lesions were counted after the front line systemic therapy, and so lesions that resolved during that therapy (i.e., were no longer visible on computed tomography [CT] or avidity had resolved on positron emission tomography [PET]/CT) were not included in the total number. Second, any positive thoracic nodes (N1-N3) including the supraclavicular fossae were counted collectively as one lesion to account for the prognostic significance of this involvement; in other words, patients with nodal involvement could have a maximum of two additional sites of disease. Finally, patients who required immediate local therapy for CNS lesions could receive this treatment before randomization, but any lesion treated in this manner would be counted towards the total number of metastases. For example, if a patient had two brain metastases and received radiosurgery to those sites, that patient could then be randomized. However, both of these lesions counted towards the three that were allowed.
The study schema is depicted in Appendix, page 2. During the first 9 months of the study, patients were enrolled after they completed front line systemic therapy (n=11 patients). Thereafter, to facilitate accrual, another step was added in which patients could be enrolled during systemic therapy. At the end of that therapy, disease progression was evaluated by (1) systemic imaging (either CT of the chest/abdomen/pelvis or PET/CT) and (2) brain imaging (either CT or magnetic resonance imaging [MRI]). Patients with disease that did not progress after front line systemic therapy were then randomly assigned to one of two arms: (1) local consolidative therapy (LCT) followed by standard maintenance or observation or (2) standard maintenance therapy or observation only. Maintenance therapy was chosen by the treating physician from a predefined set of standard-of-care options.
Randomisation and Masking
Patients were randomized to one of two arms: LCT +/− maintenance therapy versus maintenance therapy/observation. The randomization was not masked, so both the patient and the provider were aware of the randomization assignment. The data managers and data analysts were also not masked to the treatment groups, in that they had access to the database and randomization website for the purpose of entering/analyzing data.
In lieu of stratification, the randomization was balanced dynamically on five prognostic covariates related to PFS per the method of Pocock and Simon.(9) The covariates were (a) number of sites of disease (0–1 vs. 2–3); (b) response to first-line systemic therapy (stable disease vs. partial response [PR]; (c) CNS metastases (yes vs. no); (d) intrathoracic nodal status (N0/N1 vs. N2/N3), and (e) EGFR/ALK mutation (yes vs. no). After a research nurse enrolled each patient and entered the above variables, randomization was conducted using the MD Anderson Department of Biostatistics Clinical Trials Conduct (CTC) website. Note that performing randomization through dynamic balance, rather than stratification, eliminates the need for the creation of multiple randomization lists which can grow exponentially with the addition of each stratification factor, and this strategy can be implemented efficiently through the MD Anderson CTC website.
Procedures
Patients who were randomized to the LCT arm were treated with the intent to ablate all residual disease (primary tumor, lymph nodes, and metastatic sites as appropriate) with surgery, radiation therapy, or both. The type of LCT was determined in consultation with multidisciplinary teams. The choice of dose-fractionation regimen was made by the treating radiation oncologist, with curative intent when possible. Stereotactic ablative body radiation (SABR), intermediate hypofractionated radiation (e.g., 15 fractions to the mediastinum), and concurrent chemoradiation were allowed. After LCT, the choice for maintenance therapy or observation (e.g. crossover) was made at the treating physician’s discretion. Patients on the LCT arm continued to be followed on this arm until progression, regardless of whether they were treated with systemic therapy after LCT.
Observation was defined as close surveillance, with follow-up as noted below but without any cytotoxic treatment. Because no single “standard” systemic approach has been accepted, and because the choice between maintenance therapy or observation typically depends on tumor histology and mutation status, the treating physicians for both treatment arms were given several recommended current FDA-approved regimens, including observation. Acceptable options for maintenance therapy included pemetrexed and bevacizumab (for non-squamous NSCLC), erlotinib, crizotinib (for patients with ALK rearrangement), and observation. Patients in the maintenance therapy/observation arm could also crossover and be treated with LCT at the time of progression. However, if crossover to LCT occurred prior to the time of progression, the event time was censored as described in the Statistical Analysis section below.
Patients in both arms were followed for adverse events and progression (with imaging) every 6 weeks (±2 weeks) after randomization for the first year, and then at the physician’s discretion thereafter. Acceptable follow-up tests included systemic imaging with either CT or PET/CT, and brain imaging (MRI or CT) if the patient had intracranial metastases. Progression was defined according to the Response Evaluation Criteria for Solid Tumors (RECIST) 1.1(10) and assessed by the treating physician and confirmed by an investigator of the study (principal investigator [PI], co-PI, or site PI). No laboratory monitoring was performed on this trial, and no dosing criteria were present for maintenance therapy or radiation therapy.
Outcomes
Primary Endpoint
The primary outcome, PFS, was defined from the time of randomization to the time of disease progression or death, whichever occurred first. For patients whose disease did not progress, PFS was evaluated by censoring patients at their most recent imaging.
Secondary Endpoints
Secondary outcomes for the study were overall survival (OS), safety/tolerability of LCT, time to progression of new and prior metastatic lesions, and quality of life (QOL). For OS, patients who were alive at the last contact date (with or without an imaging study at that time) were censored. Time to progression of new vs. prior lesions was defined by determining if the lesions identified at progression could be previously visualized on imaging. When performing our analysis at accrual closure, the endpoint that was of most clinical interest was the time to progression of new lesions, in that we wanted to determine if LCT was prolonging the development of new lesions (and thus acting through some mechanism in a similar way as systemic therapy). Thus, we elected to report only the time to new lesions in this manuscript. This endpoint was calculated from the time of randomization to the time to development of a previously unknown lesion. Therefore, if a patient progressed only within known sites, (s)he continued to be followed until a new lesion occurred.
With regard to the QOL outcome, patients were given an assessment tool as measured by the lung cancer module of the validated MD Anderson Symptom Inventory(11). Questionnaires were completed and collected at baseline (after randomization) and at each follow-up visit for the first year. Data was stored in the study database, to be analyzed at the completion of the trial. The endpoint for this secondary outcome was differences in quality of life between the LCT and maintenance therapy arms.
Statistical Analysis
This study is registered as a randomized controlled trial with ClinicalTrials.gov, NCT01725165). It was designed to assess if immediate LCT after induction therapy for oligometastatic NSCLC would reduce the rate of disease progression compared with maintenance therapy/observation only. Progression free survival was analyzed between the LCT and maintenance therapy arms using the log-rank test and through an intention to treat (ITT) analysis. Note that the log-rank test is a suitable method for comparing the survival distributions between study groups of interest, and is preferable when the proportional hazards model may be in question. In addition to meeting the inclusion/exclusion criteria above, patients needed to have included one imaging study for assessment of disease progression to be included in the analysis for progression. Disease progression was then subcategorized as locoregional (primary tumor or N1-N3 lymph nodes) or distant, and at a known lesion site (identified by imaging, biopsy or both) or a new lesion site. Finally, we used log-rank tests for an exploratory analysis comparing the time from randomization to the appearance of new lesions between treatment groups. Scaled Schoenfeld residuals were used to assess the proportional hazards assumption when necessary.(12)
The design had a one-sided 10% type I error and 90% power. The study was powered to detect an improvement in PFS from 4 months in the standard (no LCT) arm (chosen based on prior studies of maintenance therapy(13–17)) to 7 months in the experimental (LCT) arm, based on prior evidence suggesting 3 months as the difference in time to progression in known sites of disease as opposed to the time to develop any new site of disease in metastatic NSCLC, in sites potentially amenable to local ablative therapy(1); this corresponded to a hazard ratio (HR) of 0.57, or a 75% improvement in median PFS. Given these assumptions, the design required 94 patients randomized over 37.6 months, with an additional 9 months of follow-up. Patients were followed until their time of death, thus providing complete follow-up, or until their time of last contact, thus providing incomplete follow-up. The median follow-up time was derived using both complete and incomplete follow-up times.
Descriptive statistics were used to summarize patient characteristics by treatment arm. Continuous patient characteristics were compared with t tests. Chi-square or Fisher’s exact tests were used to compare categorical patient characteristics between treatment arms. Kaplan-Meier estimates of 1-year PFS and OS rates were provided for each treatment arm and for patient characteristics of interest. Log-rank tests were used to compare the PFS and OS distributions between the two treatment arms. Cox proportional hazards regression was considered when assessing PFS in univariable and multivariable analyses. Patients were considered for inclusion in efficacy analyses if they received at least one of the intended therapies. Patients in the maintenance arm who were crossed over to the LCT arm were censored at the time of crossover. High-grade toxicity (grade ≥3 events defined in the Common Terminology Criteria for Adverse Events [CTCAE] v4.0) was assessed in both arms as well. All statistical analyses were two-sided and based on the intent-to-treat principle, and P values <0.10 were considered statistically significant.
Data Safety Monitoring Committee
The Data Safety Monitoring Committee for this study was based at MD Anderson. Established prior to the study, this Committee comprised MD Anderson faculty, faculty from neighboring institutions, and community members. Both physicians and statisticians were included (12 total members), none of whom were investigators on the current study. An interim analysis was planned after 44 events for safety and futility. Annual reviews were also done, primarily for safety but also to assess major trends in outcomes that would affect the continuation of the trial.
Role of the Funding Source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The following investigators had access to the raw data: DRG, MH, JJL, and RY. The corresponding author had full access to all data in the study and has final responsibility for the decision to submit for publication.
Results
Patient Characteristics and Enrollment
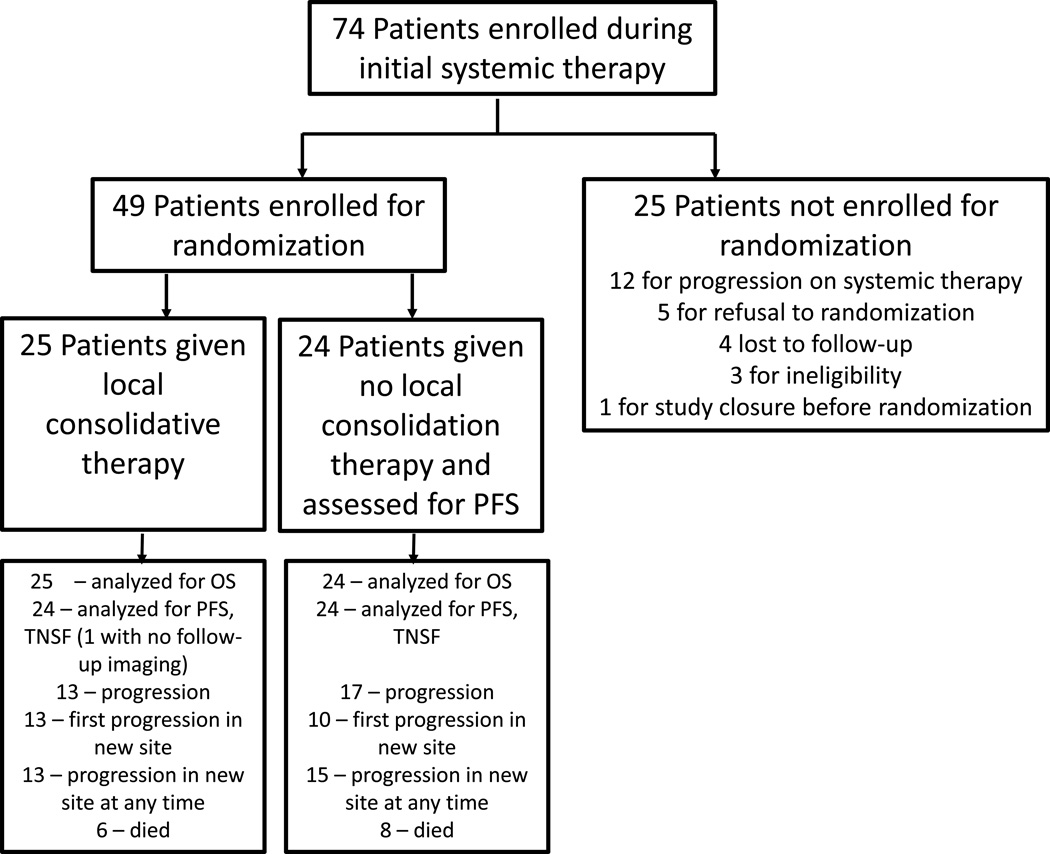
The study was open for enrollment from 11/28/12 to 1/19/16. All patients were enrolled either during or at the completion of front line systemic therapy. Data were analyzed as of April 1, 2016, and 49 evaluable patients were randomized (Figure 1) at this time. Of the 25 patients that were enrolled but not randomized, the most common reason was disease progression on systemic therapy. The study was closed as part of the annual analyses performed by the DSMB of all randomized trials at our institution, and prior to the planned interim analysis of 44 events. During the annual DSMB review, while a significant difference was not observed between arms in safety, there was a substantial efficacy improvement in the LCT arm. Specifically, the DSMB found that based on the current data, the probability of concluding in favor of the LCT arm was 99.46% if the current trend were to continue. Therefore, at this time, upon the recommendation of the DSMB, the principal investigators decided to close the study to new patient entry.
Figure 1.
CONSORT diagram.
Patient characteristics are shown in Table 1. The arms were well balanced in terms of sex, age, ethnicity, tumor histology, CNS involvement, number of non-regional metastases after front-line systemic therapy, EGFR /ALK status, response to first-line therapy, and nodal status. Three patients that were enrolled had previously treated (metachronous) primary tumors but then presented with metastases in liver, adrenal gland, and pleura.
Table 1.
Patient Characteristics
| LCT N = 25 (%) |
No LCT N = 24 (%) |
Total N = 49 (%) |
|
|---|---|---|---|
| Age | |||
| Mean ± SD | 64 ± 10 | 63 ± 10 | 63 ± 10 |
| Median (Min to Max) | 63 (43 to 83) | 61 (43 to 80) | 61 (43 to 83) |
| Sex | |||
| Male | 12 (48) | 10 (42) | 22 (45) |
| Female | 13 (52) | 14 (58) | 27 (55) |
| Ethnicity | |||
| White | 20 (80) | 18 (75) | 38 (78) |
| Black | 2 ( 8) | 3 (12) | 5 (10) |
| Hispanic | 2 ( 8) | 0 ( 0) | 2 ( 4) |
| Asian | 1 ( 4) | 3 (12) | 4 ( 8) |
| Tumor Histology | |||
| Adenocarcinoma | 21 (84) | 18 (75) | 39 (80) |
| Adenosquamous | 0 ( 0) | 1 ( 4) | 1 ( 2) |
| NSCLC, NOS | 1 ( 4) | 0 ( 0) | 1 ( 2) |
| Poorly Differentiated NSCLC, NOS | 2 ( 8) | 0 ( 0) | 2 ( 4) |
| SCC | 1 ( 4) | 4 (17) | 5 (10) |
| Sarcomatoid Carcinoma | 0 ( 0) | 1 ( 4) | 1 ( 2) |
| Synchronous | |||
| Metachronous | 1 ( 4) | 2 ( 8) | 3 ( 6) |
| Synchronous | 24 (96) | 22 (92) | 46 (94) |
| Non-Regional Metastases after Initial Systemic Therapy |
|||
| 0 to 1 | 17 (68) | 15 (62) | 32 (65) |
| 2 to 3 | 8 (32) | 9 (38) | 17 (35) |
| Response to First Line Chemotherapy | |||
| PR/CR | 9 (36) | 9 (38) | 18 (37) |
| SD | 16 (64) | 15 (62) | 31 (63) |
| CNS Metastases | |||
| No | 18 (72) | 18 (75) | 36 (73) |
| Yes | 7 (28) | 6 (25) | 13 (27) |
| Nodal Status | |||
| N0/N1 | 12 (48) | 11 (46) | 23 (47) |
| N2/N3 | 13 (52) | 13 (54) | 26 (53) |
| Mutation Type | |||
| None | 20 (80) | 21 (88) | 41 (84) |
| EGFR | 3 (12) | 3 (12) | 6 (12) |
| EML4ALK | 2 ( 8) | 0 ( 0) | 2 ( 4) |
Percentages may not add to 100% because of round-off error
Abbreviations: NSCLC, NOS, non-small cell lung cancer, not otherwise specified; SCC, squamous cell carcinoma; PR, partial response; CR, complete response; SD, stable disease; CNS, central nervous system
Patients underwent body (PET/CT or CT chest/abdomen/pelvis) and brain (MRI or CT) imaging to assess for disease progression prior to randomization. 13 patients (56%) in the LCT arm underwent PET/CT, and the rest underwent CT of the chest/abdomen/pelvis. In the maintenance therapy arm, 14 patients (52%) underwent PET/CT. Twenty patients (80%) had an MRI of the brain in the LCT arm, and the remainder had a CT scan of the brain. In the maintenance therapy arm, 21 patients (88%) underwent MRI. The location of the metastases counted at randomization for the 49 patients is shown in Appendix, page 3. The locations of metastasis by patient (not lesion) number are:: brain=13 patients, bone=10, adrenal gland=8, pleura=7, metastatic lung lesion=6, cervical lymph node=4, liver=2, spleen=2, retroperitoneal lymph nodes=1, paraspinal mass=1, kidney=1. Of the 13 patients with brain metastases, all patients except for one received treatment to the brain lesions prior to randomization. This final patient had a small, asymptomatic brain lesion that responded to systemic therapy, so it was observed. For six patients in the LCT group and five patients in the maintenance therapy arm, the only site of metastasis was the brain.
Patient treatment regimens are detailed in Appendix, page 4 and the patient numbers correspond to the patients listed in Appendix, page 3, with patients 1–25 on the LCT arm and 26–50 on the maintenance therapy/observation arm. The LCT regimens (combined primary and metastatic) were: hypofractionated RT/SABR, n=12 (48%); combination surgery and radiation, n=6 (24%); chemoradiation, n=2 (8%); combined hypofractionated RT and chemoradiation, n=3 (12%); surgery to all sites, n=1 (4%). With respect to the primary lung lesion, the local treatments were: intermediate or standard fractionated radiation (>10 fractions), n=14 (56%); SABR, n=5 (20%); surgery, n=3 (12%). Three patients did not undergo treatment to their primary site, two of which didn’t have an active primary tumor at the completion of front line systemic therapy and received treatment to only the metastatic site(s). One additional patient had been scheduled for surgery, but between randomization and preparation for surgery a CT scan revealed new metastases, and this patient therefore received systemic treatment but was analyzed on the LCT arm per intent to treat. Five patients (20%) on the LCT arm received maintenance therapy after LCT, three (with EFGR mutations) with erlotinib, one with crizotinib, and one with pemetrexed. One of the two patients with an ALK rearrangement did not receive maintenance therapy after the completion of LCT.
In the patients on the maintenance therapy/observation arm, the regimens were: pemetrexed, n=16 patients (67%); erlotinib, n=2 (8%); afatinib, n=1 (4%); bevacizumab, n=1 (4%); observation, n=4 (17%, 3 with SCC and one with sarcomatoid carcinoma).
Progression-Free Survival
At the time of data analysis, one patient had not had follow-up imaging for progression, and so 48 patients were included in the PFS analysis (24 in each arm). Three patients in the maintenance arm later received LCT because they could not tolerate maintenance therapy; these patients were analyzed in the maintenance arm until they were crossed over to LCT, at which time they were taken off study and censored. Fourteen patients had a PFS event on the LCT arm, and 17 on the maintenance therapy arm. The median follow-up time for the entire cohort was 12.39 months (interquartile range [IQR]: 5.52,20.30). The median follow-up time in the LCT arm was 13.44 months (IQR: 4.66,21.12), and in the maintenance therapy/observation arm was 11.32 months (IQR: 6.44,17.38). Follow-up time was not significantly different between the two groups (p=0.157)
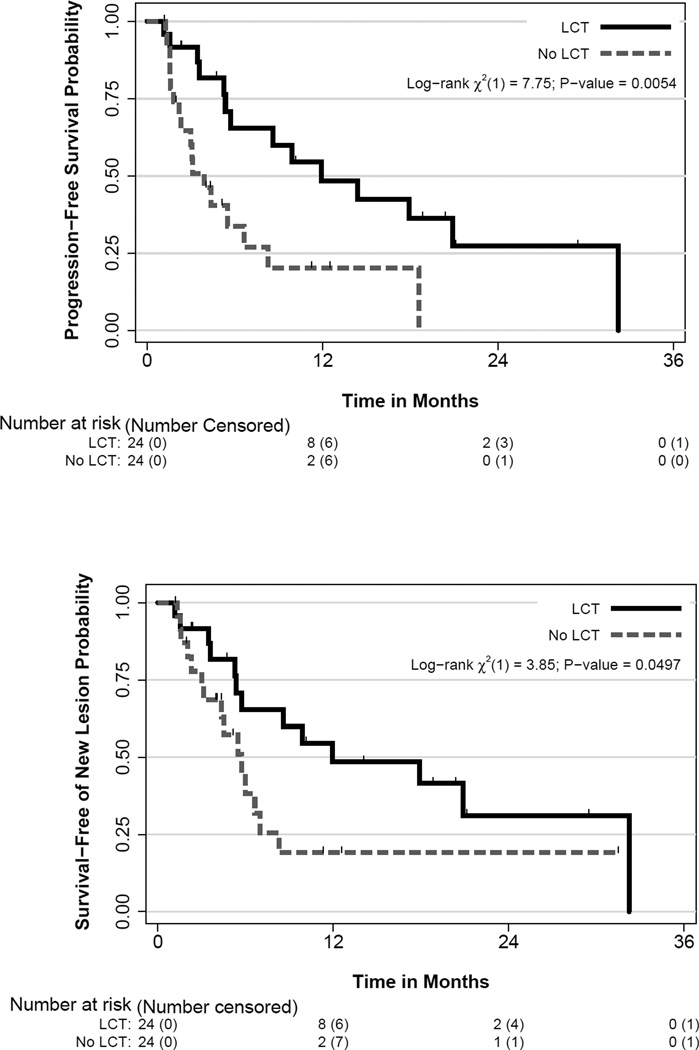
Patients in the LCT arm had significantly improved PFS: median PFS time was 11.93 months (90% CI 5.72,20.90) in the LCT arm and 3.9 months (90% CI 2.30,6.64) in the control arm, which corresponded to an HR for the LCT group of 0.35 (90% CI 0.18,0.66; log rank, p=0.005). The 1-year PFS rate was 48% in the LCT arm and 20% in the control arm (Figure 2a). Moreover, when only patients who received platinum-doublet chemotherapy were analyzed (i.e., excluding patients given anti-EGFR or -ALK), a statistically significant PFS improvement remained for the LCT arm (HR=0.41, 90% CI 0.21, 0.79, log-rank p=0.022). We did not analyze the effect of LCT on PFS in the 8 patients who did not receive platinum-based doublet chemotherapy (i.e. those with EGFR/ALK mutations) because such a small number would not be expected to yield meaningful results.
Figure 2.
(A) Progression-free survival and (B) time to appearance of disease at a new site by treatment groups. LCT, local consolidative therapy.
Overall Survival
Fourteen patients died during this study, 6 in the LCT arm and 8 in the no-LCT arm. All patients died of their lung cancer, except for one patient on the LCT arm who had a sudden cardiac death and was without evidence of disease. At this time, the OS data are immature, as the median survival time has not been reached in either group. An event chart outlining both progression events and deaths in both groups is presented in Appendix, page 7.
Patterns of Failure
Thirty patients (61%) experienced disease progression (13 in the LCT group and 17 in the no-LCT group). When examining patterns of failure by locoregional vs. distant, in the LCT arm they were: distant, n=10; locoregional, n=1; both, n=2. In the maintenance therapy arm they were: distant, n=6; locoregional, n=4; both, n=7. When evaluating patterns of failure by new vs. known (prior) lesions, in the LCT arm they were: new, n=11; both, n=2. In the maintenance therapy arm they were: known, n=7; new, n=3; both, n=7.
Salvage Therapy
Of the 17 patients with progressive disease in the no-LCT arm, 11 received local therapy at the time of progression (6 SABR, 3 surgery only, 1 surgery plus radiation, 1 SABR plus chemoradiation). Of the six patients who did not cross over to LCT, three received further systemic therapy, one chose to pursue a vaccine trial and non-cytotoxic therapy, one did not have local therapy because of poor performance status, and one experienced disease progression after two cycles of maintenance bevacizumab and thus was given palliative radiation at that time. Although the radiation was tolerated well, the disease continued to progress and the patient died approximately 3 months later.
Exploratory Predictors of Progression-Free Survival
Predictors of PFS are shown in Table 2. This analysis was done with the entire group rather than each arm separately owing to the limited number of events in each arm. The following variables were analyzed: treatment type, sex, timing of metastases, patterns of failure, sites of progression, number of non-regional metastases after front-line systemic therapy, response to front-line systemic therapy, CNS metastases, nodal status, and mutation type. Aside from treatment type, the only characteristics that correlated with PFS was EGFR/ALK status (HR=0.19, 90% CI 0.06–0.64, p=0.024).
Table 2.
Characteristics Evaluated for Association with Progression-Free Survival Outcomes
| No. of Patients** |
No. of Events |
Hazard Ratio (90% CI) |
1-Yr PFS Rate (90% CI) |
Log-Rank P Value |
|
|---|---|---|---|---|---|
| Treatment | |||||
| No LCT | 24 | 17 | Ref. | 0.20 (0.07, 0.38) | 0.005 |
| LCT | 25 | 14 | 0.35 (0.18, 0.66) | 0.48 (0.29, 0.66) | |
| Sex | |||||
| Male | 22 | 15 | Ref | 0.27 (0.11, 0.46) | 0.52 |
| Female | 27 | 16 | 0.79 (0.43, 1.45) | 0.41 (0.24, 0.58) | |
| Timing of Metastases |
|||||
| Metachronous | 3 | 2 | Ref | NE [NAR] | N/Ab |
| Synchronous | 46 | 29 | 0.83 (0.24, 2.78) | 0.36 (0.24, 0.50) | |
| Pattern of Failure | |||||
| None | 19 | 1 | Ref | 1.00 (NE [EF]) | N/Ab |
| Locoregional | 5 | 5 | 22.69 (3.73, 137.98) | 0.20 (0.02, 0.52) | |
| Distant | 16 | 16 | 19.13 (3.49, 104.83) | 0.19 (0.06, 0.37) | |
| Both | 9 | 9 | 49.57 (8.47, 290.04) | NE [NAR] | |
| Site of Progression | |||||
| None | 19 | 1 | Ref | 1.00 (NE) | N/Ab |
| Known Lesion | 7 | 7 | 40.10 ( 6.85, 234.78) | 0.14 (0.01, 0.41) | |
| New Lesion | 14 | 14 | 14.51 ( 2.62, 80.21) | 0.21 (0.07, 0.41) | |
| Both | 9 | 9 | 98.50 (16.02, 605.48) | NE [NAR] | |
| Non-Regional Metastases after Front Line Systemic Therapy |
|||||
| 0–1a | 32 | 19 | Ref | 0.38 (0.21, 0.54) | 0.24 |
| 2–3 | 17 | 12 | 1.54 (0.83, 2.85) | 0.28 (0.12, 0.48) | |
| Response to Front Line Systemic Therapy |
|||||
| PR/CR | 18 | 13 | Ref | 0.23 (0.08, 0.42) | 0.48 |
| SD | 31 | 18 | 0.77 (0.41, 1.42) | 0.42 (0.25, 0.58) | |
| CNS Metastases | |||||
| No | 36 | 24 | Ref | 0.28 (0.15, 0.43) | 0.60 |
| Yes | 13 | 7 | 0.80 (0.39, 1.64) | 0.54 (0.26, 0.75) | |
| Nodal Status | |||||
| N0–1 | 23 | 13 | Ref | 0.41 (0.20, 0.60) | 0.49 |
| N2–3 | 26 | 18 | 1.29 (0.70, 2.37) | 0.30 (0.15, 0.46) | |
| Mutation Type | |||||
| None | 41 | 29 | Ref | 0.24 (0.12, 0.38) | 0.012 |
| EGFR/ EML4ALK | 8 | 2 | 0.19 (0.06, 0.64) | 0.88 (0.50, 0.98) |
One patient in the LCT arm contributed zero follow-up time and was eliminated prior to estimating the HR
Patients categorized as having 0 metastatic lesions had had complete resolution or prior treatment to metastatic sites and thus were randomized to receive LCT to the primary tumor vs. maintenance therapy.
P values are not provided when there is insufficient data to make statistical comparisons
Abbreviations: CI, confidence interval; PFS, progression-free survival; LCT, local consolidative therapy; NE [NAR], not evaluable because no at-risk patients remained at 1 year; NE [EF], not evaluable because all patients were event-free at 1 year; PR, partial response; CR, complete response; SD, stable disease; CNS, central nervous system
We then assessed PFS within each treatment arm in the following variables: number of non-regional metastases after front line systemic therapy, response to front line systemic therapy, CNS metastases, and nodal status, even though this analysis was limited by a small number of events (Appendix, page 8). The only variable that was associated with improved PFS in this analysis was response to front line chemotherapy in the maintenance therapy/observation arm (log-rank p=0.062, HR=0.37, 90% CI=0.15–092).
Finally, the time to the appearance of a new lesion was longer among patients in the LCT arm (11.9 months vs. 5.7 months in the no-LCT arm; p=0.0497) (Figure 2B). A table displaying the relationship between the scaled Schoenfeld residuals versus time for the covariates in Table 2 is presented in Appendix, page 9 as an assessment of the proportional hazards assumption. Plots of the residuals versus time are depicted in Appendix, page 10–13. There was no evidence suggesting a strong violation of the proportional hazards assumption.
Toxicity
No patient in either arm experienced grade 4–5 toxicity. In the no-LCT arm, three patients crossed over because of toxicity (before disease progression), one with grade 2 elevations in creatinine and grade 2 fatigue, one with grade 3 fatigue, and one with grade 3 anemia. One other patient who remained on the no-LCT arm had significant bilateral lower extremity edema related to pemetrexed maintenance therapy, which resolved upon discontinuation of the pemetrexed.
Five patients in the LCT arm experienced grade ≤3 events, two with grade 3 radiation-induced esophagitis, both of which led to hospital admission and one to feeding tube placement (certainly related). One patient received radiation therapy to the spleen and developed anemia requiring transfusion 1 month after treatment was completed (likely related). One patient completed SABR for a lung lesion and 17 months later developed a pneumothorax secondary to a rib fracture (possibly related). The fifth patient had had a history of grade 2 radiation esophagitis that improved after treatment, but one month after radiation this patient was admitted for right upper quadrant pain thought to be related to gallstones (i.e., required hospitalization but not related to treatment).
Secondary Quality of Life Analysis
A total of 31 patients completed the MDASI questionnaires at baseline, 18 on the LCT arm and 13 on the maintenance therapy arm. However, by the second follow-up visit (at approximately 16 weeks), the number of patients completing these questionnaires had dropped to 6 on each arm. It was thus concluded that the results were insufficient at later time points to perform a formal analysis of the QOL data. In addition, as patients no longer completed the questionnaire when going off study, no differences could be elucidated after the time of progression, which also limited the utility of the results. Thus, no results from this secondary analysis will be reported.
Discussion
To the best of our knowledge, this is the first report of a randomized trial of aggressive LCT to all sites followed by standard maintenance therapy versus maintenance therapy alone for patients with oligometastatic NSCLC that did not progress after initial systemic therapy. The pertinent findings of this study were as follows. First, the PFS time was longer for patients who received LCT than for patients who did not, leading the Data Safety Monitoring Committee to recommend early study closure. Second, we found that LCT may also be able to prolong the time to progression of new sites of disease. Although this analysis was exploratory in nature, this finding is provocative and warrants further investigation. Third, LCT was feasible and was not associated with an increase in serious adverse events.
Several prior retrospective studies have supported a role for LCT in oligometastatic NSCLC, with selection criteria such as lymph node status,(18–20) tumor histology,(19, 21) thoracic disease bulk,(22) performance status,(8) and number of metastatic sites.(4) In addition, some studies have attempted to formulate prognostic models for survival in patients treated aggressively for oligometastatic disease. In one such study, 309 patients with ≤5 metastases who were treated aggressively with SABR were analyzed to determine which factors portended long-term survival. The authors identified several risk factors for poorer prognosis, including having nonadenocarcinoma histology, intracranial metastasis, synchronous disease, and being male.(23) However, without strong prospective results, the optimal treatment for patients with limited metastatic disease has thus been controversial. From one perspective, retrospective findings have supported this approach, and the advent over the past several years of more effective and tolerable maintenance chemotherapy regimens and technologic advances in SABR have improved the options available to such patients.
Nonetheless, retrospective non-randomized trials have many limitations that have been well described elsewhere.(24) The issue of immortal time bias (also known as survivor treatment selection bias) in particular is difficult to overcome when comparing patients who received LCT with those who did not, because the patients who were to receive LCT had to survive long enough to receive that treatment—the “immortal” period. Further, patients to be treated with LCT are often selected because of favorable risk factors, not all of which can be captured through standard survival analysis. Thus the decision of whether to treat aggressively or not is based not only on disease progression but also on whether the patient can tolerate induction chemotherapy, the presence of comorbid conditions, logistical factors, and patient/physician preferences, among others. Thus, a retrospectively observed survival benefit between those who receive LCT and those who do not may not be caused by the actual treatment, but rather may be a manifestation of the natural consequence of the disease. This is also a major reason why prospective studies are important for comparing outcomes between treatment options.
Indeed, single-arm phase II prospective trials examining aggressive therapy for oligometastatic NSCLC have been reported. In one analysis from Memorial-Sloan Kettering Cancer Center, patients with NSCLC and a solitary metastasis were treated with chemotherapy (mitomycin, vinblastine, and cisplatin) and resection of all sites of disease, with postoperative radiation therapy when appropriate. For the 23 patients enrolled on this trial, the median OS time was 11 months, and 2 patients (9%) had a survival time of at least 5 years.(25) In comparison, survival outcomes were modestly improved in another prospective study in which 39 patients with synchronous oligometastatic NSCLC received radical treatment to all sites of disease. Systemic therapy was not mandated, and solitary intracranial metastases were included. The median PFS time was 12.1 months, and the median OS time was 13.1 months. Although the treatment was well tolerated, no clinical characteristic was found to be correlated with PFS or OS.(26) Both of these trials were limited in their interpretation by the lack of a comparison arm. And although no studies have compared the role of comprehensive LCT for patients with non-progressing disease, one phase III trial examined the role of stereotactic radiosurgery to intracranial metastasis plus chemotherapy vs. chemotherapy alone in patients with up to 4 brain metastases. This trial was closed early because of slow accrual, but no statistically significant difference was found between symptomatic progression of brain metastases in the two arms.(27) It is difficult to elucidate from these prospective trials if aggressive local therapy offers a benefit or if the mixed conclusions are the result of variations in patient selection and treatment approaches.
In the current study, we found that the PFS time for the standard (maintenance therapy) group was almost exactly as had been hypothesized from prior studies (3.9 months observed vs. 4 months hypothesized). However, the PFS in the experimental (LCT) group was substantially longer than predicted (11.9 months observed vs. 7 months hypothesized), although this interval was in line with other recently reported single-arm phase II studies in similar contexts.(23, 28) Notably, our hypothesis was based on retrospective data in which sites of new disease versus sites of known disease could be followed more thoroughly for progression.(1) In addition, time to the appearance of a new lesion was longer for patients in the LCT arm than for in the no-LCT arm (11.9 months vs. 5.7 months, p=0.0497), suggesting that the LCT could be altering the natural history of the disease, either by limiting the potential for later spread or possibly by altering systemic anticancer immune responses to facilitate longer control of subclinical disease.
Other constraints on the interpretation of our trial results are as follows. First, because the study was stopped early, the overall number of patients was relatively small, thus limiting the statistical power of secondary subgroup analyses. Second, because of potential concerns with insurance approval and to make the study more pragmatic across institutions, physicians were allowed to select the imaging modality used for disease staging and follow-up from a limited number of choices (CT or PET/CT for body imaging, CT or MRI for brain imaging). Although all of these imaging approaches are acceptable for staging and surveillance, because our primary endpoint was PFS, we acknowledge that choice of imaging modality could have affected PFS time. However, given the effects of randomization and the approximately equivalent proportions of staging studies used after progression (52% received PET/CT in the LCT arm vs. 58% in the maintenance arm), we believe that any effect of differences in imaging between arms would have been minor.
Finally, the study population included patients with NSCLC of different histology and molecular subtype. These broad inclusion criteria were used because the feasibility of recruiting patients with oligometastatic disease to a prospective randomized trial had not been established, and several such trials in NSCLC over the past 5 years have been closed owing to low accrual.(29) Therefore, establishing which subgroups defined by various clinical or molecular criteria derive the greatest benefit or potential harm from this approach is not possible at this time. This question will be explored in ongoing correlative analyses of the current study and in future studies.
In conclusion, we found that LCT after initial systemic therapy was feasible, tolerable, and significantly extended PFS time (from 3.9 months in the control arm to 11.9 months) among patients with oligometastatic NSCLC. We further found that adding LCT also delayed the appearance of new lesions, implying that the benefit of consolidation may extend beyond known sites of disease. Our findings on OS are immature at this time. We recommend that LCT be further tested in larger phase III studies in which OS is the primary endpoint to further define which subgroups of patients are most likely to benefit.
Supplementary Material
Research in Context.
Evidence Before This Study
In 2011, we searched PubMed for all studies comparing local consolidative therapy to systemic therapy or observation alone in patients with oligometastatic NSCLC using the keywords “oligometastatic lung cancer” OR “oligometastatic lung cancer surgery” OR “oligometastatic lung cancer randomized” OR “limited metastasis lung cancer”. We did not limit our search to English speaking studies. In addition, we queried the abstracts of major international conferences, including ASTRO, ASCO, the World Lung Conference (IASLC), AACR, and ESTRO. We found retrospective and prospective single arm studies that assessed the role of aggressive local treatment in oligometastatic lung cancer. We also found that single-arm studies examining the role of aggressive local therapy that included many primary sites of disease. Finally, we discovered that several prospective randomized trials had been initiated and closed due to poor accrual, and that related studies were being planned but not yet close to completion. As a result of these queries, we initiated a trial that compared aggressive local therapy to maintenance therapy or observation (standard of care) in patients that did not progress after front-line systemic treatment.
Added Value of This Study
To the best of our knowledge, this is the first multi-institutional, phase II randomized trial comparing aggressive local consolidation therapy with maintenance therapy or observation for patients with oligometastatic NSCLC that did not progress on front line systemic therapy, the primary outcome of which was progression-free survival (PFS). The protocol was closed early by the institutional Data Safety Monitoring Committee when an interim analysis showed that local consolidative therapy extended the PFS time by 8 months. We further found in an exploratory analysis that local therapy prolonged the time to appearance of a new lesion, suggesting that control of known lesions can influence further metastatic spread, possibly by preventing further dissemination of known sites or by initiating a host response such as an immunologic reaction. Toxicity was no different between arms, with no grade 4 or 5 events in either arm. Overall survival data are immature, with only 14 deaths to date (8 in the standard arm, 6 in the experimental arm).
Implications of All of the Available Evidence
To our knowledge, our study is the first to demonstrate in a randomized fashion that aggressive local therapy improves time to progression in patients with oligometastatic NSCLC. Taken together with recent preclinical, retrospective, and single-arm prospective analyses, these findings provide a strong foundation to support the further exploration of this approach in phase III randomized studies, with overall survival as an endpoint and potentially with the incorporation of novel systemic agents such as immunotherapy.
Acknowledgments
Funding: MD Anderson Lung Cancer Priority Fund, MD Anderson Cancer Center Moon Shot Initiative, and Cancer Center Support (Core) Grant CA016672 from the National Cancer Institute, National Institutes of Health.
RCD reports personal fees from Loxo, Ariad, Pfizer, Clovis, and AstraZeneca, grants from Threshold, Ignyta, Mirati, , Abbott Molecular, OxOnx, and Loxo, all outside the submitted work.
We thank Suja Koshy, R.N., Denise Erdman, R.N., Monica Robischon, R.N., Albert Gratton, Qiuling Shu, Rensi Zacharia, M.D., and Monica Ramirez for their research support on the trial; Tommy Sheu, M.D., for publication support; and Christine Wogan, M.S., E.L.S., for editing this manuscript. We would also like to acknowledge the support of the generous philanthropic contributions to the University of Texas MD Anderson Lung Cancer Priority Fund and the MD Anderson Cancer Center Moon Shots Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 2016 American Society of Clinical Oncology Annual Meeting, June 3–7, Chicago, IL
Declaration of Interest: The other authors declared no conflicts of interest.
Author Contributions:
DRG - study design, data analysis, data interpretation, writing/editing
GRB - study design, data interpretation, writing/editing
JJL - study design, statistical analysis and interpretation, writing
MH - statistical analysis and interpretation, writing
RY - statistical analysis and interpretation, writing
DRC - study design, data analysis, data interpretation, writing/editing
RD - data analysis, data interpretation, writing/editing
FS - data analysis, data interpretation, writing/editing
LEG - study design, data analysis, data interpretation, writing/editing
DLG - study design, data analysis, data interpretation, writing/editing
JAK - data analysis, data interpretation, writing/editing
BDK - study design, data analysis, data interpretation, writing/editing
CT - data analysis, data interpretation, writing/editing
RK - study design, data analysis, data interpretation, writing/editing
AVL - data analysis, data interpretation, writing/editing
DAP - data analysis, data interpretation, writing/editing
AST - study design, data analysis, data interpretation, writing/editing
BS – study design, writing/editing
WNW - study design, data analysis, data interpretation, writing/editing
JZ - data analysis, data interpretation, writing/editing
QS – data analysis, data interpretation, writing/editing
XSW – study design, data analysis, data interpretation, writing/editing
SGS - study design, data analysis, data interpretation, writing/editing
JVH - study design, data analysis, data interpretation, writing/editing
References
- 1.Rusthoven KE, Hammerman SF, Kavanagh BD, Birtwhistle MJ, Stares M, Camidge DR. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol. 2009;48(4):578–583. doi: 10.1080/02841860802662722. [DOI] [PubMed] [Google Scholar]
- 2.Wong AC, Watson SP, Pitroda SP, Son CH, Das LC, Stack ME, et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT) Cancer. 2016 doi: 10.1002/cncr.30058. [DOI] [PubMed] [Google Scholar]
- 3.Lussier YA, Xing HR, Salama JK, Khodarev NN, Huang Y, Zhang Q, et al. MicroRNA expression characterizes oligometastasis(es) PLoS One. 2011;6(12):e28650. doi: 10.1371/journal.pone.0028650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salama JK, Chmura SJ, Mehta N, Yenice KM, Stadler WM, Vokes EE, et al. An initial report of a radiation dose-escalation trial in patients with one to five sites of metastatic disease. Clin Cancer Res. 2008;14(16):5255–5259. doi: 10.1158/1078-0432.CCR-08-0358. [DOI] [PubMed] [Google Scholar]
- 5.Inoue T, Katoh N, Aoyama H, Onimaru R, Taguchi H, Onodera S, et al. Clinical outcomes of stereotactic brain and/or body radiotherapy for patients with oligometastatic lesions. Jpn J Clin Oncol. 2010;40(8):788–794. doi: 10.1093/jjco/hyq044. [DOI] [PubMed] [Google Scholar]
- 6.Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer. 2010;69(3):251–258. doi: 10.1016/j.lungcan.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Khan AJ, Mehta PS, Zusag TW, Bonomi PD, Penfield Faber L, Shott S, et al. Long term disease-free survival resulting from combined modality management of patients presenting with oligometastatic, non-small cell lung carcinoma (NSCLC) Radiother Oncol. 2006;81(2):163–167. doi: 10.1016/j.radonc.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Sheu T, Heymach JV, Swisher SG, Rao G, Weinberg JS, Mehran R, et al. Propensity score-matched analysis of comprehensive local therapy for oligometastatic non-small cell lung cancer that did not progress after front-line chemotherapy. Int J Radiat Oncol Biol Phys. 2014;90(4):850–857. doi: 10.1016/j.ijrobp.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Mendoza TR, Wang XS, Lu C, Palos GR, Liao Z, Mobley GM, et al. Measuring the symptom burden of lung cancer: the validity utility of the lung cancer module of the M. D. Anderson Symptom Inventory. Oncologist. 2011;16(2):217–227. doi: 10.1634/theoncologist.2010-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therneau TGPM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 13.Patel JD, Hensing TA, Rademaker A, Hart EM, Blum MG, Milton DT, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol. 2009;27(20):3284–3289. doi: 10.1200/JCO.2008.20.8181. [DOI] [PubMed] [Google Scholar]
- 14.Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374(9699):1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 15.Perol MCB, Milleron J, Gervais R, Barlesi F, Westeel V, Crequit J, Lena A, Vergnenegre D, Perol D. Maintenance with either gemcitabine or erlotinib versus observation with predefined second-line treatmetn after cisplatin-gemcitabine induction chemotherapy in advanced NSCLC: IFCT-GFPC 0502 phase III study. J Clin Oncol. 2010;28(15s) doi: 10.1200/JCO.2011.39.9782. Abstract 7507. [DOI] [PubMed] [Google Scholar]
- 16.Paz-Ares LGFDM, Dediu M, Thomas M, Pujol J, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed SA, John WJ, Chouaki N, Zimmerman A, Visseren Grul CM, Gridelli C. PARAMOUNT: Phase III study of maintenance pemetrexed plus best supportive care versus placebo plus best supportive care immediatly following induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small cell lung cancer. J Clin Oncol. 2011;29 (abstract CRA7510) [Google Scholar]
- 17.Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 18.Billing PS, Miller DL, Allen MS, Deschamps C, Trastek VF, Pairolero PC. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg. 2001;122(3):548–553. doi: 10.1067/mtc.2001.116201. [DOI] [PubMed] [Google Scholar]
- 19.Bonnette P, Puyo P, Gabriel C, Giudicelli R, Regnard JF, Riquet M, et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest. 2001;119(5):1469–1475. doi: 10.1378/chest.119.5.1469. [DOI] [PubMed] [Google Scholar]
- 20.Hu C, Chang EL, Hassenbusch SJ, 3rd, Allen PK, Woo SY, Mahajan A, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer. 2006;106(9):1998–2004. doi: 10.1002/cncr.21818. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki A, Shirakusa T, Yoshinaga Y, Enatsu S, Yamamoto M. Evaluation of the treatment of non-small cell lung cancer with brain metastasis and the role of risk score as a survival predictor. Eur J Cardiothorac Surg. 2004;26(3):488–493. doi: 10.1016/j.ejcts.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 22.Lopez Guerra JL, Gomez D, Zhuang Y, Hong DS, Heymach JV, Swisher SG, et al. Prognostic Impact of Radiation Therapy to the Primary Tumor in Patients With Non-small Cell Lung Cancer and Oligometastasis at Diagnosis. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Vin T, Engels B, Gevaert T, Storme G, De Ridder M. Stereotactic radiotherapy for oligometastatic cancer: a prognostic model for survival. Ann Oncol. 2014;25(2):467–471. doi: 10.1093/annonc/mdt537. [DOI] [PubMed] [Google Scholar]
- 24.Palma DA, Salama JK, Lo SS, Senan S, Treasure T, Govindan R, et al. The oligometastatic state -separating truth from wishful thinking. Nat Rev Clin Oncol. 2014;11(9):549–557. doi: 10.1038/nrclinonc.2014.96. [DOI] [PubMed] [Google Scholar]
- 25.Downey RJ, Ng KK, Kris MG, Bains MS, Miller VA, Heelan R, et al. A phase II trial of chemotherapy and surgery for non-small cell lung cancer patients with a synchronous solitary metastasis. Lung Cancer. 2002;38(2):193–197. doi: 10.1016/s0169-5002(02)00183-6. [DOI] [PubMed] [Google Scholar]
- 26.De Ruysscher D, Wanders R, van Baardwijk A, Dingemans AM, Reymen B, Houben R, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450) J Thorac Oncol. 2012;7(10):1547–1555. doi: 10.1097/JTO.0b013e318262caf6. [DOI] [PubMed] [Google Scholar]
- 27.Lim SH, Lee JY, Lee MY, Kim HS, Lee J, Sun JM, et al. A randomized phase III trial of stereotactic radiosurgery (SRS) versus observation for patients with asymptomatic cerebral oligo-metastases in non-small-cell lung cancer. Ann Oncol. 2015;26(4):762–768. doi: 10.1093/annonc/mdu584. [DOI] [PubMed] [Google Scholar]
- 28.Iyengar P, Kavanagh BD, Wardak Z, Smith I, Ahn C, Gerber DE, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol. 2014;32(34):3824–3830. doi: 10.1200/JCO.2014.56.7412. [DOI] [PubMed] [Google Scholar]
- 29.Patel PR, Yoo DS, Niibe Y, Urbanic JJ, Salama JK. A call for the aggressive treatment of oligometastatic and oligo-recurrent non-small cell lung cancer. Pulm Med. 2012;2012:480961. doi: 10.1155/2012/480961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.