Abstract
Purpose
The conversion of tumor cells from an epithelial to a mesenchymal-like phenotype, via a process designated as the epithelial-mesenchymal transition (EMT), is known to mediate tumor resistance to a variety of cell death inducers, including cytotoxic effector immune cells. The goal of this study was to identify and potentially repurpose FDA-approved compounds capable of reducing mesenchymal features of human lung carcinoma cells, which could be used in combination with immunotherapies or chemotherapeutic strategies to improve clinical responses.
Experimental Design
In the present report we have utilized a quantitative high throughput screening (qHTS) of a pharmaceutical collection of more than 2,000 compounds to identify clinically approved drugs capable of augmenting the sensitivity of mesenchymal-like, lung cancer cells to immune- and chemotherapy-mediated lysis, both in vitro and in vivo.
Results
The estrogen receptor antagonist fulvestrant was shown to reduce mesenchymal features of lung carcinoma cells, resulting in tumor sensitization to the cytotoxic effect of antigen-specific T cells, natural killer (NK) effectors cells and chemotherapy both in vivo and in vitro.
Conclusions
To our knowledge, this is the first report defining a potential role for estrogenic signaling in promoting tumor resistance to immune-mediated cytotoxicity and chemotherapy in lung cancer. Our data demonstrates a robust association between the acquisition of mesenchymal attributes, therapeutic resistance of lung carcinoma cells, and the expression of estrogen receptor 1 (ESR1), supporting further investigations on the role of estrogen signaling in lung cancer progression via the induction of EMT.
Keywords: immune resistance, EMT, fulvestrant, lung cancer, brachyury
Introduction
As we improve our understanding of how epithelial tumors progress toward metastatic disease, there are new opportunities for development of therapeutic modalities aimed at countering cancer progression. One of the recently proposed mechanisms utilized by carcinoma cells to disseminate and to metastasize involves the conversion of tumor cells from an epithelial to a mesenchymal-like phenotype, via a process designated as the epithelial-mesenchymal transition (EMT) (1-3). It has now been recognized that in addition to becoming prone to metastasize, carcinoma cells undergoing EMT become resistant to cytotoxic treatments, including chemotherapy (4, 5), radiation (6) or small molecule-targeted therapies (7, 8). Interfering with, or reversing the process of EMT represents an attractive therapeutic modality against tumor dissemination and, perhaps more importantly, to minimize the occurrence of therapeutic resistance (9-11).
In a recent series of reports, the association between EMT and tumor resistance to therapies has been extended to immunotherapy, as mesenchymal-like tumor cells have also been shown to be less susceptible to the cytotoxic effect of adaptive or innate immune effector cells than their epithelial counterparts (12, 13). Data from those reports suggest that tumor EMT could have a negative impact on the various immune-based interventions against cancer that are currently being investigated in the clinic, all of which ultimately rely on the ability of effector immune cells to efficiently lyse cancer cells. We thus hypothesize that the efficacy of cancer vaccines approaches, adoptively transferred anti-tumor lymphocytes, or monoclonal antibodies against checkpoint inhibitors or those that mediate antibody-dependent cell cytotoxicity (ADCC) could all be potentially improved when used in combination with approaches that reduce tumor resistance imparted by the EMT phenomenon.
In the present work, a quantitative high-throughput screening (qHTS) assay was used to identify compounds capable of enhancing immune-mediated lysis of mesenchymal-like lung carcinoma cells. The primary screening was performed by assessing the susceptibility of a clonal population of lung carcinoma cells to the surrogate immune effector TNF-related apoptosis inducting ligand (TRAIL), following exposure to a comprehensive collection of small-molecule compounds available from the National Institutes of Health (NIH) Chemical Genomics Center (NCGC), including the NCGC Pharmaceutical Collection (NPC) (14). By using this approach, we have identified the estrogen receptor antagonist fulvestrant as being able to render mesenchymal-like lung cancer cells significantly more susceptible to immune effector cells, as well as chemotherapy. Further studies demonstrated a robust association between the acquisition of mesenchymal features by lung carcinoma cells and the expression of estrogen receptor 1 (ESR1, ER-alpha), and blockade of estrogen signaling via fulvestrant was shown to revert tumor phenotype while significantly augmenting their susceptibility to NK cells, tumor-reactive cytotoxic T cells and chemotherapy.
Although the role of estrogen signaling in lung cancer remains controversial (15), the findings from this study support further investigations on the association between active estrogen signaling and lung cancer progression via the induction of EMT. These findings also form the rationale for the potential use of combinations of fulvestrant and immune-mediated therapies for the management of advanced lung cancer patients.
Materials and Methods
Cell lines and culture conditions
H460 and H1703 cells were originally purchased from the American Type Culture Collection (ATCC) and propagated as recommended. The cells lines were authenticated by short tandem repeat (STR) analysis (Bio-Synthesis Inc. or IDEXX BioResearch) in Jan 2013, May 2014 and Dec 2015. Two single cell-derived clonal populations of H460 cells, designated as H460-M and H460-E were expanded from the parental H460 cell line. Chemo-resistant H1703 cells were generated by repeated (4-6 cycles) weekly exposure of parental cells to culture medium containing 500 ng/mL cisplatin (APP Pharmaceuticals) and 40 ng/mL vinorelbine (Tocris) for six hours. Chemo-resistant H460 cells were generated by continuous growth in the presence of 10 ng/mL cisplatin and 1 ng/mL vinorelbine.
Compound library
The NPC collection, consisting of 2816 small molecule compounds, was assembled as previously described (14). Approximately, 50% of compounds in the collection are approved for human or animal use by the United States Food and Drug Administration (FDA).
Quantitative High-Throughput Screen (qHTS)
H460-M cells were dispensed in two sets of 1536-well plates (Greiner Bio-One) at 1000 cells/well. After overnight incubation, compounds were added at multiple doses beginning at 46 μmol/L and diluted 3-fold with DMSO. After 48 hours, each set of plates received 1 μL of PBS or recombinant TRAIL (30 ng/mL final concentration, Enzo Life Sciences). Cell viability was assessed at four hours post-TRAIL addition (PBS for vehicle set) using CellTiter-Glo reagent (Promega) and luminescence (RLU) was quantified using a ViewLux (PerkinElmer). A more detailed description of the qHTS is provided as Supplemental Material and Methods.
qHTS data analysis
Activity of the hits from the qHTS screen was analyzed using the Curve Response Class (CRC) classification, in which normalized data is fitted to 4-parameter dose response curves using a custom grid-based algorithm to generate CRC score for each compound dose response (16, 17). CRC values of -1.1, -1.2, -2.1 and -2.2 are considered highest quality hits; CRC values of -1.3, -1.4, -2.3, -2.4 and -3 are inconclusive hits; and CRC values of 4 are inactive compounds. Additional parameters obtained from qHTS and used for hit selection were the Maximum Response, which is the % activity at the maximum concentration of compound tested (46 μmol/L) and the AC50, which is obtained from the curve fitting obtained using the CRC algorithm.
Cytotoxic assays
Peripheral blood from healthy donors and cancer patients was obtained under appropriate Institutional Review Board approval and informed consent. Natural Killer (NK) cells were isolated from the peripheral blood of normal donors using a CD56 positive selection kit (Miltenyi Biotec.). HLA-A24-restricted mucin-1 (MUC-1) reactive CD8+ T cells were expanded from the peripheral blood of cancer patients as previously described (18). For immune-mediated lytic assays, target cells were labeled with 20 μCi 111Indium oxine (GE Healthcare) for 15 minutes in serum-free medium at room temperature, washed and plated at 3,000 targets per well in 96-well round-bottom plates, followed by the addition of effector cells at indicated effector-to-target ratios. Following an overnight incubation at 37°C, culture supernatant (100 μl) was collected from each well and the 111In released was measured using a WIZARD2 gamma counter (Perkin Elmer). Spontaneous lysis was determined by incubating target cells with medium alone; complete lysis by incubating targets with 2.5% Triton X-100. Percentage lysis was calculated by the following formula: [(counts test well - counts spontaneous release)/(counts complete lysis - counts spontaneous lysis)] ×100 (12, 18). Where indicated, target cells were incubated with compounds (Sigma) or DMSO for 48-72 hours prior to the cytotoxic assays. Cultures were also treated with 1μmol/L β-estradiol (Sigma) 24 hours prior to the addition of fulvestrant. For chemotherapy-mediated cytotoxicity, cultures were exposed to cisplatin and vinorelbine for six hours; media was replaced and cells were allowed to grow for three days, followed by cell survival analysis by the MTT assay. Survival for treated wells was calculated as a percentage of the values representing wells of untreated cells.
Plasmids
The plasmids encoding the full-length human brachyury and ESR1, along with empty vectors were purchased from Origene Technologies. Brachyury and GAPDH promoter reporter plasmids were purchased from SwitchGear Genomics. Brachyury promoter activity was normalized to GAPDH promoter activity. A firefly luciferase reporter vector encoding a minimum promoter with a single palindromic brachyury-binding element (AATTTCACACCTAGGTGTGAAATT) (19) was generated by the Protein Expression Laboratory, NCI.
RNA interference
ON-TARGET plus SMART pool siRNA for Brachyury (L-011399-00-0020), ESR1 (L-003401-00-0005) and non-targeting control siRNA and DharmaFECT-2 transfecting reagent were purchased from GE Dharmacon. Cells were transfected with 25 nmol/L siRNA using the manufacture's recommended protocol. Assays were performed 72 hours post-transfection.
RNA expression
RNA isolation and real time PCR assays were performed as previously described (20) utilizing recommended probes (Life Technologies). Estrogen signaling qPCR array was purchased from SA Biosciences. Expression was normalized to GAPDH. ESR1/2 expression in association with various markers of EMT in lung cancer was assessed using a TCGA dataset containing data from 490 lung adenocarcinomas and 58 histologically normal lung tissues (http://cancergenome.nih.gov/; downloaded April 2014). Data were analyzed utilizing the Nexus Expression 3 analysis software package (BioDiscovery); classification of samples in high vs. low ESR1 groups was performed by comparison to the mean expression level observed in normal tissues plus or minus two standard deviations.
Western blot and immunofluorescence
Western blots were performed as previously described (12) using the following antibodies: pan-actin (clone Ab-5, Thermo Scientific), fibronectin, vimentin, ZO-1 (BD Biosciences) and brachyury (MAb 54-1). For immunofluorescence analysis, tumor cells grown on glass coverslips were rinsed with PBS and fixed for 10 minutes using 3% paraformaldehyde (Electron Microscopy Sciences). Following permeabilization using 0.05% Triton X-100 in PBS, coverslips were blocked in PBS supplemented with 1% BSA and 10% goat serum, incubated with anti-ESR1 antibody (GTX62423, GeneTex) overnight at 4°C, and subsequently stained with AlexaFluor-647 labeled goat anti-rabbit IgG (Thermo Fisher Scientific) for one hour at room temperature, according to the manufacturer's instructions. Nuclei were stained using DAPI; where indicated, coverslips were stained with phalloidin Alexa Fluor-488 (Thermo Fisher Scientific). Images were captured using a Leica Fluorescent microscope (Leica Biosystems Inc.).
Tumor xenografts
Studies involving the use of animals were carried out in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines, and under the approval of the NIH intramural animal care and use committee. Five-week old female immune-compromised mice were implanted subcutaneously with 2×106 H460 cells; when tumors became palpable, mice were treated with intraperitoneal injection of either HBSS or 20 mg/kg docetaxel every three days for three cycles. Fulvestrant-treated animals were given a single subcutaneous dose of 250 mg/kg fulvestrant five days prior to tumor collection. In the combination study, animals were implanted subcutaneously with 1×106 H460 cells; fulvestrant (250 mg/kg) was given on days 4 and 11 and docetaxel (20 mg/kg) on days 7 and 10 after tumor implantation. Tumor sections were stained using primary antibodies against ESR1 (ab32063, Abcam), brachyury (MAb 54-1) and fibronectin (GTX112794, GeneTex), and counterstained with haematoxylin (Sigma).
Results
Mesenchymal-like carcinoma cells are resistant to immune attack
We have previously demonstrated that acquisition of mesenchymal features by carcinoma cells can impart tumor resistance to immune-mediated attack (12). As established cell lines are comprised of cells with varying epithelial and mesenchymal features, in the present study we have isolated homogenous populations of epithelial vs. mesenchymal-like cancer cells by single cell-derived culture of lung carcinoma H460 cells. As shown in Fig. 1A, two clones were selected based upon their differential expression of mesenchymal and epithelial markers. H460-E cells were characterized by low levels of expression of mesenchymal brachyury and fibronectin and high levels of epithelial ZO-1; in contrast, clone H460-M was considered mesenchymal-like, with high levels of brachyury and fibronectin and very low levels of ZO-1 (Fig. 1A). As predicted, the mesenchymal H460-M clone exhibited a 2.4-fold greater IC50 in response to treatment with cisplatin than the epithelial H460-E clone (IC50 H460-E: 0.37 μg/mL; IC50 H460-M: 0.90 μg/mL, Fig. 1B). H460-M also demonstrated decreased sensitivity to both brachyury-specific CD8+ cytotoxic T cells and effector NK cells, at all effector-to-target ratios evaluated (Figs. 1C and D, respectively). H460-M cells also exhibited a marked resistance to a range of concentrations of TRAIL (Fig. 1E). As one of the known mechanisms of resistance to immune-mediated cytotoxicity is the loss of cell surface death receptors and/or the gain of corresponding decoy receptors, expression of TRAIL receptors TRAIL-R1 and TRAIL-R2, FAS and the decoy receptors DcR1, DcR2 and DcR3 were evaluated in both clones. As shown in Fig. 1F, no changes were observed that could explain the loss of immune-mediated cytotoxicity observed with H460-M cells.
Figure 1. H460-M clone exhibits resistance to cell death.
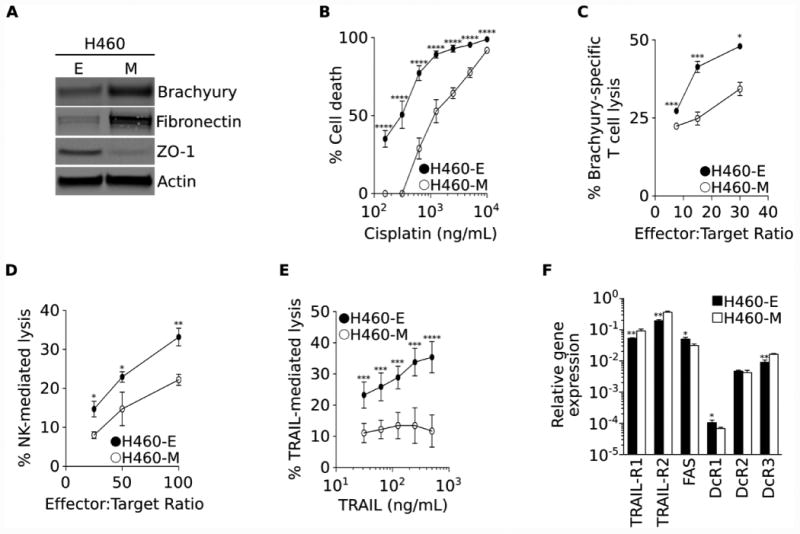
(A) Western blot analysis of indicated proteins expressed by the H460-E and H460-M clones. (B) Susceptibility of H460-E vs. -M clones to a range of indicated concentrations of cisplatin. (C) Percent of lysis mediated by brachyury-specific CD8+ T cells, (D) NK effector cells, and (E) recombinant TRAIL. (F) Expression of mRNA encoding for TRAIL, FAS, and decoy receptors (DcR1, DcR2, DcR3), relative to GAPDH. Error bars indicate the standard error of the mean (SEM) of triplicate measurements. [* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001].
Identification of compounds that enhance immune-mediated lysis via qHTS
Utilizing the H460-M clone as a model and a qHTS assay, the NCGC Pharmacological Collection was screened to identify clinically relevant compounds that could enhance the susceptibility of resistant lung cancer cells to immune-mediated lysis. The screen was aimed at identifying compounds that were cytotoxic for TRAIL-treated cells but were devoid of cell toxicity when used alone. Using these criteria, 53 hits were identified corresponding to 51 unique compounds (Fig. 2A, larger dots). These 51 hits were subsequently ranked based on 1) Δ%MaxResponse= [(MaxResponseTRAIL+compound)-(MaxResponsePBS+compound)] < -50%; 2) AC50 < 20 μmol/L for TRAIL-treated cells; and because the objective of this screen was to identify drugs that could be rapidly translated into clinical studies, the focus was on compounds that are 3) approved and available for clinical use in the US; 4) have well-known pharmacological, pharmacokinetic and toxicity profiles; and 5) show activity within a range of concentrations attainable in vivo. Based on these criteria, fulvestrant, selegiline and midazolam were selected for further analysis (Fig. 2A colored dots and Fig. 2B). As midazolam has been replaced in the clinic by newer generations of benzodiazepines, clonazepam, diazepam and lorazepam were further evaluated in secondary assays; only fulvestrant was confirmed to enhance susceptibility to TRAIL (Fig. 2C), thus being chosen as the lead compound for further studies.
Figure 2. Fulvestrant renders mesenchymal cells more sensitive to immune-mediated lysis.
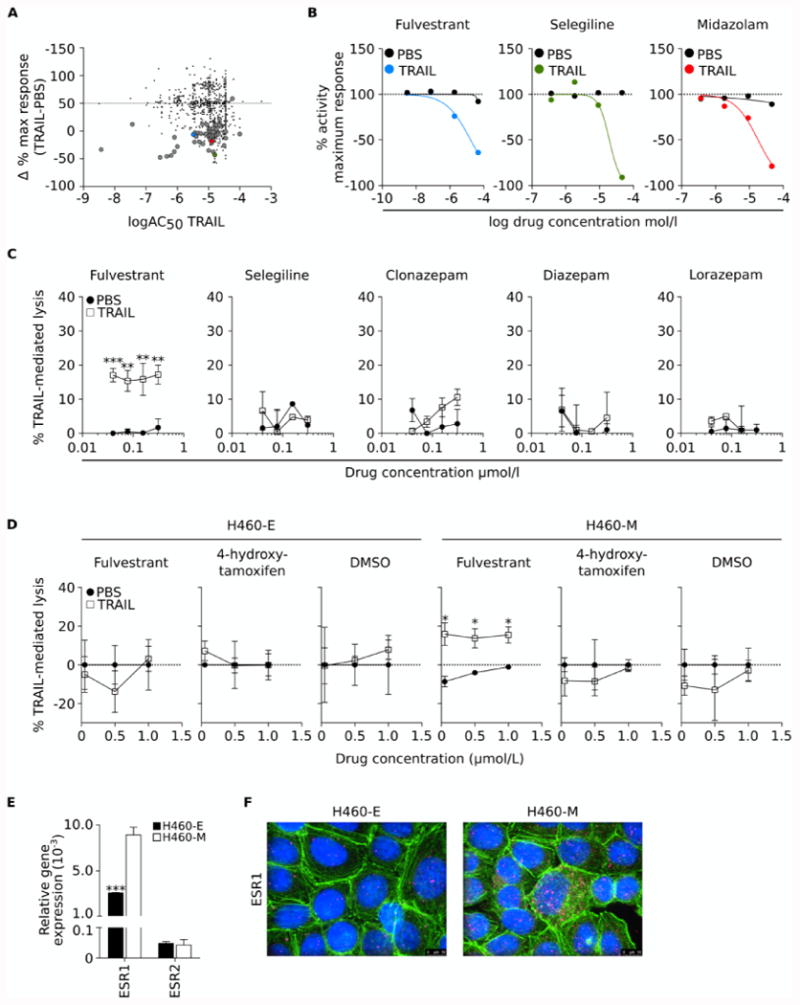
(A) Graphical depiction of compounds having measurable activity in the qHTS assay. The y-axis represents the Δ%MaxResponse= [(MaxResponseTRAIL+compound)-(MaxResponsePBS+compound)], where Maximum Response is the % activity at the maximum concentration of compound tested (46 μmol/L). The x-axis represents the logAC50 for TRAIL, calculated as indicated in the Materials and Methods section. Large circles represent 53 hits with curves class of 4 when used with PBS, and curves classes -1 or -2 when used with TRAIL. The top three ranked compounds are indicated by color circles: fulvestrant (blue), selegiline (green) and midazolam (red). (B) Maximum response (%) of the top three ranked compounds when used in combination with TRAIL vs. PBS. (C) Secondary screening of top compounds showing dose response curves when used in combination with TRAIL vs. PBS in H460 cells. (D) Dose response curves of H460-E and H460-M cells treated with indicated doses of fulvestrant, 4-hydroxytamoxifen (active metabolite of the FDA-approved estrogen receptor antagonist tamoxifen) or DMSO to TRAIL-mediated lysis. (E) ESR1 and ESR2 mRNA expression levels in the H460-E and -M clones, relative to GAPDH. (F) Immunofluorescent analysis of ESR1 (pink signal) expression in the H460-E and -M clones (100× magnification). Green signal corresponds to phalloidin staining; blue corresponds to DAPI. [* p<0.05, ** p<0.01, *** p<0.001].
Unlike tamoxifen, a widely used estrogen receptor blocker that retains agonistic activity in certain tissues, fulvestrant is a pure estrogen receptor antagonist that induces receptor degradation. To assess whether the ability of fulvestrant to enhance the sensitivity of mesenchymal-like lung tumor cells to TRAIL might be a consequence of its ability to downregulate estrogen receptor levels, we compared its activity with that of 4-hydroxytamoxifen, the active metabolite of tamoxifen, which is another FDA-approved estrogen receptor antagonist. H460-E and H460-M cells were pre-treated for 3 days with various concentrations of fulvestrant vs. 4-hydroxytamoxifen prior to the addition of TRAIL. Intriguingly, both antagonists failed to modify the cytotoxic response of the epithelial H460-E cells, while fulvestrant (and not 4-hydroxytamoxifen) was able to significantly augment the susceptibility of the mesenchymal H460-M cells to TRAIL-mediated lysis (Fig. 2D), thus suggesting that the effect observed with fulvestrant might be due to receptor downregulation. The analysis of ESR1 expression at the mRNA level demonstrated a 3.4-fold increase in ESR1 in the H460-M vs. H460-E cells, while no difference was observed with ESR2 (Fig 2E). Increased levels of ESR1 protein were also observed in the H460-M vs. H460-E cells (Fig 2F).
Fulvestrant enhances immune cytotoxicity of mesenchymal-like tumor cells
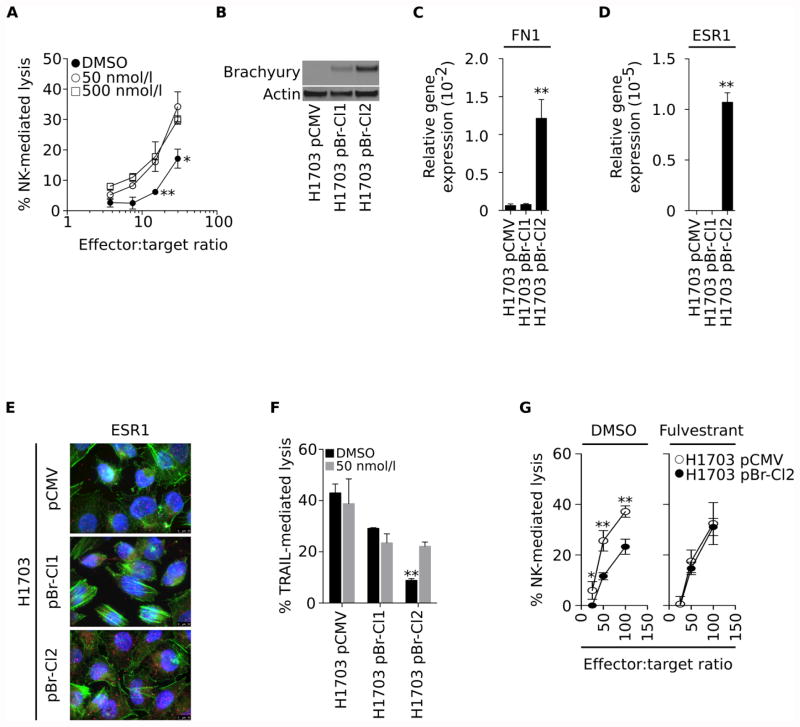
The ability of fulvestrant to improve the susceptibility of carcinoma cells to immune-mediated lysis was confirmed with additional cell line model systems. As shown in Fig. 3A, NK effector cells lysed parental H460 cells pre-treated with 50 or 500 nmol/L fulvestrant more efficiently than control H460 cells. Based on these results, all subsequent experiments, unless indicated, were conducted with 50 nmol/L fulvestrant, a dose that is comparable to the plasma Cmax (∼40 nmol/L) for multiple dose steady state observed in patients treated with the drug (21). To confirm the observations in another human lung carcinoma cell line, we generated isogenic H1703 lines stably transfected with either a control (pCMV) or a brachyury expressing (pBr) vector, from which we expanded two clonally-derived cell populations characterized by low (pBr-Cl1) or high (pBr-Cl2) levels of brachyury (Fig. 3B). As expected from our previous observations, pBr-Cl2 with the highest expression of brachyury also exhibited mesenchymal features, including high expression of fibronectin (Fig. 3C) and increased ESR1 at the mRNA and protein levels (Fig. 3D and E, respectively). When evaluated for cytotoxicity, only H1703 cells with mesenchymal features (pBr-Cl2) exhibited resistance to TRAIL (Fig 3F) or NK-mediated lysis (Fig. 3G, left panel), a phenomenon that could be alleviated by tumor pre-treatment with fulvestrant (Fig. 3G, right panel). These results demonstrated that fulvestrant treatment of mesenchymal-like (and not epithelial) lung carcinoma cells could increase immune-mediated lysis potentially by repairing defective cell death mechanisms driven by the EMT (12). Furthermore, these observations suggested that estrogen signaling might play an important role in protecting mesenchymal-like lung carcinoma cells to immune-mediated attack.
Figure 3. Fulvestrant renders mesenchymal cells more sensitive to immune-mediated lysis.
(A) Susceptibility of parental H460 cells treated with two doses of fulvestrant vs. DMSO to lysis by NK cells at various effector-to-target ratios. (B) Brachyury protein levels and expression of mRNA encoding for fibronectin (C) and ESR1 (D), relative to GAPDH, in clonally-derived H1703 cells transfected with pCMV vs. pBr (Clones 1 and 2). (E) Immunofluorescent analysis of ESR1 (pink signal) protein expression in H1703 pCMV, pBr-Cl1 and pBr-Cl2 cells (100× magnification). Green and blue correspond to phalloidin and DAPI staining, respectively. (F) Susceptibility to TRAIL-mediated lysis in cells pre-treated with fulvestrant (grey bars) vs. DMSO (black bars). (G). Susceptibility of H1703 pCMV and H1703 pBr-Cl2 cells treated with fulvestrant (right panel) vs. DMSO (left panel) to lysis by NK cells at various effector-to-target ratios. Error bars indicate the standard error of the mean (SEM) of triplicate measurements. [* p<0.05, ** p<0.01].
Upregulation of ESR1 signaling in chemo-resistant lung cancer cells
Several studies have shown that exposure of carcinoma cells to chemotherapeutic agents in vitro or in vivo can select for a population of chemo-resistant cells with mesenchymal-like features (4). As shown in Fig. 4A, H1703 cells selected in vitro in the presence of a combination of cisplatin and vinorelbine exhibited enhanced expression of T, SNAI2, FN1 and OCLN mRNA (encoding for brachyury, slug, fibronectin, and occludin protein, respectively), and had a 672-fold increase in ESR1 mRNA levels, compared to control H1703 cells, the latter confirmed at the protein level (Fig. 4B). The chemo-resistant cells were also highly resistant to immune-effector mechanisms, including lysis by TRAIL and effector NK cells (Fig. 4C). However, pre-treatment with fulvestrant effectively restored their TRAIL or NK-mediated lysis to levels observed with control H1703 cells (Fig. 4C). Interestingly, the sensitivity of the H1703 chemo-resistant cells to a combination of cisplatin and vinorelbine was also reconstituted when the tumor cells were exposed to fulvestrant prior to, and during the cytotoxic assay (Fig 4D).
Figure 4. Fulvestrant reverts immune resistance of chemo-resistant lung cancer cells.
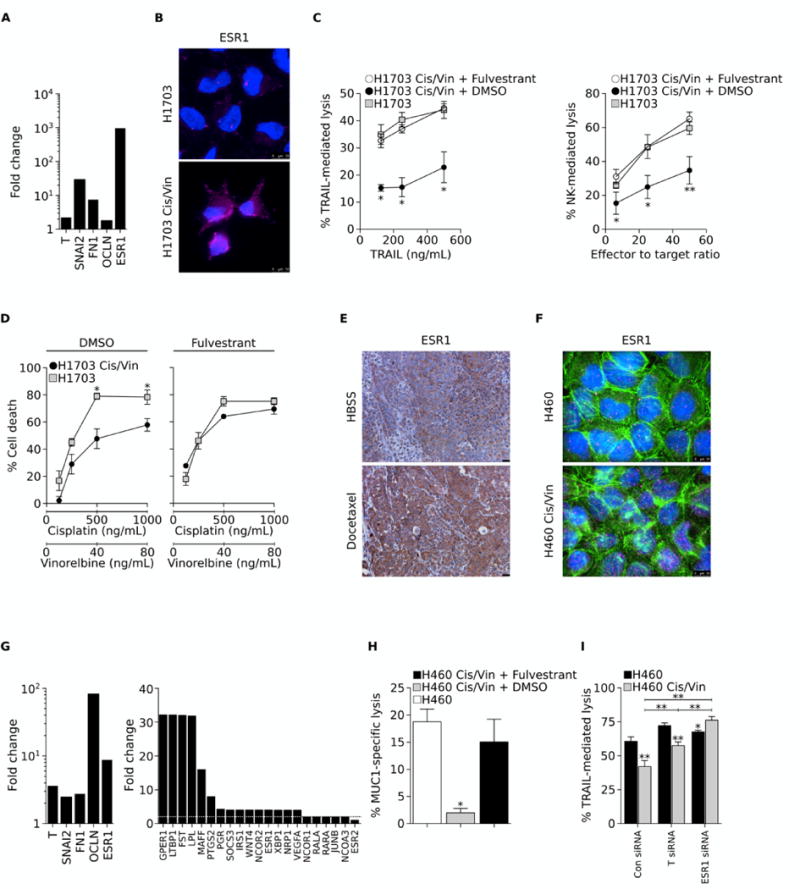
(A) Fold change in expression levels of indicated mRNA in chemo-resistant vs. control H1703 cells. (B) Immunofluorescent analysis of ESR1 (pink signal) in control and cisplatin/vinorelbine-resistant (Cis/Vin) H1703 cells. Blue signal corresponds to DAPI staining. (C) Susceptibility of control H1703 vs. Cis/Vin-resistant H1703 cells to lysis by either TRAIL (left panel) or NK cells (right panel). Chemo-resistant cells were treated with DMSO or fulvestrant for 72 hours prior to the cytotoxic assay. (D) Sensitivity of control vs. Cis/Vin-resistant H1703 cells to a combination of indicated concentrations of vinorelbine and cisplatin; tumor cells were treated with DMSO (left panel) or fulvestrant (right panel) for 72 hours prior to exposure to chemotherapy. (E) Immunohistochemical analysis of ESR1 expression in H460 xenografts from mice treated with either HBSS or docetaxel (20× magnification). (F) Immunofluorescent analysis of ESR1 expression (pink signal) in control and Cis/Vin-resistant H460 cells (100× magnification). Green and blue correspond to phalloidin and DAPI staining, respectively. (G) Fold-change in expression levels of indicated mRNA in Cis/Vin-resistant vs. control H460 cells (H) Susceptibility of parental vs. chemo-resistant H460 cells to lysis by MUC1-specific CD8+ T cells. Chemo-resistant cells were treated with DMSO or fulvestrant for 72 hours prior to the cytotoxic assay. (I) TRAIL-mediated lysis of parental H460 vs. chemo-resistant H460 cells previously transfected with a control non-targeting Con siRNA vs. a pool of siRNAs targeting either brachyury (T siRNA) or the estrogen receptor 1 (ESR1 siRNA). Error bars indicate the standard error of the mean (SEM) of triplicate measurements. [* p<0.05, ** p<0.01].
To investigate the relevance of estrogenic signaling upregulation in vivo in the context of chemotherapy, ESR1 expression was examined by immunohistochemistry in H460 xenografts of mice treated with repeated doses of docetaxel. The performance of the anti-ESR1 antibody and staining technique were first validated utilizing human invasive ductal carcinoma tissues with known ER status, as well as control IgG (Supplemental Fig. 1A and B). Utilizing this antibody, a marked increase in ESR1 protein was observed in tumors of docetaxel-treated vs. control mice (Fig. 4E), mostly in the cytoplasm of the tumor cells (Supplemental Fig. 1B).
H460 cells grown in vitro in the presence of cisplatin and vinorelbine also demonstrated increased ESR1 protein expression (Fig. 4F), along with the upregulation of T, SNAI2, FN1, and OCLN mRNA and an eight-fold increase in the expression of ESR1 mRNA (Fig. 4G, left panel), compared to control H460 cells. Further analysis of an array of 84 genes involved in estrogen receptor activation and response demonstrated that estrogenic signaling is active in these cells, as the expression of 20 out of the 84 genes analyzed was upregulated ≥ 2-fold (Fig. 4G, right panel) in chemo-resistant vs. parental H460 cells. Noteworthy, upregulation of ESR1 but not ESR2 mRNA was observed in these cells. As shown in Fig. 4H, the ability of MUC1-specific CD8+ T cells to lyse H460 chemo-resistant cells was markedly reduced compared to control cells, but their lysis was fully reconstituted by pre-treatment with fulvestrant prior to the cytotoxic assay. To ascertain a role for brachyury and ESR1 in mediating this increased resistance, we silenced each gene using specific siRNA pools in both control and chemo-resistant H460 cells. While silencing of brachyury (T) resulted in a modest but significant increase of cell death in response to TRAIL, silencing of ESR1 was able to fully reconstitute the susceptibility of the chemo-resistant cells to TRAIL-mediated lysis (Fig. 4I), confirming the central role of ESR1 signaling in the resistant phenotype of these cells.
Overexpression of ESR1 drives resistance to immune-mediated cytotoxicity
To ascertain whether ESR1 could have a direct role in the phenomenon of resistance to immune attack exhibited by mesenchymal-like lung cancer cells, H460 cells were stably modified to overexpress ESR1. As shown in Fig. 5A, high expression of ESR1 significantly decreased the response of H460 cells to NK cells. Moreover, single clonal populations of H460 selected based on the expression of ESR1 (Fig. 5B) demonstrated the direct association between ESR1 levels and resistance to immune-mediated lysis, with the H460 ESR1-High clone being completely resistant to TRAIL, compared to the H460 ESR1-Low clone (Fig. 5C). Similar results were observed in response to NK cells where the H460 ESR1-Low clone was lysed more efficiently than the ESR1-High clone, an effect that was exacerbated when using NK effector cells devoid of perforin/granzyme activity (Fig. 5D).
Figure 5. Estrogen receptor mediates resistance to immune attack.
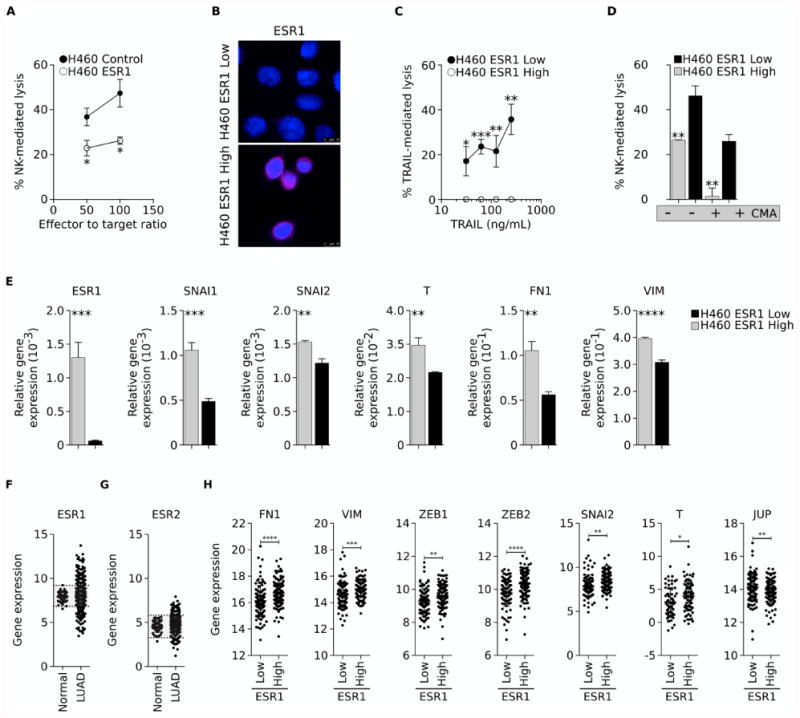
(A) H460 cells stably transfected with pCMV or a vector encoding the ESR1 gene were assessed for their sensitivity to NK-mediated lysis. (B) Immunofluorescent analysis of ESR1 expression (pink signal) in single cell clones of H460 cells with High vs. Low ESR1 expression. Blue signal corresponds to DAPI staining. (C) Clones were evaluated for their susceptibility to lysis by TRAIL (C) or NK cells that were either untreated or pre-treated with CMA to inhibit perforin-dependent lytic pathways (D). (E) Relative expression of indicated mRNA in clonal H460 ESR1-High vs. ESR1-Low cells. (F) ESR1 and (G) ESR2 mRNA in normal lung vs. lung adenocarcinoma (LUAD) tissues. Shaded areas correspond to the normal range of expression for each gene, calculated as the mean expression in normal lung tissues (± two standard deviations). (H) mRNA expression of indicated genes in lung samples categorized as either ESR1 Low or High, based on the expression in normal lung tissues. Error bars indicate the standard deviation of the mean. [* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001].
As induction of EMT was shown to associate with expression of ESR1 in lung cancer cells, we also investigated whether mesenchymal markers were differentially expressed in clonal H460 cells with High vs. Low levels of ESR1. The ESR1-High clone (Fig. 5E) had significantly higher levels of expression of mesenchymal SNAI1, SNAI2, T, FN1 and VIM mRNA (encoding for snail, slug, brachyury, fibronectin and vimentin, respectively), as compared with the ESR1-Low clone. These results prompted us to analyze whether the association between estrogen receptor expression and markers of EMT is also present in lung tumor tissues. An initial analysis of mRNA data from the lung adenocarcinoma TCGA dataset demonstrated over-expression of ESR1 and ESR2 mRNA in 18% (88/490) and 11% (53/490) of tumors, respectively, compared to normal lung tissues (Figs. 5F, and G). Further analysis of tumor samples segregated into Low vs. High ESR1 groups demonstrated statistically significant higher levels of mRNA for the mesenchymal markers FN1, VIM, ZEB1, ZEB2, SNAI2, and T in the High vs. Low ESR1 group, while the expression of the epithelial marker JUP mRNA (encoding for plakoglobin) was higher in the ESR1 Low vs. High group (Fig. 5H). No correlation, however, was observed between the levels of ESR2 and mesenchymal or epithelial markers (data not shown).
Association of estrogen signaling and EMT of lung carcinomas
The role of fulvestrant in EMT modulation was first evaluated with H460 cells treated with fulvestrant in vitro. As shown in Fig. 6A, fulvestrant markedly reduced the expression of the mesenchymal proteins brachyury, fibronectin and vimentin in a dose-dependent manner. To more directly assess the effects of fulvestrant treatment on the transcriptional activity of the brachyury protein, a luciferase reporter vector was generated containing a promoter with a single brachyury-binding site. This construct was transfected into the H460 cell line, and the effect of fulvestrant treatment on brachyury transcriptional activity was measured, resulting in a dose-dependent decrease in brachyury activity in response to fulvestrant (Fig. 6B). Further, fulvestrant was also able to reduce, on a dose-dependent fashion, the activity of a brachyury promoter reporter construct (Fig. 6C), thus demonstrating that estrogen signaling directly or indirectly regulates the transcription of the EMT transcription factor brachyury in lung cancer cells.
Figure 6. Fulvestrant treatment reduces EMT markers and increases sensitivity of lung xenografts to docetaxel.
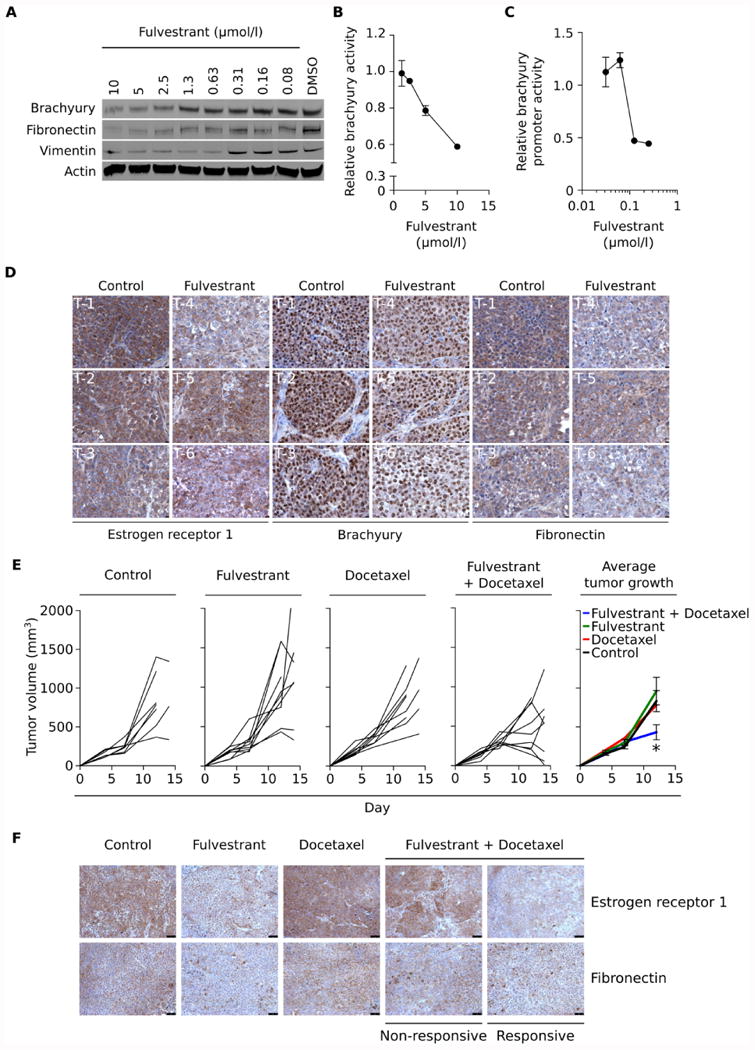
(A) Western blot analysis of brachyury, fibronectin and vimentin protein in H460 cells treated in culture for six days with indicated concentrations of fulvestrant vs DMSO. (B) Brachyury transcriptional activity in H460 cells stably transfected with a brachyury-reporter vector treated for 48 hours with indicated doses of fulvestrant and normalized to DMSO-treated cells. (C) Brachyury promoter activity in H460 cells transiently transfected with a brachyury promoter vs. a GAPDH promoter reporter vector, after treatment for six days with indicated concentrations of fulvestrant. (D) Estrogen receptor 1, brachyury and fibronectin expression in H460 tumor xenografts five days after a single injection of either HBSS or fulvestrant. (E) Tumor volume of H460 xenografts treated as indicated, with fulvestrant (250 mg/kg) given on days 4 and 11 and docetaxel (20 mg/kg) on days 7 and 10. (F) Estrogen receptor 1 and fibronectin expression in H460 tumor xenografts, treated as indicated. Error bars indicate the standard error of the mean (SEM) of triplicate measurements. [* p<0.05, ** p<0.01].
In subsequent experiments, the effect of fulvestrant was evaluated in vivo by administration of a single dose fulvestrant to mice bearing lung H460 xenografts. To assess changes on tumor phenotype, expression of estrogen receptor 1, brachyury and fibronectin were evaluated by immunohistochemistry (Fig 6D). Overall, fulvestrant was able to decrease the intensity of staining of all three proteins in tumor cells (Supplemental Table 1), with the most significant reductions of fibronectin and brachyury taking place in tumors where the highest decrease of ESR1 levels (tumors T-4 and T-6, Fig. 6D) took place. As previously shown in Fig. 4F, estrogen receptor 1 staining was primarily localized in the cytoplasm of the H460 tumor cells.
The potential effect of fulvestrant-mediated reduction of mesenchymal features on tumor sensitivity to cytotoxic treatment was evaluated in vivo. Athymic nude mice bearing H460 xenografts were treated with either docetaxel or fulvestrant alone, or a combination of both. As shown in Fig. 6E, neither treatment alone had any measurable impact on tumor growth, however, when fulvestrant was administered three days prior to docetaxel, a marked reduction of tumor volume was observed compared to single treatments (Fig. 6E, right panel). Expression of estrogen receptor 1 and fibronectin were also assessed in these tumors, with representative images from each group being shown in Fig. 6F. Fulvestrant alone markedly reduced the expression of estrogen receptor 1 and fibronectin, while docetaxel associated with increased expression of estrogen receptor 1, compared to untreated tumors. When expression of estrogen receptor 1 and fibronectin were analyzed in tumors from the combined treatment group, a marked reduction of estrogen receptor and fibronectin were observed in treatment-responsive vs. progressive (non-responsive) tumors, thus demonstrating that fulvestrant can reduce mesenchymal features in lung carcinoma cells in vivo, and that reduction of mesenchymal features associates with tumor sensitization to the cytotoxic effect of chemotherapy.
Discussion
The acquisition of therapeutic resistance associated with tumor progression is a barrier to the effective treatment of patients with advanced, metastatic tumors. In particular, carcinoma cells acquiring a mesenchymal-like phenotype via the EMT are thought to represent a population of cells with increased resistance to a variety of cytotoxic therapies. In the present study, utilizing a qHTS functional assay with a clonal population of mesenchymal-like lung carcinoma cells, the estrogen antagonist fulvestrant was identified as able to modulate tumor phenotype and sensitize mesenchymal lung cancer cells to chemotherapy and immune-mediated lysis. Activation of ESR1 signaling was also demonstrated here to associate with mesenchymal and resistant features in lung cancer cells. To our knowledge, this is the first report defining a potential role for estrogenic signaling in promoting tumor resistance to both immune-mediated cytotoxicity and chemotherapy in lung cancer.
Several reports have shown that tumor cells undergoing EMT also acquire features typically associated with cancer stem cells (CSCs), particularly resistance to cell death (3, 22). Targeting carcinoma cells with mesenchymal-like features could be an efficient strategy to prevent tumor recurrence post-therapies mediated by CSCs. Previous reports, however, have shown that EMT can negatively impact tumor susceptibility to immune-mediated lysis (12, 13, 23). Our group, for example, has shown that very high levels of expression of the EMT driver brachyury induce tumor resistance to antigen-specific CD8+ T lymphocytes or innate NK cells (12), where the defective lysis is associated with a faulty tumor caspase-mediated nuclear apoptosis. The screen performed here was designed to identify drugs capable of improving immune-mediated lysis of tumor cells undergoing EMT. To this end, a highly homogeneous population of mesenchymal-like, brachyury-high lung carcinoma cells was first exposed to a library of compounds and subsequently lysed in the presence of TRAIL. The approach utilized here, which allowed for the identification of fulvestrant as an enhancer of immune-mediated lysis of lung cancer cells, is different to other screening assays against EMT previously reported. For example, a cell-based small molecule screening was used for identification of compounds that could inhibit the initiation of EMT by EGF, HGF or IGF-1 in carcinoma cells, based on the ability of the drugs to inhibit spot cell migration (24). In another study, human mammary epithelial cells induced into an EMT via E-cadherin knockdown were used for identification of salinomycin as a compound capable of preferentially killing tumor cells that underwent EMT vs. their epithelial counterparts (25). Our study sought instead to identify compounds which themselves are not directly cytotoxic to mesenchymal-like cells, but render tumor cells more sensitive to the cytotoxic activity of TRAIL.
Fulvestrant is an FDA-approved, selective estrogen receptor antagonist used in the treatment of hormone receptor-positive breast cancer, with well-known pharmacokinetics, pharmacological and toxicity profiles (21, 26). Interestingly, we have observed here that tamoxifen, a widely used estrogen receptor modulator with agonistic activity, is unable to recapitulate the sensitizing effect observed with the pure antagonistic fulvestrant. In lung cancer, the role of estrogens remains unclear, with variable levels of expression of ESR1 or ESR2 being reported in lung cancer tissues and cell lines, particularly in adenocarcinoma, depending on the method and reagents used for the detection (15, 27, 28). It has been demonstrated for example that estrogens stimulate lung cancer cells proliferation in vitro and progression in vivo (29), an effect that can be blocked by fulvestrant.
The possible involvement of estrogen in the development of lung cancer has been suggested. For example, in one report, post-menopausal women with NSCLC have been shown to live longer than similarly aged men (30), while younger pre-menopausal women commonly presented a more aggressive form of lung cancer disease (31). Also in support of a potential role for estrogens in NSCLC tumor progression, hormone replacement therapy has been associated with a more rapid disease progression (32, 33), while the use of estrogen antagonists has been shown to reduce disease mortality (34). Furthermore, it has been reported that ESR1 expression is associated with poorer overall survival (15), and that low ESR1 mRNA levels predict responsiveness to chemotherapy in resected non-small cell lung cancer (35). Estrogen receptor signaling is also known to interact with the epidermal growth factor receptor (EGFR) pathway (36, 37), and based on this knowledge clinical trials have been conducted for the treatment of NSCLC in post-menopausal women using fulvestrant in combination with EGFR-blockade (38, 39). Our observations suggest that fulvestrant treatment of lung cancer could also be expanded to combinations with chemotherapy or immunotherapies.
In this report we have observed a marked increase in ESR1 (but not ESR2) levels as lung cancer cells acquired resistance to chemotherapy in vitro and in vivo. This elevated expression of ESR1 was concomitant to the acquisition of mesenchymal-like features by carcinoma cells, which is a known mechanism of resistance to cytotoxic drugs. Treatment with fulvestrant not only reconstituted the sensitivity of the tumor cells to chemotherapy in vitro and in vivo, but also improved their lysis by immune effector mechanisms, including TRAIL, NK cells and antigen-specific T cells. When the expression of mesenchymal proteins in lung cancer cells treated with fulvestrant was analyzed, it was observed that blockade of estrogenic signaling efficiently decreased expression of the mesenchymal markers fibronectin, vimentin and the EMT driver brachyury, therefore suggesting that fulvestrant is able to revert tumor EMT which, in turn, could result in sensitization to a variety of cytotoxic insults.
In agreement with a potential role for ESR1 signaling in lung cancer EMT, a positive correlation between higher levels of ESR1 (but not ESR2) and expression of mesenchymal markers was observed here, supporting further investigations on the association between active estrogen signaling and lung cancer progression via the EMT. Interfering with, or reversing the process of EMT represents an attractive therapeutic modality against tumor dissemination and resistance to therapies. The findings reported here support the development of future combinations of fulvestrant with chemotherapy or immune-mediated therapies for the management of advanced NSCLC cancer patients.
Supplementary Material
Translational Relevance.
The acquisition of therapeutic resistance associated with tumor progression is a barrier to the effective treatment of patients with advanced, metastatic tumors. In particular, carcinoma cells acquiring a mesenchymal-like phenotype via the EMT are thought to represent a population of cells with increased resistance to a variety of cytotoxic therapies. In the present study, estrogen receptor alpha signaling is shown to correlate with mesenchymal tumor features and with increased resistance to cell death inducers. The estrogen receptor antagonist fulvestrant was identified as being able to not only decrease mesenchymal features of lung cancer cells, but also to increase tumor sensitivity to chemotherapy and immune-mediated lysis. Our findings provide rationale for the potential use of fulvestrant in combination with immunotherapy or chemotherapy for the management of advanced lung cancer patients.
Acknowledgments
The authors wish to thank the LTIB Clinical Trials Group and Dr. Jeffrey Schlom for their input during the selection of compounds, Dr. Kwong-Yok Tsang for providing MUC-1- and brachyury-specific T cells, and Kristen McCampbell for her technical assistance. Some of the results published here are based upon data from the TCGA Research Network.
Financial support: This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, and the National Center for Advancing Translational Sciences, National Institutes of Health.
Footnotes
The authors have no conflict of interest to declare.
Bibliography
- 1.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature reviews Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 4.Huang B, Cohen JR, Fernando RI, Hamilton DH, Litzinger MT, Hodge JW, et al. The embryonic transcription factor Brachyury blocks cell cycle progression and mediates tumor resistance to conventional antitumor therapies. Cell death & disease. 2013;4:e682. doi: 10.1038/cddis.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6:10697–711. doi: 10.18632/oncotarget.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–68. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 7.Thomson S, Buck E, Petti F, Griffin G, Brown E, Ramnarine N, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455–62. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 8.Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:279–90. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palena C, Fernando RI, Litzinger MT, Hamilton DH, Huang B, Schlom J. Strategies to target molecules that control the acquisition of a mesenchymal-like phenotype by carcinoma cells. Exp Biol Med (Maywood) 2011;236:537–45. doi: 10.1258/ebm.2011.010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palena C, Fernando RI, Hamilton DH. An immunotherapeutic intervention against tumor progression: Targeting a driver of the epithelial-to-mesenchymal transition. Oncoimmunology. 2014;3:e27220. doi: 10.4161/onci.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis FM, Stewart TA, Thompson EW, Monteith GR. Targeting EMT in cancer: opportunities for pharmacological intervention. Trends in pharmacological sciences. 2014;35:479–88. doi: 10.1016/j.tips.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton DH, Huang B, Fernando RI, Tsang KY, Palena C. WEE1 inhibition alleviates resistance to immune attack of tumor cells undergoing epithelial-mesenchymal transition. Cancer Res. 2014;74:2510–9. doi: 10.1158/0008-5472.CAN-13-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P, et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res. 2013;73:2418–27. doi: 10.1158/0008-5472.CAN-12-2432. [DOI] [PubMed] [Google Scholar]
- 14.Huang R, Southall N, Wang Y, Yasgar A, Shinn P, Jadhav A, et al. The NCGC pharmaceutical collection: a comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Science translational medicine. 2011;3 doi: 10.1126/scitranslmed.3001862. 80ps16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai H, Ishii A, Washiya K, Konno T, Kon H, Yamaya C, et al. Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:5084–9. doi: 10.1158/1078-0432.CCR-05-0200. [DOI] [PubMed] [Google Scholar]
- 16.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, et al. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A. 2006;103:11473–8. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Jadhav A, Southal N, Huang R, Nguyen DT. A grid algorithm for high throughput fitting of dose-response curve data. Curr Chem Genomics. 2010;4:57–66. doi: 10.2174/1875397301004010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jochems C, Tucker JA, Vergati M, Boyerinas B, Gulley JL, Schlom J, et al. Identification and characterization of agonist epitopes of the MUC1-C oncoprotein. Cancer immunology, immunotherapy : CII. 2014;63:161–74. doi: 10.1007/s00262-013-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kispert A, Koschorz B, Herrmann BG. The T protein encoded by Brachyury is a tissue-specific transcription factor. The EMBO journal. 1995;14:4763–72. doi: 10.1002/j.1460-2075.1995.tb00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton DH, Litzinger MT, Fernando RI, Huang B, Palena C. Cancer vaccines targeting the epithelial-mesenchymal transition: tissue distribution of brachyury and other drivers of the mesenchymal-like phenotype of carcinomas. Seminars in oncology. 2012;39:358–66. doi: 10.1053/j.seminoncol.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuter I, Gee JM, Hegg R, Singer CF, Badwe RA, Lowe ES, et al. Dose-dependent change in biomarkers during neoadjuvant endocrine therapy with fulvestrant: results from NEWEST, a randomized Phase II study. Breast cancer research and treatment. 2012;133:237–46. doi: 10.1007/s10549-011-1947-7. [DOI] [PubMed] [Google Scholar]
- 22.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 23.Akalay I, Janji B, Hasmim M, Noman MZ, Thiery JP, Mami-Chouaib F, et al. EMT impairs breast carcinoma cell susceptibility to CTL-mediated lysis through autophagy induction. Autophagy. 2013;9:1104–6. doi: 10.4161/auto.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chua KN, Sim WJ, Racine V, Lee SY, Goh BC, Thiery JP. A cell-based small molecule screening method for identifying inhibitors of epithelial-mesenchymal transition in carcinoma. PloS one. 2012;7:e33183. doi: 10.1371/journal.pone.0033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–59. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson JF, Erikstein B, Osborne KC, Pippen J, Come SE, Parker LM, et al. Pharmacokinetic profile of intramuscular fulvestrant in advanced breast cancer. Clinical pharmacokinetics. 2004;43:529–38. doi: 10.2165/00003088-200443080-00003. [DOI] [PubMed] [Google Scholar]
- 27.Olivo-Marston SE, Mechanic LE, Mollerup S, Bowman ED, Remaley AT, Forman MR, et al. Serum estrogen and tumor-positive estrogen receptor-alpha are strong prognostic classifiers of non-small-cell lung cancer survival in both men and women. Carcinogenesis. 2010;31:1778–86. doi: 10.1093/carcin/bgq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niikawa H, Suzuki T, Miki Y, Suzuki S, Nagasaki S, Akahira J, et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:4417–26. doi: 10.1158/1078-0432.CCR-07-1950. [DOI] [PubMed] [Google Scholar]
- 29.Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–50. [PubMed] [Google Scholar]
- 30.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991;9:1618–26. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 31.Moore KA, Mery CM, Jaklitsch MT, Estocin AP, Bueno R, Swanson SJ, et al. Menopausal effects on presentation, treatment, and survival of women with non-small cell lung cancer. The Annals of thoracic surgery. 2003;76:1789–95. doi: 10.1016/s0003-4975(03)01024-5. [DOI] [PubMed] [Google Scholar]
- 32.Ganti AK, Sahmoun AE, Panwalkar AW, Tendulkar KK, Potti A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol. 2006;24:59–63. doi: 10.1200/JCO.2005.02.9827. [DOI] [PubMed] [Google Scholar]
- 33.Slatore CG, Chien JW, Au DH, Satia JA, White E. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J Clin Oncol. 2010;28:1540–6. doi: 10.1200/JCO.2009.25.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lother SA, Harding GA, Musto G, Navaratnam S, Pitz MW. Antiestrogen use and survival of women with non-small cell lung cancer in Manitoba, Canada. Hormones & cancer. 2013;4:270–6. doi: 10.1007/s12672-013-0149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brueckl WM, Al-Batran SE, Ficker JH, Claas S, Atmaca A, Hartmann A, et al. Prognostic and predictive value of estrogen receptor 1 expression in completely resected non-small cell lung cancer. International journal of cancer Journal international du cancer. 2013;133:1825–31. doi: 10.1002/ijc.28209. [DOI] [PubMed] [Google Scholar]
- 36.Márquez-Garbán DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids. 2007;72:135–43. doi: 10.1016/j.steroids.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pietras RJ, Márquez DC, Chen HW, Tsai E, Weinberg O, Fishbein M. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005;70:372–81. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Garon EB, Sadeghi S, Kabbinavar FF, Reckamp KL, Marquez-Garban DC, Stabile LP, et al. Interim safety analysis of a phase II study of erlotinib (E) alone or combined with fulvestrant (F) in previously treated patients with advanced non-small cell lung cancer (NSCLC) Journal of Clinical Oncology. 2008;26:19091. [Google Scholar]
- 39.Traynor AM, Schiller JH, Stabile LP, Kolesar JM, Eickhoff JC, Dacic S, et al. Pilot study of gefitinib and fulvestrant in the treatment of post-menopausal women with advanced non-small cell lung cancer. Lung Cancer. 2009;64:51–9. doi: 10.1016/j.lungcan.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.