Abstract
The habenula (Hb) is a central structure connecting forebrain to midbrain regions. This microstructure regulates monoaminergic systems, notably dopamine and serotonin, and integrates cognitive with emotional and sensory processing. Early preclinical data have described Hb as a brain nucleus activated in anticipation of aversive outcomes. Evidence has now accumulated to show that Hb encodes both rewarding and aversive aspects of external stimuli, thus driving motivated behaviors and decision-making. Human Hb research is still nascent but develops rapidly, alongside with the growth of neuroimaging and deep brain stimulation techniques. Not surprisingly Hb dysfunction has been associated with psychiatric disorders, and studies in human patients have established evidence for Hb involvement in major depression, addiction and schizophrenia, as well as in pain and analgesia. Here we summarize current knowledge from animal research, and overview the existing human literature on anatomy and function of the Hb. We also discuss challenges and future directions in targeting this small brain structure in both rodents and humans. By combining animal data and human experimental studies, this review addresses the translational potential of preclinical Hb research.
Keywords: habenula, reward, addiction, depression, human, rodent
INTRODUCTION
The habenula (Hb) is a bilateral epithalamic structure, evolutionary conserved among vertebrates (1–3). This small brain nucleus is composed of two subdivisions –the medial (MHb) and the lateral habenula (LHb)- and has a central anatomical position in the brain connecting the forebrain to the ventral midbrain and hindbrain (4, 5). The Hb regulates midbrain monoaminergic systems, notably dopamine and serotonin, and integrates cognitive with emotional and sensory processing.
A keystone study in rhesus monkeys originally described the structure as a brain nucleus that is activated in anticipation of aversive outcomes, or failure to obtain reward, and in turn suppresses motor behavior (6). Hb function has since attracted increasing attention in both neuroscience and the clinic. Preclinical data have now accumulated to show that Hb encodes both rewarding and aversive aspects of external stimuli. The general view from animal research is that habenula activity prevents behaviors leading to negative reward such as punishment, while reinforcing behaviors with positive reward value (7), thus driving motivated behaviors and decision-making (8). Consequent to this highly integrative function, Hb also contributes to learning and memory (9) as well as a range of other behaviors (8, 10). Not surprisingly, therefore, Hb dysfunction has been associated with psychiatric disorders, and studies in human patients have established evidence for Hb involvement in major depression (11, 12), addiction (11, 13) schizophrenia (14) and Attention Deficit Hyperactivity Disorder (ADHD) (15), as well as in pain and analgesia (10).
Although still limited, human Hb research is expected to develop rapidly in the next decade, while knowledge on Hb anatomy, connectivity and function in non-human primates and rodents is increasing exponentially (16). Here we briefly summarize current knowledge from animal research, and extensively review the existing human literature on Hb structure and function. Focus is on psychiatric disorders (main text), and a section on pain and analgesia is also proposed (Supplementary Materials). We will also discuss the translational potential of preclinical research to understand Hb function in humans and for psychiatry.
ANATOMY
Rodents
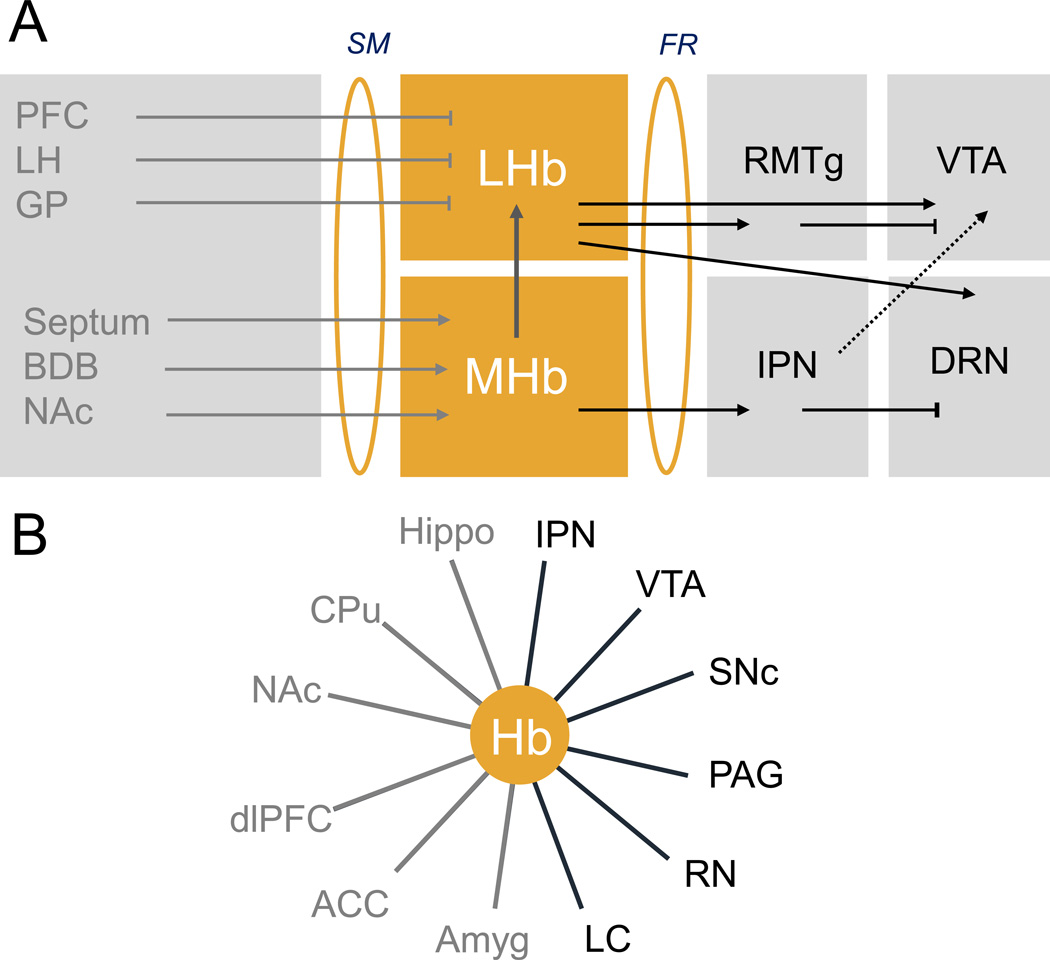
Most knowledge on Hb connectivity, as well as morphology and neurochemistry of Hb neurons, stems from studies in animals. In brief, retrograde and anterograde tracing studies in rodents (4) and electrophysiological studies in non-human primates (5) have provided a detailed description of afferent and efferent connections of the Hb complex, summarized in Figure 1. Because of their distinct input/output structures, LHb and MHb seem to form parallel channels regulating the information flow from forebrain to midbrain.
Figure 1. Habenula connectivity in rodents and humans.
Key pathways connecting medial habenula (MHb) and lateral habenula (LHb), the two subdivisions of the Hb, to other brain structures. Habenula connectivity is embedded in brain circuits classically described as reward and emotion circuits, whose dysfunction is associated to psychiatric diseases reviewed here. A. Structural connectivity in animal studies: The lateral Hb (LHb) receives inhibitory inputs from the prefrontal cortex (PFC), ventral pallidum, globus pallidus (GP) and lateral hypothalamus (LH) through the stria medullaris (SM) and, in turn, sends information to monoaminergic nuclei (5). Projections of LHb to dopaminergic neurons have been best described, and include direct (ventral tegmental area (VTA), see (100)) and indirect (tail VTA, see (101, 102)) projections. A recent tracing study further revealed an equal number of LHb projections to either dopaminergic (VTA) or serotonergic (dorsal raphe (DR) and median raphe nucleus (MnR)) nuclei, which are mostly but not exclusively segregated, indicating that LHb regulates the two monoamine nuclei either independently (the vast majority of LHb projecting neurons) or jointly (few heterogeneously distributed LHb projecting neurons) (103); both projections are excitatory (11, 104). The medial Hb (MHb) circuitry is less well known. The medial nucleus receives mainly excitatory inputs from the septum, nucleus accumbens (NAc) and broca diagonal band (BDB) (4, 5). and has excitatory projections to the rostromedial tegmental nucleus (RMTg) but mainly and massively to the interpeduncular nucleus (IPN), which in turn projects to the VTA and possibly the raphe nuclei (104). Thus, both MHb and LHb regulate in turn the VTA, DRN and possibly other midbrain and hindbrain structures such as the Locus Coereulus (LC) (103). Asymmetrical projections from MHb to LHb have been described (17). B- Functional connectivity in human studies: Hb connectivity established for both forebrain (in grey) and midbrain/hindbrain (in black) structures by fMRI (10, 21, 105). Abbreviations: CPu: caudate Putamen; Hippo: Hippocampus; Amyg: Amygdala; SNC: substantia nigra compacta; PAG: periaqueductal gray; RN: Raphe Nucleus; LC: locus coeruleus; ACC: anterior cingulate cortex.
Electrophysiology and morphological analyses of rat Hb slices show distinct intrinsic circuitries within the two nuclei, confirming different information processing at the two sites, and also reveals asymmetrical MHb projections to LHb within the Hb complex (17). The latter observation, which deserves further investigation, suggests potential interactions across the two circuitries whose functional implications remain unknown. Whether similar parallel and potentially interacting LHb/MHb networks operate in humans is unknown.
The analysis of LHb cytoarchitecture in rat brain slices shows high morphological heterogeneity, which is unrelated to electrophysiological characteristics of the neurons (18). The latter appear surprisingly homogenous throughout the LHb nucleus, and include neuron populations with silent, tonic or bursting spontaneous activities, as well as neuroglialform cells that could be interneurons (18). MHb cells are classified into only two types based on their dendritic morphology and, regardless from their anatomy, all show similar electrophysiological activity (17). Notably, the latter study also shows the existence of asymmetrical projections from MHb to LHb only (17).
Immunostaining, in situ hybridization and anterograde tracing experiments show that LHb neurons are mostly glutamatergic, with some GABAergic neurons (19). LHb neurons are also characterized by heterogeneous expression of monoaminergic receptors across sub-nuclei, mainly dopaminergic D2 receptors and serotonin 5-HT2C receptors (19). Similarly, MHb contains mainly glutamatergic neurons distributed into three phenotypically distinct populations, i. e. neurons expressing glutamate alone or co-expressing either substance P or acetylcholine (19, 20).
Humans
Anatomical description of Hb in the human brain remains limited. As for the rodent Hb, the human Hb is also located next to the third ventricle above the thalamus, and is approximately 5–9 mm in diameter with a total volume in the 30–36 mm3 range (21) (mouse Hb is 0.8 mm in height and width for comparison (22)). Histology of post-mortem human brain shows partition of Hb into medial and lateral parts, connected by the Hb commissure, similarly to the partition observed in rodents (23). Another morphologic and immunohistochemical analysis showed that, overall, the MHb subnuclear organization in humans is similar to that observed in rodents, whereas the shape, relative size, and intranuclear organization of the LHb show significant difference (24). One important difference resides in the substantially enlarged dorsal part of the human LHb that shows GABA-B receptors immunoreactive cells. This growth in size possibly indicates increased influence of limbic and striatal afferents into the LHb of humans compared to rodents (24).
Apart from these post-mortem studies and due to its particularly small size, the human Hb was difficult to investigate structurally until recently. Ultra-high resolution Magnetic Resonance Imaging (hr-MRI) at 7 Tesla (7T) now allows researchers to visualize and explore the structure non-invasively.
Using 7T hr-MRI, Strotmann et al. were able to discriminate MHb, LHb and the habenular commissure in vivo (25), and also explored structural connectivity of the Hb. Tractography analysis of diffusion weighted MRI data revealed fiber tracts connecting Hb to other brain regions for both MHb (anterior posterior direction, in the form of the retroflexus fasciculus identified in rodents) and LHb (anterior posterior direction and superior inferior direction) (25). The general topography of Hb connecting forebrain and mid/hindbrain, therefore, appears similar in rodents and humans. In another study these authors used 7T ultra hr-MRI ex vivo to further differentiate subnuclei within the Hb. High resolution T1 and T2 weighted images with 300 and 60µm isotropic resolution, respectively, revealed LHb heterogeneity with two distinct lateral and medial substructures (26). Ideally, these ex vivo results should help interpreting in vivo structural MRI data (25, 26).
Because an increasing number of functional MRI studies, performed at 3T, are reporting neural activation of human Hb (see next sections), it is critical to isolate this structure from adjacent thalamic areas. A study by Lawson et al. (27) offers a set of guidelines to anatomically define the Hb for in vivo hr-MRI at 3T in conjunction with a stereotactic atlas of the human brain. This analysis in native space, as opposed to voxel-wise approaches, aims at minimizing reductions in spatial specificity and avoiding localization errors during pre-processing (27).
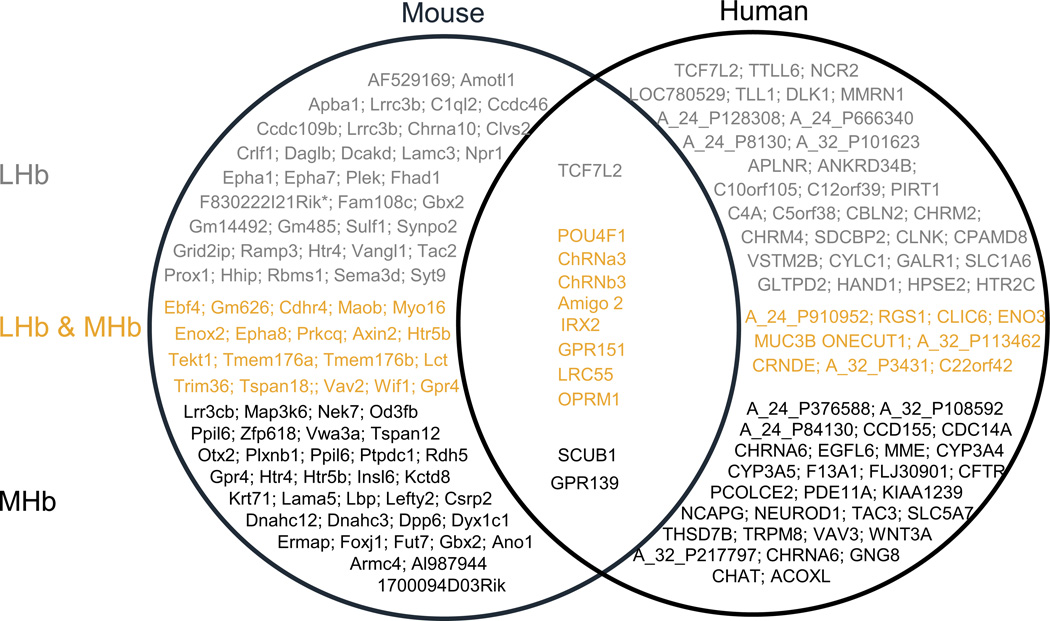
Overall, the ability to identify human Hb and its connections by using MRI and tractography has largely confirmed neuroanatomical findings in experimental animals (see Pain and Analgesia below) and, altogether, supports the notion that structural and functional habenula characteristics are essentially translatable from rodents to humans. Interestingly also, transcriptome analysis identifies genes expressed in both rodent and human MHb and/or LHb, which also have translational potential for Hb research (Figure 2).
Figure 2. Gene transcriptome in the habenula.
Genome-wide gene expression studies in rodents show differing expression patterns between LHb and MHb, as exemplified by Wagner et al. study (106) or large-scale gene mapping studies (see Allen Brain Atlas or GENSAT). In our own analysis, data extracted from both Allen Brain Atlas and Brain Star show the top-100 genes with strongest expression in mouse (left) and human (right) transcriptomes. Genes from these groups detected in lateral habenula (LHb, in grey), medial habenula (MHb, in black) or both (in yellow) are indicated. Our analysis (Figure 2) of mouse databases confirms differential gene expression in MHb and LHb, with only a small pool of common genes detected across the two Hb subdivisions. As for the mouse, our analysis of highly expressed human genes using the same AllenBrain and BrainStar databases, (Figure 2) unveils differential gene expression in LHb and MHb in the human brain, supporting the notion of separate functions for the two main Hb nuclei. Interestingly, comparison of mouse and human transcriptome data reveals a cluster of highly expressed Hb genes common to humans and rodents. This cluster includes Gpr139 encoding an orphan G protein coupled receptor and Scub1 encoding a ribosomal protein highly expressed in MHb, Tcf7l2 encoding a transcription factor best detected in LHb across species, and several other genes encoding notably the mu opioid receptor, the orphan receptor GPR151 or subunits of the nicotinic acetylcholine receptors that are well detected in both subdivisions of the Hb in rodents and humans. All these genes expressed in both species have translational value for rodent Hb research and potential clinical developments.
DEPRESSION
Rodents
In animal research, the notion that LHb hyperactivity is associated to depressive-like symptoms, while LHb inhibition improves depressive-like behaviors, is well established (reviewed in (12)). In the late 80’s, a first rat study showed elevated desoxyglucose metabolism in LHb across three behavioral models of depression (28, 29). Among main further findings, an LHb lesion study showed reduced depressive-like behaviors and increased 5-HT turnover in the DRN of rats subjected to chronic stress procedures (30). These findings were replicated using other procedures. Similar consequences of LHb lesion were reported in a 6-OHDA rat model of parkinson’s disease (31), while on the contrary, LHb activation using a 5-HT2C agonist decreased monoamine levels and increased depressive-like behaviors in hemiparkinsonian rats (32). Pharmacological inhibition of LHb by the GABA agonist muscimol had antidepressant effects in congenital helpless rats (33), and opposite metabolic alterations in Hb (high) and VTA (low) were observed in these rats (34). A very recent study also showed that enhancement of GABA-GIRK function in the LHb ameliorates depressive-like behaviors in mice (35).
Further evidence stems from Deep Brain Stimulation (DBS) experiments in rats. Repeated stimulation of LHb afferences in animals displaying learned helplessness suppressed synaptic drive onto VTA-projecting LHb neurons and increased escape behavior in an active avoidance task (36). Hb DBS also improved depressive-like behaviors and increased monoamine concentrations (dopamine and serotonin) in rats exposed to chronic mild stressors (37). Rats also showed reduced anxiety levels and increased motivation for food when LHb was stimulated, (38), substantiating the notion that DBS treatment of the LHb effectively improves depressive symptoms in rats. LHb DBS in a rat model of depression was further shown to alter signaling pathways involving Ca2+/calmodulin-dependent protein kinase, glycogen synthase kinase 3 and AMP-activated protein kinase, and the phosphorylation status of these molecules was associated with the antidepressant actions of DBS (39).
Optogenetic stimulation of GABAergic and glutamatergic neurons projecting to the LHb indicated that LHb activity is controlled by co-release of the two neurotransmitters (40). The GABA/Glu ratio was reduced in a mouse model of depression, and in contrast, mice chronically treated with an antidepressant showed a high GABA/glutamate co-release ratio. Further supporting to the notion that inhibition/activation balance of LHb activity is key to mood control and depression. Finally, a recent 18FDG-PET live imaging study in the rat showed coordinated increased metabolic activity in septum (projecting on MHb) and Hb during uncontrollable stress that correlated with subsequent learned helplessness behavior (41).
Humans
Human research has identified Hb as a brain structure contributing to mood disorders. An early PET study showed enhanced coupling between Hb and raphe activities in volunteer patients experiencing transient depressive relapse upon tryptophan depletion (42). This report provided first evidence for Hb implication in mood regulation in humans. Further data reporting structural changes in depressed patients are emerging. A post-mortem histological study showed decreased volume of both LHb and MHb in depressed patients, diagnosed with major depression disorder (MDD) or bipolar disorder (BD), showed a reduction of neuron number in the Hb (23). The authors also processed post-mortem tissues from schizophrenic patients and found no change (23), suggesting that robust structural Hb alterations are specific to depressive states. Another study using hr-MRI at 3T to analyze Hb volumes also showed a decrease of Hb volumes for unmedicated BD patients, as well as unmedicated female MDD patients (21). Another volumetric MRI study reported increased volume of Hb white matter for women with a first episode of MDD (43). Recently, a structural MRI study used grey matter MR images to predict the diagnostic status of treatment resistant depression subjects compared to healthy controls (44). In this study, major brain regions supporting the diagnosis classification were caudate, insula and Hb. Finally, a 7 T MRI study linked the increase of Hb volumes with disease severity in unmedicated major depressive patients, but not in medicated individuals, further supporting that changes in Hb volumes are linked to disease development (45). Of note is that volumetric changes of Hb have not been reported in animals to date, therefore mechanisms underlying this phenomenon have not been studied yet. Whether structural changes in Hb relate to functional modifications in depressed patients remains open.
Despite the paucity of Hb-focused human functional MRI data in the area of depression, there is evidence that the Hb is activated in aversive learning (46, 47). Using high-resolution fMRI in conjunction with a reinforcement learning paradigm, Lawson et al. (46) demonstrated positive habenula responses to the changing values of cues signaling punishments (painful electric shocks). Another study investigated the role of the dopaminergic midbrain (mainly the VTA) and Hb in the processing aversive events in humans. Using high-resolution cardiac-gated fMRI (3T), the authors measured functional activity in the VTA and Hb as well as other midbrain structures while participants were experiencing rewarding, aversive and neutral stimuli. Results showed strong Hb activation, as well as increased functional coupling between Hb and VTA in response to aversive stimuli (47). Although none of these studies directly addresses depressive states, it is possible that Hb overactivity upon chronic aversive learning contributes to the development of structural modifications observed in MDD and BD patients. A recent high resolution fMRI study examining Hb responses to potential and experienced negative outcome in MDD confirmed habenular activation during prediction of future losses in a probabilistic guessing task with healthy patients, but this was not observed in depressive patients (48). The latter finding demonstrates abnormal habenula activation in response to negative outcome, and definitely links aversive learning to MDD.
Finally a remarkable success, and perhaps the best example to date for translational Hb research, comes from DBS studies (49). Sartorius et al. tested the potential benefit of inhibiting Hb by DBS in two MDD patients with treatment-resistant depression (50, 51). DBS of the stria medullaris thalami, the major LHb afferent bundle, in a patient with treatment-resistant reached full and stable remission, and a second patient showed a 50% improvement of depression symptoms (52). Because this finding is consistent with evidence from animal studies (36–38, 50), efforts are under way to evaluate the reliability, as well as pros and cons of this potential therapy.
In the area of depression, therefore, rodent and human data converge to support the notion that Hb hyperactivity contributes to depressive-like symptoms, and that these symptoms can be relieved by inhibiting the structure, providing a strong opportunity to treat depression. Further steps towards this aim include a better understanding of molecular and cellular bases for this activity in animal studies, determining genetic and environmental factors that lead to Hb hyperactivity in mood deficits in both rodent models and human patients, and selecting molecular targets that could allow selective reduction of Hb hyperactivity by pharmacological means.
ADDICTION
In addiction research, animal studies have been extraordinarily productive to demonstrate the importance of Hb in neuroadaptations to drugs of abuse and negative consequences of drug dependence. We here summarize current knowledge with emphasis on recent studies.
Several rodent studies have proposed a role for LHb in cocaine reward and dependence. In a mouse model of cocaine conditioned place preference (CPP), c-fos immunohistochemistry showed increased neuronal activation in the LHb of mice undergoing cocaine-primed reinstatement (53). Another study investigated intrinsic properties of LHb neurons following cocaine self-administration (SA) in rats, as well as after short and long-term withdrawal from cocaine. Membrane neuron excitability was increased after short-term withdrawal and persisted at least 7 days, suggesting that sustained amplification of neuronal signaling in LHb could be implicated in the long-term negative effects of cocaine use (54). This hypothesis was strengthened through a recent study of glutamatergic transmission in LHb projections to the RMTg. Cocaine-treated mice showed synaptic potentiation of these neurons for at least 14 days, and virally-mediated blockade of GluA1 trafficking in LHb prevented cocaine-induced depressive-like phenotypes in tail suspension and forced swim tests (55). GluA1 trafficking-dependent plasticity in the LHb is a therefore critical for cocaine-driven aversive states.
While the LHb is mainly associated with cocaine studies, the MHb subdivision has become a main focus of interest in the area of nicotine research (56, 57). Several nicotinic receptors subunits are highly expressed in the MHb-interpeduncular pathway, including mainly α3β4 receptors but also α2–α6 and β2–β4 subunits (58), and knockout mouse studies addressing the role of distinct subunits in rodent models of nicotine addiction have been reviewed recently (13). Notably, nicotine acting at α3β4 receptors in MHb was shown to directly modify mesolimbic dopamine responses (59). Circuit mapping also identified α 5 nicotinic subunits at the level of the interpeduncular nucleus (IPN), the main MHb output structure, forming a possible link to serotonergic centers of the brain (60). A recent study showed that optogenetic silencing of MHb input to the IPN, and also pharmacological blockade of CRF1 receptors in the IPN, both reduce nicotine withdrawal-induced anxiety, possibly implicating a VTA-MHb-IPN circuit (61). Together therefore, a large set of rodent studies definitely establish the importance of the MHb-IPN pathway in negative aspects of nicotine dependence.
Mu opioid receptors are strongly expressed in the habenular, mainly within MHb (20), and likely interact with cholinergic transmission. In rats, blockade of α3β4 nicotinic receptors in MHb and IPN attenuates sensitization of the dopamine response to repeated morphine administration, and chronic exposure to morphine enhances cholinergic signaling in the MHb (62). Whether the MHb-IPN pathway contributes to opioid addiction, however, remains open and, more generally, a potential role for LHb and MHb in opioid and alcohol reward and dependence has not been studied in rodent as yet.
Finally, in order to potentially translate rodent research to clinical applications, DBS was used in rats to examine whether LHb stimulation would lead to decrease cocaine consumption in a set of two studies (63, 64). In this work, retrograde tracing experiments showed dose-depend degeneration of the fasciculus retroflexus (FR) after extinction and reinstatement of cocaine SA, suggesting decreased LHb-midbrain connectivity upon cocaine SA. Focusing on the LHb, the authors conducted DBS during maintenance, extinction and reinstatement of cocaine SA, and found that DBS reduced cocaine intake and seeking, at least in rats that SA low doses of cocaine. The two studies together provide support for LHb-targeted DBS in the treatment of cocaine dependence (65), but there is no reported study in humans as yet. Current studies are evaluating the efficacy of DBS in human addiction and have mainly focused on the NAc and STN, LHb may also be a target of interest in this context (66).
Humans
At present, studies in humans are scarce and will undoubtedly develop in upcoming years. Related to substance use disorders and reward processing, are studies addressing reward prediction error (RPE), a fundamental dimension of associative learning. In monkeys, a grounding electrophysiological study by Matsumoto and Hikosaka demonstrated that LHb neurons are excited by negative prediction error (unpleasant event or absence of reward) and inhibited by unexpected reward, therefore encoding RPE rather than reward per se (6). Recent studies have explored RPE in the context of drug abuse showing correlation between RPE and addiction not only in rodents but also in humans with cocaine (67), cigarette smoking (68, 69) and alcohol (70).
To date, two fMRI studies have provided evidence that RPE activates the human habenula (71, 72). A pilot study scanned subjects in a 3T MRI scanner during a juice-delivery task, and data revealed Hb activation during negative prediction error, i.e. when the juice is not delivered at the expected time (72). Another study further investigated Hb activation using fMRI together with connectivity analysis, and demonstrated correlated LHb and VTA activation during a stop-error task measuring the negative prediction error (71). Whether the Hb networks are altered in addicted individuals remains to be studied.
In a very different context, human genetics indirectly implicates Hb in nicotine addiction (73). Three meta-analyses have simultaneously found significant association between Single Nucleotide Polymorphisms (SNPs) and cigarettes smoked per day, and SNPs were included in the α5-α3-β4 nicotinic receptor subunits cluster. Nicotinic receptor subunits encoded by these genes are expressed in several brain areas, but only the MHb and its primary output, the IPN, show co-expression of all three subunits. These findings therefore integrate Hb pathways in human nicotine research.
Overall, rodent data identify Hb as a key brain site for addiction research while human Hb addiction research is still at its infancy. In the latter, an important step will be the mapping of Hb connectivity and activation in dependent and abstinent individuals, in relation to other components of reward and aversion networks. Another potential approach yet to be used is deep brain stimulation of the Hb for the treatment of craving and relapse representing the greatest challenge in the area of substance use disorders. As was done for MDD, such studies should be done in both rodents and humans using translational designs.
SCHIZOPHRENIA
Because of the complex connectivity of Hb to multiple forebrain and hindbrain circuits, similar in rodents, non-human primates and humans, it is anticipated that Hb activity impacts multiple dimensions of normal behavior, with implications for disease beyond depression and addiction. Here we focus on the possible role of Hb in schizophrenia.
Tightly linked to predicting errors are decision-making processes, and rat studies have demonstrated causal implication of LHb in subjective decision-making. Stopper and Floresco used in vivo electrophysiology to manipulate phasic dopamine signaling during a risk/reward decision-making task, and showed that LHb stimulation prior to choice redirects the selection of action away from the preferred or rewarded outcome (74). Conversely, LHb inactivation abolishes the previously described choice biases, favoring random patterns of choice behavior (14, 75). This particular function of Hb may be relevant to schizophrenia research (14), as reinforcement learning deficits and misusing feedback to appropriately guide decision-making are integral aspects of schizophrenia (76, 77).
In humans, anatomical modifications in Hb have been linked to schizophrenia. An early computed tomographic study on post-mortem human brain slices showed increased calcification in the Hb of schizophrenic patients (78). Post-mortem immunochemistry also showed reduced capillary densities specifically in the Hb of schizophrenic patients, as well as reduced density of neurons expressing ABCB1 (ATP-binding cassette transporter) whose malfunction has been associated in schizophrenia (79).
FMRI coupled to a visual-spatial match-to-sample task further showed that patients with schizophrenia lack appropriate modulation of Hb activity in adaptive response to feedback and errors (80). This finding suggests that pronounced deficits observed in schizophrenic patients in situations of problem solving and learning could result from an alteration of habenula-mediated feedback processing. Further studies are necessary to confirm this hypothesis, with perhaps selected schizophrenic patient subgroups.
Of note also is that LHb lesions in the rat induce behavioral deficits in the Morris Water Maze (81), analogous to deficits of declarative memory in humans known to be impaired in schizophrenia (82), and also lead to disturbed attention in a 5-CSRT task (83) modeling the continuous performance test of attention in the clinic where schizophrenia patients score low (84). Although Hb function in learning and memory has been less well-studied, and engages processes distinct from those underlying subjective decision-making, evidence from animal studies all support the notion that Hb research is relevant to cognition in the area of schizophrenia.
CHALLENGES AND FUTURE DIRECTIONS
In this review, we have organized rodent and human data in three major psychiatric disease areas: depression, drug dependence and other potential disease areas of psychiatry, notably schizophrenia. We have also added a section on pain in the Supplementary Materials. Table 1 summarizes functional consequences of Hb manipulations in both rodents and humans within the four categories.
Table 1. Functions mediated by habenula in rodents and human.
This table summarizes main preclinical and human studies discussed in this review. Reports for rodents and humans are categorized based on studies in area of pain and analgesia, depression, addiction and in schizophrenia (top to bottom). These studies address the medial habenula (MHb), lateral habenula (LHb) or both. Some of the studies are also reviewed in (10) for pain and analgesia, in (12) for mood and depression, in (13) for drug dependence and in (14) for schizophrenia. The parallel between rodent and human findings show promises for effective translation of preclinical research to human psychiatry.
| Humans | Functions |
|---|---|
| Pain and Analgesia MHb and LhB |
fMRI: Noxious Heat activates Hb (108) |
|
Rs-fMRI: Pediatric patients with chronic pain exhibit a reduced Hb rsFC to the rest of the brain and specifically with forebrain area (105) | |
|
Post-mortem histological study (23) and structural MRI studies (21, 44) show decreased volumes of Hb in MDD and BD patients | |
| Mood and Depression MHb and LhB |
PET study shows enhanced coupling between Hb and raphe activities in volunteer patients experiencing transient depressive relapse upon tryptophan depletion (41) |
| Mood and Depression LhB |
DBS of the stria medullaris thalami, the major LHb afferent bundle, reduces symptoms of treatment-resistant depression (50, 52) |
| Drug Dependence MHb |
Genetic meta-analyses found association between α5-α3-β4 cluster and cigarettes smoked per day. Only the MHb shows co-expression of all three subunits (73) |
| Drug Dependence & Schizophrenia LHb |
Computed tomographic study on post-mortem brain slices shows increased calcification in the Hb of schizophrenic patients (78) |
| Schizophrenia MHb and LhB |
fMRI study shows that patients with schizophrenia lack appropriate modulation of Hb activity in adaptive response to feedback and errors (80). |
| Rodents | Functions |
| Pain and Analgesia MHb |
Gene Knock down: Medical habenular RSK2 contributes to morphine analgesia (107) |
| Pain and Analgesia MHb and LHb |
Electrical stimulation of the Hb induces analgesia (109, 110) |
| Hb: integrative hub for pain control and regulating nociceptive processes (111) | |
| Mood and Depression LHb |
Activation of LHb 5-HT2C receptors increases depressive-like behaviors (32) |
| LHb lesion studies (30, 31) or pharmacological inhibition (33, 34) reduces depressive- like behaviors | |
| DBS of LHb reduces depressive-like behavior (36, 37) by suppressing synaptic drive onto VTA-projecting LHb neurons (36) and increases monoamine concentrations (37) | |
| Optogenetic stimulations of GABA and Glu neurons projecting to the LHb demonstrate that LHb activity is regulated by corelease of both neurotransmitters (40) | |
| Mood and Depression MHb and LHb |
18FDG-PET live imaging study shows increased activity of Hb that correlates with subsequent learned helplessness behavior (41) |
| Drug Dependence LHb |
A cocaine conditioned place preference study shows increased neuronal activation in the LHb of mice undergoing cocaine-primed reinstatement (53) |
| Electrophysiological studies show that cocaine induces synaptic potentiation of LHb neurons (54, 55) | |
| Drug Dependence MHb |
Optogenetic silencing of MHb input to the IPN or pharmacological blockade of CRF1 receptors in the IPN reduce nicotine withdrawal-induced anxiety (61) |
| Drug Dependence & Schizophrenia MHb |
Pharmacological Blockade of α3β4 nicotinic receptors in MHb and IPN attenuates sensitization of the dopamine response to repeated morphine administration (112). |
| Chronic exposure to morphine enhances cholinergic signaling in the MHb (62). | |
| Drug Dependence & Schizophrenia LHb |
Retrograde labeling shows dose-dependent degeneration of the fasciculus retroflexus after extinction and reinstatement of cocaine self-administration (64) |
| DBS of LHb reduces cocaine intake and seeking (63, 64) | |
| Relevant to schizophrenia: LHb stimulation prior to choice redirects the selection of action away from the preferred or rewarded outcome (14). | |
| Schizophrenia LHb |
LHb lesions in the rat induce memory (81, 113)) and attentional (81) deficits analogous to cognitive impairments in schizophrenic patients. |
Abbreviations: rsFC: resting-state functional connectivity; MDD: major depression disorder; BD: bipolar disorder; VTA: ventral tegmental area; GABA: γ-Aminobutyric acid; Glu: glutamate; DBS: deep brain stimulation.
Basic research in laboratory animals has revealed Hb as a core integration center, which influences many aspects of behavior. One current goal of rodent research is the genetic targeting of specific Hb neuron populations in order to dissect circuit mechanisms underpinning the many Hb-controlled behaviors. Main recent studies demonstrate Hb implication in emotional or cognitive responses that have not been discussed here. For example, in the area of stress, fear and anxiety, optogenetic activation of LHb efferent neurons to the RMTg induced acute and conditioned avoidance (85), ablation of projection neurons from the triangular septum to the MHb promoted deficits of anxiety-related behavior, and ablation of neurons projecting from the bed nucleus of the anterior commissure to MHb led to severe decreases in fear responses and fear learning (86, 87). Also, specific deletion of CB1 gene in MHb neurons reduced aversive-acquired responses such as freezing in cued and contextual fear conditioning experiments (88), whereas optogenetic activation of glutamatergic LHb neurons projecting to the laterodrosal tegmentum generates fear-like responses and regulates olfactory cue-induced innate fear (89). In the context of executive functions, another recent study showed that ablation of MHb neurons increases impulsivity and impairs cognition-dependent functions, including aversion to delay and effort, deficits in long-term memory and reduced flexibility (90). The LHb also contributes to behavioral flexibility through utilizing proactive and retroactive information when performing decision-making appetitive tasks (91). Finally, Hb integrity was found essential in processing positive (social play) and negative (social isolation) social information in juvenile rats, with a specific implication of the medial LHb (92). Whether and how these activities relate to psychiatric disorders in humans represents an entire field of investigation for the next decade.
On the human side, a major effort lies in overcoming technical challenges due to the small size of the Hb. On one hand, high resolution MRI and fMRI now allow accurate targeting of the structure (25, 27) and manganese-based neuroimaging with minimal toxicity may develop for patients in the future (93). Also, surgical treatment for psychiatric disorders is being rekindled and strong efforts are dedicated to deep brain stimulation in sites involving emotional and behavioral circuitry, among which the Hb (65, 94). Traps and pitfalls of the technique applied to small deep structures are being addressed, and achieving successful surgery is becoming feasible (95). Human Hb research should now focus on sharpening neuroimaging and deep brain stimulation techniques in order to increase both functional studies and clinical trials. Together, future studies will promote the use of translational techniques, that is, approaches that can either be used across species or at least have a predictive value (predict outcome in humans). A corpus of techniques applicable to both rodents and humans is developing in the field of habenular research, including deep brain stimulation, magnetic resonance imaging, functional magnetic resonance imaging and some behavioral tasks or experiments. Efforts are now required on the animal side, particularly in the area of neuroimaging techniques representing the best translatable analysis tool for brain activity (96) (97) (98).
In conclusion, Hb research in humans is still in its infancy (see also (99)), but develops at a rapid pace. Animal research, on the other hand, has become a mature field and has revealed a vast spectrum of Hb functions throughout emotional and cognitive brain processes, opening the way to multiple opportunities in terms of potential implications in the clinic. Upcoming findings in both rodents and humans will contribute to refine our understanding of habenula’s role, the foundation of which was set in 2007 (6), and perhaps assign a unique integrative role in reward and aversion processing to this intriguing brain structure. Future studies will also determine whether Hb-targeted strategies indeed prove efficient in the treatment of depression, and could perhaps surpass mood disorders for broader applications in the area of psychiatric disorders.
Supplementary Material
Acknowledgments
We thank Maria Osikowicz for comments and careful reading of the manuscript. We are grateful to the Canada Research Chairs, the Monique H. Bourgeois Chair in Pervasive Developmental Disorders (McGill University) and the National Institutes of Health (NIH-NIAAA #16658 and NIH-NIDA #005010) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Concha ML, Wilson SW. Asymmetry in the epithalamus of vertebrates. Journal of anatomy. 2001;199:63–84. doi: 10.1046/j.1469-7580.2001.19910063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aizawa H, Amo R, Okamoto H. Phylogeny and ontogeny of the habenular structure. Frontiers in neuroscience. 2011;5:138. doi: 10.3389/fnins.2011.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beretta CA, Dross N, Guiterrez-Triana JA, Ryu S, Carl M. Habenula circuit development: past, present, and future. Frontiers in neuroscience. 2012;6:51. doi: 10.3389/fnins.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neuroscience and biobehavioral reviews. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 5.Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 7.Dillon DG, Rosso IM, Pechtel P, Killgore WD, Rauch SL, Pizzagalli DA. Peril and pleasure: an rdoc-inspired examination of threat responses and reward processing in anxiety and depression. Depression and anxiety. 2014;31:233–249. doi: 10.1002/da.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nature reviews Neuroscience. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neuroscience and biobehavioral reviews. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Shelton L, Becerra L, Borsook D. Unmasking the mysteries of the habenula in pain and analgesia. Progress in neurobiology. 2012;96:208–219. doi: 10.1016/j.pneurobio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lecca S, Meye FJ, Mameli M. The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. The European journal of neuroscience. 2014;39:1170–1178. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- 12.Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nature neuroscience. 2014;17:1146–1152. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velasquez KM, Molfese DL, Salas R. The role of the habenula in drug addiction. Frontiers in human neuroscience. 2014;8:174. doi: 10.3389/fnhum.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stopper CM, Floresco SB. Dopaminergic circuitry and risk/reward decision making: implications for schizophrenia. Schizophrenia bulletin. 2015;41:9–14. doi: 10.1093/schbul/sbu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YA, Goto Y. Habenula and ADHD: convergence on time. Neuroscience and biobehavioral reviews. 2013;37:1801–1809. doi: 10.1016/j.neubiorev.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Nair SG, Strand NS, Neumaier JF. DREADDing the lateral habenula: a review of methodological approaches for studying lateral habenula function. Brain research. 2013;1511:93–101. doi: 10.1016/j.brainres.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim U, Chang SY. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. The Journal of comparative neurology. 2005;483:236–250. doi: 10.1002/cne.20410. [DOI] [PubMed] [Google Scholar]
- 18.Weiss T, Veh RW. Morphological and electrophysiological characteristics of neurons within identified subnuclei of the lateral habenula in rat brain slices. Neuroscience. 2011;172:74–93. doi: 10.1016/j.neuroscience.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 19.Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H. Molecular characterization of the subnuclei in rat habenula. The Journal of comparative neurology. 2012;520:4051–4066. doi: 10.1002/cne.23167. [DOI] [PubMed] [Google Scholar]
- 20.Gardon O, Faget L, Chu Sin Chung P, Matifas A, Massotte D, Kieffer BL. Expression of mu opioid receptor in dorsal diencephalic conduction system: new insights for the medial habenula. Neuroscience. 2014;277:595–609. doi: 10.1016/j.neuroscience.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savitz JB, Nugent AC, Bogers W, Roiser JP, Bain EE, Neumeister A, et al. Habenula volume in bipolar disorder and major depressive disorder: a high-resolution magnetic resonance imaging study. Biological psychiatry. 2011;69:336–343. doi: 10.1016/j.biopsych.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe T, Radulovic J, Boretius S, Frahm J, Michaelis T. Mapping of the habenulointerpeduncular pathway in living mice using manganese-enhanced 3D MRI. Magnetic resonance imaging. 2006;24:209–215. doi: 10.1016/j.mri.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Ranft K, Dobrowolny H, Krell D, Bielau H, Bogerts B, Bernstein HG. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychological medicine. 2010;40:557–567. doi: 10.1017/S0033291709990821. [DOI] [PubMed] [Google Scholar]
- 24.Diaz E, Bravo D, Rojas X, Concha ML. Morphologic and immunohistochemical organization of the human habenular complex. The Journal of comparative neurology. 2011;519:3727–3747. doi: 10.1002/cne.22687. [DOI] [PubMed] [Google Scholar]
- 25.Strotmann B, Heidemann RM, Anwander A, Weiss M, Trampel R, Villringer A, et al. High-resolution MRI and diffusion-weighted imaging of the human habenula at 7 tesla. Journal of magnetic resonance imaging : JMRI. 2014;39:1018–1026. doi: 10.1002/jmri.24252. [DOI] [PubMed] [Google Scholar]
- 26.Strotmann B, Kogler C, Bazin PL, Weiss M, Villringer A, Turner R. Mapping of the internal structure of human habenula with ex vivo MRI at 7T. Frontiers in human neuroscience. 2013;7:878. doi: 10.3389/fnhum.2013.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawson RP, Drevets WC, Roiser JP. Defining the habenula in human neuroimaging studies. NeuroImage. 2013;64:722–727. doi: 10.1016/j.neuroimage.2012.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caldecott-Hazard S. Interictal changes in behavior and cerebral metabolism in the rat: opioid involvement. Experimental neurology. 1988;99:73–83. doi: 10.1016/0014-4886(88)90128-8. [DOI] [PubMed] [Google Scholar]
- 29.Caldecott-Hazard S, Mazziotta J, Phelps M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:1951–1961. doi: 10.1523/JNEUROSCI.08-06-01951.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behavioural brain research. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Luo XF, Zhang BL, Li JC, Yang YY, Sun YF, Zhao H. Lateral habenula as a link between dopaminergic and serotonergic systems contributes to depressive symptoms in Parkinson's disease. Brain research bulletin. 2015;110:40–46. doi: 10.1016/j.brainresbull.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Han LN, Zhang L, Li LB, Sun YN, Wang Y, Chen L, et al. Activation of serotonin(2C) receptors in the lateral habenular nucleus increases the expression of depression-related behaviors in the hemiparkinsonian rat. Neuropharmacology. 2015;93:68–79. doi: 10.1016/j.neuropharm.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Winter C, Vollmayr B, Djodari-Irani A, Klein J, Sartorius A. Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behavioural brain research. 2011;216:463–465. doi: 10.1016/j.bbr.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 34.Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain research. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- 35.Lecca S, Pelosi A, Tchenio A, Moutkine I, Lujan R, Herve D, et al. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nature medicine. 2016;22:254–261. doi: 10.1038/nm.4037. [DOI] [PubMed] [Google Scholar]
- 36.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng H, Wang Y, Huang M, Lin W, Wang S, Zhang B. Chronic deep brain stimulation of the lateral habenula nucleus in a rat model of depression. Brain Res. 2011;1422:32–38. doi: 10.1016/j.brainres.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 38.Lim LW, Prickaerts J, Huguet G, Kadar E, Hartung H, Sharp T, et al. Electrical stimulation alleviates depressive-like behaviors of rats: investigation of brain targets and potential mechanisms. Transl Psychiatry. 2015;5:e535. doi: 10.1038/tp.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y, Morath B, Hu C, Byrne LK, Sutor SL, Frye MA, et al. Antidepressant actions of lateral habenula deep brain stimulation differentially correlate with CaMKII/GSK3/AMPK signaling locally and in the infralimbic cortex. Behavioural brain research. 2016;306:170–177. doi: 10.1016/j.bbr.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 40.Shabel SJ, Proulx CD, Piriz J, Malinow R. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science (New York, NY) 2014;345:1494–1498. doi: 10.1126/science.1250469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirrione MM, Schulz D, Lapidus KA, Zhang S, Goodman W, Henn FA. Increased metabolic activity in the septum and habenula during stress is linked to subsequent expression of learned helplessness behavior. Frontiers in human neuroscience. 2014;8:29. doi: 10.3389/fnhum.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999;10:163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- 43.Carceller-Sindreu M, de Diego-Adelino J, Serra-Blasco M, Vives-Gilabert Y, Marti NBA, Puigdemont D, et al. Volumetric MRI study of the habenula in first episode, recurrent and chronic major depression. Eur Neuropsychopharmacol. 2015 doi: 10.1016/j.euroneuro.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Johnston BA, Steele JD, Tolomeo S, Christmas D, Matthews K. Structural MRI-Based Predictions in Patients with Treatment-Refractory Depression (TRD) PLoS One. 2015;10:e0132958. doi: 10.1371/journal.pone.0132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt FM, Schindler S, Adamidis M, Strauss M, Trankner A, Trampel R, et al. Habenula volume increases with disease severity in unmedicated major depressive disorder as revealed by 7T MRI. European archives of psychiatry and clinical neuroscience. 2016 doi: 10.1007/s00406-016-0675-8. [DOI] [PubMed] [Google Scholar]
- 46.Lawson RP, Seymour B, Loh E, Lutti A, Dolan RJ, Dayan P, et al. The habenula encodes negative motivational value associated with primary punishment in humans. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:11858–11863. doi: 10.1073/pnas.1323586111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hennigan K, D'Ardenne K, McClure SM. Distinct midbrain and habenula pathways are involved in processing aversive events in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:198–208. doi: 10.1523/JNEUROSCI.0927-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furman DJ, Gotlib IH. Habenula responses to potential and actual loss in major depression: preliminary evidence for lateralized dysfunction. Social cognitive and affective neuroscience. 2016 doi: 10.1093/scan/nsw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morishita T, Fayad SM, Higuchi MA, Nestor KA, Foote KD. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11:475–484. doi: 10.1007/s13311-014-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiening K, Sartorius A. A new translational target for deep brain stimulation to treat depression. EMBO molecular medicine. 2013;5:1151–1153. doi: 10.1002/emmm.201302947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sartorius A, Hoyer C, Kiening K, Meyer-Lindenberg A. Medial forebrain bundle stimulation-speed access to an old or entry into a new depression neurocircuit? Biological psychiatry. 2013;74:e43. doi: 10.1016/j.biopsych.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 52.Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 53.Brown RM, Short JL, Lawrence AJ. Identification of brain nuclei implicated in cocaine-primed reinstatement of conditioned place preference: a behaviour dissociable from sensitization. PloS one. 2010;5:e15889. doi: 10.1371/journal.pone.0015889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neumann PA, Ishikawa M, Otaka M, Huang YH, Schluter OM, Dong Y. Increased Excitability of Lateral Habenula Neurons in Adolescent Rats following Cocaine Self-administration. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2014 doi: 10.1093/ijnp/pyu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meye FJ, Valentinova K, Lecca S, Marion-Poll L, Maroteaux MJ, Musardo S, et al. Cocaine-evoked negative symptoms require AMPA receptor trafficking in the lateral habenula. Nature neuroscience. 2015;18:376–378. doi: 10.1038/nn.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baldwin PR, Alanis R, Salas R. The Role of the Habenula in Nicotine Addiction. Journal of addiction research & therapy. 2011:S1. doi: 10.4172/2155-6105.S1-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fowler CD, Kenny PJ. Habenular signaling in nicotine reinforcement. Neuropsychopharmacology. 2012;37:306–307. doi: 10.1038/npp.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leslie FM, Mojica CY, Reynaga DD. Nicotinic receptors in addiction pathways. Molecular pharmacology. 2013;83:753–758. doi: 10.1124/mol.112.083659. [DOI] [PubMed] [Google Scholar]
- 59.McCallum SE, Cowe MA, Lewis SW, Glick SD. alpha3beta4 nicotinic acetylcholine receptors in the medial habenula modulate the mesolimbic dopaminergic response to acute nicotine in vivo. Neuropharmacology. 2012;63:434–440. doi: 10.1016/j.neuropharm.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsu YW, Tempest L, Quina LA, Wei AD, Zeng H, Turner EE. Medial habenula output circuit mediated by alpha5 nicotinic receptor-expressing GABAergic neurons in the interpeduncular nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:18022–18035. doi: 10.1523/JNEUROSCI.2927-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao-Shea R, DeGroot SR, Liu L, Vallaster M, Pang X, Su Q, et al. Increased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nature communications. 2015;6:6770. doi: 10.1038/ncomms7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neugebauer NM, Einstein EB, Lopez MB, McClure-Begley TD, Mineur YS, Picciotto MR. Morphine dependence and withdrawal induced changes in cholinergic signaling. Pharmacology, biochemistry, and behavior. 2013;109:77–83. doi: 10.1016/j.pbb.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, et al. Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology. 2010;59:452–459. doi: 10.1016/j.neuropharm.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lax E, Friedman A, Croitoru O, Sudai E, Ben-Moshe H, Redlus L, et al. Neurodegeneration of lateral habenula efferent fibers after intermittent cocaine administration: implications for deep brain stimulation. Neuropharmacology. 2013;75:246–254. doi: 10.1016/j.neuropharm.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 65.Yadid G, Gispan I, Lax E. Lateral habenula deep brain stimulation for personalized treatment of drug addiction. Frontiers in human neuroscience. 2013;7:806. doi: 10.3389/fnhum.2013.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luigjes J, van den Brink W, Feenstra M, van den Munckhof P, Schuurman PR, Schippers R, et al. Deep brain stimulation in addiction: a review of potential brain targets. Molecular psychiatry. 2012;17:572–583. doi: 10.1038/mp.2011.114. [DOI] [PubMed] [Google Scholar]
- 67.Parvaz MA, Konova AB, Proudfit GH, Dunning JP, Malaker P, Moeller SJ, et al. Impaired neural response to negative prediction errors in cocaine addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:1872–1879. doi: 10.1523/JNEUROSCI.2777-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Potts GF, Bloom EL, Evans DE, Drobes DJ. Neural reward and punishment sensitivity in cigarette smokers. Drug and alcohol dependence. 2014;144:245–253. doi: 10.1016/j.drugalcdep.2014.09.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu X, Lohrenz T, Salas R, Baldwin PR, Soltani A, Kirk U, et al. Belief about nicotine selectively modulates value and reward prediction error signals in smokers. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2539–2544. doi: 10.1073/pnas.1416639112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deserno L, Beck A, Huys QJ, Lorenz RC, Buchert R, Buchholz HG, et al. Chronic alcohol intake abolishes the relationship between dopamine synthesis capacity and learning signals in the ventral striatum. The European journal of neuroscience. 2015;41:477–486. doi: 10.1111/ejn.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ide JS, Li CS. Error-related functional connectivity of the habenula in humans. Frontiers in human neuroscience. 2011;5:25. doi: 10.3389/fnhum.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salas R, Baldwin P, de Biasi M, Montague PR. BOLD Responses to Negative Reward Prediction Errors in Human Habenula. Frontiers in human neuroscience. 2010;4:36. doi: 10.3389/fnhum.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vandenbergh DJ, Schlomer GL. Finding genomic function for genetic associations in nicotine addiction research: the ENCODE project's role in future pharmacogenomic analysis. Pharmacology, biochemistry, and behavior. 2014;123:34–44. doi: 10.1016/j.pbb.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stopper CM, Floresco SB. What's better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nature neuroscience. 2014;17:33–35. doi: 10.1038/nn.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stopper CM, Tse MT, Montes DR, Wiedman CR, Floresco SB. Overriding phasic dopamine signals redirects action selection during risk/reward decision making. Neuron. 2014;84:177–189. doi: 10.1016/j.neuron.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 76.Keri S, Kelemen O, Szekeres G, Bagoczky N, Erdelyi R, Antal A, et al. Schizophrenics know more than they can tell: probabilistic classification learning in schizophrenia. Psychol Med. 2000;30:149–155. doi: 10.1017/s0033291799001403. [DOI] [PubMed] [Google Scholar]
- 77.Culbreth AJ, Gold JM, Cools R, Barch DM. Impaired Activation in Cognitive Control Regions Predicts Reversal Learning in Schizophrenia. Schizophr Bull. 2015 doi: 10.1093/schbul/sbv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandyk R. Pineal and habenula calcification in schizophrenia. The International journal of neuroscience. 1992;67:19–30. doi: 10.3109/00207459208994773. [DOI] [PubMed] [Google Scholar]
- 79.Bernstein HG, Hildebrandt J, Dobrowolny H, Steiner J, Bogerts B, Pahnke J. Morphometric analysis of the cerebral expression of ATP-binding cassette transporter protein ABCB1 in chronic schizophrenia: Circumscribed deficits in the habenula. Schizophrenia research. 2016 doi: 10.1016/j.schres.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 80.Shepard PD, Holcomb HH, Gold JM. Schizophrenia in translation: the presence of absence: habenular regulation of dopamine neurons and the encoding of negative outcomes. Schizophrenia bulletin. 2006;32:417–421. doi: 10.1093/schbul/sbj083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lecourtier L, Neijt HC, Kelly PH. Habenula lesions cause impaired cognitive performance in rats: implications for schizophrenia. The European journal of neuroscience. 2004;19:2551–2560. doi: 10.1111/j.0953-816X.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- 82.Perry W, Light GA, Davis H, Braff DL. Schizophrenia patients demonstrate a dissociation on declarative and non-declarative memory tests. Schizophr Res. 2000;46:167–174. doi: 10.1016/s0920-9964(99)00229-7. [DOI] [PubMed] [Google Scholar]
- 83.Lecourtier L, Kelly PH. Bilateral lesions of the habenula induce attentional disturbances in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:484–496. doi: 10.1038/sj.npp.1300595. [DOI] [PubMed] [Google Scholar]
- 84.Chudasama Y, Robbins TW. Psychopharmacological approaches to modulating attention in the five-choice serial reaction time task: implications for schizophrenia. Psychopharmacology (Berl) 2004;174:86–98. doi: 10.1007/s00213-004-1805-y. [DOI] [PubMed] [Google Scholar]
- 85.Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nature neuroscience. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okamoto H, Aizawa H. Fear and anxiety regulation by conserved affective circuits. Neuron. 2013;78:411–413. doi: 10.1016/j.neuron.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 87.Yamaguchi T, Danjo T, Pastan I, Hikida T, Nakanishi S. Distinct roles of segregated transmission of the septo-habenular pathway in anxiety and fear. Neuron. 2013;78:537–544. doi: 10.1016/j.neuron.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soria-Gomez E, Busquets-Garcia A, Hu F, Mehidi A, Cannich A, Roux L, et al. Habenular CB1 Receptors Control the Expression of Aversive Memories. Neuron. 2015;88:306–313. doi: 10.1016/j.neuron.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 89.Yang H, Yang J, Xi W, Hao S, Luo B, He X, et al. Laterodorsal tegmentum interneuron subtypes oppositely regulate olfactory cue-induced innate fear. Nature neuroscience. 2016;19:283–289. doi: 10.1038/nn.4208. [DOI] [PubMed] [Google Scholar]
- 90.Kobayashi Y, Sano Y, Vannoni E, Goto H, Suzuki H, Oba A, et al. Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice. Frontiers in behavioral neuroscience. 2013;7:17. doi: 10.3389/fnbeh.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baker PM, Raynor SA, Francis NT, Mizumori SJ. Lateral habenula integration of proactive and retroactive information mediates behavioral flexibility. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 92.van Kerkhof LW, Damsteegt R, Trezza V, Voorn P, Vanderschuren LJ. Functional integrity of the habenula is necessary for social play behaviour in rats. The European journal of neuroscience. 2013;38:3465–3475. doi: 10.1111/ejn.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Silva AC, Bock NA. Manganese-enhanced MRI: an exceptional tool in translational neuroimaging. Schizophrenia bulletin. 2008;34:595–604. doi: 10.1093/schbul/sbn056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sartorius A, Henn FA. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses. 2007;69:1305–1308. doi: 10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 95.Schneider TM, Beynon C, Sartorius A, Unterberg AW, Kiening KL. Deep brain stimulation of the lateral habenular complex in treatment-resistant depression: traps and pitfalls of trajectory choice. Neurosurgery. 2013;72:ons184–ons193. doi: 10.1227/NEU.0b013e318277a5aa. discussion ons193. [DOI] [PubMed] [Google Scholar]
- 96.Wang L, Lu H, Brown PL, Rea W, Vaupel B, Yang Y, et al. Manganese-Enhanced MRI Reflects Both Activity-Independent and Activity-Dependent Uptake within the Rat Habenulomesencephalic Pathway. PloS one. 2015;10:e0127773. doi: 10.1371/journal.pone.0127773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harsan LA, David C, Reisert M, Schnell S, Hennig J, von Elverfeldt D, et al. Mapping remodeling of thalamocortical projections in the living reeler mouse brain by diffusion tractography. Proc Natl Acad Sci U S A. 2013;110:E1797–E1806. doi: 10.1073/pnas.1218330110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mechling AE, Hubner NS, Lee HL, Hennig J, von Elverfeldt D, Harsan LA. Fine-grained mapping of mouse brain functional connectivity with resting-state fMRI. Neuroimage. 2014;96:203–215. doi: 10.1016/j.neuroimage.2014.03.078. [DOI] [PubMed] [Google Scholar]
- 99.Benarroch EE. Habenula: Recently recognized functions and potential clinical relevance. Neurology. 2015;85:992–1000. doi: 10.1212/WNL.0000000000001937. [DOI] [PubMed] [Google Scholar]
- 100.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. The Journal of comparative neurology. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- 102.Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. The Journal of comparative neurology. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bernard R, Veh RW. Individual neurons in the rat lateral habenular complex project mostly to the dopaminergic ventral tegmental area or to the serotonergic raphe nuclei. The Journal of comparative neurology. 2012;520:2545–2558. doi: 10.1002/cne.23080. [DOI] [PubMed] [Google Scholar]
- 104.Viswanath H, Carter AQ, Baldwin PR, Molfese DL, Salas R. The medial habenula: still neglected. Frontiers in human neuroscience. 2013;7:931. doi: 10.3389/fnhum.2013.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Erpelding N, Sava S, Simons LE, Lebel A, Serrano P, Becerra L, et al. Habenula functional resting-state connectivity in pediatric CRPS. Journal of neurophysiology. 2014;111:239–247. doi: 10.1152/jn.00405.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wagner F, French L, Veh RW. Transcriptomic-anatomic analysis of the mouse habenula uncovers a high molecular heterogeneity among neurons in the lateral complex, while gene expression in the medial complex largely obeys subnuclear boundaries. Brain structure & function. 2014 doi: 10.1007/s00429-014-0891-9. [DOI] [PubMed] [Google Scholar]
- 107.Darcq E, Befort K, Koebel P, Pannetier S, Mahoney MK, Gaveriaux-Ruff C, et al. RSK2 signaling in medial habenula contributes to acute morphine analgesia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1288–1296. doi: 10.1038/npp.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shelton L, Pendse G, Maleki N, Moulton EA, Lebel A, Becerra L, et al. Mapping pain activation and connectivity of the human habenula. Journal of neurophysiology. 2012;107:2633–2648. doi: 10.1152/jn.00012.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cohen SR, Melzack R. Morphine injected into the habenula and dorsal posteromedial thalamus produces analgesia in the formalin test. Brain research. 1985;359:131–139. doi: 10.1016/0006-8993(85)91420-9. [DOI] [PubMed] [Google Scholar]
- 110.Mahieux G, Benabid AL. Naloxone-reversible analgesia induced by electrical stimulation of the habenula in the rat. Brain Res. 1987;406:118–129. doi: 10.1016/0006-8993(87)90776-1. [DOI] [PubMed] [Google Scholar]
- 111.Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Progress in neurobiology. 2013;109:1–27. doi: 10.1016/j.pneurobio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taraschenko OD, Shulan JM, Maisonneuve IM, Glick SD. 18-MC acts in the medial habenula and interpeduncular nucleus to attenuate dopamine sensitization to morphine in the nucleus accumbens. Synapse (New York, NY) 2007;61:547–560. doi: 10.1002/syn.20396. [DOI] [PubMed] [Google Scholar]
- 113.Mathis V, Cosquer B, Avallone M, Cassel JC, Lecourtier L. Excitatory Transmission to the Lateral Habenula is Critical for Encoding and Retrieval of Spatial Memory. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.