Abstract
Introduction
Although catheter ablation (CA) for atrial fibrillation (AF) is commonly used to improve symptoms, AF recurrence is common and new tools are needed to better inform patient selection for CA. Left Atrial Function Index (LAFI), an echocardiographic measure of atrial mechanical function, has shown promise as a non-invasive predictor of AF. We hypothesized that LAFI would relate to AF recurrence after CA.
Methods and Results
All AF patients undergoing index CA were enrolled in a prospective institutional AF Treatment Registry between 2011 and 2014. LAFI was measured post-hoc from pre-ablation clinical echocardiographic images in 168 participants. Participants were mostly male (33% female), middle-aged (60±10 years), obese and had paroxysmal AF (64%). Mean LAFI was 25.9±17.6. Over 12 months of follow-up, 78 participants (46%) experienced a late AF recurrence. In logistic regression analyses adjusting for factors known to be associated with AF, lower LAFI remained associated with AF recurrence after CA [OR 0.04 (0.01 – 0.67), p= 0.02]. LAFI discriminated AF recurrence after CA slightly better than CHADS2 (C-statistic 0.60 LAFI, 0.57 CHADS2). For participants with persistent AF, LAFI performed significantly better than CHADS2 score (C statistic= 0.79 LAFI, 0.56 CHADS2, p= 0.02).
Conclusion
LAFI, an echocardiographic measure of atrial function, is associated with AF recurrence after CA and has improved ability to discriminate AF recurrence as compared to the CHADS-2 score, especially among persistent AF patients. Since LAFI can be calculated using standard two-dimensional echocardiographic images, it may be a helpful tool for predicting AF recurrence.
Keywords: Left atrial function index, atrial fibrillation, catheter ablation, arrhythmia recurrence, atrial remodeling
Background
One in four Americans over the age of 40 years will likely develop atrial fibrillation (AF).1 AF contributes significantly to death and disability through its associations with diminished quality of life as well as risk for ischemic stroke, heart failure, and hospitalization.2 Over the past decade, catheter ablation (CA) for AF has emerged as a useful therapy to reduce arrhythmia burden and to manage AF-related symptoms, particularly in those who fail or do not tolerate pharmacological therapy.3 Not surprisingly, rates of CA are increasing rapidly in the US and worldwide;4 however, only a few predictors for outcome after CA exist to help inform appropriate patient selection for CA. Presently, clinical characteristics, such as age and comorbidities (e.g., CHADS2 score), as well as patterns of AF, can help to guide therapeutic decision making, due to their associations with adverse outcomes and AF recurrence. In spite of these predictors, rates of AF recurrence vary widely, with reported estimates of recurrence in the contemporary era ranging between 10-60% in the literature.3 Clinical, laboratory, and electrophysiological predictors for pre-procedural risk stratification for recurrence are of limited value.3,5,6 Nevertheless, an emerging literature supports the use of stroke risk prediction instruments, including the CHADS2 score (Congestive heart failure= 1 point, hypertension= 1 point, age>75= 1 point, diabetes mellitus= 1 point, prior stroke= 2 points), as the best available predictor of AF recurrence after CA.7
Atrial structural remodeling is thought to contribute to the generation of a substrate vulnerable to incident and recurrent AF. Therefore, echocardiographic measures of atrial structure, including left atrial (LA) anteroposterior dimension and LA volume, have been proposed and examined as predictors of AF recurrence after cardioversion and CA.5 LA dimension relates to AF recurrence after CA, but it does not significantly reclassify the risk of AF recurrence versus the traditional factors of clinical characteristics and pattern of AF7 Moreover, recent data suggests that LA dimension as measured by 2D echocardiography does not correlate with the gold standard of Magnetic Resonance Imaging (MRI) measured LA volumes and thus routine reporting of LA dimension is now falling out of favor in most echocardioghraphy laboratories.8
Our group and others have shown that advanced echocardiographic measures of atrial function, including left atrial strain, independently relate to AF recurrence.9 This finding suggests that LA function may reflect AF vulnerability. Nevertheless, measurement of atrial strain remains impractical for routine clinical use due to the need for dedicated tissue Doppler or speckle tracking measures.9 Traditional hemodynamic assessments of LA function with peak velocity and velocity-time integral of the A wave of transmitral pulsed Doppler flow and A′ of tissue Doppler are also limited because they cannot be calculated in patients in AF.10,11 To address this limitations, Thomas et al developed the left atrial function index (LAFI), which incorporates information gathered from a routine echocardiogram, including cardiac output, atrial reservoir function, and LA volume, as a measure of atrial mechanical function.11 Unlike traditional measures of non-invasive echocardiographic measures of left atrial function, LAFI can be calculated in both sinus rhythm and AF. LAFI is significantly lower in patients with AF compared to those with sinus rhythm and improves after successful cardioversion or CA.11,12,13 We hypothesized that baseline LAFI would relate to AF recurrence after CA and would discriminate AF recurrence better than the traditional clinical risk score.
Methods
Study Population
Between April 2011 and October 2014, 339 patients with AF underwent a clinically indicated index CA and were enrolled in the UMass Memorial Center (UMMC) AF Treatment Registry. Each study participant underwent a pre-procedural transthoracic echocardiogram as part of the standard clinical work-up. Late AF recurrences (from 3 months to 12 months post-procedure) were adjudicated for 224 participants.7 Among these, 168 participants had echocardiographic images available and of sufficient quality to allow LAFI calculation, and thus 168 participants were included in the final study sample.
Demographic, clinical, and laboratory characteristics of participants were abstracted from the UMMC AF Treatment Registry as well as from hospital electronic medical records by trained study staff. Twelve-lead electrocardiograms (ECGs) were reviewed by one of the electrophysiologists (CB, CE, JKD, LR, KCF, DDM) to validate the diagnosis of AF. Paroxysmal AF was defined AF that terminates spontaneously or with intervention within 7 days. Persistent AF was defined as continuous AF that is sustained beyond 7 days. Echocardiographic images were manually reviewed by three experienced operators (MS, OA, GS) to extract the data required for calculation of LAFI. EchoPac (v110.0.0, General Electric Medical Systems, Horten, Norway) software was used for the echocardiography measurements. This study was approved by the University of Massachusetts Medical School Institutional Review Board (IRB #H00003865).
Echocardiographic methods
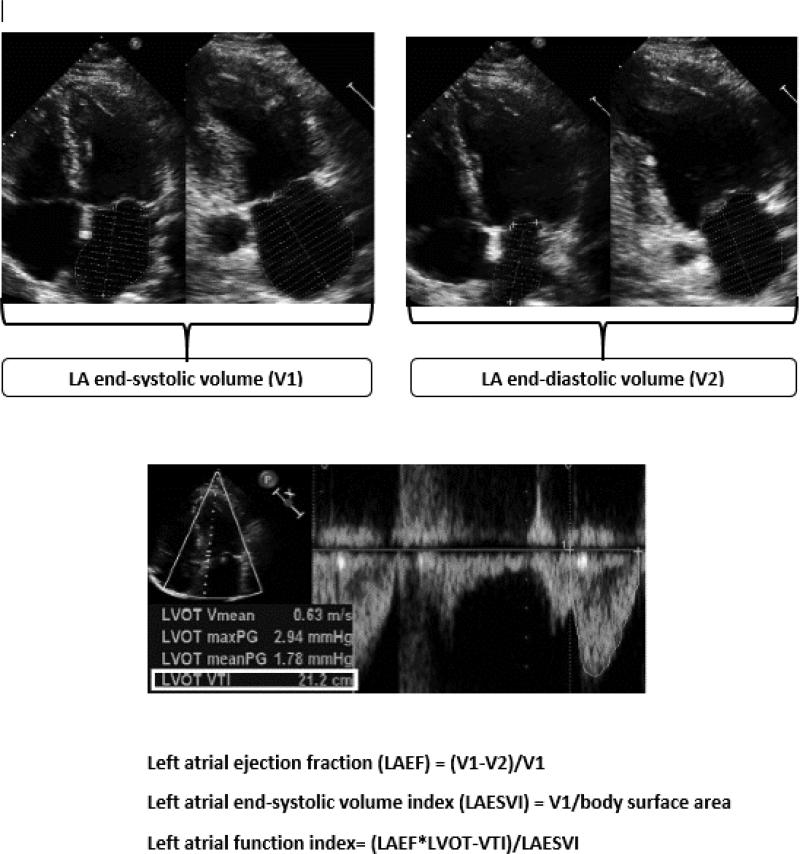
LAFI was calculated by the previously validated formula: LAFI = LA emptying fraction*LVOT-VTI / LAESVI11 (figure 1). LA volumes were calculated by the area-length method in apical 4 and 2-chamber views. LA emptying volume was calculated by subtracting LA end-systolic volume (V1) from LA end-diastolic volume (V2) and LA emptying fraction was calculated by dividing LA emptying volume by LA end systolic volume. LVOT-VTI (Left ventricular outflow tract velocity time integral) was measured by manually tracing pulsed Doppler velocities in the left ventricular outflow tract in apical 5-chamber or apical 3-chamber views.14 For participants in AF at the time of echocardiogram, LVOT-VTI was averaged over 3-5 beats.
FIGURE 1.
Calculation of left atrial function index. From top to bottom: measurement of left atrial end-systolic volume (V1) and end-diastolic volume (V2) in in apical 4 and 2 chamber views, tracing left ventricular outflow tract – velocity time integral (LVOT-VTI) in apical 5 chamber view, calculation of left atrial ejection fraction, calculation of left atrial end-systolic volume index, calculation of left atrial function index
Catheter Ablation for Atrial Fibrillation
One of 5 clinical electrophysiologists at University of Massachusetts Medical Center (a tertiary care medical center) performed CA using either radiofrequency ablation15 or cryoablation16. A standardized CA approach as described below was followed in almost all cases. For radiofrequency ablation procedures, wide area circumferential ablation was performed with an open-irrigated or 8-mm Radiofrequency Ablation catheter. For cryoablation procedures, an Arctic Front cryoballoon catheter (28 or 23 mm, Medtronic Inc.) was utilized in performing PVI. In each vein, cryoablation was done twice for 150 to 240 seconds. In all patients, assessment of entrance and exit blockage was determined with a circular mapping catheter. For patients with persistent AF, additional linear lesions were made in the LA roof, the basal posterior wall, and the LA isthmus, at the discretion of the performing electrophysiologist. After CA, subjects were treated with proton pump inhibitors for 4 weeks, and antiarrhythmic medications were typically stopped at a routine 3-month follow-up visit if no symptomatic AF recurrences were reported or seen on routine post-procedural electrocardiographic examinations. Oral anticoagulation was prescribed for 3 to 6 months after ablation with long-term use determined by stroke risk as predicted by CHADS2 or CHA2DS2-VASc scores, based on the judgment of the treating physician.
Outcome Definitions and Adjudication
All participants enrolled in the current study had follow-up for at least 12 months after CA, and AF recurrence was adjudicated by careful review of clinical notes, 12-lead ECGs, and event monitor data. All participants underwent a 12-lead ECG at 1, 3, 6 and 12 months in addition to having a 7-day cardiac event monitor at 1-month after the procedure. When participants presented with symptoms suggestive of AF (such as palpitations, shortness of breath, etc.), additional ECGs and Holter monitor recordings were obtained. AF recurrence was defined by the presence of AF on a 12-lead ECG or any AF episode lasting for more than 20 seconds on event monitor. One of the electrophysiologists (CB, CE, JKD, LR, KCF, DDM) reviewed the recordings to confirm the diagnosis of AF. Additionally, if any participant underwent a cardioversion or repeat AF ablation procedure, this was considered as AF recurrence. Late AF recurrences were defined as any AF recurrence occurring after a traditional blanking period of 3 months after CA.3
Statistical Analysis
Baseline statistics are presented as mean ± standard deviation (continuous variables) or as proportions (binary and categorical variables). Differences in means were tested by t-test and difference in proportions using the chi-square (χ2) test. Multivariate logistic regression was used to examine the relationships of baseline predictors with AF recurrence. Covariates included in the multivariable models were selected a priori based on clinical relevance to AF, CA procedure and AF recurrence (age, sex, body mass index, hypertension, diabetes, coronary artery disease, congestive heart failure, prior stroke, obstructive sleep apnea, chronic kidney disease, CHADS2 score, AF type, LAFI, LA dimension, LA end-systolic volume index, left ventricular ejection fraction, mitral inflow E/e’ lateral tissue Doppler velocity ratio, B-type natriuretic peptide (BNP), C-reactive protein (CRP), discharge medications, ablation type, ablation time). Only factors associated with AF recurrences with a P value of less than 0.2 in univariate analyses were entered into the final model and a 2-sided P value of less than 0.05 was considered significant. Receiver operating characteristic (ROC) curves were generated to show the ability of LAFI to discriminate late AF recurrence after CA. Cut-points for LAFI were identified for the whole group and the subgroups based on the rhythm at the time of echo and type of AF. Sensitivity, specificity, positive and negative predictive values for these cut-points were calculated to discriminate AF recurrence. Bland-Altman analysis was performed to determine the inter- and intra-observer variability in measurement of LAFI. To better understand the potential mechanisms underlying associations between atrial function and AF recurrence, correlation analyses were attempted to determine the relationship of LAFI with BNP and CRP, which are biomarkers of subclinical cardiac dysfunction and inflammation, respectively.17 The C-statistic from ROC curve for LAFI was compared against the CHADS2 score for discrimination of late AF recurrence.
Prior studies have shown that the patients with persistent AF have a high propensity for recurrence after CA.5 We performed a stratified analysis of participants with persistent AF to determine the role of LAFI in risk stratification before CA in this high risk group. Statistical analysis was performed using MedCalc (v16.2.1.0 – 64bit, MedCalc software, Mariakerke, Belgium) and SPS (IBM SPSS version 24, Chicago IL) softwares.
RESULTS
The baseline characteristics of all registry patients and current study participants are shown in Table 1. The mean age of participants was 60±10 years, one-third were women, nearly two-thirds were obese and the majority of participants had a moderate-to-severe burden of cardiovascular comorbidities (Table 1). Most of the participants had paroxysmal AF (64%) and nearly 3 out of 4 were treated with an antiarrhythmic drug prior to CA, which reflects the fact that most participants had a consensus class IA indication for CA.3 Approximately half the participants underwent radiofrequency ablation, whereas the remainder underwent cryoablation. Over 12 months of standardized and intensive follow-up, 78 participants (46%) experienced a late AF recurrence (3 – 12 months after CA). Mean LAFI was 25.9±17.6. LAFI was lower in participants with persistent AF (16.7±14.6, n=61) as compared to the participants with paroxysmal AF (31.1±17.6, n=107, p<0.01).
TABLE 1.
Baseline Characteristics of All Registry Patients and Study Participants
| Variable | All Registry Patients (N = 339) | Study Participants AF Recurrence (N = 78) | Study Participants No AF Recurrence (N = 90) |
|---|---|---|---|
| Age (years) | 60 ± 10 | 61 ± 10 | 59 ± 11 |
| Female sex | 112 (33) | 28 (35) | 28 (30) |
| Body mass index (kg/m2) | 31.7 ± 6.1 | 31.8 ± 5.9 | 31.6 ± 6.4 |
| Clinical characteristics | |||
| Paroxysmal AF | 227 (67) | 55 (71) | 54 (59) |
| Persistent AF | 112 (33) | 23 (29) | 38 (41) |
| CHADS2 score† | 1.3 ± 0.9 | 1.4 ± 0.9 | 1.1 ± 0.9 |
| Congestive heart failure | 52 (15) | 12 (15) | 12 (13) |
| Hypertension | 251 (74) | 61 (78) | 60 (65) |
| Diabetes mellitus | 70 (21) | 20 (26) | 20 (22) |
| Stroke | 17 (5) | 5 (6) | 3 (3) |
| Coronary artery disease | 66 (19) | 18 (23) | 20 (22) |
| Obstructive sleep apnea | 118 (35) | 29 (37) | 39 (42) |
| Chronic kidney disease | 24 (7) | 7 (9) | 4 (4) |
| Chronic obstructive Pulmonary disease | 32 (9) | 9 (12) | 6 (6) |
| Laboratory characteristics‡ | |||
| B-type natriuretic peptide (pg/dL) | 131.2 ± 146.3 | 114.1 ± 147.3 | 1212 ± 123 |
| C-reactive protein (mg/dL) | 4.3 ± 6 | 5.3 ± 7.9 | 42 ± 5.7 |
| Total cholesterol (mg/dL) | 181.1 ± 45.9 | 179.7 ± 46 | 1802 ± 46.6 |
| Echocardiographic characteristics | |||
| Left atrial anteroposterior diameter (mm) | NA | 41.7 ± 5.8 | 42 ± 5.5 |
| Left atrial end-systolic volume index (mL/m2) | NA | 38.9 ± 13.6 | 362 ± 11.7 |
| Left atrial function index | NA | 22.6 ± 17.2 | 28.7 ± 18.2 |
| E/e’ ratio§ | NA | 7.9 ± 32 | 8.4 ± 2.9 |
| LVOT-VTI (cm)¶ | NA | 19.4 ± 5.9 | 19.8 ± 5.7 |
| Left ventricular ejection fraction (%) | NA | 59.7 ± 7.4 | 59.6 ± 7.4 |
| Left ventricular mass index (g/m2) | NA | 80.9 ± 20.9 | 81.5 ± 16.9 |
| Medications (after ablation) | |||
| Class I or III antiarrhythmics | 241 (71) | 51 (65) | 69 (75) |
| β-Blockers | 164 (48) | 36 (46) | 41 (45) |
| Anticoagulants | 326 (96) | 72 (92) | 86 (93) |
| Radiofrequency ablation | 144 (43) | 40 (51) | 42 (46) |
| Cryoablation | 195 (57) | 38 (49) | 50 (54) |
CHADS2 scons = Congestive heart failure (1 point), hypertension (1 point), age > 75 (1 point). Diabetes mellitus (1 point), prior stroke (2 points).
B-type natriuretic peptide measurements were available for 78% of participants. C-reactive protein for 72% of participants, and total cholesterol for 88% of participants.
Mitral inflow E velocity/Lateral tissue Doppler e’ velocity ratio.
Left ventricular outflow tract-velocity time integral.
Variability and Time Required for Measurement of Left Atrial Function Index
There was an excellent correlation between the operators for the calculation of LAFI (r= 0.95, MS vs OA; r= 0.93, MS vs GS). Intra-observer variability was 4.2+/−3.6%. Although we manually measured all of the individual components required for the calculation of LAFI from echocardiographic images, LVOT-VTI, LA End-systolic Volume and BSA are reported for all patients as a standard of practice at the UMMC Echocardiography Laboratory. The only additional measurement required for LAFI calculation is LA End-diastolic Volume. For 10 randomly selected study participants, we measured the time required for measuring LAEDV and calculating LAFI when the values for other parameters were provided to the operators. Whereas the mean time for reporting LAFI for a new operator was 69+/−8 seconds, mean time for an experienced operator (more than 100 prior measurements) was 50+/−7 seconds.
Clinical and Laboratory Factors Associated With AF Recurrence
Logistic regression was used to analyze relations of candidate clinical and laboratory predictors with AF recurrence (table 2). In univariate analysis, LAFI, LA end-systolic volume index, hypertension, CHADS2 score, and AF type were associated with late AF recurrence. Because CHADS2 score includes hypertension and age, these variable were not included separately in the multivariable model. In multivariate analysis (model 1, table 2), LAFI (OR= 0.04 (0.01 – 0.67), p= 0.02) was associated with decrease in the risk of AF recurrence. Although CHADS2 score showed a trend towards association with AF recurrence, it did not reach statistical significance (OR= 1.36 (0.96 – 1.94), p= 0.08). Notably, other traditional echocardiographic markers with previously demonstrated association with AF, including LA anteroposterior dimension, were not associated with the AF recurrence.
Table 2.
Predictors of Late Atrial Fibrillation Recurrence*
| Predictor Variables | odds ratio (95% CI) | odds ratio (95% CI) | odds ratio (95% CI) | odds ratio (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Age+ | 1.02 (0.99 – 1.05) | 0.23 | NA | NA | NA | NA | NA | NA |
| Female sex | 1.23 (0.65 – 2.36) | 0.52 | 1.34 (0.67 – 2.70) | 0.41 | 1.43 (0.70 – 2.93) | 0.32 | 1.36 (0.60 – 3.12) | 0.46 |
| Hypertension+ | 1.88 (0.94 – 3.76) | 0.07 | NA | NA | NA | NA | NA | NA |
| CHADS2 score | 1.33 (0.96 – 1.84) | 0.08 | 1.36 (0.96 – 1.94) | 0.08 | 1.45 (1.01 – 2.10) | 0.04 | 1.71 (1.12 – 2.61) | 0.01 |
| AF type (persistent vs paroxysmal) | 0.57 (0.30 – 1.09) | 0.09 | 0.32 (0.15 – 0.69) | <0.01 | 0.48 (0.21 – 1.07) | 0.07 | 0.32 (0.13 – 0.80) | 0.01 |
| Left atrial function index | 0.13 (0.02 – 0.85) | 0.03 | 0.04 (0.01 – 0.67) | 0.02 | 0.01 (0.01 – 0.13) | <0.01 | 0.01 (0.01 – 0.29) | <0.01 |
| Left atrial end-systolic volume index | 1.02 (0.99 – 1.04) | 0.19 | 1.00 (0.97 – 1.04) | 0.96 | 1.00 (0.96 – 1.04) | 0.70 | 1.01 (0.96 – 1.05) | 0.79 |
| Left atrial anteroposterior dimension++ | 0.99 (0.94 – 1.04) | 0.72 | NA | NA | NA | NA | NA | NA |
| Rhythm at time of echo (AF vs sinus) | NA | NA | NA | NA | 0.24 (0.10 – 0.60) | <0.01 | NA | NA |
| B-type natriuretic peptide | 0.99 (0.99-1.00) | 0.75 | NA | NA | NA | NA | 0.99 (0.99 – 1.00) | 0.07 |
LEGEND: Model 1: Multivariable adjusted model including variables with p≤0.2 in univariate analysis; Model 2: Model 1 + rhythm at time of echocardiogram; Model 3: Model 1 + B-type natriuretic peptide
Other variables included in univariate analysis were: body mass index, diabetes, coronary artery disease, congestive heart failure, prior stroke, obstructive sleep apnea, chronic kidney disease, left ventricular ejection fraction, mitral inflow E/e' lateral tissue Dopplervelocity ratio, C-reactive protein, discharge medications, ablation type, ablation time
Age and Hypertension were not included in the multivariable model as they are part of CHADS2 score
Left atrial anteroposterior dimension was not included in the multivariable analysis.
Left Atrial Function Index Relates to Atrial Fibrillation Recurrence
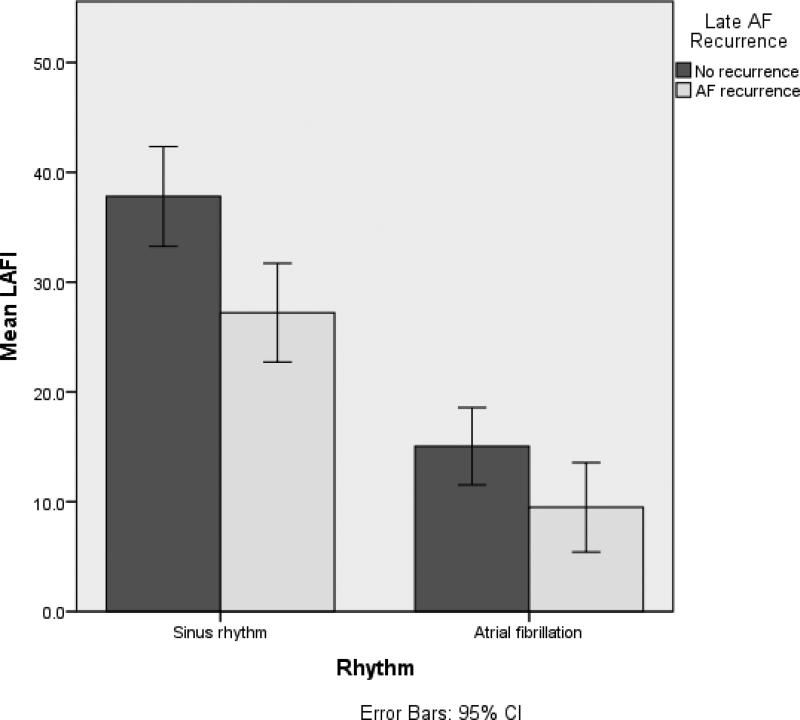
LAFI values were significantly lower in participants who developed late AF recurrence when compared to participants with no late recurrence (22.6±17.2 vs 28.7±18.2, p= 0.03). LAFI was lower in participants who were in AF (13.1±10, n= 56) at the time of pre-ablation echocardiogram compared to those in sinus rhythm (32.3±17.5, n= 112, p<0.01) (figure 2). On inclusion of the rhythm in the multivariable model (model 2, table 2), the association of LAFI with AF recurrence continued to be significant (OR= 0.01 (0.01 – 0.13), p<0.01).
FIGURE 2.
Distribution of Left atrial function index by rhythm at the time of echocardiogram and recurrence.
Left Atrial Function Index is Inversely Related to B-Type Natriuretic Peptide Levels
In light of prior data showing that BNP levels relate to new-onset and recurrent AF, we examined associations between LAFI with BNP. We observed an inverse non-linear relationship of LAFI with baseline BNP levels (supplemental figure 1). Association with BNP remained significant after adjustment for ejection fraction, left ventricular diastolic dimension and mitral inflow E/lateral e’ velocity on tissue Doppler imaging. We did not find a significant association between LAFI and baseline CRP levels using linear and non-linear models. BNP itself was not associated with late AF recurrence in the univariate analysis. Prior studies, however, have shown its association with late AF recurrence.7,18 On inclusion of BNP in the multivariable model (model 3, table 2), the association of LAFI with AF recurrence continued to be significant (OR= 0.01 (0.01 – 0.29), p<0.01).
Left Atrial Function Index Compared to Clinical Predictors of AF Recurrence
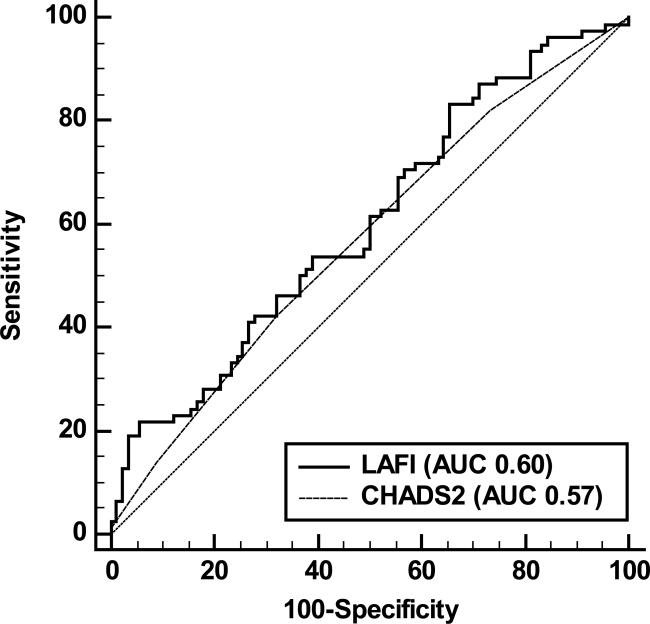
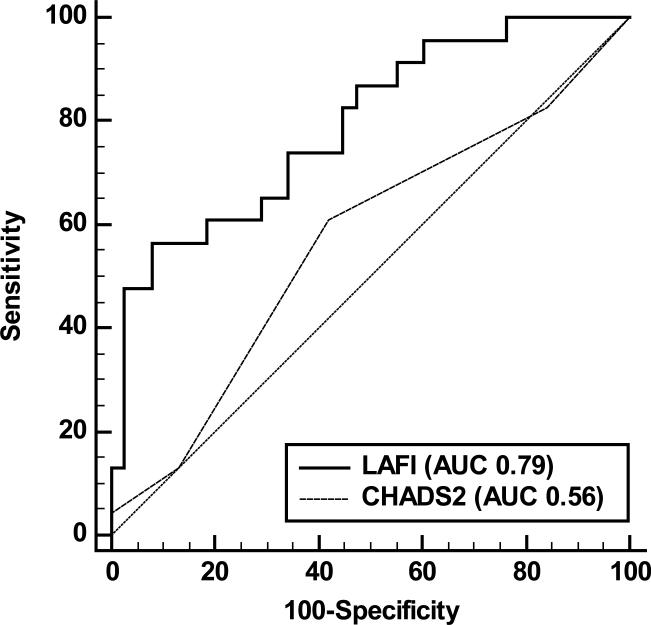
ROC curves were plotted for CHADS2 score and LAFI as discriminators for AF recurrence. The area under the receiver operating characteristic curve for LAFI was slightly larger than that for CHADS2 score (0.60 vs 0.57, p= 0.60; figure 3). However, in pre-specified stratified analyses including only participants with persistent AF, LAFI exhibited a significantly superior performance in discriminating AF recurrence than the CHADS2 score (C-statistic 0.79 vs 0.56, p= 0.02; figure 4). LAFI≤33.9 discriminated AF recurrence with a negative predictive value of 70% and positive predictive value of 52% (supplemental table 1). Lower cut-points were identified for participants with AF at the time of echo (5.9) and those with persistent AF (6.3) (supplemental table 1). LAFI≤6.3 had high negative and positive predictive values (78% and 81%, respectively) to discriminate AF recurrence in participants with persistent AF. Similarly, for participants in AF at the time of echo, LAFI≤5.9 had high negative and positive predictive values (79% and 73%, respectively) in discriminating AF recurrence.
FIGURE 3.
Receiver Operator Characteristic curves for discrimination of late atrial fibrillation recurrence in all study participants. Solid line represents the performance of LAFI (Area under the curve 0.60) and dotted line represents the performance of CHADS2 score (Area under the curve 0.57). Diagonal line of reference is also displayed.
FIGURE 4.
Receiver Operator Characteristic curves for discrimination of late atrial fibrillation recurrence in participants with persistent atrial fibrillation. Solid line represents the performance of LAFI (Area under the curve 0.79) and dotted line represents the performance of CHADS2 score (Area under the curve 0.56). Diagonal line of reference is also displayed.
Discussion
In our analysis of 168 participants undergoing index CA for symptomatic AF and enrolled in our prospective AF Treatment Registry, we found that lower LAFI was associated with increased risk for late AF recurrence. Our findings suggest that this novel echocardiographic measure of atrial function might help identify patients at high risk for AF recurrence after CA, thereby providing a useful tool to help guide therapeutic decision-making around use of CA in symptomatic AF patients.
Left Atrial Function Index, Electrical Remodeling and AF recurrence
Thomas et al introduced LAFI as a measure of atrial mechanical function. We believe LAFI is an indicator of complex electromechanical function of atrium.11 Similar to our study, Thomas et al observed that LAFI was the lower in participants with AF (a state of chaotic electromechanical activity) when compared to patients with sinus rhythm (a state of organized electromechanical activity). They observed an immediate improvement in LAFI after cardioversion to sinus rhythm in patients with AF. Multiple prior studies have proven that the mechanical recovery lags behind the electrical recovery after cardioversion.19 Thus immediate improvement in LAFI parallels the early electrical recovery of LA function. Nagase et al recently observed similar improvement in LAFI after CA.12
Subtle changes in P wave morphology20, reduced atrial conduction time21, and the presence of non-PV foci22 are associated with increased risk of AF recurrence after CA. These observations support the role of electrical remodeling as mediator of AF recurrence. It is possible that the strong relation of LAFI to AF recurrence after CA is because of the ability of LAFI to capture these facets of electrical remodeling not previously captured by traditional non-invasive imaging measures.
Left Atrial Function Index, Mechanical Remodeling and AF recurrence
Left atrial mechanical function is divided into reservoir (during left ventricular systole and isovolumetric relaxation), conduit (passive passage of blood from left atrium to left ventricle), and active phases (atrial contraction).23 The relationship of mechanical remodeling of LA with recurrence has been studied extensively with several advanced imaging techniques. Dodson et al utilized cardiac MRI to calculate LA passive ejection fraction (during conduit phase) in patients referred for CA.24 The rate of AF recurrence at 2 years increased from the highest quintile (17%) to the lowest quintile (60%) of LA passive ejection fraction. Habibi et al recently studied the association of various phases of atrial emptying (passive, active, total) with rate of recurrence after CA using cardiac MRI.25 After adjusting for various clinical and imaging parameters (including late-gadolinium enhancement as a marker of atrial fibrosis), lower total LA emptying fraction was associated with increased risk for recurrent AF. Motoki et al also showed that mechanical atrial dysfunction as detected by impairment of reservoir left atrial strain is associated with increased risk of AF recurrence after CA.26 Our group has also shown a similar association of left atrial strain with AF recurrence in participants undergoing cardioversion. Unfortunately, the cost and logistical challenges of cardiac MRI and strain measurement currently make them impractical for widespread clinical use. LAFI is also a measure of the reservoir function of left atrium. Measured from conventional, clinical two-dimensional echocardiographic images, LAFI has performed similarly to other, less widely available, measures of atrial mechanical function in discriminating AF recurrence.26 The LAFI formula accounts for not only for LA ejection fraction but also for the stroke volume and left atrial size. This minimizes the impact of conditions affecting heart rate or preload on LAFI, making it a refined indicator of atrial mechanical function as compared to LA ejection fraction. LA end-systolic volume index is the currently recommended method of left atrial size measurement by the American Society of Echocardiography.8 LAFI was significantly associated with late AF recurrence despite inclusion LA end-systolic volume index in the multivariable model. This observation suggests that LAFI reflects atrial substrate better than the measures of atrial size alone and its association with AF recurrence is independent of atrial size. Our finding that LAFI is a clinically useful discriminator for AF recurrence after CA is in line with these prior mechanistic studies and, if replicated, our findings suggest a relevant use-case for LAFI as a factor to be considered when considering CA as a therapeutic option for symptomatic patients with AF.
Performance of LAFI based on Rhythm at Time of Echocardiogram
Concordant with prior studies, we observed lower LAFI values for participants in AF at the time of echo. (figure 2).11 When we performed a pre-specified subgroup analysis by rhythm at the time of echocardiogram, C-statistic for discriminative value of LAFI for late AF recurrence improved from 0.60 for whole group to 0.70 for both AF subgroup and 0.69 for the sinus rhythm subgroup. Similarly, there was a parallel improvement seen in the positive and negative predictive values of LAFI cut-points in these subgroups (supplemental table 1). These observations suggest that the true impact of LAFI on AF recurrence was dampened by over-representation of participants in AF at the time of echocardiogram in the non-recurrence group.
Performance of LAFI in Participants with Persistent AF
To best of our knowledge, ours is the first study to identify the presence of lower LAFI in the participants with persistent AF. D'Ascenzo et al performed a meta-analysis of 19 studies to study the effect of various baseline clinical and echocardiographic parameters on AF recurrence after CA.5 They concluded that persistent AF was associated with significantly increased risk of AF recurrence (OR 1.78 [1.14 – 2.77]). Identifying a marker to predict AF recurrence in this group of patients could greatly impact therapeutic decision-making. LAFI performed exceedingly well in this subgroup as a discriminative tool (C-statistic 0.79 (0.67 – 0.88)) when compared to the other clinical tools such as CHADS2 score. Moreover, discrimination based on LAFI had a high specificity and positive predictive value for AF recurrence (supplemental table 1).
Presence of persistent AF, in contrast to other studies5, was not associated with increased risk of AF recurrence in our study. When we included all the participants with adjudicated recurrence data in UMMC AF Registry (including participants whose echocardiograms were not available at UMMC), persistent AF was associated with increased risk of late AF recurrence (OR= 1.94, CI= 1.13 – 3.31, p= 0.01, n= 224). We believe that lack of association seen in our study sample (n= 168) is related to over-representation of participants with persistent AF in the non-recurrence group.
Strengths and Limitations
Our study is the first to demonstrate that LAFI is associated with late AF recurrence in patients undergoing CA. LAFI calculation does not require any additional echocardiographic views and can easily be performed in under one minute by an experienced operator. Moreover, LAFI is a refined measure of left atrial mechanical and possibly electrical function. These characteristics present LAFI as a stronger, faster and unique discriminating tool for AF recurrence when compared to other advanced imaging parameters. Our study is also strengthened by the use of a prospective Registry with comprehensive, standardized and contemporary clinical, electrophysiological and laboratory data and with longitudinal intensive monitoring for AF recurrence. However, our study has certain limitations. First, although participants underwent per protocol 12-lead ECGs, 7-day cardiac event monitors, and symptom-triggered assessments for AF after CA, it is possible that asymptomatic episodes of AF were missed. However, these limitations would likely bias our study toward the null hypothesis and are unlikely to invalidate observed associations. Study participants were predominantly white. As such, our results might not be generalizable to other racial or ethnic groups.
Conclusion
In our cohort of nearly 200 participants with AF undergoing index CA ablation, we observed that LAFI was associated with presence of AF during imaging and AF recurrence after CA. Moreover, we demonstrate that LAFI is superior to traditional clinical AF recurrence prediction tools, especially in patients with persistent AF. LAFI correlated with other known laboratory markers of atrial functional impairment, including BNP and persistent AF. Since LAFI can be easily calculated from almost a standard echocardiographic recording, our findings suggest that LAFI may be a helpful additional tool to guide therapeutic decision-making regarding CA, particularly among patients with symptomatic persistent AF, in whom recurrence rates are especially high. Further data are needed to validate our findings in other diverse cohorts of AF patients undergoing CA.
Supplementary Material
Acknowledgments
D.D. McManus was supported by KL2RR031981, 5R01HL126911-02, 1R15HL121761-01A1, and 1UH2TR000921-02 from the National Heart, Lung and Blood Institute of the National Institutes of Health, Bethesda, MD
D.D. McManus has been supported by grants from Biotronik, Philips, and Otsuka Pharmaceuticals. He is also an equity holder in Mobile Biosense and ATRIA, Inc.
Footnotes
Other authors: No disclosures.
References
- 1.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation the Framingham heart study. Circulation. 2004;110.9:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr., Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart rhythm. 2012;9:632–696. [Google Scholar]
- 4.Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K, Grover P, Singh V, Vallurupalli S, Savani GT, Badheka A. Inhospital complications associated with catheter ablation of atrial fibrillation in the united states between 2000-2010: Analysis of 93,801 procedures. Circulation. 2013;128:2104–2112. doi: 10.1161/CIRCULATIONAHA.113.003862. [DOI] [PubMed] [Google Scholar]
- 5.D'Ascenzo F, Corleto A, Biondi-Zoccai G, Anselmino M, Ferraris F, di Biase L, Natale A, Hunter RJ, Schilling RJ, Miyazaki S, Tada H, Aonuma K, Yenn-Jiang L, Tao H, Ma C, Packer D, Hammill S, Gaita F. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation? A meta-analysis. International journal of cardiology. 2013;167:1984–1989. doi: 10.1016/j.ijcard.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Kornej J, Hindricks G, Kosiuk J, Arya A, Sommer P, Husser D, Rolf S, Richter S, Huo Y, Piorkowski C, Bollmann A. Comparison of CHADS2, R2CHADS2, and CHA2DS2-VASc scores for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol. 2014;7:281–287. doi: 10.1161/CIRCEP.113.001182. [DOI] [PubMed] [Google Scholar]
- 7.Shaikh AY, Esa N, Martin-Doyle W, Kinno M, Nieto I, Floyd KC, Browning C, Ennis C, Donahue JK, Rosenthal LS, McManus DD. Addition of b-type natriuretic peptide to existing clinical risk scores enhances identification of patients at risk for atrial fibrillation recurrence after pulmonary vein isolation. Critical pathways in cardiology. 2015;14:157–165. doi: 10.1097/HPC.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography. 2015;28.1:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Shaikh AY, Maan A, Khan UA, Aurigemma GP, Hill JC, Kane JL, Tighe DA, Mick E, McManus DD. Speckle echocardiographic left atrial strain and stiffness index as predictors of maintenance of sinus rhythm after cardioversion for atrial fibrillation: a prospective study. Cardiovascular ultrasound. 2012;10.1:1. doi: 10.1186/1476-7120-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JJC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 aha/acc/hrs guideline for the management of patients with atrial fibrillation: Executive summarya report of the american college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. Journal of the American College of Cardiology. 2014;64:2246–2280. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Thomas L, Hoy M, Byth K, Schiller NB. The left atrial function index: A rhythm independent marker of atrial function. European Heart Journal - Cardiovascular Imaging. 2008;9:356–362. doi: 10.1016/j.euje.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Nagase T, Kato R, Nakano S, Shiki Y, Tanaka S, Ikeda Y, Iwanaga S, Nishimura S, Matsumoto K. Prediction of improvement in left atrial function index after catheter ablation for atrial fibrillation. Journal of interventional cardiac electrophysiology. 2015;44:151–160. doi: 10.1007/s10840-015-0043-z. [DOI] [PubMed] [Google Scholar]
- 13.Li T, Li B, Duan C, Hu X, Zhang X. Gw26-e3524 value of the left atrial function index for assessing left atrial function in patients with atrial fibrillation. J Am Coll Cardiol. 2015;66.16_S [Google Scholar]
- 14.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;1:1–23. doi: 10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Eitel C, Hindricks G, Sommer P, Gaspar T, Kircher S, Wetzel U, Dagres N, Esato M, Bollmann A, Husser D, Hilbert S. Circumferential pulmonary vein isolation and linear left atrial ablation as a single-catheter technique to achieve bidirectional conduction block: the pace-and-ablate approach. Heart Rhythm. 2010;7:157–164. doi: 10.1016/j.hrthm.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Packer DL, Kowal RC, Wheelan KR, Irwin JM, Champagne J, Guerra PG, Dubuc M, Reddy V, Nelson L, Holcomb RG, Lehmann JW. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–1723. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ. Multiple biomarkers for the prediction of first major cardiovascular events and death. New England Journal of Medicine. 2006;355(25):2631–9. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 18.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M, Jais P. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 19.Manning WJ, Leeman DE, Gotch PJ, Come PC. Pulsed Doppler evaluation of atrial mechanical function after electrical cardioversion of atrial fibrillation. Journal of the American College of Cardiology. 1989;13.3:617–23. doi: 10.1016/0735-1097(89)90602-5. [DOI] [PubMed] [Google Scholar]
- 20.Kizilirmak F, Demir GG, Gokdeniz T, Gunes HM, Cakal B, Guler E, Karaca İO, Omaygenç MO, Yılmaz F, Olgun FE, Kilicaslan F. Changes in Electrocardiographic P Wave Parameters after Cryoballoon Ablation and Their Association with Atrial Fibrillation Recurrence. Ann Noninvasive Electrocardiol. 2016 doi: 10.1111/anec.12364. doi: 10.1111/anec.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.den Uijl DW, Gawrysiak M, Tops LF, Trines SA, Zeppenfeld K, Schalij MJ, Bax JJ, Delgado V. Prognostic value of total atrial conduction time estimated with tissue Doppler imaging to predict the recurrence of atrial fibrillation after radiofrequency catheter ablation. Europace. 2011;13.11:1533–1540. doi: 10.1093/europace/eur186. [DOI] [PubMed] [Google Scholar]
- 22.Lo Lw, Lin Yj, Chang Sl, Hu Yf, Chao Tf, Chung Fp, Liao Jn, Chiou Cw, Tsao Hm, Chen Sa. Predictors and Characteristics of Multiple (more Than 2) Catheter Ablation Procedures for Atrial Fibrillation. J Cardiovasc Electrophysiol. 2015;26.10:1048–56. doi: 10.1111/jce.12748. [DOI] [PubMed] [Google Scholar]
- 23.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63.6:493–505. doi: 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 24.Dodson JA, Neilan TG, Shah RV, Farhad H, Blankstein R, Steigner M, Michaud GF, John R, Abbasi SA, Jerosch-Herold M, Kwong RY. Left atrial passive emptying function determined by cardiac magnetic resonance predicts atrial fibrillation recurrence after pulmonary vein isolation. Circulation: Cardiovascular Imaging. 2014;7.4:586–92. doi: 10.1161/CIRCIMAGING.113.001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habibi M, Lima JA, Ipek EG, Zimmerman SL, Zipunnikov V, Spragg D, Ashikaga H, Rickard J, Marine JE, Berger RD, Calkins H. The Association of Baseline Left Atrial Structure and Function Measured with Cardiac Magnetic Resonance and Pulmonary Vein Isolation Outcome in Patients with Drug Refractory Atrial Fibrillation. Heart Rhythm. 2016;13.5:1037–44. doi: 10.1016/j.hrthm.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Motoki H, Negishi K, Kusunose K, Popović ZB, Bhargava M, Wazni OM, Saliba WI, Chung MK, Marwick TH, Klein AL. Global left atrial strain in the prediction of sinus rhythm maintenance after catheter ablation for atrial fibrillation. Journal of the American Society of Echocardiography. 2014;27.11:1184–92. doi: 10.1016/j.echo.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.