Abstract
We investigated relationships between outdoor air pollution and pterygium in Korean adults. This study includes 23,276 adults in population-based cross-sectional data using the Korea National Health and Nutrition Examination Survey 2008–2011. Pterygium was assessed using slit lamp biomicroscopy. Air pollution data (humidity, particulate matter with aerodynamic diameter less than 10 μm [PM10], ozone [O3], nitrogen dioxide [NO2], and sulfur dioxide levels [SO2]) for 2 years preceding the ocular examinations were acquired. Associations of multiple air pollutants with pterygium or pterygium recurrence after surgery were examined using multivariate logistic models, after adjusting for several covariates. Distributed lag models were additionally used for estimating cumulative effects of air pollution on pterygium. None of air pollution factors was significantly associated with pterygium or pterygium recurrence (each P > 0.05). Distributed lag models also showed that air pollution factors were not associated with pterygium or pterygium recurrence in 0-to-2 year lags (each P > 0.05). However, primary pterygium showed a weak association with PM10 after adjusting for covariates (odds ratio [OR] 1.23; [per 5 μg/m3 PM10 increase]; P = 0.023). Aging, male sex, and greater sun exposure were associated with pterygium, while higher education level and myopia were negatively associated with pterygium (each P ≤ 0.001). Male sex and myopia were negatively associated with pterygium recurrence (each P < 0.05). In conclusion, exposure to higher PM10 levels was associated with primary pterygium, although this study observed no significant association between air pollution and overall pterygium or pterygium recurrence in Korean adults.
Keywords: Air Pollution, Pterygium, Association, Recurrence, PM10
Graphical Abstract
INTRODUCTION
According to the World Health Organization (WHO), air pollution is of major public health concern, and its four common air pollutants include particulate matter (PM), ozone (O3), nitrogen dioxide (NO2), and sulfur dioxide (SO2) (1). Outdoor air pollution may induce both acute and chronic adverse effects on human health, affecting a number of different systems and organs (2). Although ocular surface is exposed to multiple air pollutants all of time, effects of outdoor air pollutants on ocular surface diseases have not been well-investigated. Recently, we revealed that higher tropospheric O3 levels were associated with dry eye disease in the Korean adult population (3), and suggested a potential mechanism that air pollution-induced oxidative stress and subclinical inflammation may be related with dry eye disease.
Pterygium is a common ocular surface disease characterized by fibrovascular subconjunctival connective tissue and hypertrophy of overlying conjunctival epithelium. Although the precise pathogenesis of pterygium is unknown, reactive oxygen species (ROS) and chronic inflammation induced by ultraviolet (UV) light, low humidity, and dust are mainly considered to cause pterygium (4,5). Therefore, oxidative stress and subclinical inflammation from outdoor air pollutants may affect pterygium occurrence. To the best of our knowledge, there have been no large scale studies to consider multiple air pollutants in assessing the relationship between outdoor air pollution and pterygium. We investigated the relationships between multiple outdoor air pollutants and pterygium in Korean adults, while controlling for potential risk factors and using distributed lag models.
MATERIALS AND METHODS
Study population and data collection
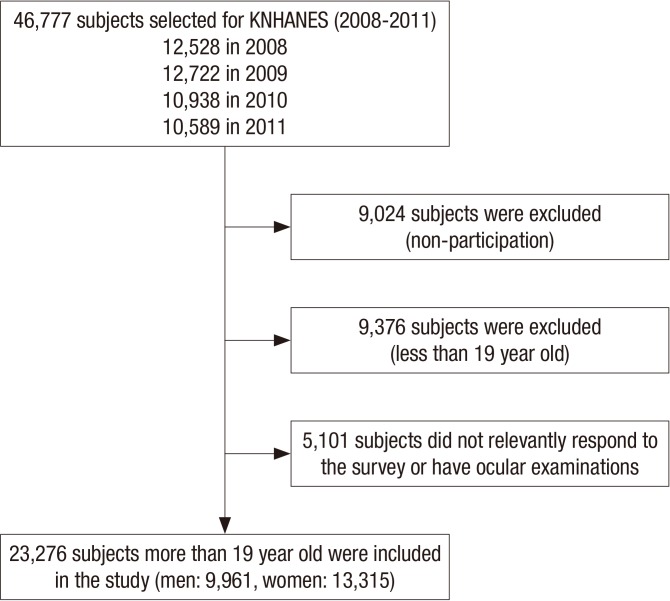
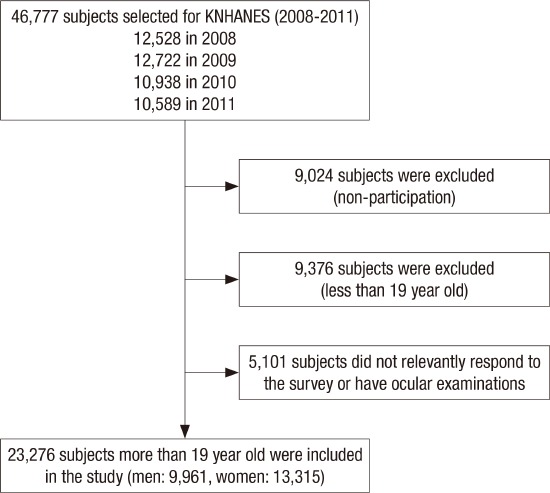
The Korea National Health and Nutrition Examination Survey (KNHANES) is a nationwide, population-based, cross-sectional epidemiological survey conducted by the Korean Ministry of Health and Welfare. Between 2008 and 2009 (from July 1, 2008 to December 31, 2009), 8,000 households from 400 survey districts were enrolled in KNHANES IV using the stratified, multistage clustered sampling method based on 2005 National Resident demographics; between 2010 and 2011 (from January 1, 2010 to December 31, 2011), 11,400 households from 576 survey districts were enrolled in KNHANES V using the aforementioned method based on 2009 National Resident Demographics. Among 46,777 participants from 19,400 households, 37,753 subjects finally participated in this survey with 80.7% response rate. After excluding the subjects who did not take ocular examinations or did not have its related variables’ information, 23,276 adults who were more than 19 years old were included for the statistical analyses (Fig. 1).
Fig. 1.
Flow diagram presenting the selection of study participants.
KNHANES = Korea National Health and Nutrition Examination Survey.
Outdoor air pollutants data
We obtained air pollutants data for 6 years between January 1, 2006 and December 31, 2011 from the Korea Ministry of Environment. The data were available from 283 atmospheric monitoring stations across South Korea. Air pollution factors of ambient PM10 (PM with an aerodynamic diameter less than 10 μm), O3, NO2, and SO2 concentrations and relative humidity were considered in this study. Outdoor relative humidity was considered together as an air pollution factor because PM10 and NO2 were significantly correlated with humidity in the previous study (3). We calculated annual average concentration to each air pollutant prior to the ocular examinations (present, 1 year ago, and 2 years ago) for the total 16 administrative divisions of South Korea, and additionally calculated average concentration of each air pollutant for 2 years. The detailed monitoring methods were specified in the previous study (3).
Pterygium examination
Ocular examinations were performed using a BQ 900 slitlamp (Haag-Streit AG, Koeniz, Switzerland) by ophthalmologists. Pterygium was defined as a radially oriented fibrovascular lesion crossing the nasal or temporal limbus. Outcome measure for pterygium was defined whether or not the subjects had a pterygium regardless of laterality: (pterygium [+/−]). The subjects which had undergone pterygium surgery were classified as pterygium (+). Pterygium recurrence after surgical excision was additionally investigated (pterygium recurrence [+/−]). To avoid confounding bias, outcome for primary pterygium was additionally defined whether or not the subjects had a pterygium without pterygium surgery history (primary pterygium [+/−]).
Covariates
Based on literature review of various epidemiological studies on pterygium (6,7,8,9,10), we considered their common risk factors as covariates including sociodemographic and clinical factors. Sociodemographic factors included age, sex, region of residence (urban or rural), education level (high school or less or and university or higher), income level (high [1st or 2nd quartile] or low [3rd or 4th quartile]). Also, we considered sun exposure (< vs. ≥ 5 hours per a day and myopia [+/−]) as covariates, because previous studies using KNHANES data suggested that pterygium was associated with sun exposure and myopia (7,10).
Statistical analysis
We analyzed the data using SPSS Complex Samples procedures (SPSS Statistics version 18; IBM Inc., Chicago, IL, USA) according to the SPSS manual from the Korea Centers for Disease Control and Prevention. Multivariate logistic models were performed to determine whether each air pollution factors were associated with a pterygium (n = 23,276). Because each air pollutants have high correlation (3), we also applied multi-pollutant models in the analysis; Model 1 controlled sociodemographic factors, and Model 2 controlled sociodemographic, clinical factors as covariates. Moreover, we analyzed associations between air pollution factors and the presence of pterygium with two different ways: 1) a model using average concentrations for previous 2 years before the ocular examination; and 2) distributed lag models using annual average concentrations by up to lagged 2 years before the ocular examination (year lag 0 through year lag 2), to account for the cumulative and/or delayed effects of environmental exposures (11,12,13). Primary pterygium was also analyzed according to aforementioned statistical methods (n = 22,216). Furthermore, we examined associations between air pollution and pterygium recurrence using multivariate logistic models among the subjects who experienced pterygium surgery (n = 1,060).
P values of different year lags were controlled by Bonferroni’s correction.
Ethics statement
This study was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention and complied with the tenets of the Declaration of Helsinki. Informed consent was waived by the board.
RESULTS
The baseline characteristics of 23,276 participants (9,961 men and 13,315 women) were shown in Table 1. The mean age ± standard deviation (SD) of overall participants was 49.7 ± 16.6 years. A total of 1,060 subjects had a history of pterygium surgery, and 101 subjects of 1,060 subjects experienced pterygium recurrence after the surgery (recurrence rate: 10.3%, weights adjusted). The prevalence of pterygium was 5.3% (weights adjusted). Pterygium was significantly more prevalent in men and subjects with rural residence, lower education and lower income, greater sun exposure, myopia, and significantly different across age (χ2 test, each P < 0.001). Between 2006 and 2011, the mean value (± SD) of humidity, PM10, O3, NO2, and SO2 were 66.21% ± 4.19%, 51.70 ± 6.26 µg/m3, 0.024 ± 0.004 ppm, 0.022 ± 0.006 ppm, and 0.005 ± 0.001 ppm, respectively. There were no significant changes in air pollutants over 6 years (repeated measure analysis of variance [ANOVA], each P > 0.05). Multicollinearity between each air pollution factors was examined by variance inflation factor (VIF), and we confirmed they all did not exceed 10.
Table 1. Characteristics of the study population.
| Demographics | Pterygium (+/−) (%)* | P value† | No. of participants (Total 23,276) |
|---|---|---|---|
| Sex | < 0.001 | ||
| Men | 784/9,177 (6.5) | 9,961 | |
| Women | 882/12,433 (5.2) | 13,315 | |
| Age, yr | < 0.001 | ||
| 19–29 | 2/2,976 (0.1) | 2,978 | |
| 30–39 | 37/4,418 (0.7) | 4,455 | |
| 40–49 | 149/4,248 (3.4) | 4,397 | |
| 50–59 | 299/3,897 (7.3) | 4,196 | |
| 60–69 | 525/3,300 (13.5) | 3,825 | |
| 70≤ | 654/2,771 (18.9) | 3,425 | |
| Region of residence | < 0.001 | ||
| Urban | 539/9,907 (3.8) | 10,446 | |
| Rural | 1,127/11,703 (6.7) | 12,830 | |
| Education level‡ | < 0.001 | ||
| University graduation or higher | 87/6,324 (1.1) | 6,411 | |
| High school graduation or less | 1,546/15,012 (7.2) | 16,558 | |
| Income level‡ | < 0.001 | ||
| High (1st, 2nd quartile group) | 542/11,911 (3.6) | 12,453 | |
| Low (3rd, 4th quartile group) | 1,102/9,401 (7.7) | 10,503 | |
| Sun exposure‡ | < 0.001 | ||
| 5 hours or more | 672/4,344 (9.7) | 5,016 | |
| Less | 996/17,064 (4.4) | 18,050 | |
| Myopia‡ | < 0.001 | ||
| (+) | 365/9,921 (2.5) | 10,286 | |
| (−) | 1,192/11,120 (7.9) | 12,312 |
*Calculated after applying weights; †χ2 test; ‡Except for non-respondents.
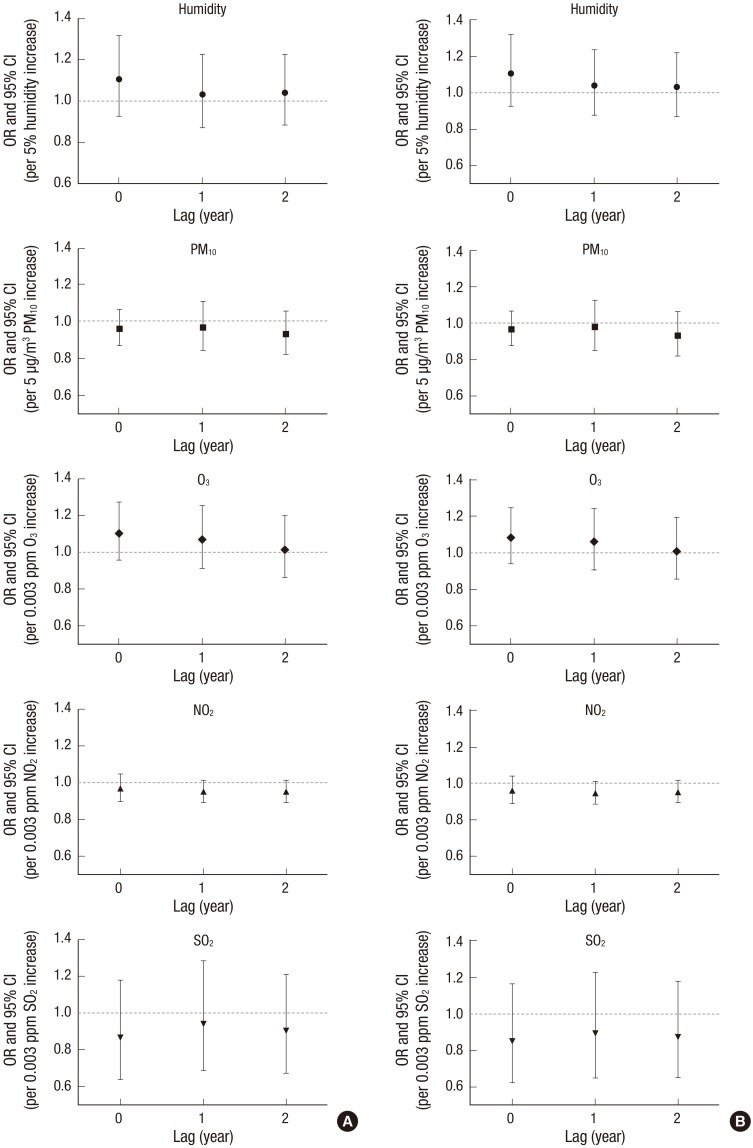
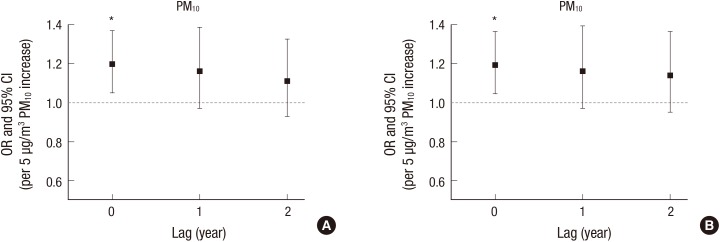
Table 2 presents the results for the presence of pterygium using multivariate logistic models. None of outdoor air pollution factors (2 years’ average concentrations of humidity, PM10, O3, NO2, and SO2) were significantly associated with the risk of pterygium in Models 1 and 2 (each P > 0.05). Older age, men, and sun exposure were significantly associated with higher risk of pterygium (10 year increased—Model 1 [OR 1.90; P < 0.001], Model 2 [OR 1.86; P < 0.001], men vs. women—Model 1 [OR 1.32; P < 0.001], Model 2 [OR 1.31; P < 0.001], sun exposure vs. non-exposure—Model 2 [OR 1.31; P = 0.001]), and higher education level and myopia were significantly associated with the lower risk of pterygium (University level education vs. less—Model 1 [OR, 0.33; P < 0.001], Model 2 [OR 0.38; P < 0.001], myopia vs. non-myopia—Model 2 [OR 0.68; P < 0.001]). Fig. 2 showed the results for the overall pterygium using distributed lag models with year lags from 0 to 2 years (annual average concentrations). Humidity, PM10, O3, NO2, and SO2 were not associated with pterygium in all year lags (each P > 0.05). However, primary pterygium was associated with PM10 level (Model 1 [OR 1.22; P = 0.029], Model 2 [OR, 1.23; P = 0.023], Table 3). Distributed lag models presented that PM10 in year lag 0 was only associated with primary pterygium (Model 1 [OR 1.20; P = 0.008], Model 2 [OR, 1.19; P = 0.009], Fig. 3). Other air pollutants were not associated with primary pterygium (each P > 0.05).
Table 2. Association of outdoor air pollutants with pterygium: multivariate logistic regression (n = 23,276).
| Variables | Model 1 Multivariate OR (95% CI, P value) |
Model 2 Multivariate OR (95% CI, P value) |
|---|---|---|
| Air pollution factors (average during 2 years) | ||
| Humidity, % (5% increase) | 1.15 (1.02–1.29, 0.436) | 1.08 (0.89–1.30, 0.444) |
| PM10, µg/m3 (5 µg/m3 increase) | 0.99 (0.87–1.11, 0.553) | 0.97 (0.85–1.11, 0.621) |
| O3, ppm (0.003 ppm increase) | 1.08 (0.91–1.28, 0.443) | 1.06 (0.89–1.27, 0.511) |
| NO2, ppm (0.003 ppm increase) | 0.93 (0.86–0.99, 0.231) | 0.96 (0.89–1.03, 0.219) |
| SO2, ppm (0.003 ppm increase) | 0.89 (0.64–1.29, 0.438) | 0.83 (0.58–1.20, 0.324) |
| Sociodemographic factors | ||
| Age (10-year increase)* | 1.90 (1.82–1.99, < 0.001) | 1.86 (1.77–1.94, < 0.001) |
| Sex (men/women)* | 1.32 (1.17–1.50, < 0.001) | 1.31 (1.15–1.51, < 0.001) |
| Region of residence (urban/rural) | 0.84 (0.59–1.20, 0.333) | 0.88 (0.62–1.26, 0.494) |
| Education level (university or higher/high school or less)* | 0.33 (0.25–0.44, < 0.001) | 0.38 (0.29–0.51, < 0.001) |
| Income level (high/low) | 0.99 (0.85–1.14, 0.826) | 1.01 (0.88–1.17, 0.876) |
| Other factors | ||
| Sun exposure (more/less than 5 hours a day)* | NA | 1.31 (1.12–1.54, 0.001) |
| Myopia (+/−)* | NA | 0.68 (0.58–0.80, < 0.001) |
Model 1: sociodemographic factors were modeled as covariates; Model 2: sociodemographic factors, sun exposure, and myopia were modeled as covariates.
OR = odds ratio, CI = confidence interval, PM10 = particulate matter with aerodynamic diameter less than 10 µm, O3 = ozone, NO2 = nitrogen dioxide, SO2 = sulfur dioxide, NA = not available.
*Associated with pterygium in multivariate logistic regression.
Fig. 2.
Distributed lag models between outdoor air pollutants (humidity, PM, O3, NO2, and SO2) and pterygium. (A) Model 1, (B) Model 2.
Model 1: sociodemographic factors were included as covariates; Model 2: sociodemographic factors, sun exposure, and myopia were included as covariates.
PM10 = particulate matter with aerodynamic diameter less than 10 µm, O3 = ozone, NO2 = nitrogen dioxide, SO2 = sulfur dioxide, OR = odds ratio, CI = confidence interval.
Table 3. Association of outdoor air pollutants with primary pterygium: multivariate logistic regression (n = 22,216).
| Variables | Model 1 Multivariate OR (95% CI, P value) |
Model 2 Multivariate OR (95% CI, P value) |
|---|---|---|
| Air pollution factors (average during 2 years) | ||
| Humidity, % (5% increase) | 1.21 (0.93–1.57, 0.162) | 1.19 (0.91–1.56, 0.206) |
| PM10, µg/m3 (5 µg/m3 increase)* | 1.22 (1.02–1.45, 0.029) | 1.23 (1.03–1.47, 0.023) |
| O3, ppm (0.003 ppm increase) | 1.09 (0.86–1.37, 0.479) | 1.09 (0.87–1.38, 0.439) |
| NO2, ppm (0.003 ppm increase) | 0.93 (0.84–1.02, 0.115) | 0.94 (0.85–1.04, 0.198) |
| SO2, ppm (0.003 ppm increase) | 0.89 (0.59–1.19, 0.292) | 0.91 (0.61–1.20, 0.301) |
| Sociodemographic factors | ||
| Age (10-year increase)* | 1.80 (1.69–1.92, < 0.001) | 1.74 (1.62–1.86, < 0.001) |
| Sex (men/women)* | 1.56 (1.24–1.95, < 0.001) | 1.44 (1.13–1.82, 0.003) |
| Region of residence (urban/rural) | 1.16 (0.72–1.85, 0.548) | 1.21 (0.75–1.94, 0.434) |
| Education level (university or higher/high school or less)* | 0.29 (0.18–0.45, < 0.001) | 0.34 (0.21–0.54, < 0.001) |
| Income level (high/ low) | 0.98 (0.77–1.24, 0.837) | 1.01 (0.79–1.29, 0.964) |
| Other factors | ||
| Sun exposure (more/less than 5 hours a day)* | NA | 1.54 (1.20–1.96, 0.001) |
| Myopia (+/−)* | NA | 0.62 (0.48–0.81, < 0.001) |
Model 1: sociodemographic factors were modeled as covariates; Model 2: sociodemographic factors, sun exposure, and myopia were modeled as covariates.
OR = odds ratio, CI = confidence interval, PM10 = particulate matter with aerodynamic diameter less than 10 µm, O3 = ozone, NO2 = nitrogen dioxide, SO2 = sulfur dioxide, NA = not available.
*Associated with pterygium in multivariate logistic regression.
Fig. 3.
Distributed lag models between PM and primary pterygium. (A) Model 1, (B) Model 2.
Model 1: sociodemographic factors were included as covariates; Model 2: sociodemographic factors, sun exposure, and myopia were included as covariates.
PM10 = particulate matter with aerodynamic diameter less than 10 µm, OR = odds ratio, CI = confidence interval.
*Associated with primary pterygium in multivariate logistic regression.
Table 4 presents the results for pterygium recurrence using multivariate logistic models, among the subjects who had a history of pterygium surgery (n = 1,060). No associations between outdoor air pollution factors (2 years’ average concentrations) with pterygium recurrence were observed (each P > 0.05). Men and myopia were negatively associated with pterygium recurrence (men/women—Model 1 [OR 0.35; P < 0.001], Model 2 [OR 0.32; P < 0.001], myopia/non-myopia—Model 2 [OR 0.44; P = 0.029]).
Table 4. Association of outdoor air pollutants with pterygium recurrence after surgery: multivariate logistic regression (n = 1,060).
| Variables | Model 1 Multivariate OR (95% CI, P value) |
Model 2 Multivariate OR (95% CI, P value) |
|---|---|---|
| Air pollution factors (average during 2 years) | ||
| Humidity, % (5% increase) | 1.59 (0.87–2.91, 0.129) | 1.50 (0.82–2.76, 0.191) |
| PM10, µg/m3 (5 µg/m3 increase) | 0.99 (0.67–1.46, 0.971) | 0.93 (0.63–1.37, 0.700) |
| O3, ppm (0.003 ppm increase) | 0.94 (0.63–1.40, 0.749) | 0.92 (0.61–1.39, 0.704) |
| NO2, ppm (0.003 ppm increase) | 1.10 (0.90–1.35, 0.333) | 1.10 (0.89–1.36, 0.375) |
| SO2, ppm (0.003 ppm increase) | 0.42 (0.17–1.05, 0.062) | 0.45 (0.17–1.18, 0.103) |
| Sociodemographic factors | ||
| Age (10-year increase) | 0.94 (0.76–1.15, 0.718) | 0.88 (0.69–1.10, 0.268) |
| Sex (men/women)* | 0.35 (0.20–0.60, < 0.001) | 0.32 (0.18–0.57, < 0.001) |
| Region of residence (urban/rural) | 1.70 (0.61–4.73, 0.308) | 1.54 (0.53–4.51, 0.431) |
| Education level (university or higher/high school or less) | 0.51 (0.11–2.25, 0.370) | 0.57 (0.13–2.51, 0.456) |
| Income level (high/low) | 1.14 (0.65–2.01, 0.638) | 0.87 (0.50–1.51, 0.615) |
| Other factors | ||
| Sun exposure (more than 5 hours a day/less) | NA | 0.75 (0.39–1.43, 0.379) |
| Myopia (+/−)* | NA | 0.44 (0.21–0.91, 0.029) |
Model 1: sociodemographic factors were modeled as covariates; Model 2: sociodemographic factors, sun exposure, and myopia were modeled as covariates.
OR = odds ratio, CI = confidence interval, PM10 = particulate matter with aerodynamic diameter less than 10 µm, O3 = ozone, NO2 = nitrogen dioxide, SO2 = sulfur dioxide, NA = not available.
*Associated with pterygium in multivariate logistic regression.
DISCUSSION
This study revealed that outdoor air pollution factors were not associated with overall pterygium or pterygium recurrence after various risk factors were controlled and distributed lag models were applied. In subgroup analysis, primary pterygium was associated with increased PM10 level. Aging, men, and greater sun exposure were associated with pterygium, while higher education and myopia were negatively associated with pterygium. Men and myopia were negatively associated with pterygium recurrence.
Although a precise pathogenesis of pterygium is still not fully understood, oxidative stress is well-known to be play an important role in its pathogenesis (14,15,16). Oxidative stress from UV-B exposure causes upregulation of many potential mediators related with pterygium and increases reactive oxygen and nitrogen species leading to DNA damage (15). Additionally, inflammatory mediators are known to be associated with the development of pterygium (15). Dust is one of the risk factors in pterygium and it is known that mechanical irritation and inflammation by dust particles may have a role in the pathogenesis of pterygium (17). Outdoor air pollution could induce oxidative damage and inflammation to various biomolecules in ocular surface (18), which may result in the development of pterygium. Several studies suggested that dry eye disease is a risk factor for pterygium and pterygium itself may also lead to dry eye disease (19,20,21). Our previous study presented that higher O3 and NO2 levels were associated with dry eye disease (3), and explained their major potential pathway may be air pollution-induced oxidative stress and inflammation. The current study could not observe a relationship between outdoor air pollution and overall pterygium, however primary pterygium showed weak association with PM10. Microtrauma and inflammation by PM seem to be related. Distributed lag models did not show cause and effect relationship between PM10 and primary pterygium. Therefore, it is difficult to affirm that PM10 is a risk factor in pterygium. Because annual mean concentrations of air pollutants were not extremely high for the period between 2006 and 2011 in Korea and the general population was subject to investigation, the effects of air pollution on pterygium may be masked in this study. The exposure to higher levels may provide a significant effect on pterygium. Further clinical and experimental studies about the exposure of higher air pollutants levels and pterygium are needed.
The pterygium prevalence has been reported 6.2%–6.7% (7,10), however the recurrence rate of pterygium was not reported in the Korean population. This study presented the prevalence and recurrence rate of pterygium were 5.3 and 10.3% in the Korean population respectively. The prevalence can be lower than those in previous studies because overall population was included in the study (7,10). Recurrence was reported 1%–40% according to surgical methods (22). Our study presented that aging, male sex, and greater sun exposure, lower education level, and myopia were associated with pterygium. These results are consistent with previous studies (7,9,10,14). Myopia was negatively associated with both pterygium and its recurrence. The protective effect of myopia on pterygium or its recurrence may be related with wearing spectacles which can protect UV light (10). Interestingly, men were negatively associated with pterygium recurrence. Indeed, major factors inducing pterygium recurrence include sun exposure, surgical technique, postoperative inflammation, and increased vascularity in pterygium (23,24). It is known that estrogen stimulates immune responses, whereas androgens suppress inflammatory reactions (25). Hormonal difference between men and women may influence postoperative inflammation after pterygium excision. Further studies are needed to confirm these results.
This study had some limitations. First, fine PM (PM2.5) was not investigated as air pollutants, because PM2.5 data were not available. Second, seasonal and daily variations of air pollutants were not considered. Third, the subjects that had undergone pterygium surgery were classified as those with pterygium, which may induce bias. Fourth, the duration of the pterygium in each patient was not available in KNHANES data. However, this study seems to be meaningful because we used well-organized population-based data and distributed lag models, and applied multiple air pollutants data for 2 years to take into consideration temporal exposure to air pollution on the ocular surface, although 2 years were not a long time enough to explain a cause-and-effect relationship.
In conclusion, we cannot observe significant associations between outdoor air pollution and the presence of pterygium or recurrence of pterygium in the Korean population. However, primary pterygium was weakly associated with PM10 level. Additional longitudinal studies are needed to confirm the associations between outdoor air pollution and pterygium.
ACKNOWLEDGMENT
We thank the Epidemiologic Survey Committee of the Korean Ophthalmologic Society. The Epidemiologic Survey Committee of the Korean Ophthalmologic Society mainly participated in making and processing Korea National Health and Nutrition Examination Survey (KNHANES) data about ophthalmologic questionnaire and examinations, and helped us to access KNHANES data.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Research conception and design: Kim DH. Data analysis and interpretation: Lee KW, Choi YH, Hwang SH, Kim DH. Statistical analysis: Lee KW, Choi YH, Hwang SH, Kim DH. Drafting of the manuscript: Lee KW, Choi YH, Kim DH. Critical revision of the manuscript: Paik HJ, Kim MK, Wee WR. Approval of final manuscript: all authors.
References
- 1.World Health Organization. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005. Geneva: World Health Organization; 2006. [Google Scholar]
- 2.Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151:362–367. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Hwang SH, Choi YH, Paik HJ, Wee WR, Kim MK, Kim DH. Potential importance of ozone in the association between outdoor air pollution and dry eye disease in South Korea. JAMA Ophthalmol. 2016;134:503–510. doi: 10.1001/jamaophthalmol.2016.0139. [DOI] [PubMed] [Google Scholar]
- 4.Livingston PM, McCarty CA, Taylor HR. Visual impairment and socioeconomic factors. Br J Ophthalmol. 1997;81:574–577. doi: 10.1136/bjo.81.7.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill JC, Maske R. Pathogenesis of pterygium. Eye (Lond) 1989;3:218–226. doi: 10.1038/eye.1989.31. [DOI] [PubMed] [Google Scholar]
- 6.Marmamula S, Khanna RC, Rao GN. Population-based assessment of prevalence and risk factors for pterygium in the South Indian state of Andhra Pradesh: the Andhra Pradesh eye disease study. Invest Ophthalmol Vis Sci. 2013;54:5359–5366. doi: 10.1167/iovs.13-12529. [DOI] [PubMed] [Google Scholar]
- 7.Rim TH, Nam J, Kim EK, Kim TI. Risk factors associated with pterygium and its subtypes in Korea: the Korean National Health and Nutrition Examination Survey 2008-2010. Cornea. 2013;32:962–970. doi: 10.1097/ICO.0b013e3182801668. [DOI] [PubMed] [Google Scholar]
- 8.Tano T, Ono K, Hiratsuka Y, Otani K, Sekiguchi M, Konno S, Kikuchi S, Onishi Y, Takegami M, Yamada M, et al. Prevalence of pterygium in a population in Northern Japan: the locomotive syndrome and health outcome in Aizu cohort study. Acta Ophthalmol. 2013;91:e232–6. doi: 10.1111/aos.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L, You QS, Xu L, Ma K, Wang YX, Yang H, Jonas JB. 10-year incidence and associations of pterygium in adult Chinese: the Beijing eye study. Invest Ophthalmol Vis Sci. 2013;54:1509–1514. doi: 10.1167/iovs.12-11183. [DOI] [PubMed] [Google Scholar]
- 10.Lim CY, Kim SH, Chuck RS, Lee JK, Park CY. Risk factors for pterygium in Korea: the Korean National Health and Nutrition Examination Survey V, 2010-2012. Medicine (Baltimore) 2015;94:e1258. doi: 10.1097/MD.0000000000001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welty LJ, Peng RD, Zeger SL, Dominici F. Bayesian distributed lag models: estimating effects of particulate matter air pollution on daily mortality. Biometrics. 2009;65:282–291. doi: 10.1111/j.1541-0420.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 12.Lim YH, Kim H, Kim JH, Bae S, Park HY, Hong YC. Air pollution and symptoms of depression in elderly adults. Environ Health Perspect. 2012;120:1023–1028. doi: 10.1289/ehp.1104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dabass A, Talbott EO, Venkat A, Rager J, Marsh GM, Sharma RK, Holguin F. Association of exposure to particulate matter (PM2.5) air pollution and biomarkers of cardiovascular disease risk in adult NHANES participants (2001-2008) Int J Hyg Environ Health. 2016;219:301–310. doi: 10.1016/j.ijheh.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Liu T, Liu Y, Xie L, He X, Bai J. Progress in the pathogenesis of pterygium. Curr Eye Res. 2013;38:1191–1197. doi: 10.3109/02713683.2013.823212. [DOI] [PubMed] [Google Scholar]
- 15.Bradley JC, Yang W, Bradley RH, Reid TW, Schwab IR. The science of pterygia. Br J Ophthalmol. 2010;94:815–820. doi: 10.1136/bjo.2008.151852. [DOI] [PubMed] [Google Scholar]
- 16.Chui J, Di Girolamo N, Wakefield D, Coroneo MT. The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf. 2008;6:24–43. doi: 10.1016/s1542-0124(12)70103-9. [DOI] [PubMed] [Google Scholar]
- 17.Coroneo MT. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993;77:734–739. doi: 10.1136/bjo.77.11.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poljšak B, Fink R. The protective role of antioxidants in the defence against ROS/RNS-mediated environmental pollution. Oxid Med Cell Longev. 2014;2014:671539. doi: 10.1155/2014/671539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozsutcu M, Arslan B, Erdur SK, Gulkilik G, Kocabora SM, Muftuoglu O. Tear osmolarity and tear film parameters in patients with unilateral pterygium. Cornea. 2014;33:1174–1178. doi: 10.1097/ICO.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 20.Ishioka M, Shimmura S, Yagi Y, Tsubota K. Pterygium and dry eye. Ophthalmologica. 2001;215:209–211. doi: 10.1159/000050860. [DOI] [PubMed] [Google Scholar]
- 21.Rajiv MS, Mithal S, Sood AK. Pterygium and dry eye--a clinical correlation. Indian J Ophthalmol. 1991;39:15–16. [PubMed] [Google Scholar]
- 22.Kaufman SC, Jacobs DS, Lee WB, Deng SX, Rosenblatt MI, Shtein RM. Options and adjuvants in surgery for pterygium: a report by the American Academy of Ophthalmology. Ophthalmology. 2013;120:201–208. doi: 10.1016/j.ophtha.2012.06.066. [DOI] [PubMed] [Google Scholar]
- 23.Han SB, Jeon HS, Kim M, Lee SJ, Yang HK, Hwang JM, Kim KG, Hyon JY, Wee WR. Risk factors for recurrence after pterygium surgery: an image analysis study. Cornea. 2016;35:1097–1103. doi: 10.1097/ICO.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 24.Sul S, Korkmaz Ş, Novruzlu Ş. Seasonal effects on pterygium surgery outcome: implications for the role of sunlight exposure. Cornea. 2014;33:504–506. doi: 10.1097/ICO.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 25.Cutolo M, Wilder RL. Different roles for androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum Dis Clin North Am. 2000;26:825–839. doi: 10.1016/s0889-857x(05)70171-x. [DOI] [PubMed] [Google Scholar]