Abstract
Background/objective
The dietary inflammatory index (DII) measured at one time point is associated with risk of several chronic diseases but disease risk may change with longitudinal changes in DII scores. Data are lacking regarding changes in DII scores over time, therefore we assessed changes in the DII in the Women's Health Initiative (WHI).
Methods
DII scores were calculated using data from repeated food frequency questionnaires in the WHI Observational Study (OS; n=76,671) at baseline and Year 3, and the WHI Dietary Modification trial (DM; n=48,482) at three time points. Lower DII scores represent more anti-inflammatory diets. We used generalized estimating equations to compare mean changes in DII over time, adjusting for multiple comparisons; and multivariable linear regression to determine predictors of DII change.
Results
In the OS, mean DII decreased modestly from −1.14 at baseline to −1.50 at Year 3. In the DM, DII was −1.32 in Year 1, −1.60 in Year 3, and −1.48 in Year 6 in the intervention arm, and was −0.65 in Year 1, −0.94 in Year 3 and −0.96 in Year 6 in the control arm. These changes were modified by BMI, education, and race/ethnicity. A prediction model explained 22% of the variance in the change in DII scores in the OS.
Conclusion
In this prospective investigation of postmenopausal women, reported dietary inflammatory potential decreased modestly over time. Largest reductions were observed in normal weight, highly educated women. Future research is warranted to examine whether reductions in DII are associated with decreased chronic disease risk.
Keywords: dietary inflammatory index, changes over time, Women's Health Initiative, prediction, longitudinal trends
Introduction
Dietary index or pattern analysis can produce more intuitively appealing results that may improve prediction of disease risk as compared to examining individual foods or nutrients separately.1-4 Despite the growing use of dietary index or pattern analysis,5-7 relatively few studies have investigated the stability of dietary indices or patterns over time,8-14 or the factors influencing stability.15-17 To the best of our knowledge, this evaluation has not been conducted in relation to the inflammatory potential of diet.
Dietary behaviours are subject to change over time,11, 12 and they may influence chronic disease risk when they persist over time.8 Knowledge of the longitudinal stability of dietary patterns could aid researchers in planning follow-up measurements or, as Weismayer et al. indicated,11 the cost of maintaining such cohorts could be reduced if diet is proven to be stable over time (e.g., by reducing the necessity for frequent data collection).
The dietary inflammatory index (DII) was developed18 and construct validated19, 20 based on the evidence that many dietary factors have anti- or pro-inflammatory properties and the idea that no nutrient or food is consumed alone, but rather in conjunction with other nutrients. In the current study, we calculated the DII based on the food frequency questionnaire (FFQ) data collected in the Women's Health Initiative (WHI) Observational Study (OS) and WHI Dietary Modification trial (DM).21 Our goal was to examine the stability of the inflammatory potential of diet, and the predictors of change in DII scores over time.
Methods
Participants
The WHI was designed to address the major causes of morbidity and mortality among postmenopausal women. The design of the WHI has been described.22 Briefly, WHI investigators enrolled 161,808 postmenopausal women 50 to 79 years old with a predicted >3-year survival, in 40 sites in the United States between 1993 and 1998. Subjects were enrolled into the OS (n= 93,676) or one or more of four Clinical Trial (CT) groups (n=68,132) that included the DM (n=48,835). Women were excluded from the DM if their self-reported diets by FFQ at baseline were assessed to have <32% energy from fat.21 The WHI protocol was approved by the institutional review boards at the Clinical Coordinating Center (CCC) at the Fred Hutchinson Cancer Research Center (Seattle, WA) and at each of the 40 Clinical Centers.22
Dietary Assessment
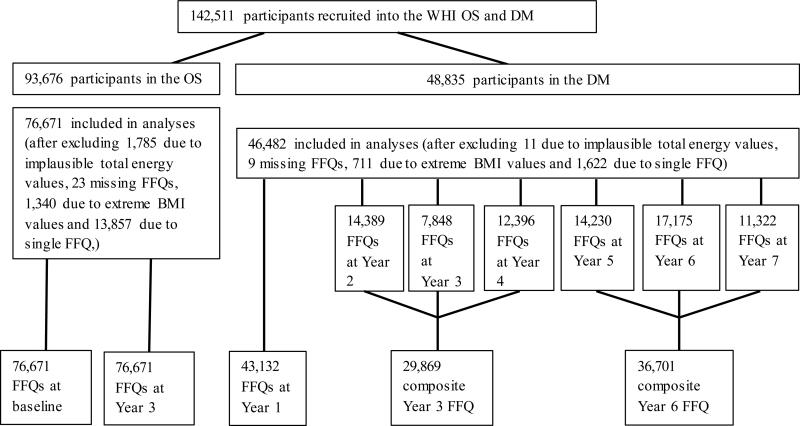
During screening, all participants completed a standardized self-administered 122-item FFQ developed for the WHI to estimate average daily nutrient intake over the previous three-month period, which served as the baseline measure. Figure 1 describes the administration of the WHI FFQ in the OS and DM. Follow-up measures included: an FFQ completed by all DM participants in Year 1; an FFQ completed annually from Year 2 until study end (approximately ten years) in a random third of DM participants; and an FFQ completed at Year 3 for ≈90% of OS participants. There was an average of two FFQs per participant in the OS and three FFQs per participant in the DM. In the DM, we created composite FFQs for Year 3 (by averaging the FFQs in Years 2, 3 and 4) and composite Year 6 (by averaging the FFQs administered in Years 5, 6 and 7). We excluded FFQs for Years 8, 9 and 10 due to small sample sizes after Year 7. We did not include baseline FFQ data from DM participants in the analyses due to the upwardly biased baseline mean percent energy from fat as a result of the >32% energy-from-fat eligibility criterion.23-25 FFQ data were considered complete if all adjustment questions, all summary questions, 90% of the foods, and at least one-half of every food group section was completed.22, 26 The nutrient database, linked to the University of Minnesota Nutrition Data System for Research (NDSR®),27 is based on the US Department of Agriculture Standard Reference Releases and manufacturer information. The WHI FFQ has produced results comparable to those obtained from four 24-hour dietary recall interviews and four days of food diaries recorded within the WHI.21
Figure 1.
Participant flow in the administration of food frequency questionnaires in the Women's Health Initiative (WHI) Observational Study (OS) and Dietary Modification trial (DM)
Description of the DII
The main outcome of interest is longitudinal change in DII scores. Details of the development18 and construct validation19, 20 of the DII have been described. Briefly, an extensive literature search was performed to identify articles published in peer-reviewed journals reporting on the association between specific foods and nutrients (components of the DII) and six inflammatory markers [interleukin (IL)-1β, IL-4, IL-6, IL-10, tumor necrosis factor alpha (TNFα), and C-reactive protein (CRP)]. A total of 1,943 eligible articles published through 2010 identified 45 food parameters (including whole foods, nutrients, and other constituents). All 1943 articles were indexed and scored to derive component-specific inflammatory effect scores.
Actual dietary intake data derived from baseline WHI FFQ were standardized to a representative global diet database constructed based on 11 datasets from diverse populations in different parts of the world. The standardized dietary intake data were then multiplied by the literature-derived inflammatory effect scores for each DII component, and summed across all components, to obtain the overall DII.18 A higher DII score indicates a more pro-inflammatory diet and a lower (i.e., more negative) DII score indicates a more anti-inflammatory diet. In the WHI FFQ, 32 of the 45 original DII components were available for inclusion in the overall DII. See Table 1 for the list of the 32 DII components included in the DII calculation. The DII has been construct validated in the WHI and found to significantly predict concentrations of IL-6 and TNFαR2.20
Table 1.
Characteristics of study participants, Women's Health Initiative
| Characteristic – N (%) for Categorical Variables | Observational Study (n=76,671) | Dietary Modification Trial intervention arm (n=18,604) | Dietary Modification Trial control arm (n=27,878) |
|---|---|---|---|
| Age groups (years) | |||
| <50-59 | 24144 (31.5) | 6832 (36.7) | 10203 (36.6) |
| 60-69 | 34293 (44.7) | 8681 (46.7) | 13033 (46.7) |
| 70-79 | 18234 (23.8) | 3091 (16.6) | 4642 (16.7) |
| Body mass index (kg/m2) | |||
| Normal ( <25) | 30577 (39.9) | 5230 (28.1) | 6820 (24.5) |
| Overweight (25.0 - <30) | 26605 (34.7) | 6534 (35.1) | 9940 (35.7) |
| Obese (≥30) | 19489 (25.4) | 6840 (36.8) | 11118 (39.9) |
| Race/ethnicity | |||
| Asian or Pacific Islander | 2102 (2.7) | 421 (2.3) | 645 (2.3) |
| African American | 4697 (6.1) | 1932 (10.4) | 2836 (10.2) |
| Hispanic/Latino | 2253 (3.0) | 661 (3.6) | 999 (3.6) |
| European American | 66331 (86.8) | 15263 (82.2) | 22916 (82.3) |
| Other | 1078 (1.4) | 286 (1.5) | 430 (1.6) |
| Educational level | |||
| Some high school or lower educational level | 814 (1.1) | 186 (1.0) | 332 (1.2) |
| High school graduate/some college or associate degree | 21209 (27.9) | 5703 (30.8) | 8609 (31.1) |
| ≥4 years of college | 54067 (71.0) | 12604 (68.2) | 18761 (67.7) |
| Smoking status | |||
| Never | 38661 (50.1) | 9502 (51.7) | 14386 (52.1) |
| Former | 32813 (43.3) | 7715 (50.0) | 11370 (41.2) |
| Current | 4242 (5.6) | 1169 (6.3) | 1842 (6.7) |
| Physical activity (PA), minutes/week | |||
| Not meeting PA recommendations | 39636 (52.2) | 10860 (65.2) | 16421 (65.7) |
| Meeting PA recommendations | 36254 (47.8) | 5797 (34.8) | 8567 (34.3) |
| NSAIDs use | |||
| No | 36819 (48.0) | 6687 (35.9) | 9732 (34.9) |
| Yes | 39852 (52.0) | 11917 (64.1) | 18146 (65.1) |
| DII* (baseline in OS, Year 1 in DM) - Average (SD) | −1.14 (2.58) | −1.32 (2.63) | −0.65 (2.64) |
NSAIDs=Non-steroidal Anti-inflammatory Drugs
DII components available in the WHI FFQ were: alcohol, beta carotene, caffeine, carbohydrates cholesterol, total energy, total fat, saturated fat, fiber, folic acid, iron, magnesium, niacin, riboflavin, thiamin, zinc, monounsaturated fatty acid(fa) polyunsaturated fa, omega 3 fa, omega 6 fa, trans fat, protein, selenium, vitamins B12, B6, A, C, D, E, onion, green/black tea, isoflavones; while the following components were not available in the WHI FFQ: ginger, turmeric, garlic, oregano, pepper, rosemary, eugenol, saffron, flavan-3-ol, flavones, flavonols, flavonones, anthocyanidins
Statistical analysis
We excluded participants with reported total energy intake judged to be implausible (<600kcal/d or >5000kcal/d) or with extreme body mass index (BMI) (<15kg/m2 or >50kg/m2) as well as those with ≤1 FFQ, leaving 76,671 in the OS and 46,482 in the DM for the final analyses (Figure 1). We computed mean DII scores at baseline and Year 3 in the OS and at three different time points in the DM (Year 1, composite Years 3 and 6); and used these measures to describe changes over time in the OS, and plotted DII scores on graphs for a visual appraisal of the longitudinal trend, separately for the intervention and control arms of the DM. Analyses were stratified by BMI, education, and race/ethnicity. To determine if there were significant differences between mean DII scores calculated at different time points, we constructed marginal linear regression models using generalized estimating equations (GEE) that adjusted for within-subject correlation in the DII measurements in order to calculate and compare all pair-wise contrast estimates between mean DII scores. The GEE was fit as a univariate model with time-from-baseline as the only independent variable and changes in the DII over time as the dependent variable. Adjustment for multiple comparisons was made using the Bonferroni approach. Within the DM, results were stratified by intervention arm.
Next, we utilized stepwise linear regression to construct the most parsimonious predictive multivariable model for change in DII from baseline to Year 3 in the OS. A previous WHI study investigated predictors of dietary change and maintenance in the DM and included intrapersonal, interpersonal, intervention characteristics and clinical center characteristics as predictors.15 The DM intervention moved participants toward an anti-inflammatory diet; therefore, predictors of dietary change investigated by Tinker et al. are likely to predict DII change in the DM. We therefore focused mainly on the potential predictors of DII change in the OS. We used a p-value of 0.10 for entry into and retention in the model. The stepwise approach identified variables that were included in a multivariable linear regression model to calculate beta (β) coefficients, corresponding p-values, and the R2 for the model's overall predictive ability. Participants with missing data in the predictors (n=3,438) were further excluded, leaving a final sample of 73,233 OS participants for the prediction model.
Analyses were conducted using SAS® version 9.3 (SAS Institute). All tests were 2-sided and p<0.05 was used as the cutpoint to signify statistical significance of parameter estimates.
Results
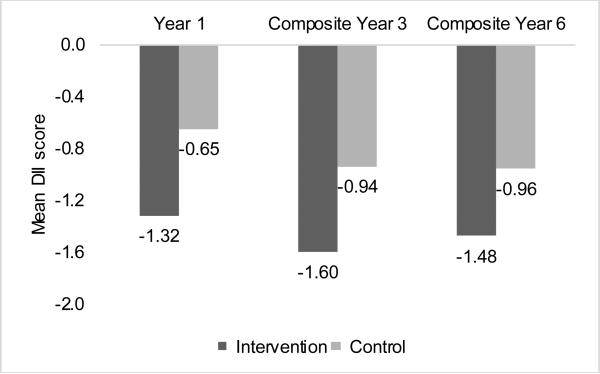
Participant characteristics were similar between OS and DM for race/ethnicity, educational level and smoking status. More OS (23.8%) than DM (16.7%) participants were ≥70 years; a higher proportion of participants in the DM (38.4%) than OS (25.4%) were obese (Table 1). In the total study population, the mean (±SD) DII at Year 3 was −1.32 (±2.71), and ranged from −7.30 to 5.78. In the OS, the mean overall DII decreased from −1.14 (±2.58) at baseline to −1.50 (±2.60) at Year 3. Figure 2 presents the overall mean DII scores over time in the DM. In the intervention arm, scores decreased from −1.32 (±2.63) at Year 1 to −1.60 (±2.59) at Year 3, then slightly increased to −1.48 (±2.63) at Year 6. In the control arm, the trend of DII decrease over time was similar to that observed in the intervention arm. Table 2 presents the means from multiple comparisons of DII scores across years of follow-up. While mean DII estimates were statistically significantly different across time in both intervention and control arms, except for Years 3 and 6 in the intervention arm, the average change in DII score was never greater than - 0.33, which represents a small fraction (≈2%) of the overall range in DII change in the DM (−9.14 to +8.83).
Figure 2.
Average dietary inflammatory index (DII) scores across years of follow-up in the Women's Health Initiative Dietary Modification trial, by study arm. The P-value for the difference in DII scores between intervention and control was <0.0001 at each time point. Numbers of participants were as follows: intervention: 17383, 11,895, and 14,399; control: 25749, 17,974, and 22,302; for Year 1, composite Year 3, and composite Year 6 respectively.
Table 2.
Pair-wise comparisons of the DII means across years of follow-up in the Dietary Modification Trial; Women's Health Initiative
| Visit year | Visit year | LS mean estimate (SE) | p-value | Bonferroni-adjusted p-value |
|---|---|---|---|---|
| Intervention arm | ||||
| 1 | 3 | −0.22 (0.020) | <0.0001 | <0.0001 |
| 1 | 6 | −0.17 (0.020) | <0.0001 | <0.0001 |
| 3 | 6 | 0.049 (0.021) | 0.0208 | 0.12 |
| Control arm | ||||
| 1 | 3 | −0.22 (0.016) | <0.0001 | <0.0001 |
| 1 | 6 | −0.33 (0.016) | <0.0001 | <0.0001 |
| 3 | 6 | −0.11 (0.017) | <0.0001 | <0.0001 |
NB: 1=year 1, 3=composite year 3, 6=composite year 6;
There was evidence for interaction between DII change and BMI, education, and race/ethnicity; analyses were further stratified by these variables. In the OS, normal-weight women experienced the largest decrease in DII between baseline and Year 3 [−1.39 (±2.55) to −1.81 (±2.54)] compared to obese women [−0.78 (±2.61) to −1.04 (±2.67)]; while women with ≥4y of college education showed the greatest change in DII [−1.39 (±2.51) to −1.77 (±2.52)] compared to women with less than a high school education, whose DII scores were more pro-inflammatory [0.26 (±2.71) to 0.06 (±2.71)]. Asians/Pacific Islanders experienced the largest change in DII [−1.76 (±2.53) to −2.04 (±2.51)], followed by European Americans [−1.25 (±2.52) to −1.63 (±2.53)].
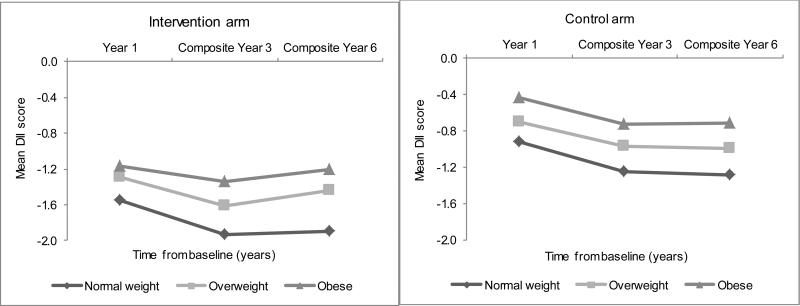
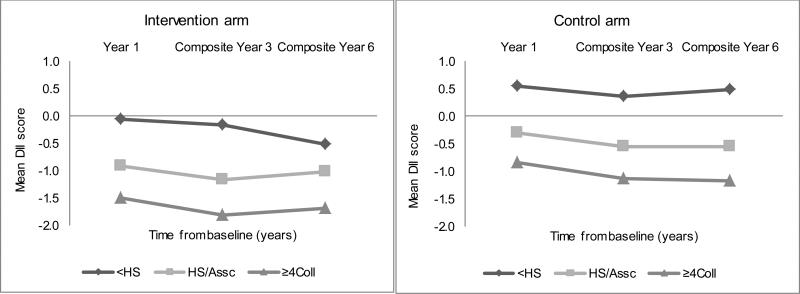
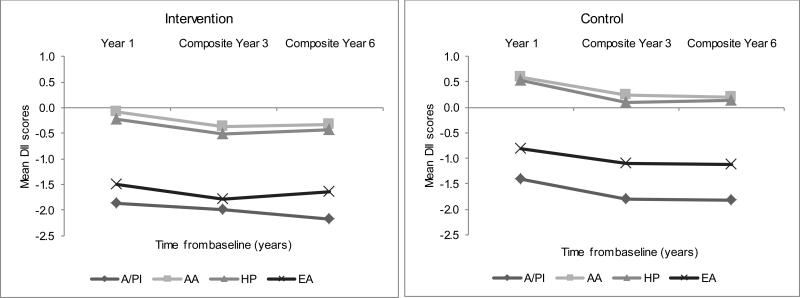
Figures 3, 4 and 5 present the corresponding longitudinal trends in the DM intervention and control arms, which paralleled those in the OS upon stratification by BMI, education, and race/ethnicity. Normal-weight women consistently experienced the largest decrease in DII scores over time, followed by overweight women, while obese women showed the smallest decrease in DII scores over time (Figure 3). Highly educated women experienced the most anti-inflammatory changes over time (Figure 4). Asians/Pacific Islanders showed the largest DII decreases consistent with a more anti-inflammatory diet over time, while African Americans and Hispanics showed the smallest changes, over time (Figure 5).
Figure 3.
Average dietary inflammatory index scores over time by body mass index category and Dietary Modification trial arm in the Women's Health Initiative.
Figure 4.
Average dietary inflammatory index (DII) over time by educational level and Dietary Modification trial arm in the Women's Health Initiative. <HS= High school graduate/some college or associate degree, HS/Assc= High school graduate/some college or associate degree, ≥4Coll = ≥4 years of college.
Figure 5.
Average dietary inflammatory index (DII) over time by race/ethnicity and Dietary Modification trial arm in the Women's Health Initiative. A/PI=Asian/Pacific Islander, AA=African American, HP=Hispanic, EA=European American
The final predictive ability of the model presented in Table 3 explained 22% of the variance in DII changes between baseline and Year 3 in the OS. Decreases in DII over time were predicted by baseline DII (having a higher baseline DII predicted a larger decrease in DII), being Asians/Pacific Islanders or European American, having BMI<25kg/m2, being more highly educated, being a nonsmoker, and meeting public health recommendations for physical activity.
Table 3.
Multivariable predictive model of change in dietary inflammatory index over time in the Observational Study; Women's Health Initiative
| Predictors | β (SE) | P-value (β) |
|---|---|---|
| Baseline DII | −0.44 (0.00) | <0.0001 |
| Body mass index (kg/m2) | ||
| Normal weight (>25) | referent | |
| Overweight(25 - <30) | 0.25 (0.02) | <0.0001 |
| Obese(>30) | 0.10 (0.02) | <0.0001 |
| Race/ethnicity | ||
| European American | referent | |
| African American | 0.48 (0.03) | <0.0001 |
| Asian or Pacific Islander | −0.17 (0.05) | <0.0001 |
| Hispanic | 0.68 (0.05) | <0.0001 |
| Other | 0.07 (0.06) | 0.28 |
| Educational level | ||
| ≥4 years of college | referent | |
| High school graduate/some college or associate degree | 0.31 (0.02) | <0.0001 |
| Some high school or lower educational level | 0.47 (0.10) | <0.0001 |
| Use of NSAIDs | ||
| Yes | referent | |
| No | 0.08 (0.01) | <0.0001 |
| Age group (years) | ||
| 50-59 | referent | |
| 60-69 | −0.04 (0.01) | <0.0001 |
| 70-79 | 0.02 (0.01) | 0.18 |
| Physical activity (minutes/week) | ||
| Meeting PA recommendation | referent | |
| Not meeting PA recommendation | 0.26 (0.02) | <0.0001 |
| Smoking status | ||
| Never | referent | |
| Former | −0.07 (0.02) | <0.0001 |
| Current | 0.24 (0.03) | <0.0001 |
| Hypertension status | ||
| No | referent | |
| Yes | 0.06 (0.01) | <0.0001 |
| Diabetes | ||
| No | referent | |
| Yes | 0.10 (0.02) | <0.0001 |
| Use of estrogen & progesterone | ||
| None | referent | |
| < 5y | 0.00 (0.02) | 0.91 |
| 5 to <10y | −0.10 (0.02) | 0.0001 |
| 10 to <15y | −0.09 (0.02) | 0.0002 |
| ≥15y | 0.10 (0.04) | 0.02 |
NB: Starting model for prediction include: baseline DII, education, BMI, race/ethnicity, study year, age, physical activity, smoking status, use of NSAIDs, statins, anti-depressants, history of hypertension, diabetes, cancer, use of estrogen, combined of use of estrogen and progesterone. The final model included: baseline DII, education, BMI, race/ethnicity, age, physical activity, smoking status, diabetes, and combined of use of estrogen and progesterone.
Discussion
Using data from both the WHI OS and DM, we described changes over time in the inflammatory potential of diet using DII scores. The DII score in the OS decreased modestly from baseline to Year 3, with an average change of −0.36 ± 2.35, representing about 2% of the full range of change in DII scores in the OS (−9.52 to 10.71). In women randomized to the DM intervention who had already made changes in diet from baseline to Year 1, DII scores remained relatively stable across the rest of the intervention from Year 1 to Years 3 and 6, and were similarly uniform in the control arm. In both the OS and DM, participants who reported the largest DII decrease (representing a transition toward an anti-inflammatory diet) had a normal BMI, a high educational level, and were more likely to be Asian/Pacific Islanders or European Americans. Those who reported the smallest decrease were obese, had less than high school education, and were more likely to be Hispanic or African Americans.
Highly educated women could be more exposed to information about healthier food choices and have better financial access to a wider variety of healthier food choices than women with lower educational levels. In a study on the longitudinal trends in diet over a 20-year period, diet quality improved with higher educational attainment.28 Chaix et al. observed that poorly educated participants shopping in supermarkets whose catchment areas included more poorly educated residents had higher BMIs or waist circumferences.29 Additionally, Drewnowski et al. found lower levels of education and incomes, among other factors, to be associated with higher obesity risk.30 These findings could partially explain our result showing that obese and less-educated participants experienced the smallest decreases in DII. The low DII scores in Asian/Pacific Islanders and European Americans compared to other race/ethnic groups may be due to different dietary patterns inherent in the cultures of racial/ethnic groups. For example, diets of most Asian populations contain numerous anti-inflammatory constituents and lack many of the pro-inflammatory substances in Western diets.31, 32 In the WHI, European American women have higher educational attainment33 and may be more willing to change their diets in keeping with recommendations.30
While the modest decrease in the dietary inflammatory potential from baseline to Year 3 in participants in the OS provided insights into changes in dietary behavior over time, the follow-up period was insufficient to draw conclusions regarding long-term changes in dietary behavior in an observational setting. Participants in the control arm of the DM were not asked to make dietary changes and were observed throughout the follow-up period; however, the trend in dietary behavior change over time in this group was similar, though smaller, to that observed in the intervention arm. Participants randomized to the control arm may have been motivated to change their diets prior to joining the study, and thus made personal efforts to improve their diets over time.
Some studies have examined the stability of dietary patterns over time;8-14 however, this is the first to study the stability of a dietary index describing the inflammatory potential over time. Previous studies reported inconsistent results on the stability of dietary behaviors over time, with some indicating stable behaviors after a short follow-up period of about 2 to 4 years,10, 14 and others reporting significant changes only after a moderately long follow up (e.g., ≥7 years).11, 13 Changes in diet over time may be due, in part, to the response to frequent updates to dietary guidelines, changes over time in the availability of different foods in some communities, and disease diagnosis that may alter dietary intake (e.g., diabetes or hypertension). Methodologic differences between studies would include differences in duration of follow-up, frequency and method of dietary assessment, and sample composition and size.
This study has several strengths including the relatively large population-based sample in the OS and DM, good regional and racial/ethnic representation, and inclusion of a large number of potential predictors of DII change. The DM had a relatively long follow-up duration with diet assessed annually on random subsamples of the study population.
Our study also had some limitations: FFQ data were not available in the OS after Year 3; thus we were not able to compare changes in dietary behaviour between the OS and DM beyond the first three years of follow up. The decrease in dietary inflammatory potential in the first three years may have been due to survey learning effects, in part attributed to social desirability bias, rather than a real improvement in diet quality. This limitation might have been mitigated had social desirability, an established source of bias of dietary self-report data, been measured in the WHI.34, 35 In our DM sample, not every participant had FFQ data at all three time points, which could have reduced the effect of survey learning as participants did not complete the FFQ every year. Although WHI enrolled only postmenopausal women, 23, 24 average DII scores in the WHI were comparable to other US populations that have been examined.19, 36
All of the 13 unavailable DII components are anti-inflammatory. Though we showed previously that reasonable predictive ability was retained when replacing 24-hour recall-derived DII scores with those derived from a structured questionnaire,19 there still may be a reduction in predictive ability in a population that was actively trying to change to a more healthful diet and therefore might be more likely to begin consuming these food items that are not on the FFQ list. Despite this limitation, in the construct validation of the DII in the WHI, the DII computed based on the 32 components available in the FFQ significantly predicted concentrations of inflammatory markers.20 Also, there was no reduction in the ability of the DII to predict interval changes in CRP levels in SEASONS study participants when using the 44 DII components available from up to fifteen 24-hour recall interviews to the 28 components available from five administrations of the 7DDR, a structured dietary assessment instrument.19 The DII score calculated from the 32 available components in the WHI validation study ranged from −7.30 to +5.78, which is higher than the range of −5.4 to +5.8 obtained in the SEASONS study using 44 of the 45 DII components,19 indicating that the range of DII may be more dependent on the amount of foods actually consumed rather than on the number of components available for scoring.
After including a comprehensive list of demographic, lifestyle and health-related factors, our final prediction model explained 22% of the variation in DII change in the OS. This represents reasonable explanatory ability when one considers that a change score is accompanied by large overall variance owing to the fact that the variance of a difference is the sum of the variance of the individual components37 (while the absolute difference can often be quite small). Other potential predictors of DII change that are outside the scope of the current study may include behavioural factors, such as those investigated by Tinker et al. in the prediction of dietary change and maintenance in the DM.15
In this population of postmenopausal women, the average DII decreased modestly over time in both the OS and in the DM (intervention and control group) participants from Year 1 to composite Year 6. In all three study groups, the extent of decrease was modified by BMI, education, and race/ethnicity. Baseline DII and several demographic, lifestyle and clinical factors significantly predicted changes in the inflammatory potential of diet in the first three years of follow up in an observational setting. Future research is warranted to examine whether reductions in DII scores over time are associated with decreased chronic disease risk.
Supplementary Material
Acknowledgements
Dr. Tabung and Dr. Hebert were supported by National Cancer Institute grants numbers F31CA177255 and K05CA136975, respectively. The WHI program was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no conflict of interest.
DISCLOSURE: Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings.
References
- 1.Hu FB. Dietary Pattern Analysis: A New Direction in Nutritional Epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Randall E, Marshall JR, Graham S, Brasure J. Patterns in Food Use and their Associations with Nutrient Intakes. Am J Clin Nutr. 1990;52(4):739–745. doi: 10.1093/ajcn/52.4.739. [DOI] [PubMed] [Google Scholar]
- 3.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean Diet Attenuates Inflammation and Coagulation Process in Healthy Adults: The Attica Study. J Am Coll Cardiol. 2004;44(1):152–158. doi: 10.1016/j.jacc.2004.03.039. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Jacques PF, Tucker KL. Are Dietary Patterns Useful for Understanding the Role of Diet in Chronic Disease? Am J Clin Nutr. 2001;73(1):1–2. doi: 10.1093/ajcn/73.1.1. [DOI] [PubMed] [Google Scholar]
- 5.McCullough ML. Diet Patterns and Mortality: Common Threads and Consistent Results. J Nutr. 2014 doi: 10.3945/jn.114.192872. doi: 10.3945/jn.114.192872. [DOI] [PubMed] [Google Scholar]
- 6.Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF. Higher Diet Quality is Associated with Decreased Risk of All-Cause, Cardiovascular Disease, and Cancer Mortality among Older Adults. J Nutr. 2014 doi: 10.3945/jn.113.189407. doi: 10.3945/jn.113.189407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller PE, Cross AJ, Subar AF, Krebs-Smith SM, Park Y, Powell-Wiley T, Hollenbeck A, Reedy J. Comparison of 4 Established DASH Diet Indexes: Examining Associations of Index Scores and Colorectal Cancer. Am J Clin Nutr. 2013;98(3):794–803. doi: 10.3945/ajcn.113.063602. doi: 10.3945/ajcn.113.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulder M, Ranchor AV, Sanderman R, Bouma J, van den Heuvel WJ. The Stability of Lifestyle Behaviour. Int J Epidemiol. 1998;27(2):199–207. doi: 10.1093/ije/27.2.199. doi: 10.1093/ije/27.2.199. [DOI] [PubMed] [Google Scholar]
- 9.Mishra GD, McNaughton SA, Bramwell GD, Wadsworth ME. Longitudinal Changes in Dietary Patterns during Adult Life. Br J Nutr. 2006;96(4):735–744. [PubMed] [Google Scholar]
- 10.Borland SE, Robinson SM, Crozier SR, Inskip HM. SWS Study Group. Stability of Dietary Patterns in Young Women over a 2-year Period. Br J Clin Nutr. 2008;62(1):119–126. doi: 10.1038/sj.ejcn.1602684. [DOI] [PubMed] [Google Scholar]
- 11.Weismayer C, Anderson JG, Wolk A. Changes in the Stability of Dietary Patterns in a Study of Middle-Aged Swedish Women. J Nutr. 2006;136(6):1582–1587. doi: 10.1093/jn/136.6.1582. [DOI] [PubMed] [Google Scholar]
- 12.Bertheke PG, de Vente W, Kemper HC, Twisk JW. Longitudinal Trends in and Tracking of Energy and Nutrient Intake over 20 Years in a Dutch Cohort of Men and Women Between 13 and 33 Years of Age: The Amsterdam Growth and Health Longitudinal Study. Br J Nutr. 2001;85(3):375–385. doi: 10.1079/bjn2000249. [DOI] [PubMed] [Google Scholar]
- 13.Newby PK, Weismayer C, Akesson A, Tucker KL, Wolk A. Long-Term Stability of Food Patterns Identified by use of Factor Analysis among Swedish Women. J Nutr. 2006;136(3):626–633. doi: 10.1093/jn/136.3.626. [DOI] [PubMed] [Google Scholar]
- 14.Northstone K, Emmett PM. Are Dietary Patterns Stable Throughout Early and Mid-childhood? A Birth Cohort Study. Br J Nutr. 2008;100(05):1069–1076. doi: 10.1017/S0007114508968264. doi: doi:10.1017/S0007114508968264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tinker LF, Rosal MC, Young AF, Perri MG, Patterson RE, Van Horn L, et al. Predictors of Dietary Change and Maintenance in the Women's Health Initiative Dietary Modification Trial. J Am Diet Assoc. 2007;107(7):1155–1165. doi: 10.1016/j.jada.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Davis NJ, Ma Y, Delahanty LM, Hoffman HJ, Mayer-Davis E, Franks PW, et al. Diabetes Prevention Program Research Group Predictors of Sustained Reduction in Energy and Fat Intake in the Diabetes Prevention Program Outcomes Study Intensive Lifestyle Intervention. J Acad Nutr Diet. 2013;113(11):1455–1464. doi: 10.1016/j.jand.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hébert JR, Wirth M, Davis L, Davis B, Harmon BE, Hurley TG, et al. C-Reactive Protein Levels in African Americans: A Diet and Lifestyle Randomized Community Trial. Am J Prev Med. 2013;45(4):430–440. doi: 10.1016/j.amepre.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and Developing a Literature-derived, Population-based Dietary Inflammatory Index. Public Health Nutrition. 2013;17(8):1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutrition. 2013;17(8):1825–1833. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. 2015;25(6):398–405. doi: 10.1016/j.annepidem.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement Characteristics of the Women's Health Initiative Food Frequency Questionnaire. Ann Epidemiol. 1999;9(3):178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 22.Women's Health Initiative Study Group Design of the Women's Health Initiative Clinical Trial and Observational Study. Con Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 23.Prentice RL, Mossavar-Rahmani Y, Huang Y, Van Horn L, Beresford SA, Caan B, et al. Evaluation and Comparison of Food Records, Recalls, and Frequencies for Energy and Protein Assessment by Using Recovery Biomarkers. Am J Epidemiol. 2011;174(5):591–603. doi: 10.1093/aje/kwr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuhouser ML, Tinker L, Shaw PA, Schoeller D, Bingham SA, Horn LV, et al. Use of Recovery Biomarkers to Calibrate Nutrient Consumption Self-Reports in the Women's Health Initiative. Am J Epidemiol. 2008;167(10):1247–1259. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- 25.Ritenbaugh C, Patterson RE, Chlebowski RT, Caan B, Fels-Tinker L, Howard B, Ockene J. The women's health initiative dietary modification trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9, Supplement):S87–S97. doi: 10.1016/s1047-2797(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 26.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103(3):323–328. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- 27.Nutrition Coordinating Center at the University of Minnesota M, MN Nutrition Data System for Research (NDSR) University of Minnesota, Minneapolis, MN; Minneapolis, MN: 2013. [Google Scholar]
- 28.Sijtsma FP, Meyer KA, Steffen LM, Shikany JM, Van Horn L, Harnack L, et al. Longitudinal Trends in Diet and Effects of Sex, Race, and Education on Dietary Quality Score Change: The Coronary Artery Risk Development in Young Adults Study. Am J Clin Nutr. 2012;95(3):580–586. doi: 10.3945/ajcn.111.020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaix B, Bean K, Daniel M, Zenk SN, Kestens Y, Charreire H, et al. Associations of Supermarket Characteristics with Weight Status and Body Fat: A Multilevel Analysis of Individuals within Supermarkets (RECORD Study). PLoS One. 2012;7(4):e32908. doi: 10.1371/journal.pone.0032908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drewnowski A MA, Jiao J, Aggarwal A, Charreire H, Chaix B. Food Environment and Socioeconomic Status Influence Obesity Rates in Seattle and in Paris. Int J Obes (Lond) 2014;38(2):306–314. doi: 10.1038/ijo.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenner DR, Boucher BA, Kreiger N, Jenkins D, El-Sohemy A. Dietary Patterns in an Ethnoculturally Diverse Population of Young Canadian Adults. Can J Diet Pract Res. 2011;72(3):e161–168. doi: 10.3148/72.3.2011.e161. [DOI] [PubMed] [Google Scholar]
- 32.Garduño-Diaz SD, Khokhar S. South Asian Dietary Patterns and their Association with Risk Factors for the Metabolic Syndrome. J Hum Nutr Diet. 2013;26(2):145–155. doi: 10.1111/j.1365-277X.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- 33.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women's Health Initiative Recruitment Methods and Results. Ann Epidemiol. 2003;13(9, Supplement):S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 34.Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social Desirability Bias in Dietary Self-report May Compromise the Validity of Dietary Intake Measures. Int J Epidemiol. 1995;24:389–398. doi: 10.1093/ije/24.2.389. [DOI] [PubMed] [Google Scholar]
- 35.Hebert JR, Ma Y, Clemow L, Ockene IS, Saperia G, Stanek EJ, 3rd, et al. Gender Differences in Social Desirability and Social Approval Bias in Dietary Self-report. Am J Epidemiol. 1997;146(12):1046–1055. doi: 10.1093/oxfordjournals.aje.a009233. [DOI] [PubMed] [Google Scholar]
- 36.Wirth MD, Burch J, Shivappa N, Steck SE, Hurley TG, Vena JE, Hébert JR. Dietary Inflammatory Index Scores Differ by Shift Work Status: NHANES 2005 to 2010. J Occup Environ Med. 2014;56(2):145–148. doi: 10.1097/JOM.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snedecor GW, Cochran WG. Statistical Methods. 8 edn Vol. 276. Iowa State University Press; Ames Iowa: 1989. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.