Abstract
Objectives
This review outlines a conceptual framework adapted from the biopsychosocial model of pain to examine the relationship between adverse childhood events (ACEs) and chronic pain in youth in order to highlight the state of current research and guide future efforts.
Methods
A review of the literature was performed in the areas of ACEs and health outcomes with general adult and pediatric populations in addition to studies within the pain literature. Potential relationships between ACEs, chronic pain, and its impact in youth are outlined and discussed.
Results
The literature suggests an association between adverse outcomes of ACEs and chronic pain in children and adolescents although causal links have not been confirmed. However, ACEs are associated with multiple risk factors identified in the biopsychosocial model of pain, and may serve to exacerbate or confer heightened risk for pain and poor outcomes.
Discussion
Adverse experiences in childhood may be associated with greater risk for the development/maintenance of chronic pain in youth. More research is needed on ACEs and how they uniquely affect the biopsychosocial mechanisms underlying chronic pain in children throughout the lifespan.
Keywords: adverse childhood experiences, pediatric pain, youth
Adverse experiences in childhood have been associated with increased risk for a variety of negative health outcomes (e.g., cancer, heart disease, chronic illness)1–4. Prior studies have defined adverse childhood experiences (ACEs) as physical, mental, or sexual abuse, emotional or physical neglect, a violent home environment, household substance abuse, exposure to parent mental illness, parental separation or divorce, and parental incarceration4. In the pain literature, the relationship between ACEs and increased risk for pain and maladaptive pain outcomes have mostly been investigated in the context of retrospective accounts in adulthood, usually restricted to abuse or trauma4–6; and a few studies in youth have suggested that ACEs are reported more often by children and adolescents with pain syndromes (e.g., migraine headaches, fibromyalgia) than their healthy peers7,8. However, little is known about how ACEs may impact concurrent or long-term outcomes in youth with chronic pain and much work is needed in this area. Drawing from the existing literature, this review outlines a conceptual framework, adapted from the biopsychosocial model of pain9, to highlight the state of current research and guide future efforts to examine the relationship between ACEs and chronic pain in youth populations. A review of the literature was performed in the areas of ACEs and health outcomes with general adult and pediatric populations, in addition to studies within the pain literature. Search terms included such phrases as: “Adverse childhood experiences”, “chronic pain”, “health outcomes”, “pediatric”, and “stress and pain”. Relevant articles were categorized using the biopsychosocial model as a guide.
ACEs in Chronic Pain
ACEs in Adults with Chronic Pain
In a landmark study of 13,494 adults from a population based sample, Felitti and colleagues4 examined the relationship between exposure to ACEs and health outcomes. Results from this study indicated that the number of exposures to ACEs in childhood was significantly associated with the later development of serious health concerns in adulthood, including cancer, lung disease, heart disease, as well as chronic pain conditions such as headache and painful musculoskeletal conditions. Another compelling finding was that the association between ACEs and chronic conditions was frequency dependent, indicating that a higher number of ACEs leads to greater severity of chronic health concerns (e.g., both pain related and other) as an adult4. Several additional studies have subsequently been carried out examining the relationship between ACEs and chronic pain in adults. For example, evidence from multiple population-based studies suggests that a history of ACEs in childhood is significantly associated with the existence of various pain conditions as an adult (e.g., migraine headaches, back pain)1,3,5,10. Although these preliminary findings are compelling, the majority of research in adult pain populations to date has been cross-sectional and/or retrospective. Thus, these findings may be significantly influenced by recall bias (i.e., false negatives) in reporting11,12. Further, few prospective, longitudinal, population-based studies on the relationship between ACEs and pain exist to date. Of those (e.g., The British Birth Cohort Study13), methodological concerns were found regarding the operationalization of ACEs, as the examination of sexual and/or physical abuse (which are critical and frequently occurring ACEs14) was not included. Thus, generalizability of these results to pediatric chronic pain samples and predictive inferences are limited.
ACEs in Youth with Chronic Pain
The relationship between ACEs and chronic pain conditions in child and adolescent populations is less researched and primarily based on studies relying on retrospective report. The lack of empirical research in this area is particularly concerning, as chronic pain conditions are highly prevalent (approximately 20–25% of youth) and can be disabling15,16. Although an abundance of research has examined the role of various psychosocial factors in youth with chronic pain that may be proxies for ACEs, including psychological comorbidities17–19, parenting factors20,21, and social functioning22, targeted research on the relationship between chronic pain and ACEs in children remains limited, since exposure to ACEs is rarely assessed in a systematic way in the context of clinical care or research. However, preliminary evidence is compelling with recent research indicating that youth with a history of ACEs such as abuse are 21% more likely to hold the diagnosis of a chronic medical condition including a chronic pain condition (e.g., migraines, rheumatic disease) than their same-aged peers with no ACE history2.
Evidence also suggests that children and adolescents (up to age 20) with certain chronic pain conditions (e.g., fibromyalgia, abdominal pain, recurrent headache) report a higher number of ACEs experienced (≥1) across adolescence than healthy comparison cohorts7,8,23. There may also be differences within subpopulations such as adolescents with widespread pain more likely to report more frequent experience of abuse/trauma than those with chronic migraine24. However, given the cross-sectional nature of all of these studies, it is unclear if ACEs increases risk for the development or maintenance of chronic pain and/or pain related impairment. Pain outcomes may also vary depending on frequency of experiences, with multiple ACEs (e.g., complex trauma) during childhood associated with greatest impairment8,25,26 as compared to a single ACE. However, similar to adult research, most of these studies are limited inferentially, as the existing literature on youth populations includes predominantly cross-sectional data on only a subset of pain disorders (e.g., migraine, functional abdominal pain, fibromyalgia). Prospective longitudinal research, including a comprehensive assessment of ACEs, in these conditions, in addition to other pain populations that have yet to be systematically examined in youth (e.g., back pain, sickle cell disease, complex regional pain, etc.) are needed to further explicate the current understanding of this relationship.
Biopsychosocial Model of Pain: Relationship with ACEs
It is well known that biological mechanisms relating to pain do not by themselves adequately explain or predict magnitude of risk for pain-severity and/or disability both immediately and over time27. Accordingly, the biopsychosocial model offers an integrative view of pain by incorporating the biological (e.g., genetic, neurobiological, HPA axis, neuroendocrine), psychological (e.g., subjective experience of pain, affective components, coping abilities), and social (e.g., peer and family environment, social learning) factors impacting the pain experience9,28. Interestingly, in a parallel line of research, many of the above noted constructs are also implicated as relating to ACEs in youth2. In the context of evidence suggesting a relationship between ACEs and increased risk for chronic pain, more attention needs to be focused on the potential complex interplay within ACEs and biopsychosocial factors to better understand the specific nature and magnitude of risk that ACEs may have on the development and/or exacerbation of chronic pain in child and adolescent populations.
The Proposed Framework
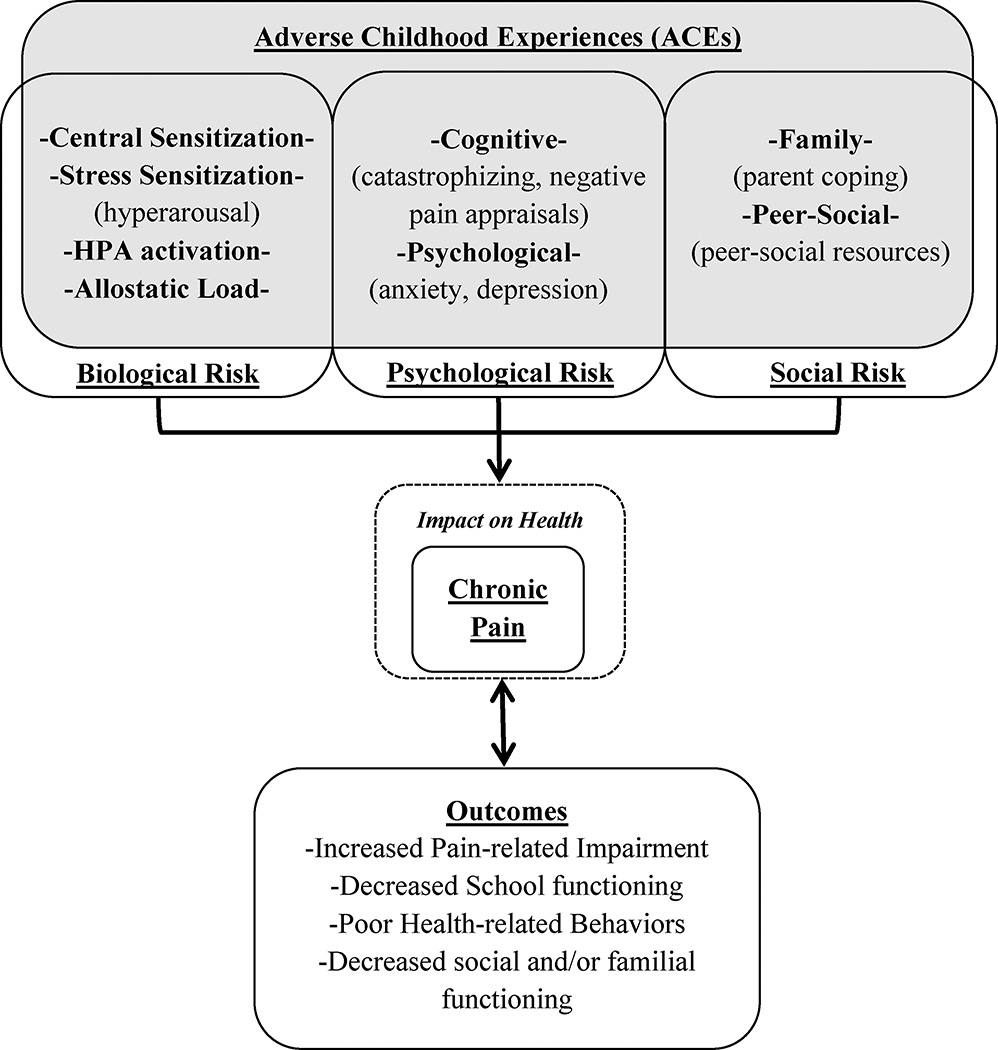
The proposed conceptual framework in this paper (Figure 1) is adapted from the biopsychosocial model of pain28, and highlights the potential exacerbating influence that ACEs may have on the development of chronic pain9,28. Specifically, biological (i.e., allostatic load), psychological (i.e., cognitive processes, psychological comorbidities), and social (i.e., family and peer/social environment) risk factors are reviewed. Despite the fact that some of these risk factors have been studied extensively in adult pain populations, commonalities between ACEs and pediatric chronic pain within each of these areas have not been systematically investigated. Thus, potential relationships and avenues for future research will be delineated. Although it is recognized that ACEs may also be related to broader health outcomes4,5,29 (which is reflected in the model), the current study is specifically focused on chronic pain and its impact.
Figure 1.
Conceptual framework for ACEs and chronic pain in youth
Biological factors: Allostatic Load
A number of biological mechanisms that underlie chronic pain have been identified30,31 including central sensitization (i.e., a state of increased neuronal activity within the central nervous system leading to the amplification of nociceptive signaling)32. Additionally, the stress model of chronic pain asserts that persistent pain may develop from sustained endocrine response (e.g., increased cortisol in the brain) and reduced hippocampal volume following repeated stressors33,34. Relatedly, several biological processes have been implicated in the exposure to stressful events, regardless of pain function. Specifically, when individuals experience multiple and/or repeated stressful experiences, their body is frequently exposed to several stress hormones (e.g., cortisol), thus predisposing them towards maintaining a constant state of arousal, which is often referred to as “sensitization” in the stress literature35,36. With repeated exposure to ACEs, significant wear and tear on the body’s sympathetic and parasympathetic response systems (e.g., “allostatic load”) may occur34,37–39.
Allostatic load and over-activation of the HPA axis (a major element of allostatic load) has been directly implicated in increasing risk for the development and/or maintenance of chronic pain (in child and adult populations) irrespective of history of ACEs28,34,40,41. Although much of the research examining allostatic load as a function of chronic stress in relation to chronic health conditions (e.g., diabetes, migraines, fibromyalgia) has been performed with adult populations, more recent efforts to examine the concept of repeated exposure to stressful events (i.e., complex trauma) in relation to allostatic load have indicated that these processes may also be significantly involved in youth29,34,42. Similarly, the experience of social stressors such as peer rejection (i.e., termed “social pain” by the authors) may activate neural pathways implicated in the processing of physical pain43,44. Taken together, predominant theories on the neurobiology of ACEs (e.g., chronic stress) appear to overlap with processes involved with altered pain processing in chronic pain. For example, dysregulation caused by allostatic load can manifest in several different areas related to chronic pain (e.g., endocrine, immune, etc.) Thus, individuals with both chronic pain and chronic stress conditions may experience greater impairment in their neurological and neuroendocrine function. More research needs to examine the relationship between neuroendocrine processes and chronic pain in youth with a history of ACEs.
Psychological Factors
Psychological comorbidities
Cross-sectional and longitudinal research indicates that youth with a history of ACEs are more likely to experience higher rates of mental health problems, including anxiety and depressive symptoms2,14,45. Similarly, an abundance of research has demonstrated the significant link between emotional difficulties (e.g., anxiety, depression) and increased pain and disability in pediatric pain patients46–48. As such, a large number of youth with chronic pain and a history of stressful experiences may be especially vulnerable to experiencing disturbances in mood and/or behavioral functioning, potentially leading to decreased coping efficacy and increased disability. Several longitudinal studies have been carried out examining the developmental trajectories of mental health concerns and chronic pain conditions in youth19,49. While evidence in youth specifically suggests a strong link between psychological symptoms and chronic pain conditions, varying trajectories even within the same pain condition have been found (e.g., some evidence indicates that anxiety precedes the development of functional abdominal pain50, while other studies evidence anxiety as an outcome of this diagnosis51). It is indeed possible that this may be bidirectional relationship. Greater understanding of this association is needed, particularly in youth with a history of ACEs, to better understand whether exposure to early ACEs, through psychological comorbidities or otherwise, puts them at greater risk for chronic pain or pain-related disability.
Cognitive factors
According to the Lazarus and Folkman model of stress and coping, when individuals are faced with a stressor, they first perform a primary appraisal of the severity of the stressor (i.e., threat), immediately followed by a secondary appraisal - their perceived ability to cope with the stressor52. This model has been frequently applied to pain populations, in that youth with chronic pain likely appraise pain as a stressful experience (often called pain catastrophizing, which is defined as the interpretation of pain as inherently negative53) and may subsequently appraise their ability to handle this stress (i.e., pain) more negatively than children without pain54,55. In pain populations many of these negative appraisals, most notably pain catastrophizing are associated with increased long-term disability56–58. Irrespective of pain, youth with a history of ACEs frequently appraise innocuous or ambiguous experiences as more dangerous or threatening than they actually are, which can lead to negative effects on their perceived ability to cope59. Within the context of chronic pain, this tendency to engage in negative appraisals may lead youth with a history of ACEs to engage in cognitive distortions surrounding threat appraisal of perceived stressors including pain, thus potentially influencing their coping efficacy and subsequent impairment. This area has potentially very important implications for behavioral intervention and yet the influence of ACEs on pain appraisals and coping has not been systematically studied.
Social Factors
Family environment
In conjunction with one’s internal coping resources, individuals utilize external coping networks when faced with a stressful experience. In children, this network is often immediately found in the home (e.g., parents/primary caregivers). It is well documented that chronic pain tends to cluster in families60,61 with parents of children with chronic pain also suffering from chronic pain62. As a consequence, these families may be at higher risk for ACEs resulting from parental disability, job loss, marital conflict, etc. In addition to these potential stressors, families with children suffering from chronic pain frequently exhibit more conflictual and/or enmeshed relational (e.g., parental overprotection or monitoring of child pain symptoms) and communication styles with parents63. Research also indicates that how a parent copes with their child’s pain is significantly related with the child’s own ability to cope with their pain64, in addition to pediatric pain outcomes65. In turn, many children and adolescents with chronic pain engage in ineffective coping strategies to manage their pain64,66. Similarly, children and adolescents with a history of ACEs, which may include pain and non-pain related ACEs (e.g., parental health concerns, parent incarceration, abuse/neglect, etc.), often live in dysfunctional and/or conflictual home environments59. Living in stressful social environments significantly increases risk for disrupted social functioning (e.g., interpersonal attachment, family relational styles)67. Accordingly, these children may not possess or be able to appropriately utilize the positive support systems (e.g., parental modeling of coping) that can help ameliorate the stress of everyday demands (e.g., school, social)68,69. More research is needed on the influence that family environment may have on functioning in youth with chronic pain and a history of ACEs.
Peer/social environment
Studies have identified social supports as a significant mediator in the relationship between stressful events in childhood and later psychosocial functioning, including the development of posttraumatic stress symptoms70–72. Evidence also suggests that children and adolescents with a history of ACEs hold greater risk for decreased prosocial behavior (e.g., sharing, cooperating, taking-turns), which can immediately affect their peer relationships and continue to influence their relational styles as they age into adulthood73. In children with chronic pain, there is evidence that social support systems are disrupted, especially with respect to peer relationships. Specifically, evidence indicates that children and adolescents with chronic pain are more socially isolated and may experience bullying and/or loss of friendships74–76. Relevant to both pain and ACEs, adolescence is a critical developmental period in which youth increasingly develop more complex social and/or peer relational skills (e.g., awareness and understanding one’s own thoughts, ability to adapt to varying peer groups)77. With these processes disrupted, youth with a history of ACEs and/or chronic pain may be at increased risk for impaired social functioning long-term. A greater understanding on how ACEs and chronic pain together can influence a child’s ability to successfully establish and maintain healthy social relationships (and subsequently use these resources to cope with stress) is crucial to understanding long-term adjustment and coping.
Potential Bidirectional Relationships within Conceptual Framework
A variety of factors related to both chronic pain and a history of ACEs (e.g., negative cognitions, ineffective coping strategies and external resources) put children and adolescents at risk for a host of ill effects. Although the model highlights the potential risk factors for various chronic pain outcomes (i.e., biopsychosocial) as separate, constructs relating to the experience of pain and subsequent outcomes may be interrelated; for example, youth with anxiety (identified as a significant factor above) may experience heighted HPA activation and/or allostatic load. There is also the potential for a bi-directional relationship between maladaptive outcomes and chronic pain (see figure). This notion is supported by research indicating that the negative impact of pain on physical and psychosocial outcomes may in turn further exacerbate pain and impairment over time76,78. This has specifically been highlighted in several sections above in relation to psychological46,47 and social functioning57,65.
Aside from these outcomes, other areas impacted by chronic pain include school attendance15,48,79, health-related behaviors including diet, sleep and exercise habits, and adherence to treatment78,80–83. School refusal/avoidance is a frequent consequence of pain conditions, and when this is prolonged, return to school can become a major source of stress in and of itself, creating a barrier to full recovery in function84. Poor health-related behaviors may also play a significant role in the maintenance of pain conditions, as many of these behaviors have been known to directly relate to increased impairment and disability (e.g., poor diet, inadequate exercise and/or sleep, etc.)78,81,83. Regardless of pain-status, children with a history of ACEs also exhibit many of the same negative outcomes59,85–87. However, little is known about how these constructs (i.e., chronic pain, ACEs) jointly influence outcomes of children and adolescents in various domains.
Finally, poor outcomes of chronic pain may directly influence the biopsychosocial risk factors for chronic pain identified in the model (thus, indicating a bidirectional relationship). For example, poor health-related behaviors and increased pain-related disability may exacerbate psychopathology (e.g., anxiety, depression)88, thus, potentially compounding risk for chronic pain, etc.
Future Directions
Research
There are several additional areas of research that require investigation in the effort to understand the nature of the relationship (e.g., temporal) between ACEs and chronic pain conditions in youth. Prospective, longitudinal examinations of the relationships outlined in this framework using the appropriate methodology would serve to significantly advance the understanding of pain outcomes in youth with a history of ACEs. These studies may also serve to increase our understanding of the actual incidence and nature of ACEs in childhood as recall bias would likely be limited. Moreover, evidence remains lacking on specific neurobiological processes (e.g., allostatic load, cortisol secretion, sympathetic/ parasympathetic nervous system activation) related to the presence and severity of chronic pain in those with a history of ACEs. For example, examining these processes in an experimental or laboratory paradigm (e.g., stress-induction task with measurement of cortisol) may serve to significantly inform our understanding of the neurobiological mechanisms behind pain and ACEs. More research on this topic would also allow healthcare providers to better predict and treat those who may be at greater risk for psychological and/or pain-related impairment. Additionally, tailored psychological treatment approaches for youth with both chronic pain and a history of ACEs do not yet exist even though there are evidence-based interventions such as pain-focused cognitive-behavioral therapy89 and trauma-focused cognitive-behavioral therapy59 which deal with each of these separately. More research, specifically with systematic randomized clinical trials, on evidence based treatments to address the potential outcomes of both chronic pain and ACEs is needed.
More generally, it would also be of interest to assess for adaptive coping or resilience factors in pediatric pain patients with a history of stressful experiences. With a better idea of how to capitalize on adaptive coping following ACEs in pain populations, mental health and medical providers can enhance their provision of services to improve long-term outcomes for this subset of youth.
Practice
Many children and families struggle with the idea of pursuing psychological services90 and the medical professional’s role in the appropriate identification and referral to psychotherapy services is becoming increasingly important. Inclusion of assessment tools [such as the Traumatic Event Screening Inventory for Children (TESI-C), a simple, free, and publicly available measure (http://www.ptsd.va.gov/professional/assessment/child/tesi.asp)] to assess for the presence of stressful or traumatic events may be considered. Additionally, early education for medical providers (e.g., during medical school) on when and how to assess trauma/stress exposure in youth presenting with pain would be beneficial. This could include a discussion on appropriate techniques for how medical providers may handle any embarrassment and/or apprehension (from parent and/or child) during the assessment process and what to do if trauma/ACEs are identified. Additional resources on identification/referral, including empirically-trained psychological providers across the country, can be found at the National Child Traumatic Stress Network (NCTSN) website (www.nctsn.org).
Summary
A significant number of youth with chronic pain have a history of adverse experiences (ACEs), yet little is known about the effects that these experiences may have on pain conditions in children and adolescents. To date, much of the research examining the relationship between ACEs and chronic pain has been carried out on adults. Drawing from the current literature, authors propose a conceptual framework, adapted from the biopsychosocial model of pain, designed to outline the potential interactional processes of ACEs and chronic pain in pediatric populations. Main elements of the model include the biological, psychological, and social risk factors for pain and the subsequent development of various outcomes, discussed in the context of youth with a history of ACEs. Recommendations for medical treatment providers include greater education on the appropriate assessment of ACEs in pediatric pain populations. A number of areas of future research have been identified that are necessary for the field to gain a more comprehensive understanding of the impact of ACEs on biopsychosocial risk for chronic pain and short- and long-term pain related outcomes.
Acknowledgments
Authors would like to gratefully acknowledge Christopher King, Ph.D., for providing feedback and comments this paper.
Sources of Funding: Preparation of this paper was supported in part by NIH grant # K24 AR056687, a midcareer mentorship award to the last author (Kashikar-Zuck).
Footnotes
Conflicts of Interests: There are no conflicts of interest.
References
- 1.Anda R, Tietjen G, Schulman E, Felitti V, Croft J. Adverse childhood experiences and frequent headaches in adults. Headache: The Journal of Head and Face Pain. 2010;50(9):1473–1481. doi: 10.1111/j.1526-4610.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 2.Kerker BD, Zhang J, Nadeem E, et al. Adverse Childhood Experiences and Mental Health, Chronic Medical Conditions, and Development in Young Children. Academic pediatrics. 2015;15(5):510–517. doi: 10.1016/j.acap.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tietjen GE, Khubchandani J, Herial NA, Shah K. Adverse childhood experiences are associated with migraine and vascular biomarkers. Headache: The Journal of Head and Face Pain. 2012;52(6):920–929. doi: 10.1111/j.1526-4610.2012.02165.x. [DOI] [PubMed] [Google Scholar]
- 4.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 5.Brennenstuhl S, Fuller-Thomson E. The painful legacy of childhood violence: migraine headaches among adult survivors of adverse childhood experiences. Headache: The Journal of Head and Face Pain. 2015;55(7):973–983. doi: 10.1111/head.12614. [DOI] [PubMed] [Google Scholar]
- 6.Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature. Clinical Journal of Pain. 2005;21(5):398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- 7.Seng JS, Graham-Bermann SA, Clark MK, McCarthy AM, Ronis DL. Posttraumatic stress disorder and physical comorbidity among female children and adolescents: results from service-use data. Pediatrics. 2005;116(6):e767–e776. doi: 10.1542/peds.2005-0608. [DOI] [PubMed] [Google Scholar]
- 8.Stensland SO, Dyb G, Thoresen S, Wentzel-Larsen T, Zwart JA. Potentially traumatic interpersonal events, psychological distress and recurrent headache in a population-based cohort of adolescents: the HUNT study. BMJ Open. 2013;3(7) doi: 10.1136/bmjopen-2013-002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatchel RJ. Comorbidity of chronic pain and mental health disorders: the biopsychosocial perspective. American Psychologist. 2004;59(8):795. doi: 10.1037/0003-066X.59.8.795. [DOI] [PubMed] [Google Scholar]
- 10.Lampe A, Doering S, Rumpold G, et al. Chronic pain syndromes and their relation to childhood abuse and stressful life events. Journal of Psychosomatic Research. 2003;54(4):361–367. doi: 10.1016/s0022-3999(02)00399-9. [DOI] [PubMed] [Google Scholar]
- 11.Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. Journal of Child Psychology and Psychiatry. 2004;45(2):260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 12.Fergusson DM, Horwood LJ, Woodward LJ. The stability of child abuse reports: a longitudinal study of the reporting behaviour of young adults. Psychological medicine. 2000;30(03):529–544. doi: 10.1017/s0033291799002111. [DOI] [PubMed] [Google Scholar]
- 13.Jones GT, Power C, Macfarlane GJ. Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain. 2009;143(1):92–96. doi: 10.1016/j.pain.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Schilling EA, Aseltine RH, Gore S. Adverse childhood experiences and mental health in young adults: a longitudinal survey. BMC Public Health. 2007;7(1):30. doi: 10.1186/1471-2458-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152(12):2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Coffelt TA, Bauer BD, Carroll AE. Inpatient characteristics of the child admitted with chronic pain. Pediatrics. 2013;132(2):e422–e429. doi: 10.1542/peds.2012-1739. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham NR, Cohen MB, Farrell MK, Mezoff AG, Lynch-Jordan A, Kashikar-Zuck S. Concordant parent-child reports of anxiety predict impairment in youth with functional abdominal pain. J Pediatr Gastroenterol Nutr. 2015;60(3):312–317. doi: 10.1097/MPG.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham NR, Tran ST, Lynch-Jordan AM, et al. Psychiatric Disorders in Young Adults Diagnosed with Juvenile Fibromyalgia in Adolescence. J Rheumatol. 2015 doi: 10.3899/jrheum.141369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsson B, Sund AM. Emotional/behavioural, social correlates and one-year predictors of frequent pains among early adolescents: influences of pain characteristics. Eur J Pain. 2007;11(1):57–65. doi: 10.1016/j.ejpain.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Van Der Veek SM, Derkx HH, De Haan E, Benninga MA, Plak RD, Boer F. Do parents maintain or exacerbate pediatric functional abdominal pain? A systematic review and meta-analysis. J Health Psychol. 2012;17(2):258–272. doi: 10.1177/1359105311410513. [DOI] [PubMed] [Google Scholar]
- 21.Sil S, Lynch-Jordan A, Ting TV, Peugh J, Noll J, Kashikar-Zuck S. Influence of family environment on long-term psychosocial functioning of adolescents with juvenile fibromyalgia. Arthritis Care Res (Hoboken) 2013;65(6):903–909. doi: 10.1002/acr.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forgeron PA, King S, Stinson JN, McGrath PJ, MacDonald AJ, Chambers CT. Social functioning and peer relationships in children and adolescents with chronic pain: A systematic review. Pain Research & Management: The Journal of the Canadian Pain Society. 2010;15(1):27. doi: 10.1155/2010/820407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greene JW, Walker LS, Hickson G, Thompson J. Stressful life events and somatic complaints in adolescents. Pediatrics. 1985;75(1):19–22. [PubMed] [Google Scholar]
- 24.Sil S, Kashikar-Zuck S. Understanding why cognitive-behavioral therapy is an effective treatment for adolescents with juvenile fibromyalgia. Int J Clin Rheumtol. 2013;8(2) doi: 10.2217/IJR.13.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook A, Spinazzola J, Ford J, Lanktree C, Blaustein M, Cloitre M. Complex trauma. Psychiatric annals. 2005;35(5):390–398. [Google Scholar]
- 26.Ford JD, Courtois CA. Defining and understanding complex trauma and complex traumatic stress disorders. Treating complex traumatic stress disorders: An evidence-based guide. 2009:13–30. [Google Scholar]
- 27.Turk DC, Monarch ES. Biopsychosocial perspective on chronic pain. Psychological approaches to pain management: A practitioner’s handbook. 1996:3–32. [Google Scholar]
- 28.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological Bulletin. 2007;133(4):581. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 29.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & behavior. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Tramullas M, Dinan TG, Cryan JF. Chronic psychosocial stress induces visceral hyperalgesia in mice. Stress. 2012;15(3):281–292. doi: 10.3109/10253890.2011.622816. [DOI] [PubMed] [Google Scholar]
- 31.Berry A, Bellisario V, Capoccia S, Francia N, Alleva E, Cirulli F. Long-Term Changes in Pain Sensitivity in an Animal Model of Social Anxiety. Veterinary Sciences. 2014;1(2):77–95. [Google Scholar]
- 32.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vachon-Presseau E, Roy M, Martel M-O, et al. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136(3):815–827. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- 34.McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism. 2010;59:S9–S15. doi: 10.1016/j.metabol.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Kendall-Tackett KA. Physiological correlates of childhood abuse: chronic hyperarousal in PTSD, depression, and irritable bowel syndrome. Child Abuse Negl. 2000;24(6):799–810. doi: 10.1016/s0145-2134(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 36.Perry BD, Pollard RA, Blakley TL, Baker WL, Vigilante D. Childhood trauma, the neurobiology of adaptation, and “use-dependent” development of the brain: How “states” become “traits”. Infant Mental Health Journal. 1995;16(4):271–291. [Google Scholar]
- 37.Rogosch FA, Dackis MN, Cicchetti D. Child maltreatment and allostatic load: consequences for physical and mental health in children from low-income families. Dev Psychopathol. 2011;23(4):1107–1124. doi: 10.1017/S0954579411000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lupien SJ, Ouellet-Morin I, Hupbach A, et al. Beyond the stress concept: Allostatic load--a developmental biological and cognitive perspective. Developmental psychopathology. (2nd) 2006;2:578–628. Developmental neuroscience. [Google Scholar]
- 39.Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 40.McEwen BS. Brain on stress: how the social environment gets under the skin. Proceedings of the National Academy of Sciences. 2012;109(Supplement 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grunau RE, Tu MT. Long-term consequences of pain in human neonates. Pain in neonates and infants. 2007;3:45–55. [Google Scholar]
- 42.Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental psychology. 2007;43(2):341. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- 43.Eisenberger NI. Broken Hearts and Broken Bones A Neural Perspective on the Similarities Between Social and Physical Pain. Current Directions in Psychological Science. 2012;21(1):42–47. [Google Scholar]
- 44.Eisenberger NI. The neural bases of social pain: evidence for shared representations with physical pain. Psychosomatic Medicine. 2012;74(2):126. doi: 10.1097/PSY.0b013e3182464dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pulvino S, Mbise-Floyd L, Patel N, Patel T, Davis A, Homan S. Adverse Child Experiences and Their Effects on Child Behavior and Mental Health. 2015 [Google Scholar]
- 46.Gauntlett-Gilbert J, Eccleston C. Disability in adolescents with chronic pain: Patterns and predictors across different domains of functioning. Pain. 2007;131(1–2):132–141. doi: 10.1016/j.pain.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 47.Kashikar-Zuck S, Parkins IS, Graham TB, et al. Anxiety, mood, and behavioral disorders among pediatric patients with juvenile fibromyalgia syndrome. Clin J Pain. 2008;24(7):620–626. doi: 10.1097/AJP.0b013e31816d7d23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kashikar-Zuck S, Lynch AM, Slater S, Graham TB, Swain NF, Noll RB. Family factors, emotional functioning, and functional impairment in juvenile fibromyalgia syndrome. Arthritis Rheum. 2008;59(10):1392–1398. doi: 10.1002/art.24099. [DOI] [PubMed] [Google Scholar]
- 49.Stanford EA, Chambers CT, Biesanz JC, Chen E. The frequency, trajectories and predictors of adolescent recurrent pain: a population-based approach. Pain. 2008;138(1):11–21. doi: 10.1016/j.pain.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 50.Campo JV, Bridge J, Ehmann M, et al. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics. 2004;113(4):817–824. doi: 10.1542/peds.113.4.817. [DOI] [PubMed] [Google Scholar]
- 51.Campo JV, Di Lorenzo C, Chiappetta L, et al. Adult outcomes of pediatric recurrent abdominal pain: Do they just grow out of it? Pediatrics. 2001;108(1):e1–e1. doi: 10.1542/peds.108.1.e1. [DOI] [PubMed] [Google Scholar]
- 52.Lazarus RS, Folkman S. Stress, appraisal, and coping. Springer publishing company; 1984. [Google Scholar]
- 53.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. 2009 doi: 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker LS, Smith CA, Garber J, Claar RL. Appraisal and coping with daily stressors by pediatric patients with chronic abdominal pain. Journal of Pediatric Psychology. 2007;32(2):206–216. doi: 10.1093/jpepsy/jsj124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker LS, Smith CA, Garber J, Claar RL. Testing a model of pain appraisal and coping in children with chronic abdominal pain. Health Psychology. 2005;24(4):364. doi: 10.1037/0278-6133.24.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lynch AM, Kashikar-Zuck S, Goldschneider KR, Jones BA. Psychosocial risks for disability in children with chronic back pain. Journal of Pain. 2006;7(4):244–251. doi: 10.1016/j.jpain.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Wilson AC, Lewandowski AS, Palermo TM. Fear-avoidance beliefs and parental responses to pain in adolescents with chronic pain. Pain Research & Management: The Journal of the Canadian Pain Society. 2011;16(3):178. doi: 10.1155/2011/296298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. PAIN®. 2012;153(9):1798–1806. doi: 10.1016/j.pain.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen JA, Mannarino AP, Deblinger E. Treating trauma and traumatic grief in children and adolescents. Guilford Press; 2006. [Google Scholar]
- 60.Arnold LM, Hudson JI, Hess EV, et al. Family study of fibromyalgia. Arthritis & Rheumatism. 2004;50(3):944–952. doi: 10.1002/art.20042. [DOI] [PubMed] [Google Scholar]
- 61.Van Hecke O, Torrance N, Smith B. Chronic pain epidemiology and its clinical relevance. British journal of anaesthesia. 2013;111(1):13–18. doi: 10.1093/bja/aet123. [DOI] [PubMed] [Google Scholar]
- 62.Hoftun GB, Romundstad PR, Rygg M. Association of parental chronic pain with chronic pain in the adolescent and young adult: family linkage data from the HUNT Study. JAMA pediatrics. 2013;167(1):61–69. doi: 10.1001/jamapediatrics.2013.422. [DOI] [PubMed] [Google Scholar]
- 63.Palermo TM, Chambers CT. Parent and family factors in pediatric chronic pain and disability: an integrative approach. Pain. 2005;119(1–3):1–4. doi: 10.1016/j.pain.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 64.Compas BE, Boyer MC, Stanger C, et al. Latent variable analysis of coping, anxiety/depression, and somatic symptoms in adolescents with chronic pain. Journal of consulting and clinical psychology. 2006;74(6):1132. doi: 10.1037/0022-006X.74.6.1132. [DOI] [PubMed] [Google Scholar]
- 65.Simons LE, Claar RL, Logan DL. Chronic pain in adolescence: Parental responses, adolescent coping, and their impact on adolescent's pain behaviors. Journal of Pediatric Psychology. 2008;33(8):894–904. doi: 10.1093/jpepsy/jsn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huguet A, Miró J. The severity of chronic pediatric pain: an epidemiological study. The Journal of Pain. 2008;9(3):226–236. doi: 10.1016/j.jpain.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 67.Holt S, Buckley H, Whelan S. The impact of exposure to domestic violence on children and young people: A review of the literature. Child abuse & neglect. 2008;32(8):797–810. doi: 10.1016/j.chiabu.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Vranceanu A-M, Hobfoll SE, Johnson RJ. Child multi-type maltreatment and associated depression and PTSD symptoms: The role of social support and stress. Child abuse & neglect. 2007;31(1):71–84. doi: 10.1016/j.chiabu.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huth-Bocks AC, Hughes HM. Parenting stress, parenting behavior, and children’s adjustment in families experiencing intimate partner violence. Journal of family violence. 2008;23(4):243–251. [Google Scholar]
- 70.Sperry DM, Widom CS. Child abuse and neglect, social support, and psychopathology in adulthood: A prospective investigation. Child abuse & neglect. 2013;37(6):415–425. doi: 10.1016/j.chiabu.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stevens NR, Gerhart J, Goldsmith RE, Heath NM, Chesney SA, Hobfoll SE. Emotion regulation difficulties, low social support, and interpersonal violence mediate the link between childhood abuse and posttraumatic stress symptoms. Behavior therapy. 2013;44(1):152–161. doi: 10.1016/j.beth.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Lamis DA, Wilson CK, King NM, Kaslow NJ. Child Abuse, Social Support, and Social Functioning in African American Children. Journal of Family Violence. 2014;29(8):881–891. [Google Scholar]
- 73.Alink LR, Cicchetti D, Kim J, Rogosch FA. Longitudinal associations among child maltreatment, social functioning, and cortisol regulation. Developmental psychology. 2012;48(1):224. doi: 10.1037/a0024892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carter B, Lambrenos K, Thursfield J. A pain workshop: an approach to eliciting the views of young people with chronic pain. Journal of clinical nursing. 2002;11(6):753–762. doi: 10.1046/j.1365-2702.2002.00642.x. [DOI] [PubMed] [Google Scholar]
- 75.Forgeron P, McGrath PJ. Self-identified needs of youth with chronic pain. Journal of Pain Management. 2009 [Google Scholar]
- 76.Kashikar-Zuck S, Lynch AM, Graham TB, Swain NF, Mullen SM, Noll RB. Social functioning and peer relationships of adolescents with juvenile fibromyalgia syndrome. Arthritis Rheum. 2007;57(3):474–480. doi: 10.1002/art.22615. [DOI] [PubMed] [Google Scholar]
- 77.Crone EA, Dahl RE. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- 78.Long AC, Krishnamurthy V, Palermo TM. Sleep disturbances in school-age children with chronic pain. Journal of Pediatric Psychology. 2008;33(3):258–268. doi: 10.1093/jpepsy/jsm129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cohen LL, Vowles KE, Eccleston C. The impact of adolescent chronic pain on functioning: disentangling the complex role of anxiety. J Pain. 2010;11(11):1039–1046. doi: 10.1016/j.jpain.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 80.Lemanek KL, Kamps J, Chung NB. Empirically supported treatments in pediatric psychology: regimen adherence. Journal of Pediatric Psychology. 2001;26(5):253–275. doi: 10.1093/jpepsy/26.5.253. [DOI] [PubMed] [Google Scholar]
- 81.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. Journal of pediatric psychology. 2008;33(6):590–611. doi: 10.1093/jpepsy/jsm128. [DOI] [PubMed] [Google Scholar]
- 82.Quittner AL, Modi AC, Lemanek KL, Ievers-Landis CE, Rapoff MA. Evidence-based assessment of adherence to medical treatments in pediatric psychology. Journal of pediatric psychology. 2008;33(9):916–936. doi: 10.1093/jpepsy/jsm064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roth-Isigkeit A, Thyen U, Stoven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: restrictions in daily living and triggering factors. Pediatrics. 2005;115(2):e152–e162. doi: 10.1542/peds.2004-0682. [DOI] [PubMed] [Google Scholar]
- 84.Logan DE, Simons LE, Stein MJ, Chastain L. School impairment in adolescents with chronic pain. The Journal of Pain. 2008;9(5):407–416. doi: 10.1016/j.jpain.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 85.Cloitre M, Miranda R, Stovall-McClough KC, Han H. Beyond PTSD: Emotion regulation and interpersonal problems as predictors of functional impairment in survivors of childhood abuse. Behavior Therapy. 2005;36(2):119–124. [Google Scholar]
- 86.McLean CP, Rosenbach SB, Capaldi S, Foa EB. Social and academic functioning in adolescents with child sexual abuse-related PTSD. Child abuse & neglect. 2013;37(9):675–678. doi: 10.1016/j.chiabu.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kovachy B, O'Hara R, Hawkins N, et al. Sleep disturbance in pediatric PTSD: Current findings and future directions. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2013;9(5):501. doi: 10.5664/jcsm.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sil S, Arnold LM, Lynch-Jordan A, et al. Identifying treatment responders and predictors of improvement after cognitive-behavioral therapy for juvenile fibromyalgia. Pain. 2014;155(7):1206–1212. doi: 10.1016/j.pain.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palermo TM, Eccleston C, Lewandowski AS, Williams ACdC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. PAIN®. 2010;148(3):387–397. doi: 10.1016/j.pain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gopalan G, Goldstein L, Klingenstein K, Sicher C, Blake C, McKay MM. Engaging families into child mental health treatment: Updates and special considerations. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2010;19(3):182. [PMC free article] [PubMed] [Google Scholar]