Abstract
Adding either macrolide or fluoroquinolone (FQ) to β-lactam has been recommended for patients with severe community-acquired pneumonia (CAP). However, due to the limited evidence available, there is a question as to the superiority of the two combination therapies. The MEDLINE, EMBASE, Cochrane Central Register, Scopus, and Web of Science databases were searched for systematic review and meta-analysis. A total of eight trials were analyzed. The total number of patients in the β-lactam plus macrolide (BL-M) and β-lactam plus fluoroquinolone (BL-F) groups was 2,273 and 1,600, respectively. Overall mortality of the BL-M group was lower than that of the BL-F group (19.4% vs. 26.8%), which showed statistical significance (odds ratio [OR], 0.68; 95% confidence interval [CI], 0.49 to 0.94; P = 0.02). Length of hospital stay was reduced in the BL-M group compared to the BL-F group (mean difference, −3.05 days; 95% CI, −6.01 to −0.09; P = 0.04). However, there was no significant difference in length of intensive care unit (ICU) stay between the two groups. Among patients with severe CAP, BL-M therapy may better reduce overall mortality and length of hospital stay than BL-F therapy. However, we could not elicit strong conclusions from the available trials due to high risk of bias and methodological limitations.
Keywords: Pneumonia, Intensive Care Units, Mortality, Macrolides, Fluoroquinolone
INTRODUCTION
Community-acquired pneumonia (CAP) is a leading cause of infectious death worldwide (1). Severe CAP has generally been defined as CAP requiring admission to the intensive care unit (ICU) due to invasive mechanical ventilation or septic shock requiring vasopressors (2). Approximately 10% of patients hospitalized with CAP require admission to the ICU, and the rate of mortality ranges from 11% to 56% (3,4,5). Therefore, it is important for critically ill patients with severe CAP to receive appropriate antibiotic regimens.
Recent prospective studies have shown non-inferiority of β-lactam monotherapy in comparison with a β-lactam plus macrolide (BL-M) combination therapy in patients with non-severe CAP (6,7). However, a β-lactam-based combination therapy is preferred for patients with severe CAP (5,8). Additionally, recent pooled analyses showed that addition of macrolides, which has been often used to cover atypical pathogens of CAP, was associated with reduction of mortality (9,10).
Currently, the official guideline from the Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) recommends either BL-M or a β-lactam plus fluoroquinolone (BL-F) combined therapy for patients with severe CAP, unless there is concern for Pseudomonas or methicillin-resistant Staphylococcus aureus (MRSA) infection (2). Guidelines from several local medical societies as well as British Thoracic Society (BTS) have also recommended regimens similar to those of the IDSA/ATS guideline for patients with higher pneumonia severity (11,12).
Since the publication of several official guidelines, there has been a question with regard to the relative superiority of BL-M vs. BL-F. However, there is a scarcity of conclusive data from clinical trials. Accordingly, through a systematic review of data from available clinical trials, we assessed the relative efficacy of BL-M and BL-F treatments.
MATERIALS AND METHODS
Data sources and search strategy
To identify potentially relevant articles, a comprehensive search of five electronic databases (MEDLINE, EMBASE, Cochrane Central Register, Scopus, and Web of Science) was performed. Articles published prior to December 2015 were included. Search results were limited to human studies. A highly sensitive search strategy was adopted using the following words and medical subject headings (MeSH) terms: “beta-lactams,” “Macrolides,” “Fluoroquinolones,” “Community-Acquired Infections,” and “Pneumonia, Bacterial.” In addition, we performed a manual search of the references cited by relevant review articles. As this study was a systematic review of published articles, informed consent and ethics approval were not required.
Inclusion criteria
A systematic review and meta-analysis was performed of studies that met the following criteria: 1) randomized controlled or observational cohort studies that targeted severe CAP patients over 18 years of age; 2) exposure to BL-M or BL-F combination therapy; 3) the presence of clinical outcomes including mortality (total, in-hospital, ICU or 30-day) and length of stay (hospital or ICU). Studies targeting outpatients, non-severe CAP patients, or patients with healthcare-associated pneumonia (HCAP), hospital acquired pneumonia (HAP), and ventilator-acquired pneumonia (VAP) were excluded.
Study selection and data extraction
Two pulmonologists (JHL and YHK) independently retrieved potentially relevant studies, reviewed each study according to the predefined criteria for eligibility, and extracted data. Any disagreement in the process of study selection or data extraction was resolved through consensus. A predefined form was used to extract data from each study. We used only officially published data. The primary outcome was overall mortality. We also assessed changes in 30-day mortality, ICU mortality, length of hospital stay, and length of ICU stay.
Quality assessment
As recommended by the Cochrane Collaboration, we used the Newcastle-Ottawa quality assessment scale (NOS) to assess the risk of bias in the observational studies (13). NOS uses a star system to evaluate nonrandomized studies in the following three domains: selection, comparability and exposure/outcome. Studies that received a star in each of the three domains were considered to be of high quality.
The quality of randomized controlled trials (RCTs) was assessed using the Cochrane Handbook for Systemic Reviews of Interventions “risk of bias” tool (14). A term of “low,” “high,” or “unclear” for risk of bias was assigned to the following domains: sequence generation/allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. Agreement between reviewers was achieved through a consensus.
Statistical analysis
We analyzed data using Review Manager Software, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Random-effects models were applied. For dichotomous variables, treatment effects were presented as odds ratios (OR) with 95% confidence intervals (CI) via the Mantel–Haenszel method. Statistical estimates for continuous variables were expressed as raw mean differences. Heterogeneity was assessed using I2 statistics on a scale of 0%–100%, with I2 > 50%, indicating a substantial level of between-study heterogeneity. To explore the robustness of the pooled effect, we removed each study in turn to determine the influence of an individual study on the overall effect estimates. Subgroups were analyzed as necessary. A P value < 0.05 was considered statistically significant.
RESULTS
Study search
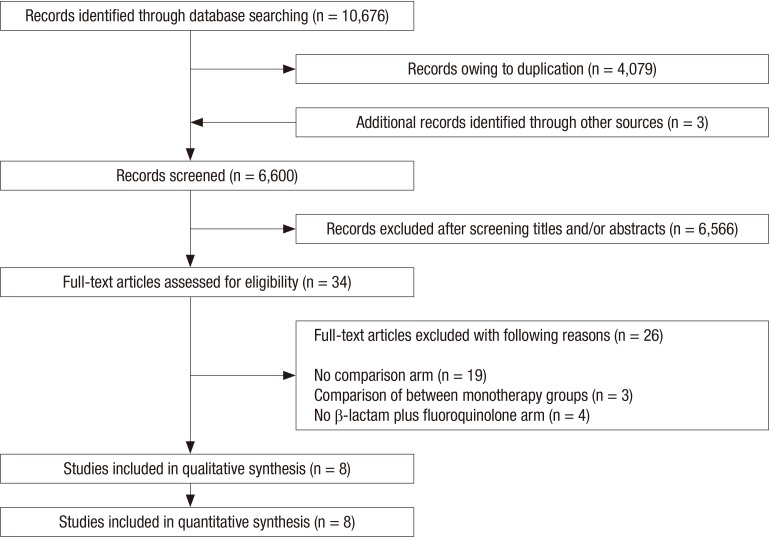
A total of 10,676 published articles were identified. After removing duplicated articles, we screened 6,600 eligible articles and added 3 potentially eligible articles from authors’ reference lists. Of these articles, 6,566 were excluded based on the title and abstract, and the remaining 34 articles underwent a full-text review. Twenty-six articles were excluded for the reasons presented in Fig 1. Finally, a total of 8 articles were included in the current analysis (15,16,17,18,19,20,21,22). Of these trials, seven trials used an observational cohort study design (15,16,17,18,19,20,21), and only one trial was an RCT (22). Six trials were performed in two or more centers. All were published between 1994 and 2013 (15,16,18,19,21,22). The features of studies included are shown in Table 1. The number of patients in each trial ranged from 61 to 1,989. The total number of patients for our systematic review and meta-analysis was 3,873, among whom 2,273 received BL-M therapy and 1,600 received BL-F therapy.
Fig. 1.
Flow chart of study selection.
Table 1. Characteristics of the studies included in the meta-analysis.
| Study (yr) | Design | Enrolled patients | Study period | Age (mean or median*), yr / male, % | Disease severity score (mean) | Mechanical ventilation, % | Severe sepsis or septic shock, % | The isolation rate of Streptococcus pneumoniae / MDR bacteria, % | Mortality, % | Primary objective of study | Risk of bias * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adrie (2013) | Multicenter, prospective observational cohort study | 394, exclusion of COPD | 1997–2010 | 64*/69.5 | CURB-65 score (3.17); % of CURB-65 score 4–5 (43.4) | Invasive (52.5), noninvasive (14.7) | Severe sepsis (91.4), septic shock (38.6) | 17.5/11.2 | 27.2% (60-day mortality) | To compare the impact on 60-day mortality in patients admitted to the ICU for CAP using one (β-lactam†) or two antibiotics (β-lactam + macrolide, β-lactam + fluoroquinolone) | Low |
| Bratzler (2008) | Multicenter, retrospective observational cohort study | 880 | 1998–1999 and 2000–2001 | NR/NR | NR | NR | NR | NR | 12.8% (30-day mortality) | To evaluate associations between various antimicrobial regimens including 2nd or 3rd β-lactam + macrolide and 2nd or 3rd β-lactam + fluoroquinolone and risk-adjusted mortality in patients admitted with CAP | High |
| Gaillat (1994) | Multicenter, prospective randomized study | 102 | 1990–1991 | 62.7/NR | SAPS score (10.5) | 24.5 | NR | 34.3/1.9 | 11.7% (total hospital mortality) | To compare of the efficacy of penicillin G/ofloxacin vs. erythromycin/amoxicillin-clavulanate in patients with severe CAP. | High |
| Karhu (2013) | Single center, retrospective observational cohort study | 210 | 2000–2010 | 55*/64.7 | % of number of patients with IDSA/ATS severe CAP criteria (76.1) | 52.3 | 47.1 | 23.8/NR | 20.4% (30-day mortality), 24.2 (60-day mortality), 10.9 (ICU mortality) | To compare the outcome of patients with severe CAP treated with the combination of 2nd or 3rd β-lactam + fluoroquinolone vs. 2nd or 3rd β-lactam + macrolide | Low |
| Martin-Loeches (2010) | Multicenter, prospective observational cohort study | 100 | 2007–2008 | 57.6/61 | SAPS II score (46.9); SOFA score (7.68) | 100 | 92 | NR | 37% (ICU mortality) | To access the effect on survival of 3rd or 4th β-lactam + macrolides or 3rd or 4th β-lactam + fluoroquinolones in intubated patients admitted to the ICU with severe CAP | High |
| Mortensen (2006) | Two center, retrospective observational cohort study | 137 | 1999–2002 | 63/88 | % of pneumonia severity index class IV/V (76.7) | NR | NR | 36.5/NR | 21.8% (30-day mortality) | To compare the mortality of patients with severe CAP treated with either 2nd or 3rd β-lactam + fluoroquinolone or other recommended antibiotics | High |
| Waterer (2001) | Single center, retrospective observational cohort study | 61 | 1996–2000 | 61.1/NR | Pneumonia severity index score (101) | NR | NR | NR | 8.1% (total hospital mortality) | To evaluate the outcome of various combination of antibiotics including 3rd β-lactam + macrolide and 3rd β-lactam + fluoroquinolone in patients with severe bacteremic CAP | High |
| Wilson (2012) | Multicenter, retrospective observational cohort study | 1,989 | 2001–2007 | 74/98.5 | NR | 39.3 | 23.6 | NR | 25.6% (30-day mortality) | To compare the outcome of elderly patients (aged > 65 yr) with severe CAP treated with the combination of either 2nd or 3rd β-lactam + fluoroquinolone or 2nd or 3rd β-lactam + macrolide | Low |
NR = not reported, COPD = chronic obstructive pulmonary disease, CURB-65 = confusion, blood urea nitrogen, respiratory rate, blood pressure, age > 65, ICU = intensive care unit, CAP = community-acquired pneumonia, SAPS = simplified acute physiology score, IDSA/ATS = Infectious Diseases Society of America/American Thoracic Society, SOFA = sequential organ failure assessment.
*The risk of bias in observational trials and randomized controlled trials was assessed using the Newcastle-Ottawa quality assessment scale (NOS) and Cochrane risk of bias tool, respectively; †β-lactam included penicillin and cephalosporin. When we could find the generation of cephalosporin from original articles, we added ‘2nd,’ ‘3rd or 4th’ into β-lactam. When original articles did not describe anything about the generation of cephalosporin, we used only ‘β-lactam’ without addition of ‘2nd,’ ‘3rd or 4th.’ 2nd, 3rd, and 4th means second generation, third generation, and fourth generation, respectively.
Quality assessment and risk of bias
A summary of the methodological quality assessment and risk of bias for each non-randomized observational trial is shown in Table 2. According to the NOS system described above, four studies were determined to be of low quality, primarily due to the absence of direct comparability between treatment groups or insufficient baseline data of each study (16,18,19,20). In contrast, three recent trials received relatively high scores as determined by NOS (15,17,21).
Table 2. Risk of bias within non-randomized trials using the Newcastle-Ottawa scale.
| Study (yr) | Selection | Comparability | Exposure/outcome | |||||
|---|---|---|---|---|---|---|---|---|
| Is the case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cohorts | Ascertainment of exposure | Same methods of ascertainment for cases and controls | Non-response rate | |
| Adrie (2013) | ★ | ★ | ★ | NA | ★ | ★ | ★ | NA |
| Bratzler (2008) | ★ | ★ | ★ | NA | NA | ★ | ★ | NA |
| Karhu (2013) | ★ | ★ | ★ | NA | ★★ | ★ | ★ | NA |
| Martin-Loeches (2010) | ★ | NA | ★ | NA | NA | ★ | ★ | NA |
| Mortensen (2006) | ★ | ★ | ★ | NA | NA | ★ | ★ | NA |
| Waterer (2001) | ★ | ★ | ★ | NA | NA | ★ | ★ | NA |
| Wilson (2012) | ★ | ★ | ★ | NA | ★★ | ★ | ★ | NA |
A maximum of one star for the selection and exposure/outcome domains and two stars for the comparability domain were assigned. Studies with stars in all domains (excluding comparability) were considered high quality.
NA = not applicable.
With regards to RCTs, we evaluated the study quality of the one RCT conducted by Gaillat et al. (22) according to recommendations by the Cochrane Collaboration. Assessment according to this recommendation showed that the RCT demonstrated selection bias, performance bias, and detection bias. Due to the low number of RCTs, we could not estimate potential publication bias with a funnel plot for all outcomes.
Mortality
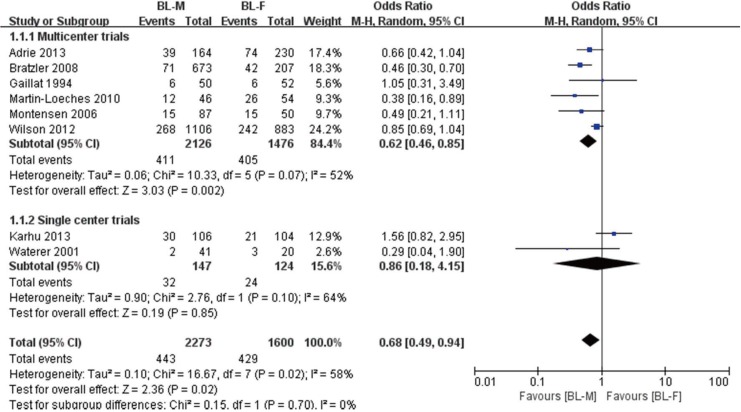
Overall mortality was reported by all 8 studies (15,16,17,18,19,20,21,22). Overall mortality rates were 19.4% (443/2,273) and 26.8% (429/1,600) for the BL-M and BL-F groups, respectively (Fig. 2). Overall, a random effect model showed that BL-M therapy was significantly associated with reduced overall mortality (OR, 0.68; 95% CI, 0.49 to 0.94; P = 0.02; I2 = 58.0%). As described above, subgroup analyses were performed according to the number of centers involved in each trial (multicenter vs. single center). Most patients belonged to six multicenter trials (n = 3,602; 93.0%) (15,16,18,19,21,22). A pooled analysis from multicenter trials demonstrated superiority of BL-M therapy (OR, 0.62; 95% CI, 0.46 to 0.85; P = 0.002; I2 = 52.0%), whereas a pooled analysis of two single center trials did not (17,20). In addition, a pooled estimate from three observational trials with a relatively high quality of study and one RCT did not reach statistical significance between the two treatment groups (OR, 0.89; 95% CI, 0.66 to 1.20; P = 0.44; I2 = 38.0%, not shown) (15,17,21,22).
Fig. 2.
Pooled results of adjusted odds ratio for overall mortality among patients with severe CAP treated with BL-M vs. BL-F.
CAP = community-acquired pneumonia, M–H = Mantel–Haenszel, CI = confidence interval, df = degrees of freedom, BL-M = β-lactam plus macrolide, BL-F = β-lactam plus fluoroquinolone.
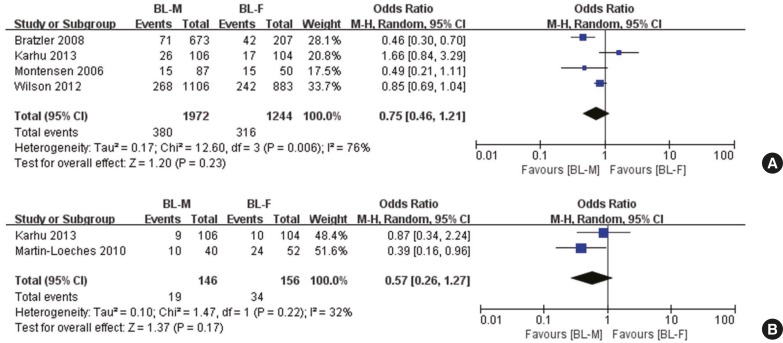
Four trials reported 30-day mortality (Fig. 3A) (16,17,19,21). A random effect showed a trend toward superiority of BL-M therapy, but its estimate did not reach statistical significance, and heterogeneity between trials was high (OR, 0.75; 95% CI, 0.46 to 1.21; P = 0.23; I2 = 76.0%). Similar results were observed for ICU mortality (OR, 0.57; 95% CI, 0.26 to 1.27; P = 0.22; I2 = 32%; Fig. 3B) (17,18).
Fig. 3.
Pooled results of adjusted odds ratio for overall mortality among the patients with severe CAP treated with BL-M vs. BL-F. (A) Thirty-days mortality. (B) ICU mortality.
CAP = community-acquired pneumonia, M–H = Mantel–Haenszel, CI = confidence interval, df = degrees of freedom, BL-M = β-lactam plus macrolide, BL-F = β-lactam plus fluoroquinolone, ICU = intensive care unit.
Length of stay
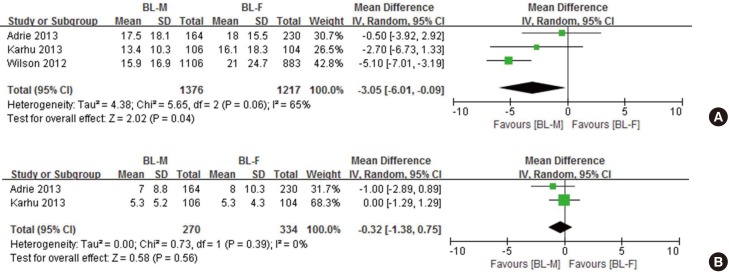
We retrieved data on the length of hospital stay from three observational trials (Fig. 4A) (15,17,21). Overall, BL-M therapy was significantly associated with shorter length of hospital stay (mean difference, −3.05 days; 95% CI, −6.01 to −0.09; P = 0.04; I2 = 65.0%). Since a trial conducted by Wilson et al. (21) included only elderly patients (mean age, 74 years) and, at the same time, extremely favored BL-M therapy, an additional analysis was performed except for this study. Its estimate was not statistically significant. However, there was a trend toward greater benefits of BL-M therapy (mean difference, −1.42 days; 95% CI, −4.03 to 1.18; P = 0.29; I2 = 0.0%, not shown). With respect to the length of ICU stay, there was no significant difference between the two groups (mean difference, −0.32 days; 95% CI, −1.38 to 0.75; P = 0.56; I2 = 0.0%; Fig. 4B) (15,17).
Fig. 4.
Pooled results of mean difference for length of stay among critically ill patients with severe CAP treated with BL-M vs. BL-F. (A) Length of hospital stay in days.(B) Length of ICU stay in days.
CAP = community-acquired pneumonia, SD = standard difference, IV = inverse variance, CI = confidence interval, df = degrees of freedom, BL-M = β-lactam plus macrolide, BL-F = β-lactam plus fluoroquinolone, ICU = intensive care unit.
DISCUSSION
Although recent trials have suggested that β-lactam monotherapy is not inferior to BL-M combination therapy or fluoroquinolone (FQ) monotherapy among patients with clinically suspected CAP admitted to non-ICU wards (6,7), a combination therapy consisting of a β-lactam with either a FQ or a macrolide has been officially recommended for patients with severe CAP (2).
There are several points of observational evidence that demonstrate combination therapy with macrolides reduces mortality rates compared to other non-macrolide combination therapies (10,23). It is well known that macrolides have immunomodulatory effects on inflammatory and epithelial cells, which can lead to attenuation of inflammatory response (8). In addition, a retrospective observational cohort study reported that combined BL-F therapy as an empirical therapy for severe CAP was associated with increased 30-day mortality when compared with other guideline-concordant antimicrobial regimens (19). On the basis of these previous results, a recent review inferred that macrolide combination may be associated with better outcomes (6). However, it is still unclear whether there is a benefit or if one combined therapy regime has superior efficacy. One reason why this question has yet to be conclusively answered is that existing clinical studies are either observational or limited in their design.
Against this backdrop, we aimed to determine the superiority between both regimes through systematic review of previous trials. Our predefined algorithm identified a total of eight trials (seven non-randomized observational trials and one RCT). Among the possible outcomes, mortality is the most important parameter when evaluating efficacy of an intervention for patients with critical illness such as severe CAP. Accordingly, we pooled overall mortality data from all eight trials. The pooled estimate showed that combined BL-M therapy was more effective at reducing overall mortality (Fig. 2). A subgroup analysis from six multicenter trials, which included most of the patients (84.4%), demonstrated a similar outcome. In contrast, quantitative analyses were possible only in four and two trials for 30-day and ICU mortality, respectively. Pooled estimates based on these data were not significant. However, we were able to observe a trend towards a greater beneficial effect of BL-M combination therapy (Fig. 3). Accordingly, we cannot exclude the probability that if a greater number of trials were available for analyses, pooled estimates of 30-day and ICU mortality would, similar to overall mortality, reach statistical significance.
Length of hospital stay is also a critical parameter that has been evaluated in clinical trials investigating antibiotic therapies. Our assessment demonstrated that, compared to patients who received BL-F, patients who received BL-M combination therapy were discharged from hospital approximately 3 days earlier (Fig. 4A). However, length of ICU stay did not differ between the two treatment groups. These data, when considered along with the mortality results, could indicate that BL-M combination therapy has greater beneficial effects among patients with relatively less severe CAP. However, to determine these results conclusively, additional trials are needed.
Overall, our systematic review and meta-analysis showed that BL-M therapy may be superior to BL-F therapy among patients with severe CAP. The superiority of BL-M therapy may be due to the mechanisms through the addition of a macrolide to BL. First, macrolides provide broader antibacterial spectrum for CAP because macrolides are generally effective against the main atypical pathogens such as Mycoplasma pneumoniae and Legionella (24). Second, as described above, macrolides exert immunomodulatory effects on inflammatory and epithelial cells (8). Third, antimicrobial synergism is attained by addition of a macrolide to β-lactam. A recent retrospective cohort study demonstrated that azithromycin was associated with a beneficial effect on 28-day ICU-free days even in severe sepsis patients without pneumonia as well as those with pneumonia (25). In univariate analysis, severe sepsis patients receiving azithromycin had 5.47 more ICU-free days on average than did those not receiving azithromycin (P = 0.005) (25).
The development of antibiotics resistance is one of the most important issues in antibiotic trials. However, we could not perform a pooled analysis because drug resistance data was reported by only one trial (15). This trial reported that the rate of acquisition of multidrug-resistant pathogen was similar between both groups.
Since FQs have a broad-spectrum antimicrobial activity, they have been widely used for the treatment of a variety of bacterial infections (26). In addition, FQs have good in vitro and in vivo activity against Mycobacterium tuberculosis (M. tuberculosis) (26) and the use of FQs could result in a delayed diagnosis of pulmonary TB (27). Therefore, BL-M can be selected as a preferential regimen in patients with severe CAP in a TB endemic area, when considering our results and activity of FQs against M. tuberculosis. On the other hand, careful monitoring of patients taking macrolides is needed due to the potential risk for sudden cardiac death or ventricular tachyarrhythmias associated with macrolide use (28).
Our study has limitations. First, since most studies included in this meta-analysis were observational in design, and so results should be interpreted with caution. Additional large-scale RCTs should be performed to overcome this limitation. Second, for ICU patients without risk factors for infection with drug-resistant pathogens, the official guidelines recommend treatment with β-lactams such as cefotaxime, ceftriaxone, or ampicillin-sulbactam. However, several of the pooled studies included a β-lactam other than recommended, while other trials did not provide information on the class of β-lactam use. Therefore, some pooled patients received guideline-discordant antibiotic regimens. Third, IDSA/ATS consensus guidelines indicated two major criteria for direct admission to ICU: septic shock requiring vasopressor support and requirement for mechanical ventilation (2). These guidelines also noted that the need for ICU care is suggested by the presence of at least three minor criteria (2). However, rather than these objective parameters, the patients of clinical trials included in our review were mostly admitted to the ICU according to clinical judgment. Fourth, we should mention antimicrobial resistance. The resistance to macrolides is increasing (29). Unfortunately, all of trials included our meta-analysis were published before 2010 and did not describe the resistance to macrolides. So, we could not perform additional analyses according to resistance. If additional analyses based on antimicrobial resistance were possible or well-designed prospective controlled trials were published, we could get a more concrete conclusion. The limitations mentioned above prohibited us from drawing strong conclusions.
In conclusion, our systemic review and meta-analysis revealed that BL-M combination therapy compared to BL-F combination therapy for severe CAP may be more effective in reducing overall mortality and length of hospital stay. However, the methodological limitations of the included trials and the scarcity of available clinical studies prevented a definitive conclusion. Accordingly, further large-scale, well-designed RCTs are needed to clarify which regimen is more effective for severe CAP.
Footnotes
Funding: This research was supported by the 2016 scientific promotion program funded by Jeju National University.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Design of the study: Lee JH, Kim YH. Data analysis: Lee JH, Kim HJ. Writing the 1st manuscript: Lee JH, Kim YH. Revision of the manuscript: Kim YH. Final approval: all authors.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez A, Mendia A, Sirvent JM, Barcenilla F, de la Torre-Prados MV, Solé-Violán J, Rello J, CAPUCI Study Group Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007;35:1493–1498. doi: 10.1097/01.CCM.0000266755.75844.05. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Ryu YJ, Chun EM, Chang JH. Outcomes and prognostic factors for severe community-acquired pneumonia that requires mechanical ventilation. Korean J Intern Med. 2007;22:157–163. doi: 10.3904/kjim.2007.22.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sligl WI, Marrie TJ. Severe community-acquired pneumonia. Crit Care Clin. 2013;29:563–601. doi: 10.1016/j.ccc.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postma DF, van Werkhoven CH, van Elden LJ, Thijsen SF, Hoepelman AI, Kluytmans JA, Boersma WG, Compaijen CJ, van der Wall E, Prins JM, et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med. 2015;372:1312–1323. doi: 10.1056/NEJMoa1406330. [DOI] [PubMed] [Google Scholar]
- 7.Garin N, Genné D, Carballo S, Chuard C, Eich G, Hugli O, Lamy O, Nendaz M, Petignat PA, Perneger T, et al. β-lactam monotherapy vs β-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med. 2014;174:1894–1901. doi: 10.1001/jamainternmed.2014.4887. [DOI] [PubMed] [Google Scholar]
- 8.Emmet O’Brien M, Restrepo MI, Martin-Loeches I. Update on the combination effect of macrolide antibiotics in community-acquired pneumonia. Respir Investig. 2015;53:201–209. doi: 10.1016/j.resinv.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Asadi L, Sligl WI, Eurich DT, Colmers IN, Tjosvold L, Marrie TJ, Majumdar SR. Macrolide-based regimens and mortality in hospitalized patients with community-acquired pneumonia: a systematic review and meta-analysis. Clin Infect Dis. 2012;55:371–380. doi: 10.1093/cid/cis414. [DOI] [PubMed] [Google Scholar]
- 10.Sligl WI, Asadi L, Eurich DT, Tjosvold L, Marrie TJ, Majumdar SR. Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis. Crit Care Med. 2014;42:420–432. doi: 10.1097/CCM.0b013e3182a66b9b. [DOI] [PubMed] [Google Scholar]
- 11.Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, Macfarlane JT, Read RC, Roberts HJ, Levy ML, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl 3):iii1–55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 12.Song JH, Jung KS, Kang MW, Kim DJ, Pai H, Suh GY, Shim TS, Ahn JH, Ahn CM, Woo JH, et al. Treatment guidelines for community-acquired pneumonia in Korea: an evidence-based approach to appropriate antimicrobial therapy. Tuberc Respir Dis (Seoul) 2009;67:281–302. [Google Scholar]
- 13.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] [accessed on 27 December 2015]. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 14.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adrie C, Schwebel C, Garrouste-Orgeas M, Vignoud L, Planquette B, Azoulay E, Kallel H, Darmon M, Souweine B, Dinh-Xuan AT, et al. Initial use of one or two antibiotics for critically ill patients with community-acquired pneumonia: impact on survival and bacterial resistance. Crit Care. 2013;17:R265. doi: 10.1186/cc13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bratzler DW, Ma A, Nsa W. Initial antibiotic selection and patient outcomes: observations from the National Pneumonia Project. Clin Infect Dis. 2008;47(Suppl 3):S193–201. doi: 10.1086/591404. [DOI] [PubMed] [Google Scholar]
- 17.Karhu J, Ala-Kokko TI, Ohtonen P, Syrjälä H. Severe community-acquired pneumonia treated with beta-lactam-respiratory quinolone vs. beta-lactam-macrolide combination. Acta Anaesthesiol Scand. 2013;57:587–593. doi: 10.1111/aas.12081. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Loeches I, Lisboa T, Rodriguez A, Putensen C, Annane D, Garnacho-Montero J, Restrepo MI, Rello J. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med. 2010;36:612–620. doi: 10.1007/s00134-009-1730-y. [DOI] [PubMed] [Google Scholar]
- 19.Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The impact of empiric antimicrobial therapy with a beta-lactam and fluoroquinolone on mortality for patients hospitalized with severe pneumonia. Crit Care. 2005;10:R8. doi: 10.1186/cc3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med. 2001;161:1837–1842. doi: 10.1001/archinte.161.15.1837. [DOI] [PubMed] [Google Scholar]
- 21.Wilson BZ, Anzueto A, Restrepo MI, Pugh MJ, Mortensen EM. Comparison of two guideline-concordant antimicrobial combinations in elderly patients hospitalized with severe community-acquired pneumonia. Crit Care Med. 2012;40:2310–2314. doi: 10.1097/CCM.0b013e31825151a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaillat J, Bru JP, Sedallian A. Penicillin G/ofloxacin versus erythromycin/amoxicillin-clavulanate in the treatment of severe community-acquired pneumonia. Eur J Clin Microbiol Infect Dis. 1994;13:639–644. doi: 10.1007/BF01973989. [DOI] [PubMed] [Google Scholar]
- 23.Metersky ML, Ma A, Houck PM, Bratzler DW. Antibiotics for bacteremic pneumonia: Improved outcomes with macrolides but not fluoroquinolones. Chest. 2007;131:466–473. doi: 10.1378/chest.06-1426. [DOI] [PubMed] [Google Scholar]
- 24.Arnold FW, Summersgill JT, Lajoie AS, Peyrani P, Marrie TJ, Rossi P, Blasi F, Fernandez P, File TM, Jr, Rello J, et al. A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Respir Crit Care Med. 2007;175:1086–1093. doi: 10.1164/rccm.200603-350OC. [DOI] [PubMed] [Google Scholar]
- 25.Afshar M, Foster CL, Layden JE, Burnham EL. Azithromycin use and outcomes in severe sepsis patients with and without pneumonia. J Crit Care. 2016;32:120–125. doi: 10.1016/j.jcrc.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginsburg AS, Grosset JH, Bishai WR. Fluoroquinolones, tuberculosis, and resistance. Lancet Infect Dis. 2003;3:432–442. doi: 10.1016/s1473-3099(03)00671-6. [DOI] [PubMed] [Google Scholar]
- 27.Long R, Chong H, Hoeppner V, Shanmuganathan H, Kowalewska-Grochowska K, Shandro C, Manfreda J, Senthilselvan A, Elzainy A, Marrie T. Empirical treatment of community-acquired pneumonia and the development of fluoroquinolone-resistant tuberculosis. Clin Infect Dis. 2009;48:1354–1360. doi: 10.1086/598196. [DOI] [PubMed] [Google Scholar]
- 28.Cheng YJ, Nie XY, Chen XM, Lin XX, Tang K, Zeng WT, Mei WY, Liu LJ, Long M, Yao FJ, et al. The role of macrolide antibiotics in increasing cardiovascular risk. J Am Coll Cardiol. 2015;66:2173–2184. doi: 10.1016/j.jacc.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Hong KB, Choi EH, Lee HJ, Lee SY, Cho EY, Choi JH, Kang HM, Lee J, Ahn YM, Kang YH, et al. Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000-2011. Emerg Infect Dis. 2013;19:1281–1284. doi: 10.3201/eid1908.121455. [DOI] [PMC free article] [PubMed] [Google Scholar]