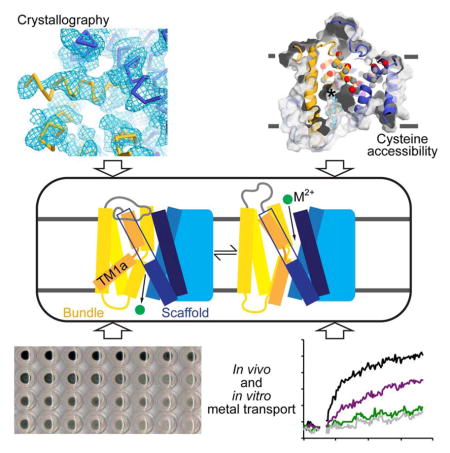
Summary
The widely-conserved natural resistance associated macrophage protein (Nramp) family of divalent metal transporters enables manganese import in bacteria and dietary iron uptake in mammals. We determined the crystal structure of the Deinococcus radiodurans Nramp homolog (DraNramp) in an inward-facing apo state, including the complete transmembrane (TM) segment 1a—absent from a previous Nramp structure. Mapping our cysteine accessibility scanning results onto this structure, we identified the metal permeation pathway in the alternate outward-open conformation. We investigated the functional impact of two natural anemia-causing glycine-to-arginine mutations, which impaired transition metal transport in both human Nramp2 and DraNramp. The TM4 G153R mutation perturbs the closing of the outward metal permeation pathway and alters the selectivity of the conserved metal-binding site. In contrast, the TM1a G45R mutation prevents conformational change by sterically blocking the essential movement of that helix, thus locking the transporter in an inward-facing state.
ETOC blurb
Bozzi, Bane, and Weihofen et al. determined the inward-open structure of a bacterial Nramp transition metal transporter with a LeuT fold. Using biochemical experiments, the authors provide mechanistic explanations for how two anemia-causing mutations impede function through altering the protein’s conformational landscape in unique ways.
Introduction
Nearly all organisms require iron to survive. Iron’s oxidation state cycle is ideal both to catalyze essential redox reactions as a cofactor in numerous enzymes and to provide a pathway for electron transport across membranes. In addition, heme iron is used for oxygen transport and storage, enabling aerobic respiration. Organisms have thus evolved mechanisms to acquire, traffic, and safely store this crucial transition metal (Aisen et al., 2001; Andrews, 2008). The natural resistance associated macrophage protein (Nramp) family of divalent metal transporters plays a vital role in mammalian iron homeostasis (Mackenzie and Hediger, 2004). Expressed in phagosomal membranes, Nramp1 both helps macrophages kill engulfed pathogens by extracting iron and other essential transition metals (Cellier et al., 2007), and enables iron recycling from dying erythrocytes (Soe-Lin et al., 2009). Mammals abundantly express a second homolog, Nramp2—also known as divalent metal transporter 1 (DMT1)—in intestinal enterocytes to enable absorption of dietary iron (Gunshin et al., 1997). Nramp2 is also expressed at lower levels in endosomal membranes of most somatic cells (Mackenzie et al., 2007), where it enables extraction of transferrin-bound iron from vesicles, an especially important process in erythropoiesis (Canonne-Hergaux et al., 2001; Gunshin et al., 2005).
To maintain homeostasis, mammals tightly regulate iron uptake and transport (Lieu et al., 2001), primarily through translation and localization of Nramps (Gunshin et al., 2001; Hubert and Hentze, 2002). An overabundance of free iron generates free radicals that cause tissue damage and increases susceptibility to infection (Ganz, 2009). In contrast, iron deficiency causes anemia (Abbaspour et al., 2014). Accordingly, mutations in Nramp2 are implicated in anemia in humans and rodents (Iolascon and De Falco, 2009; Shawki et al., 2012). The same glycine-to-arginine mutation (G185R) causes microcytic anemia in both mice (Fleming et al., 1997) and Belgrade rats (Fleming et al., 1998; Veuthey and Wessling-Resnick, 2014), and altered protein localization in enterocytes (Canonne-Hergaux et al., 2000). This mutation reduces iron transport when expressed in mammalian cell lines (Su et al., 1998; Touret et al., 2004; Xu et al., 2004), with a concomitant increase in permeability to calcium—typically a poor Nramp substrate—in G185R-transfected cells compared to WT counterparts (Xu et al., 2004). A glycine-to-arginine mutation (G75R) in human anemia patients may abrogate iron-transport function (Barrios et al., 2012; Blanco et al., 2009; Shawki et al., 2012). However, the molecular mechanisms by which these mutations perturb Nramp metal transport and cause anemia remain unknown.
The Nramp family spans the tree of life, with homologs that perform a range of essential divalent transition metal transport functions, likely as secondary transporters that harness a proton gradient (Courville et al., 2006). Based on sequence analyses, Nramp homologs form four major phylogenetic clades: the eukaryotic Nramps and the prokaryotic A, B, and C clades (Cellier et al., 2001). The crystal structure of Staphylococcus capitis Nramp (ScaNramp), belonging to clade C, confirmed a LeuT fold—named for the bacterial sodium/amino acid symporter (Yamashita et al., 2005)—for Nramps, as previously predicted (Cellier, 2012; Ehrnstorfer et al., 2014).
By the alternating access model for membrane transport proteins (Jardetzky, 1966), Nramps should cycle between at least two stable conformations: outward-facing to bind its metal substrate, and inward-facing to release its cargo into the cytosol. The ScaNramp structure, in an inward-facing state, revealed a metal-binding site, which consists of conserved aspartate, asparagine and methionine residues, and a backbone carbonyl from transmembrane segments (TMs) 1 and 6 that coordinate a range of divalent metal substrates (Ehrnstorfer et al., 2014), with the methionine providing a selective preference for transition metals over alkaline earth metals (Bozzi et al., 2016).
Here we present the crystal structure of the Deinococcus radiodurans Nramp homolog (DraNramp) from prokaryotic clade A. The DraNramp structure represents an inward-facing apo conformation, with TM1a, absent from the ScaNramp crystallization construct, swung up to open a large intracellular vestibule. We use this structure along with extensive cysteine accessibility and metal transport measurements to propose a model for conformational change in this LeuT-fold transporter and explain the mechanistic effects of two anemia-causing mammalian Nramp2 mutations. In our model, reaching the outward-facing state requires TM1a to approach the protein core to close the intracellular vestibule. This motion is prevented by an N-terminal glycine to arginine mutation that mimics an anemia causing mutation in human Nramp2. The second disease-mimicking glycine-to-arginine mutation alters the extracellular vestibule and metal-binding site, resulting in reduced transport activity and altered selectivity.
Results
DraNramp inward-facing structure shows a highly kinked transmembrane helix 1
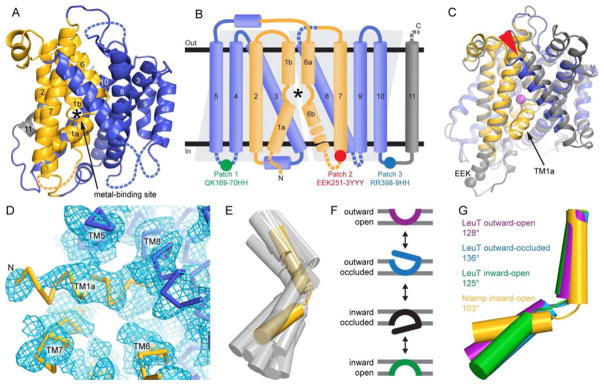
We determined the crystal structure of detergent-solubilized DraNramp (38% sequence identity with ScaNramp) in complex with a monoclonal antibody fragment (Fab) to 3.9-Å resolution (Table 1). Crystallization was facilitated by intracellular surface mutations (Figure 1B; see below). We used a ScaNramp-based homology model and the Fab fragment crystal structure as molecular replacement search models, with additional phasing provided by single-wavelength anomalous signal from three osmium ions bound to the Fab or at crystal contacts (Figure S1). Refinement was facilitated by using xMDFF (McGreevy et al., 2014; Singharoy et al., 2015). In particular, we used a combination of xMDFF and steered molecular dynamics to optimize our TM1a model, exploring different TM1a positional registries corresponding to a screw axis rotation of approximately one helical turn. The assigned TM1a registry (Figure 1D) both yielded the lowest R factor and best agreement with cysteine accessibility data (see Figures 6 and 7 below). The final asymmetric unit comprises one DraNramp transporter, one Fab bound to the DraNramp periplasmic face, and three osmium ions, with crystal packing interactions between the Fab and the DraNramp cytoplasmic face (Figure S1). All 11 DraNramp TMs are visible in the electron density, including TM1a, which was truncated in ScaNramp (Ehrnstorfer et al., 2014).
Table 1.
Data collection and refinement statistics.
| Fab | DraNramp | |
|---|---|---|
| PDB ID | n.a. | 5KTE |
| SBGrid Data Bank ID | 335 | 332, 333, 334 |
|
| ||
| Data Collection | ||
| Wavelength (Å) | 0.97917 | 1.139 |
| Resolution range (Å) | 30.00 – 3.00 (3.11 – 3.00) | 46.47 - 3.94 (4.08 - 3.94) |
| Space group | P212121 | I222 |
| Unit cell (a, b, c) | 116.17, 183.66, 299.88 | 113.13, 132.08, 221.0 |
| Number of crystals | 1 | 3 |
| Total reflections | 558353 | 245867 |
| Unique reflections | 119718 | 11791 (462) |
| Redundancy | 4.7 (4.8) | 16.6 (11.4) |
| Completeness (%) | 99.5 (100.0) | 95 (96) |
| Mean I/σ(I) | 8.4 (2.2) | 6 (.61) |
| Rmerge | 0.151 | 0.171 |
| Rmeas | 0.177 | |
| Rpim | 0.047 | |
| CC1/2 | .99 (.189) | |
| Refinement | ||
| Resolution range (Å) | 46.47 - 3.94 (4.12 - 3.94) | |
| Rwork | 0.2666 (0.2672) | |
| Rfree | 0.3128 (0.3656) | |
| Number of atoms | 5625 | |
| Protein | 5622 | |
| Ions (Os) | 3 | |
| Protein residues | 762 | |
| Ramachandran plot | ||
| Favored (%) | 692 (90.8) | |
| Allowed (%) | 66 (8.7) | |
| Outliers (%) | 4 (0.52) | |
| RMS(bonds) | 0.005 | |
| RMS(angles) | 1.12 | |
| Average B-factor | 89.8 | |
| Protein | 89.8 | |
| Ions (Os) | 190 | |
Figure 1.
DraNramp structure in the inward-facing state shows a highly kinked TM1. (A) Cartoon representation of DraNramp with TM helices labeled; the bundle (TMs 1, 2, 6, and 7) is gold, scaffold (TMs 3, 4, 5, 8, 9, and 10) blue, and TM11 gray. Dashed loops are disordered in the structure. (B) DraNramp topology diagram, with helices as cylinders, and gray trapezoids highlighting the inverted structural repeats (TMs 1-5 and TMs 6-10). Intracellular loop mutations Patch 1 to 3 in the crystallized construct are indicated. (C) Superposition of DraNramp (blue and gold) and ScaNramp (4WGW; gray and Mn2+ magenta) indicates a similar overall fold. The main differences are the position of TM5 (red arrowhead) and the presence of TM1a in DraNramp. Grey spheres mark the ScaNramp EEK motif corresponding to Patch 2, disordered in DraNramp. (D) Final 2Fo-Fc electron density map at 0.8σ showing density for TM1a. DraNramp is represented as a Cα trace. (E) Comparison of TM1 kink angle of DraNramp (yellow) with published LeuT-fold structures (gray; LeuT 2A65, 3TT1, 3TT3, 5JAE; Mhp1 2JLN, 4D1B, 2×79; vSGLT 2XQ2, 3DH4; BetP 4LLH, 4AIN, 4DOJ, 4C7R). The scaffolds of the corresponding structures were superimposed and oriented as in (A). (F) Conformational states in a transport cycle, color-coded as in (G). (G) The TM1b helices were aligned for DraNramp and three distinct LeuT conformations, highlighting the common kink at the unwound substrate-binding region in the middle of TM1. The kink is even more pronounced in inward-open DraNramp than any LeuT structure. See also Figure S1.
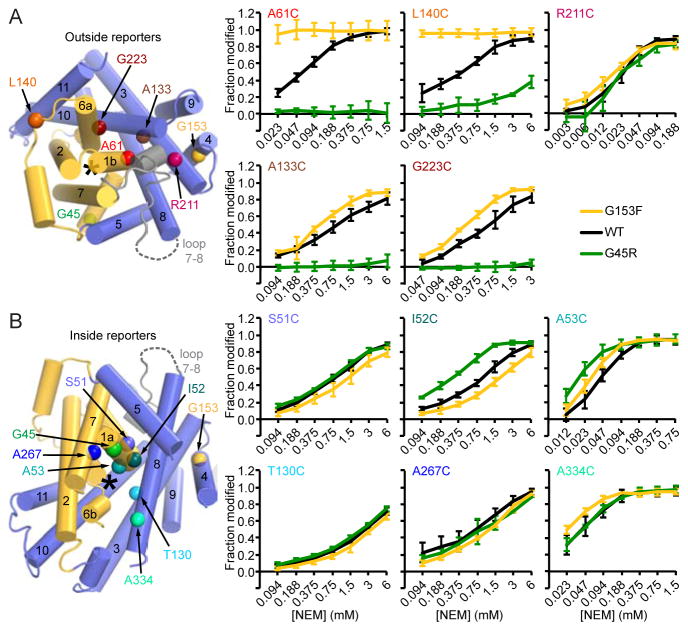
Figure 6.
Mutations of conserved glycines shift DraNramp’s conformational landscape. (A) Cysteine modification as a function of NEM concentration for five extracellularly-accessible reporters, mapped on a top view of the structure (left). * indicates the metal-binding site. A61C data are repeated from Figures 3C and 4C for comparison. (B) NEM modification for five intracellularly-accessible cysteine reporters as well as T130C, which could not clearly be assigned as intracellular or extracellularly accessible. Reporter positions are indicated on the structure, viewed down the cytoplasmic vestibule. All data are averages ± s.d. (n ≥ 4). See also Figure S5.
Figure 7.
TM1a movement is essential to the conformational change that opens the outward metal permeation pathway, thus enabling Nramp metal transport. (A) Initial (6 min) in vivo Co2+ transport (minus EV control) for accessible single-cysteine mutants along TM1 that were either left unmodified (black bars) or pre-reacted with NEM (3 mM; green bars). NEM modification greatly impaired transport for six out of the seven TM1a positions where cysteine mutants had high transport activity. Data are averages ± s.d. (n = 3). Western blots show all introduced cysteines were efficiently NEM-labeled, as preincubation with NEM (+) prevented formation of PEG5K-maleimide DraNramp [upper band in the (−) lanes]. R211C on extracellular loop 5-6 was readily NEM-modified without affecting activity. Endogenous C382 on TM10 in WT was not modified by NEM, and thus was fully modified by PEG5K-maleimide. The cysteine-less C382S is not labeled by either NEM or 5KPEG-maleimide. (B) Model of the conformational change process in DraNramp. Our metal transport and cysteine accessibility results demonstrated that the unencumbered TM1a movement is essential for conformational change into the outward-facing state, including evicting loop 7-8 which caps the outside metal permeation pathway and opening the interface between the bundle (TM1b and 6a) and scaffold (TM3, 8, and 10), thus allowing periplasmic metal ions to reach the binding site in the unwound regions of TM1 and 6 at the center of the membrane plane. See also Figure S6.
Like ScaNramp, DraNramp has a LeuT fold with two pseudosymmetry-related structural repeats comprising TMs 1-5 and TMs 6-10, respectively (Figure 1A and 1B)(Yamashita et al., 2005). As in other LeuT folds, TMs 1, 2, 6, and 7 form a “rocking bundle” whose putative movements relative to the “scaffold”, made up of the remaining TMs, likely effects the switch in active site accessibility from extracellular to intracellular (Forrest and Rudnick, 2009). TM1 and 6 are unwound in the center of the membrane plane, providing substrate-binding residues as observed in ScaNramp (Ehrnstorfer et al., 2014). In DraNramp, the metal-binding site is unoccupied and exposed to the intracellular side (see Figure 2A). Thus the structure represents a substrate-free inward-facing conformation. Accordingly, DraNramp and ScaNramp superimpose well with a root mean square distance of 1.54 Å over 279 Cα atoms. Clade A members like DraNramp have a 4-residue deletion near the TM9 N-terminus in comparison to Clade C members like ScaNramp, resulting in a shorter helix (Figure S1D)(Cellier, 2012). The main difference between the two structures is the position of scaffold helix TM5 (Figure 1C), more angled relative to the membrane plane in DraNramp, perhaps influenced by the presence of TM1a in the structure.
Figure 2.
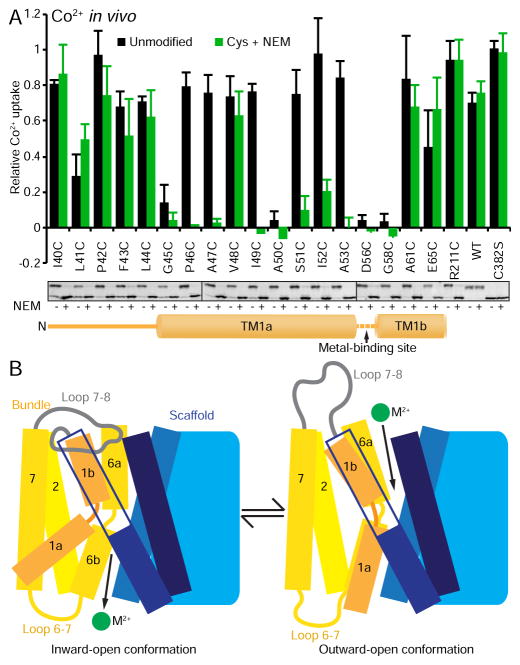
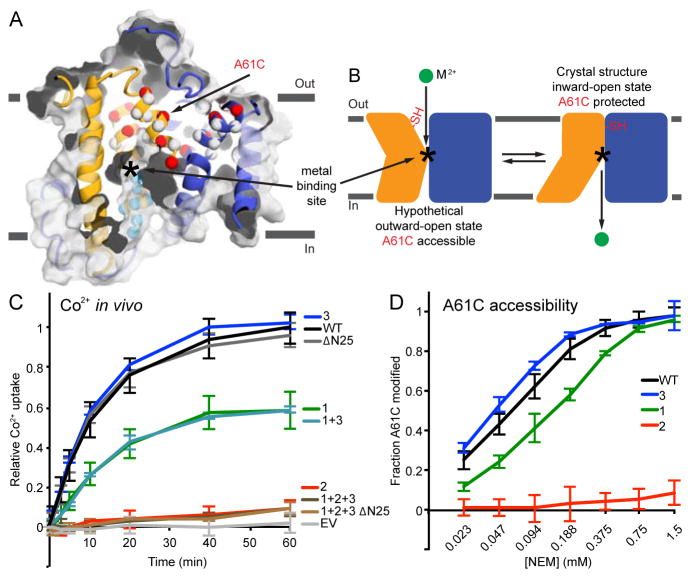
Cysteine accessibility scanning reveals the outward metal permeation pathway that is sealed shut in our crystallization construct. (A) Internal slice of the inward-facing DraNramp structure, including solvent accessibility of a panel of cysteine mutants spanning TM1, 3, and 6 using NEM. Spheres show Cα positions of highly NEM-protected (gray), outward-accessible (also MTSET and MTSEA-modified; red), inward-accessible (also MTSEA- but not MTSET- modified; cyan), or only NEM-accessible (black) residues. Accessibility is assessed as >50% NEM-modification in at least two separate experiments. Many outward-accessible residues, including A61C, are buried in our inward-open structure, suggesting they line an aqueous passage to the metal-binding site (approximate location labeled *) in an alternate outward-open conformation. (B) DraNramp’s proposed conformational equilibrium, in which A61C is solvent-accessible in the outward-open state, but buried (and thus NEM-protected) in the inward-open conformation. (C) The patch mutants in the crystallized DraNramp construct, tested alone or in combinations, have varying effects on in vivo Co2+ transport. While the 25-residue N-terminal truncation and patch 3 (RR398-9HH) did not impair function, patch 1 (QK169-70HH) reduced transport and patch 2 (EEK251-3YYY) completely eliminated transport. (D) While the transport-competent patch 1 and patch 3 mutants retained A61C accessibility (patch 1 at a reduced level), the transport-dead patch 2 mutant eliminated A61C accessibility, suggesting it locks the protein in the inward-open state. All data are averages ± s.d. (n ≥ 3). For reference, WT and EV Co2+ uptake time course and WT A61C accessibility data are repeated in subsequent figures. See also Figure S2 and Table S1.
As in other LeuT-fold proteins (Figure 1E), DraNramp TM1a is bent outwards and lies nearly parallel to the membrane between the pillars of scaffold helix TM5 and bundle helix TM7, creating a large aqueous vestibule on the cytoplasmic side of the transporter. The angle between TM1a and TM1b is 103°, smaller than that observed in the inward-facing LeuT structure (125°; (Krishnamurthy and Gouaux, 2012)), and outward-occluded LeuT (136°; (Yamashita et al., 2005)) (Figure 1F and 1G). Correspondingly this deep bend in TM1 yields a larger vestibule than in LeuT, which could facilitate water coordination of transported metal ions.
Cysteine accessibility scanning reveals outward-facing metal permeation pathway
To identify a metal-permeation pathway, we created a panel of sequential single-cysteine mutants spanning DraNramp TM1, 3, and 6, which typically line the inward- and/or outward-facing permeation pathway in LeuT-fold transporters (Shi, 2013). The mutations were introduced in the C382S background, which removed the lone endogenous cysteine while retaining full activity (Figure S2E). We measured in vivo accessibility to thiol-specific modifier N-ethylmaleimide (NEM) or inner membrane-impermeable 2-(trimethylammonium)ethyl methane thiosulfonate bromide (MTSET) (Figure S2A and S2B), which both specifically react with aqueous-exposed cysteines (Kaback et al., 2007; Karlin and Akabas, 1998). We classified the cysteine positions in three groups: (i) NEM- and MTSET-reactive residues were deemed as extracellularly exposed in at least one DraNramp conformation; (ii) NEM-reactive but MTSET-protected residues as intracellularly accessible; and (iii) NEM-protected as buried (Figure S2C and S2D and Table S1). We observed high accessibility all along TM1a and TM6b, consistent with the large aqueous vestibule below the metal-binding residues in our structure. Between the metal-binding site and the extracellular face, we observed a helical pattern of accessible positions on TM1b, 3, and 6a that line the bundle-scaffold interface (Figure S2D). As many of these NEM-reactive positions are buried in the inward-facing structure (Figure 2A), we conclude that they face an aqueous pathway for periplasmic metal ions to reach the binding site in an alternate outward-open conformation. Thus our cysteine accessibility measurements, rather than describing a single state, instead provide a composite picture of multiple conformations that DraNramp cycles through in the native membrane.
Crystallization construct mutation locks transporter in inward-open state
We exploited this knowledge of the extracellular metal-permeation pathway to select A61C as a reporter to assess the conformational preferences of various DraNramp mutants (Figure 2A and 2B). A61C was accessible to inner membrane-impermeable modifier MTSET (Figure S2B), indicating it faces a periplasmic aqueous environment, and A61C/C382S showed WT-level Co2+ transport (Figure S2E). In contrast, our crystallization construct, which contained entropy-reducing mutation patches in three intracellular loops: (1) QK169-70HH, (2) EEK251-3YYY, and (3) RR398-9HH, as well as an N-terminal 25-residue deletion (Figure S2F), did not transport Co2+ (Figure 2C). Patch 2 on its own completely eliminated Co2+ uptake (Figure 2C). This mutation is in intracellular loop 6-7, far from the metal-binding site; thus we tested A61C accessibility over a range of NEM concentrations to probe for conformational preference. Unlike the concentration-dependent increase in NEM-modification of A61C for WT DraNramp, A61C was essentially not modified in the patch 2 background (Figures 2D and S2G). Therefore the patch 2 mutant protein cannot switch to the outward-open state, which explains its loss-of-function phenotype, as substrate ions cannot reach the binding site (below A61C) from the outside. The patch 3 mutant had WT-level Co2+ transport and A61C modification, while the patch 1 mutant had both slightly reduced Co2+ transport and A61C accessibility (Figure 2C and 2D). This clear correlation of impaired metal uptake with loss of A61C accessibility further implicates the opening of the interface between the bundle and scaffold as an essential conformational change within the Nramp transport cycle.
Anemia-causing mutations impair metal transport in human Nramp2 and DraNramp
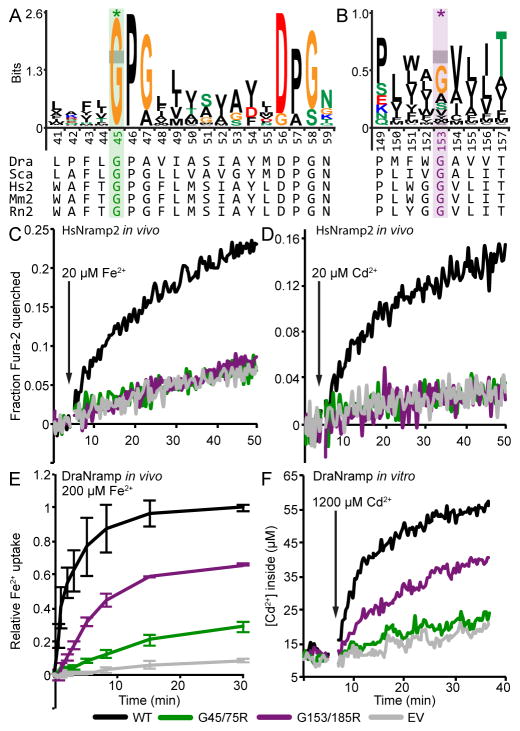
Naturally occurring glycine-to-arginine mutations G75R in TM1a in human Nramp2 and G185R in TM4 in mouse and rat Nramp2 both cause anemia (Blanco et al., 2009; Fleming et al., 1998; Fleming et al., 1997). The TM1a glycine is absolutely conserved; the TM4 glycine is generally conserved as a small residue (Figure 3A and 3B). When introduced in human Nramp2, both G-to-R mutations abrogated transport of the physiological substrate Fe2+ (Figure 3C), as well as Cd2+, Co2+, and Mn2+ (Figures 3D, S3A and S3B), as detected in transfected HEK cells using the metal-binding fluorescent dye Fura-2. Similarly, the analogous disease-mutant mimics in DraNramp significantly impaired Fe2+ transport as detected colorimetrically in DraNramp-expressing E. coli (Figure 3E), and reduced Cd2+ (Figure 3F) and Mn2+ transport (Figure S3C) as detected with Fura-2 using purified DraNramp reconstituted into proteoliposomes. In both homologs, the TM1a G-to-R mutant expressed similarly to WT, while TM4 G-to-R mutant expression was reduced (Figure S3D and S3E), which could contribute to the loss-of-function phenotype in the in vivo assays. However, the DraNramp G153R mutation clearly impaired both Cd2+ and Mn2+ transport in proteoliposomes with normalized protein concentrations (Figure S3F). Notably, the G185R mutation in mouse Nramp2 similarly impaired Fe2+ transport (Su et al., 1998). These consistent loss-of-function data for the analogous mutants indicate that DraNramp is a useful model to further investigate how these disease-causing mutations inhibit transport.
Figure 3.
Glycine-to-arginine mutations impair transition metal transport in both human Nramp2 and DraNramp. (A, B) Sequence logos of the TM1a region (A) showing that G45 (G75 in human Nramp2) on TM1a is absolutely conserved in Nramps, and a TM4 segment (B) showing that G153 (G185 in human/mouse/rat Nramp2) is generally a small amino acid. Logos were generated from a HMMER alignment of 2691 sequences using DraNramp to search the UniprotRef database, with an E-value cut-off of 1 × 10−9. Dra = D. radiodurans MntH, Sca = S. capitis MntH, Hs2 = Homo sapiens Nramp2, Mm2 = Mus musculus Nramp2, Rn2 = Rattus norvegicus Nramp2. (C, D) Fura-2 fluorescence quenching traces showing severe loss of function (no transport activity above baseline) for both G-to-R mutants compared to WT human Nramp2 for transport of the transition metals Fe2+ (C) and Cd2+ (D) in transfected HEK cells. Traces are representative of at least three independent transfection experiments. (E) Relative Fe2+ uptake of E. coli expressing the analogous G-to-R DraNramp mutants also showed significantly decreased transport activity compared to WT. Plotted are averages ± s.d. (n = 3). (F) Both G-to-R DraNramp mutants had decreased Cd2+ transport when reconstituted into proteoliposomes. Traces are representative of three experiments. EV = empty vector/vesicle. See also Figure S3.
The G153R mutation alters the conformational equilibrium and metal selectivity
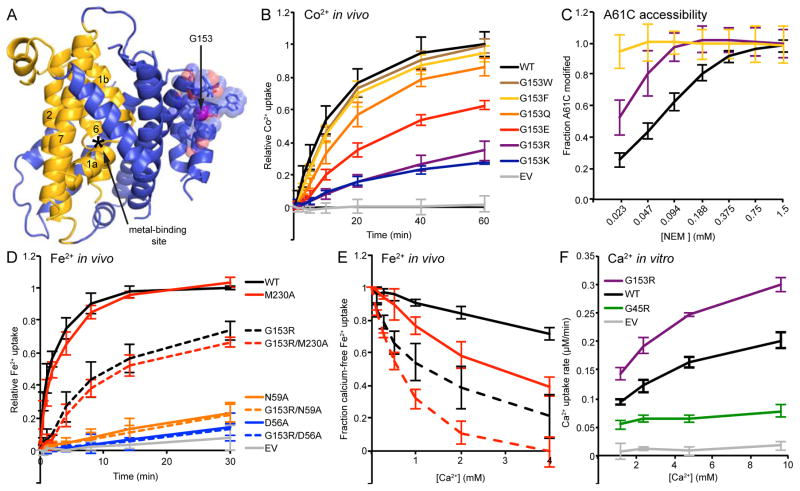
G153 is far—~20 Å—from the metal-binding site, near the top of TM4 within the scaffold (Figure 4A). We tested in vivo Co2+ uptake activity of various substitutions at this position. G153R, and the similarly large and positively charged G153K, were most impaired (Figure 4B). In contrast, replacing G153 with large aromatics (G153F and G153W) did not significantly alter Co2+ uptake (although Fe2+ uptake by G153F was somewhat reduced; Figure S4B). A negatively charged glutamate (G153E) reduced uptake much more than a polar glutamine (G153Q), although less than G153R. Overall, charged sidechains caused the largest reductions in transport activity.
Figure 4.
G153R mutation perturbs outward-facing state and alters metal selectivity of binding site to favor Ca2+. (A) In the inward-facing DraNramp structure, G153 is located on the extracellular end of TM4 tightly packed in the back of the scaffold, far from the conserved metal-binding site. The G153 Cα is a magenta sphere; nearby residues are shown as transparent spheres with sidechains as sticks. (B) Co2+ uptake data showing charged bulky substitutions at G153 reduced transport, while aromatic substitutions retained WT-level transport. (C) G153R showed increased accessibility to NEM modification of the A61C outward-open conformational reporter, and G153F showed a further increase. (D) In in vivo Fe2+ uptake, G153R showed little additional effect when combined with metal-binding site mutations, and its residual Fe2+ transport still uses the conserved metal-binding site as double mutations G153R/D56A and G153R/N59A both severely reduced transport. (E) Competing Ca2+ reduced in vivo Fe2+ uptake more for G153R than WT, and double mutant G153R/M230A was even more susceptible to Ca2+ competition. The Ca2+-free Fe2+ uptake level (2 min uptake for WT/M230A; 15 min uptake for G153 mutants) was set to 100% for each variant to facilitate direct % inhibition comparison. (F) G153R transported more Ca2+ (as detected by Fura-2) than WT or the G45R mutant in an in vitro proteoliposome assay. All data are averages ± s.d. (n ≥ 3). See also Figure S4.
In our inward-open structure, modeling a bulky substitution at position 153 leads to major steric clashes with nearby residues such as I142 on TM3, indicating that G153R or similarly large substitutions likely alters DraNramp conformation at least locally. We therefore compared outward-open reporter A61C accessibility for this G153X panel (Figure 4C and Figure S4C). All mutations resulted in increased NEM modification of A61C, with even more pronounced effects for G153F and G153W than G153R. These data alone cannot distinguish between two scenarios: (i) increased occupancy of the outward-open state with concomitant decreased inward-open state occupancy; or (ii) local structural changes that increase A61C accessibility in a protein that can still undergo conformational cycling. We further investigated this effect in Figure 6 below. Previous work showed that in mouse Nramp2 the G185R mutation decreased Fe2+ transport, but surprisingly enabled Ca2+ transport (Xu et al., 2004). This could occur through either distortion of the conserved metal-binding site or opening of an alternative metal-permeation pathway. We first tested whether the residual Fe2+ transport by G153R DraNramp relied on the canonical metal-binding site, introducing an alanine substitution for each binding-site sidechain into the WT or G153R background. Either a D56A or N59A substitution abolished Fe2+ transport in both backgrounds, while M230A had negligible effects in both cases (Figure 4D), consistent with our previous results (Bozzi et al., 2016). Thus G153R still relies on the conserved metal-binding residues for Fe2+ transport. We next tested whether G153R perturbed the selectivity of this binding site by measuring Fe2+ transport in the presence of competing Ca2+ for WT, G153R, M230A, and G153R/M230A (Figure 4E), as well as G153F (Figure S4D). As expected, M230A, lacking the methionine “selectivity filter” that excludes alkaline earth metals (Bozzi et al., 2016), was more susceptible to Ca2+ competition than WT. Interestingly, G153R was even more susceptible to Ca2+, and the G153R/M230A double mutant was most susceptible, as 4 mM Ca2+ effectively abolished Fe2+ uptake. This suggests that G153R indeed perturbs the metal-binding site in favor of Ca2+ in DraNramp in a manner that is complementary rather than redundant with removing the conserved methionine. We observed a similar pattern using a plate-based toxicity assay in which we assess the growth of E. coli expressing DraNramp or its variants on various metal concentrations: G153R or M230A increased Ca2+ susceptibility, and G153R/M230A again had an additive effect (Figure S4F). This synergy was not observed for the similar alkaline earth metal Mg2+, as G153R/M230A had higher Mg2+ tolerance than M230A alone (Figure S4G), while G153R and WT grew similarly, suggesting that the G153R perturbation may be somewhat specific for Ca2+. G153R also had a reduced susceptibility to Cd2+ compared to WT (Figure S4H), but was more susceptible than M230A. To directly demonstrate the G153R mutant’s improved Ca2+ transport, we purified and reconstituted it into proteoliposomes, and observed decreased Cd2+ transport (Figure S4E) but increased Ca2+ transport compared to WT (Figure 4F). Of note, the other disease-mutant mimic, G45R, impaired both Ca2+ and Cd2+ transport (Figure 4F), thus the metal specificity perturbation is unique to G153R. In summary, the G153R mutation in our bacterial DraNramp model system alters the conserved metal-binding site in a manner that both improves Ca2+ transport and impairs transition metal transport, analogously to mouse Nramp2.
G45R mutation wedges transporter in inward-facing conformation
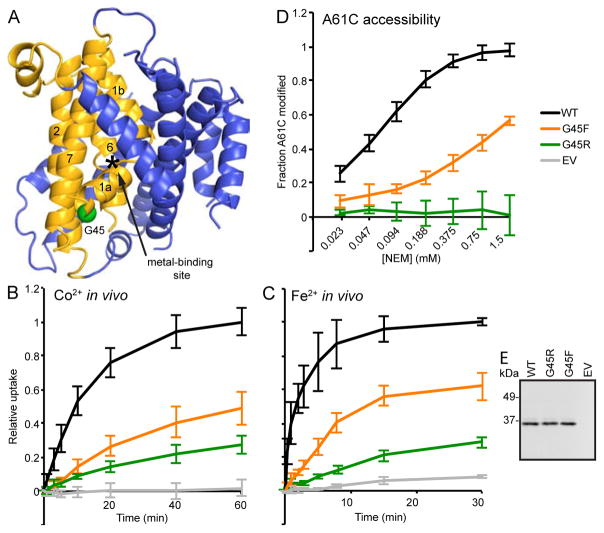
Like G153, the completely conserved G45 is ~20 Å away from the metal-binding site at the N-terminus of TM1a, which juts out at an angle to provide high cytoplasmic solvent access to the metal-binding residues (Figure 5A). Modeling an arginine at this position in our inward-facing structure yields no steric clash. We thus hypothesized that the mutation is not tolerated in the alternate outward-open state: a bulky residue at this position may act as a wedge forcing the protein to remain in the inward-open state, thus explaining G45R’s severe metal transport impairment (Figure 3). Consistently, the bulky uncharged G45F substitution impaired in vivo uptake of Co2+ (Figure 5B) and Fe2+ (Figure 5C) nearly as much as G45R. Furthermore, single-cysteine reporter A61C was essentially fully protected in G45R, indicating that the outward-open state was rarely if ever sampled, while A61C accessibility in G45F suggested a shifted conformational preference, strongly favoring the inward-open state relative to WT (Figure 5D). Thus decreased A61C accessibility of G45 substitutions correlated with decreased metal transport. This is similar to the patch mutant phenotypes (Figure 2C and 2D), especially patch 2, which just like G45 is located in the intracellular half of the bundle, indicating the importance of proper conformational cycling for protein function.
Figure 5.
G45R mutation inhibits transport by locking Nramp in outward-closed conformation. (A) G45 (green sphere) is on TM1a on the intracellular side of the bundle, 11 residues below the metal-binding D56. (B) G45R and G45F were significant impaired in in vivo Co2+ transport. (C) G45F also impaired in vivo Fe2+ transport (WT, G45R and EV data reproduced from Figure 3E for comparison). (D) G45R and G45F drastically decreased accessibility of outside-open conformational reporter A61C, indicating a strong preference for the inward-open state. (E) Western blot showing G45R and G45F expressed similarly to WT DraNramp. All data are averages ± s.d. (n ≥ 3).
Mutations at glycine positions illustrate conformational change process in DraNramp
To further explore the DraNramp conformational cycle, we investigated how G45R and G153F altered solvent accessibility for a panel of inward- or outward-accessible single-cysteine substitutions that retained significant transport activity (Figure S5A and S5B). Accessibility of outward reporters A61C and L140C was correlated; G45R greatly decreased while G153F greatly increased accessibility (Figure 6A). This suggests G153F favors a conformational state in which part of the bundle-scaffold interface above the metal-binding site is highly exposed—perhaps by dislodging loop 7-8 that blocks solvent access to A61 and L140 in the inward-open structure. Closer to the metal-binding site within the bundle-scaffold interface, outward-accessible G223C and A133C were also fully protected in G45R, further supporting our inward-locked model for G45R (Figure 6A). However, at those cysteine positions G153F only had a modest effect, slightly increasing accessibility. Finally, neither G45R nor G153F altered accessibility of R211C on extracellular loop 5-6.
Accessibility was also unaltered at T130C, on TM3 adjacent to the unwound regions of TM1 and 6, indicating that the metal-binding site remains solvent-accessible in both G153F and G45R (Figure 6B).
To probe for water-excluding structural changes in the cytoplasmic vestibule, we compared NEM accessibility of five cysteines located below the metal-binding site in the WT, G45R, and G153F backgrounds (Figure 6B). Accessibility was the same in all backgrounds for S51C on TM1a, A267C on TM7, and A334C on TM8. For I52C and A53C on TM1a just below the metal-binding D56, accessibility was higher in G45R, suggesting these positions form part of the cytoplasmic gate regulating access to the metal-binding site. We observed only minor perturbations at all inward-reporter positions for G153F. Thus this mutation likely does not lock the protein in the outward-facing state with a closed intracellular gate, consistent with its high metal-transport activity (Figures 4B and S4B). In summary, the intracellular gate likely includes the TM1a C-terminus, although a more complete picture awaits a truly outward-locked mutant. On the extracellular side, our crystal structure and cysteine accessibility results clearly demonstrate a ~15 Å thick, solvent-excluding gate that remains firmly closed in the G45R mutant.
TM1a movement is essential to the DraNramp transport cycle
To determine the functional importance of the DraNramp N-terminus, we designed a truncation series that eliminated 25 to 49 residues (Figure S6A–C). Co2+ uptake activity was at WT levels up through the ΔN34 construct, and slight reductions in transport accompanied each further truncation for ΔN37, ΔN40, and ΔN43 (Figure S6). In contrast, ΔN46 and ΔN49, which both truncate beyond the TM1a N-terminus at the invariant G45, showed pronounced drops in Co2+ uptake activity.
To further assess the importance of TM1a movement for metal transport, we compared Co2+ uptake for single-cysteine mutants, either unmodified or pre-modified with NEM (Figure 7A). For cysteine-less mutant C382S, WT (with the inaccessible C382), and extracellular loop control R211C, NEM treatment did not impair transport. However, for six of seven TM1a positions where the cysteine mutant retained transport activity, NEM modification greatly impaired transport. Thus while those cysteines were highly accessible in at least one conformation, they must move into a more congested environment as part of the metal transport cycle, which NEM modification sterically blocks. This phenomenon was not observed for positions preceding TM1a (residues 40-44, disordered in the structure). Cysteine substitution alone impaired transport at four positions: G45C, consistent with the importance of this conserved glycine and loss-of-function of G45R and G45F (Figures 3 and 5); D56C and G58C, which disrupt the metal-binding site; and A50C—a surprising result given the subtle size increase—which may reflect how close this face of TM1a must approach TM7 and/or the scaffold (TM8) as the inside gate closes in the outward-open state. Surprisingly, we did not observe NEM-dependent impairment for the two accessible TM1b positions A61C and E65C, the first of which clearly lines the outward metal permeation pathway and is buried in the inward-open state. An A61W mutant similarly retained WT-level Co2+ transport, showing that DraNramp can indeed tolerate added bulk at this position (Figure S6D). However, pre-modifying A61C with the positively charged MTSET selectively inhibited Co2+ uptake (Figure S6G), thus some perturbation of the outward metal permeation pathway at position A61 can indeed disrupt transport.
Finally, to further test the functional importance of TM1a movement, we substituted tryptophan—an alternative way of adding steric bulk—at each of the six TM1a positions where NEM modification of cysteine mutants drastically impaired transport. Analogously to G45R, these tryptophan mutations greatly impaired Co2+ transport and fully protected the A61C outward-open reporter (Figure S6D–F), indicating that bulky modifications on TM1a lock the protein in an inward-facing conformation. In summary, these results indicate that TM1a movement is an integral part of Nramp conformational rearrangement and thus required for metal transport. From all of our results, we propose a model of Nramp conformational change in which significant inward movement of TM1a toward the scaffold is crucial to opening the outside interface between the bundle (TM1b and 6a) and scaffold (TM3, 8, and 10) to allow metal ions (and bulk water) to access the binding site from the outside (Figure 7B).
Discussion
Using X-ray crystallography, cysteine accessibility, and metal transport measurements, we developed DraNramp as a model to understand the conformational change process for the Nramp family of divalent metal transporters. The DraNramp structure, while also a LeuT fold in an inward-facing conformation, complements the ScaNramp structure (Ehrnstorfer et al., 2014) by identifying the location of the functionally-important TM1a and (with cysteine-scanning mutagenesis) the external metal permeation pathway. It also provides a structural example of a second distinct evolutionary clade within the Nramp family. Furthermore, our structure showed the locations of two highly conserved glycines where mutations to arginine cause anemia in either rodents (Fleming et al., 1998; Fleming et al., 1997) or humans (Blanco et al., 2009) and impair transport in both human Nramp2 and DraNramp (Figure 3).
The G153R mutation on DraNramp TM4 mimics the phenotype first observed in mouse Nramp2 (Xu et al., 2004), altering metal transport selectivity, despite these two homologs having only 29% sequence identity. G153R perturbs the conserved metal-binding site, thus reducing transport of Nramp’s typical transition metal substrates and increasing Ca2+ transport (Figure 4). A bulky residue cannot be accommodated at position 153 in our inward-facing structure. Our data are consistent with the G153R mutation distorting the scaffold in a manner that interferes with closing of the extracellular metal permeation pathway (Figure 6). Precisely how a mutation 20 Å from the binding site increases Ca2+ permeability remains undetermined, but interestingly G153R and M230A, which removes the sulfur ligand responsible for excluding alkaline earth metals (Bozzi et al., 2016), have additive effects (Figures 4 and S4). Other residues besides the four previously identified may be important to metal binding and/or selectivity, and in at least one other Nramp homolog mutations distant from the metal-binding site affect substrate preference (Pottier et al., 2015).
G45R on TM1a forms a steric wedge that prevents the protein from reaching the outward-open state (Figure 5). Moreover, our results imply that the unfettered movement of TM1a is essential to the conformational rearrangement that must occur to allow metal transport (Figures 7 and S6). Our study adds to a wealth of structural and functional information available for LeuT-fold secondary transporters (Penmatsa and Gouaux, 2014; Shi, 2013) that expanded on the original “rocking bundle” model of alternating access, in which the bundle (TM1, 2, 6, and 7) moves relative to the scaffold (Forrest and Rudnick, 2009; Forrest et al., 2008). Comparison of outward- and inward-open LeuT structures (Krishnamurthy and Gouaux, 2012) showed TM1a swinging up by 45° and a smaller TM6b motion to expose the substrate-binding site to the cytosol. However, the physiological relevance of this large TM1a movement in LeuT is disputed: while some movement is essential to substrate release (Zhao et al., 2011), the Y268A mutation on TM6b in the crystallized construct disrupts a hydrogen-bonding network in the inward-facing state and stabilizes a conformation not highly sampled by the WT protein (Kazmier et al., 2014b), with MD simulations also supporting a less drastic tilt in a lipid bilayer environment (Grouleff et al., 2015). In DraNramp, our inward-locking patch 2 mutant and/or crystal-packing interactions with the Fab could analogously stabilize the observed profound TM1a kink. Additional Nramp structures will help clarify this issue.
We nevertheless predict that TM1a undergoes significant displacement during the conformational change process, given its functional importance in DraNramp. In addition, the LeuT structures showed that movement of TM1b and 6a away from the scaffold opens an aqueous pathway to the binding site, in agreement with our DraNramp cysteine accessibility data. Furthermore, these LeuT structures and functional studies (Claxton et al., 2010) illustrated the contribution of loop 7-8 to closing off extracellular access to the substrate-binding site, which we also observe in DraNramp.
Structural information on other sodium-coupled symporters of small organic molecules hints at mechanistic diversity within the LeuT-fold family. Structures of the sodium/galactose transporter vSGLT show a TM1a movement (subtler than in LeuT) to open the inside gate in the transition from the inward-occluded to the inward-open state (Watanabe et al., 2010). In the sodium/betaine transporter BetP, outward- and inward-open structures also show TM1a motion as part of the inside gate, whereas loop 7-8 is similarly positioned above the bundle-scaffold interface but does not appear to move much (Perez et al., 2012). Crystallographic (Shimamura et al., 2010) and electron paramagnetic resonance (Kazmier et al., 2014a) studies of the sodium/nucleobase symporter Mhp1 showed a mechanism to similar LeuT albeit with subtle differences: TM5 rather than TM1a bends to open the inner gate and TM10 (and to a lesser extent loop 7-8) changes conformation to close the outer gate.
Our combined structural and functional results thus suggest that Nramp’s conformational change process may be most similar to LeuT’s. However, Nramp’s proposed proton co-substrate(s) would require a unique transport mechanism and lead to different environmental controls of its conformational landscape, perhaps with a protonation event substituting for the Na+ coordination that governs LeuT conformational preference (Malinauskaite et al., 2014; Tavoulari et al., 2016). Ultimately, a complementary outward-open Nramp crystal structure will help answer outstanding questions regarding the Nramp transport cycle.
Experimental Procedures
Cloning of Nramp constructs
The DraNramp sequence was inserted into the NdeI and NotI sites of pET21-N8H and Human Nramp2 into pCDNA3 vectors as previously described (Bozzi et al., 2016). Mutagenesis was performed using QuikChange protocols (Stratagene), and confirmed by sequencing.
DraNramp protein purification
DraNramp C41(DE3) cells were grown at 37°C in 12 L terrific broth with 10% (w/v) glycerol and 100 mg/L ampicillin, inoculating with 1:50 overnight culture to OD600=1.0, induced with 100 μM IPTG for 4 h. Proteins were purified at 4°C. Cells were lysed by sonication in three volumes of lysis buffer (20 mM NaPO4 pH 7.0, 75 mM imidazole-HCl pH 7.0, 500 mM NaCl, 10% glycerol) plus 1 mM PMSF, 1 mM benzamidine, 0.3 mg/mL each DNAse I and lysozyme. Debris was removed by 20 min centrifugation at 27,000 × g, membranes were pelleted in 70 min at 230,000 × g, solubilized in 35 mL lysis buffer + 1% w/v β-dodecylmaltoside (DDM) for 1 hr, and clarified by 35-min centrifugation at 140,000 × g, filtered through a 0.45 μm filter, loaded onto 5 mL Ni-Sepharose (GE Healthcare) pre-equilibrated with lysis buffer + 0.03% β-DDM, and washed thrice with 50 mL lysis + 0.03% β-DDM. Protein was eluted in 25 mL increasing imidazole to 450 mM, and concentrated to ~1 mL in a 50 kDa cutoff centrifugal concentrator. Following buffer exchange on a Superdex S200 (GE Healthcare) to 20 mM HEPES pH 7.5, 150 mM NaCl, and 0.1% (w/v) β-decylmaltoside (DM), protein-containing fractions were identified using SDS-PAGE, pooled, and concentrated to ~5 mg/mL.
Fab production
Balb/C mice were immunized four times with DraNRAMP reconstituted into proteoliposomes using E. coli lipids (Avanti Polar Lipids). Hybridomas were generated and cultured using established protocols (Yokoyama, 2008) and secreted monoclonal antibodies (mAbs) were tested in ELISA by capturing on anti-mouse IgG-coated 96-well plates. Binding mAbs were identified using detergent-solubilized biotinylated DraNramp coupled to streptavidin-HRP. Cells in ELISA-positive wells underwent four rounds of recloning by limiting dilution to ensure monoclonality and stable mAb expression. Conformation-specific mAb clones were selected as non-binders in slot blot assays for binding to SDS-denatured DraNramp. The cocrystallized mAb was purified by capture from filtered hybridoma supernatant on protein A-agarose pre-equilibrated with binding buffer (BB; 20 mM NaPO4 pH 8.0, 150 mM NaCl). After a 10 column-volume BB wash, mAbs were eluted in 7 column volumes 100 mM MES pH 6.5, 3.6 M MgCl2, dialyzed against BB, and concentrated using a 50 kDa cutoff concentrator. Purified mAbs (15 mg at 1.5 mg/mL) in 20 mM NaPO4, 20 mM EDTA pH 6.0 were digested with 5 units of ficin suspension (Sigma Aldrich) at room temperature (RT) for 5 hr. After adding 50 mg iodacetamide and 50 mg N-ethyl maleimide, and concentrated, the digest was run on a Superdex S200 16/600 (GE Healthcare) in BB. Fab-containing fractions were passed through 5-mL Protein A-agarose to remove any Fc fragment or uncleaved mAb, and concentrated to ~10 mg/mL. The Fab sequence was obtained using standard protocols (Verma, 2000).
Fab crystallization and structure determination
Fab crystals were grown by sitting drop vapor diffusion, mixing 0.5 μL 10 mg/mL Fab with 0.5 μL reservoir (0.1 M CAPS pH 9.7, 0.1 M MgCl2, 30% PEG 3350), cryoprotected in reservoir plus 25% glycerol, and frozen in liquid nitrogen. Diffraction data to 3.1 Å was collected at Advanced Photon Source beamline ID24-E (Table 1). An Igg2b Fab structure (PDBID 1KNO) was used as a molecular replacement (MR) search model, and the Fab structure was refined to Rwork/Rfree of 0.22/0.30 using REFMAC.
DraNramp-Fab crystallization and structure determination
The DraNramp crystallization construct was truncated by 25 residues at the N-terminus, and mutations were introduced in intracellular loops (QK169-170HH, EEK251-3YYY, and RR398-9HH). DraNramp was incubated for 2 hr with Fab in a 1:1.2 molar ratio, and run on a Superdex S200 10/300 (GE Healthcare) in 10 mM HEPES pH 7.5, 150 mM NaCl, and 0.1% DM. DraNramp-Fab-containing fractions were pooled and concentrated to ~5–10 mg/mL using a 50 or 100 kDa cutoff concentrator. DraNramp-Fab crystals were grown by vapor diffusion against 0.1 M sodium acetate pH 4.5, 0.05 M magnesium acetate, 24% PEG400 and 0.4–1 % β-octylglucoside in 1:1 volume ratio sitting drops at 4°C. Crystals derivatized with 1 mM OsCl3 for one day turned dark brown and were frozen in liquid nitrogen.
Diffraction data were collected from three crystals at Advanced Photon Source ID24-E and -C beamlines, using vector scanning and full transmission, processed with HKL2000 (Otwinowski and Minor, 1997), and merged to improve completeness. For initial phasing, a ScaNramp-based homology DraNramp model built using SWISS-MODEL (Biasini et al., 2014) and the Fab structure were used as MR search models in Phaser (McCoy et al., 2007). Experimental phase information from single-wavelength anomalous diffraction from the osmium-soaked crystals was determined using AutoSol in PHENIX (Adams et al., 2010). The resulting osmium sites were combined with the MR solution and data containing the experimental phase information were used for refinement. Coot (Emsley and Cowtan, 2004) was used for model building. Initial rounds of refinement used xMDFF, with input files prepared in VMD (Humphrey et al., 1996) using the MDFF-GUI (McGreevy et al., 2016) with default molecular dynamics parameters (McGreevy et al., 2014), improving Rwork/Rfree from 0.50/0.54 to 0.41/0.45. The model was further refined using PHENIX with additional local refinements using xMDFF. TM1a was placed using a combination of xMDFF and steered molecular dynamics (SMD) simulations. As with the CiVSP voltage sensor protein (Li et al., 2014), a helix-screw motion was induced in SMD simulations using the orientation quaternion (Jiang et al., 2014) of the helix as a collective variable, while simultaneously using electron density restraints. The TM1a helix position featuring the lowest R-value was identified, resulting in a 2% increase in the local cross correlation. It is also consistent with cysteine accessibility data (Figure 6). After final rounds of refinement in PHENIX, the final model includes residues 43-165, 176-236, 259-304, 310-341, 353-428 for DraNramp, 1-129+132-213 and 1-213 for the Fab heavy and light chains, respectively, and three osmium ions. Figures were made in PyMOL (Schrödinger).
Cysteine accessibility measurements
Adapting the cysteine-labeling protocol from (Tetsch et al., 2011), E. coli expressing single-cysteine DraNramp variants were exposed to thiol modifiers in 100 mM Tris pH 7, 60 mM NaCl, 10 mM KCl, 0.5 mM MgCl2, 0.75 mM CaCl2. All incubations were at 37°C. To determine overall accessibility, 3 mM NEM was applied for 60 min. To determine inside vs outside accessibility, 3 mM MTSEA or MTSET was applied for 30 min followed by 1.5 mM NEM for 30 min. For all NEM-gradient experiments, the indicated NEM concentration was applied for 15 min at RT. Excess cysteine was added to quench reactions. Cells were washed twice, resuspended and incubated 1 hr in 100 mM Tris pH 7, 6M urea, 0.5% SDS, 0.5 mM DTT, incubated with a two-fold excess of 5kDa-PEG maleimide (Creative PEGWorks), then quenched with sample buffer containing β-mercaptoethanol. Protein was detected in Western blots using an Alexa-647-conjugated anti-His-tag antibody (Qiagen) and a Typhoon Imager (GE Amersham), and background-subtracted band intensities (I) were measured using ImageJ64. The upper (5K-PEG modified) to lower band (NEM-modified) ratio was determined and compared to the no-NEM sample ratio (defined as maximal upper-to-lower band ratio = 0% NEM-modified) to calculate the NEM-modified cysteine fraction using: modified-cysteine fraction = 1 − [Iupper/(Ilower + Iupper)]/[Iupper-no NEM/(Ilower-no NEM + Iupper-no NEM)].
Metal uptake assays
Metal transport experiments in proteoliposomes, E. coli, and HEK293T cells were performed as described previously (Bozzi et al., 2016). See the Supplementary Information for additional description.
Supplementary Material
Highlights.
Deinococcus radiodurans Nramp structure reveals TM1a location in inward-open state
Unfettered movement of TM1a is essential to the metal transport cycle
G153R disease mutant mimic alters selectivity of conserved metal-binding site
G45R disease mutant mimic sterically locks protein in inward-open state
Acknowledgments
We thank Alexandra Rojek for selecting and cloning several single-cysteine DraNramp mutants, Brandon Lee for help with developing the NEM pre-modification cobalt uptake assay, Jack Nicoludis, Christina Zimanyi and members of the Gaudet Lab for discussions. The work was funded in part by a Basil O’Connor Starter Scholar Research Award from the March of Dimes Foundation to R.G., grant NIH 9P41GM104601 to K.S., and a Beckman Postdoctoral Fellowship to A.S. We gladly acknowledge supercomputer time from the Texas Advanced Computing Center via Extreme Science and Engineering Discovery Environment grant NSF-MCA93S028. We thank the NE-CAT beamline staff at the Advanced Photon Source (Argonne, IL, USA) for help with data collection. NE-CAT is funded by NIH (P41 GM103403 and S10 RR029205), and the Advanced Photon Source by the U.S. Department of Energy (DE-AC02-06CH11357). We declare no conflict of interest.
Footnotes
Author Contributions
R.G. oversaw and designed the research with W.A.W., L.B.B., and A.T.B; W.A.W. and E.R.G. generated mAbs, which H.L.P. supervised; W.A.W obtained DraNramp-Fab crystals and all diffraction data; L.B.B. purified DraNramp mutants and determined the DraNramp-Fab structure; L.B.B. and A.T.B. developed in vitro metal transport assays; A.T.B. made most Nramp mutants, performed all cysteine accessibility and metal transport experiments, and analyzed the resulting data; A.S. performed the xMDFF and SMD simulations, which K.S. supervised; A.T.B., L.B.B., and R.G. wrote the manuscript, with input from all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19:164–174. [PMC free article] [PubMed] [Google Scholar]
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001;33:940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–230. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios M, Moreno-Carralero MI, Cuadrado-Grande N, Baro M, Vivanco JL, Moran-Jimenez MJ. The homozygous mutation G75R in the human SLC11A2 gene leads to microcytic anaemia and iron overload. Br J Haematol. 2012;157:514–516. doi: 10.1111/j.1365-2141.2012.09043.x. [DOI] [PubMed] [Google Scholar]
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco E, Kannengiesser C, Grandchamp B, Tasso M, Beaumont C. Not all DMT1 mutations lead to iron overload. Blood Cells Mol Dis. 2009;43:199–201. doi: 10.1016/j.bcmd.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Bozzi AT, Bane LB, Weihofen WA, McCabe AL, Singharoy A, Chipot CJ, Schulten K, Gaudet R. Conserved methionine dictates substrate preference in Nramp-family divalent metal transporters. Proc Natl Acad Sci U S A. 2016;113:10310–10315. doi: 10.1073/pnas.1607734113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonne-Hergaux F, Fleming MD, Levy JE, Gauthier S, Ralph T, Picard V, Andrews NC, Gros P. The Nramp2/DMT1 iron transporter is induced in the duodenum of microcytic anemia mk mice but is not properly targeted to the intestinal brush border. Blood. 2000;96:3964–3970. [PubMed] [Google Scholar]
- Canonne-Hergaux F, Zhang AS, Ponka P, Gros P. Characterization of the iron transporter DMT1 (NRAMP2/DCT1) in red blood cells of normal and anemic mk/mk mice. Blood. 2001;98:3823–3830. doi: 10.1182/blood.v98.13.3823. [DOI] [PubMed] [Google Scholar]
- Cellier MF. Nramp: from sequence to structure and mechanism of divalent metal import. Curr Top Membr. 2012;69:249–293. doi: 10.1016/B978-0-12-394390-3.00010-0. [DOI] [PubMed] [Google Scholar]
- Cellier MF, Bergevin I, Boyer E, Richer E. Polyphyletic origins of bacterial Nramp transporters. Trends Genet. 2001;17:365–370. doi: 10.1016/s0168-9525(01)02364-2. [DOI] [PubMed] [Google Scholar]
- Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect. 2007;9:1662–1670. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Claxton DP, Quick M, Shi L, de Carvalho FD, Weinstein H, Javitch JA, McHaourab HS. Ion/substrate-dependent conformational dynamics of a bacterial homolog of neurotransmitter:sodium symporters. Nat Struct Mol Biol. 2010;17:822–829. doi: 10.1038/nsmb.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courville P, Chaloupka R, Cellier MF. Recent progress in structure-function analyses of Nramp proton-dependent metal-ion transporters. Biochem Cell Biol. 2006;84:960–978. doi: 10.1139/o06-193. [DOI] [PubMed] [Google Scholar]
- Ehrnstorfer IA, Geertsma ER, Pardon E, Steyaert J, Dutzler R. Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nat Struct Mol Biol. 2014;21:990–996. doi: 10.1038/nsmb.2904. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MD, Trenor CC, 3rd, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- Forrest LR, Rudnick G. The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology (Bethesda) 2009;24:377–386. doi: 10.1152/physiol.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest LR, Zhang YW, Jacobs MT, Gesmonde J, Xie L, Honig BH, Rudnick G. Mechanism for alternating access in neurotransmitter transporters. Proc Natl Acad Sci U S A. 2008;105:10338–10343. doi: 10.1073/pnas.0804659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Iron in innate immunity: starve the invaders. Curr Opin Immunol. 2009;21:63–67. doi: 10.1016/j.coi.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouleff J, Sondergaard S, Koldso H, Schiott B. Properties of an inward-facing state of LeuT: conformational stability and substrate release. Biophys J. 2015;108:1390–1399. doi: 10.1016/j.bpj.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, Hediger MA. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001;509:309–316. doi: 10.1016/s0014-5793(01)03189-1. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Hubert N, Hentze MW. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proc Natl Acad Sci U S A. 2002;99:12345–12350. doi: 10.1073/pnas.192423399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Iolascon A, De Falco L. Mutations in the gene encoding DMT1: clinical presentation and treatment. Semin Hematol. 2009;46:358–370. doi: 10.1053/j.seminhematol.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- Jiang W, Phillips JC, Huang L, Fajer M, Meng Y, Gumbart JC, Luo Y, Schulten K, Roux B. Generalized Scalable Multiple Copy Algorithms for Molecular Dynamics Simulations in NAMD. Comput Phys Commun. 2014;185:908–916. doi: 10.1016/j.cpc.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback HR, Dunten R, Frillingos S, Venkatesan P, Kwaw I, Zhang W, Ermolova N. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci U S A. 2007;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A, Akabas MH. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- Kazmier K, Sharma S, Islam SM, Roux B, McHaourab HS. Conformational cycle and ion-coupling mechanism of the Na+/hydantoin transporter Mhp1. Proc Natl Acad Sci U S A. 2014a;111:14752–14757. doi: 10.1073/pnas.1410431111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmier K, Sharma S, Quick M, Islam SM, Roux B, Weinstein H, Javitch JA, McHaourab HS. Conformational dynamics of ligand-dependent alternating access in LeuT. Nat Struct Mol Biol. 2014b;21:472–479. doi: 10.1038/nsmb.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2012;481:469–474. doi: 10.1038/nature10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wanderling S, Paduch M, Medovoy D, Singharoy A, McGreevy R, Villalba-Galea CA, Hulse RE, Roux B, Schulten K, et al. Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat Struct Mol Biol. 2014;21:244–252. doi: 10.1038/nsmb.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu PT, Heiskala M, Peterson PA, Yang Y. The roles of iron in health and disease. Mol Aspects Med. 2001;22:1–87. doi: 10.1016/s0098-2997(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Hediger MA. SLC11 family of H+-coupled metal-ion transporters NRAMP1 and DMT1. Pflugers Arch. 2004;447:571–579. doi: 10.1007/s00424-003-1141-9. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Takanaga H, Hubert N, Rolfs A, Hediger MA. Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1) Biochem J. 2007;403:59–69. doi: 10.1042/BJ20061290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinauskaite L, Quick M, Reinhard L, Lyons JA, Yano H, Javitch JA, Nissen P. A mechanism for intracellular release of Na+ by neurotransmitter/sodium symporters. Nat Struct Mol Biol. 2014;21:1006–1012. doi: 10.1038/nsmb.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreevy R, Singharoy A, Li Q, Zhang J, Xu D, Perozo E, Schulten K. xMDFF: molecular dynamics flexible fitting of low-resolution X-ray structures. Acta Crystallogr D Biol Crystallogr. 2014;70:2344–2355. doi: 10.1107/S1399004714013856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreevy R, Teo I, Singharoy A, Schulten K. Advances in the molecular dynamics flexible fitting method for cryo-EM modeling. Methods. 2016;100:50–60. doi: 10.1016/j.ymeth.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Elsevier; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- Penmatsa A, Gouaux E. How LeuT shapes our understanding of the mechanisms of sodium-coupled neurotransmitter transporters. J Physiol. 2014;592:863–869. doi: 10.1113/jphysiol.2013.259051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez C, Koshy C, Yildiz O, Ziegler C. Alternating-access mechanism in conformationally asymmetric trimers of the betaine transporter BetP. Nature. 2012;490:126–130. doi: 10.1038/nature11403. [DOI] [PubMed] [Google Scholar]
- Pottier M, Oomen R, Picco C, Giraudat J, Scholz-Starke J, Richaud P, Carpaneto A, Thomine S. Identification of mutations allowing Natural Resistance Associated Macrophage Proteins (NRAMP) to discriminate against cadmium. Plant J. 2015;83:625–637. doi: 10.1111/tpj.12914. [DOI] [PubMed] [Google Scholar]
- Shawki A, Knight PB, Maliken BD, Niespodzany EJ, Mackenzie B. H(+)-coupled divalent metal-ion transporter-1: functional properties, physiological roles and therapeutics. Curr Top Membr. 2012;70:169–214. doi: 10.1016/B978-0-12-394316-3.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. Common folds and transport mechanisms of secondary active transporters. Annu Rev Biophys. 2013;42:51–72. doi: 10.1146/annurev-biophys-083012-130429. [DOI] [PubMed] [Google Scholar]
- Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010;328:470–473. doi: 10.1126/science.1186303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singharoy A, Venkatakrishnan B, Liu Y, Mayne CG, Lee S, Chen CH, Zlotnick A, Schulten K, Flood AH. Macromolecular Crystallography for Synthetic Abiological Molecules: Combining xMDFF and PHENIX for Structure Determination of Cyanostar Macrocycles. J Am Chem Soc. 2015;137:8810–8818. doi: 10.1021/jacs.5b04407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe-Lin S, Apte SS, Andriopoulos B, Jr, Andrews MC, Schranzhofer M, Kahawita T, Garcia-Santos D, Ponka P. Nramp1 promotes efficient macrophage recycling of iron following erythrophagocytosis in vivo. Proc Natl Acad Sci U S A. 2009;106:5960–5965. doi: 10.1073/pnas.0900808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su MA, Trenor CC, Fleming JC, Fleming MD, Andrews NC. The G185R mutation disrupts function of the iron transporter Nramp2. Blood. 1998;92:2157–2163. [PubMed] [Google Scholar]
- Tavoulari S, Margheritis E, Nagarajan A, DeWitt DC, Zhang YW, Rosado E, Ravera S, Rhoades E, Forrest LR, Rudnick G. Two Na+ Sites Control Conformational Change in a Neurotransmitter Transporter Homolog. J Biol Chem. 2016;291:1456–1471. doi: 10.1074/jbc.M115.692012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsch L, Koller C, Donhofer A, Jung K. Detection and function of an intramolecular disulfide bond in the pH-responsive CadC of Escherichia coli. BMC Microbiol. 2011;11:74. doi: 10.1186/1471-2180-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touret N, Martin-Orozco N, Paroutis P, Furuya W, Lam-Yuk-Tseung S, Forbes J, Gros P, Grinstein S. Molecular and cellular mechanisms underlying iron transport deficiency in microcytic anemia. Blood. 2004;104:1526–1533. doi: 10.1182/blood-2004-02-0731. [DOI] [PubMed] [Google Scholar]
- Verma R. PCR of the v region. Methods Mol Med. 2000;40:453–459. doi: 10.1385/1-59259-076-4:453. [DOI] [PubMed] [Google Scholar]
- Veuthey T, Wessling-Resnick M. Pathophysiology of the Belgrade rat. Front Pharmacol. 2014;5:82. doi: 10.3389/fphar.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Choe S, Chaptal V, Rosenberg JM, Wright EM, Grabe M, Abramson J. The mechanism of sodium and substrate release from the binding pocket of vSGLT. Nature. 2010;468:988–991. doi: 10.1038/nature09580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Jin J, DeFelice LJ, Andrews NC, Clapham DE. A spontaneous, recurrent mutation in divalent metal transporter-1 exposes a calcium entry pathway. PLoS Biol. 2004;2:E50. doi: 10.1371/journal.pbio.0020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Yokoyama WM. Production of monoclonal antibody supernatant and ascites fluid. Curr Protoc Mol Biol. 2008;Chapter 11(Unit 11):10. doi: 10.1002/0471142727.mb1110s83. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Terry DS, Shi L, Quick M, Weinstein H, Blanchard SC, Javitch JA. Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue. Nature. 2011;474:109–113. doi: 10.1038/nature09971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.