Abstract
Different signaling pathways are implicated in proliferation and differentiation of stem cells. Bone Morphogenesis Pathway (BMP) signaling was known to display an important function in osteogenic and adipogenic differentiation of mesenchymal stem cells (MSCs). In the present study, the authors investigated whether blocking BMP signaling was associated with down regulation of Nestin expression as neural stem cell marker in peripheral blood derived mesenchymal stem cells (PB-MSCs). At first, MSCs were isolated from peripheral blood by plastic adherent ability and flow cytometry analysis. After reaching the confluence, the cells were treated with medium containing Noggin as antagonist of BMP signaling upon 8 days. Real time PCR analysis indicated that the expression of Nestin was diminished in PB-MSCs by attenuating BMP signaling. The obtained results suggested that BMP signaling might have a regulatory function on the Nestin expression in mesenchymal stem cells.
Keywords: Mesenchymal stem cells (MSCs), Nestin, Noggin, BMP signaling
Introduction
Nestin is an intermediate filament protein that is found in different tissues (Krupkova et al. 2010; Gilyarov 2008). Nestin is known to involve in remodeling the cytoskeleton, assembly-disassembly of intermediate filaments (Chou et al. 2003; Sahlgren et al. 2001). Nestin has been described as a progenitor cell marker. The studies indicated that the progenitor cells exhibit down regulation of Nestin as they undergo differentiation (Méndez-Ferrer et al. 2010; Tondreau et al. 2004).
Nestin was expressed in neural progenitor cells (NPCs) and neural stem cells (NSCs) during early stages of central nervous system (CNS) development (Wislet-Gendebien et al. 2003), displaying an important function in self-renewal and survival of these cells (Park et al. 2010). The differentiation of neural progenitor cells (NPCs) to neuronal lineage cells has been revealed to be associated with the replacement of Nestin expression with neural or glial specific genes (Hendrickson et al. 2011).
Nestin expression was regulated by different factors such as hypoxia and threombin (Wong et al. 2014). Several signaling pathways have been also known to influence the expression of Nestin. For example, Notch signaling could activate the Nestin expression in the undifferentiated progenitor cells (Stockhausen et al. 2010; Shih and Holland 2006).
Mesenchymal stem cells (MSCs) were found to express Nestin (Tondreau et al. 2004). These cells had the ability to differentiate into neuronal lineage cells as well as osteocytes, adipocytes and endothelial cells (Foudah et al. 2013; Heino and Hentunen 2008; Oswald et al. 2004; Jaiswal et al. 2000). Numerous signaling pathways have identified in mesenchymal stem cells (MSCs). These signaling pathways display the important functions in differentiation and proliferation of mesenchymal stem cells (James 2013; Ng et al. 2008). BMP signaling has been known in organogenesis and stem cell differentiation including the osteogenic and adipogenic differentiation and also bone formation (Huang et al. 2009, Gazzerro et al. 2007; Okamoto et al. 2006; Varga and Wrana 2005; Zhang and Li 2005; Mishina et al. 2004; Hogan 1996). Some studies supported the role of BMP family members in differentiation of mesenchymal stem cells into different cell lineages (Chen et al. 2012; Lin and Hankenson 2011; Luther et al. 2011). For example, BMP9 plays a critical role in the promotion of osteogenic differentiation of mesenchymal stem cells (Luu et al. 2007; Cheng et al. 2003) and induction of chondrogenic differentiation by up-regulation of Sox9 expression (Blunk et al. 2003; Majumdar et al. 2001). Furthermore, several studies have reported that delivery of BMP2 to MSCs was associated with osteogenic differentiation (Cheng et al. 2001). BMP signaling is also important in mesoderm formation and cardiac development during embryogenesis. BMP4 has been demonstrated to display a major role in developmental stages including mesoderm differentiation (Wang et al. 2014).
Different studies were performed to evaluate the function of BMP signaling on Nestin expression in different cell types. For example, Torres et al. have demonstrated that BMP4 caused the differentiation of embryonic stem (ES) cells into Nestin-expressing cells (Torres et al. 2012). The blockage of BMP signaling with DMH2 was accompanied with significant growth inhibition and augmentation of cell death in Lung cancer cells expressing Nestin (Langenfeld et al. 2013). The expression of Nestin increased in dental pulp cells and odontoblasts by BMP4 (About et al. 2000). Furthermore, it has been documented that the Nestin expression was not dependent on BMP-2 in embryoid bodies (EBs) (Talavera-Adame et al. 2013). The studies indicated that the proportion of ES colonies showing Nestin expression greatly increased after treatment with Noggin (Pera et al. 2004). Also, the nestin expression was higher in the Noggin-induced ES cells than the ES cells (Zhang et al. 2013). The previous studies have also revealed that the small molecule inhibitor of BMP signaling (Dorsomorphin) supported the survival and neuronal differentiation ability of the ES cells. It has been demonstrated that Dorsomorphin alone did not influence the percentage of Nestin + colonies. However, the combination of Dorsomorphin and TGFβ/activin/ nodal inhibitor (SB431542) increased the number of Nestin + cells in the culture of (induced Pluripotent Stem) iPS cells, indicating the initial differentiation of these cells into neural stem cells (Morizane et al. 2011). The present study investigated whether the inhibition of BMP signaling can influence the Nestin expression in peripheral blood-mesenchymal stem cells (PB-MSCs). For this purpose, Noggin was used as known antagonist of BMP signaling (Zimmerman et al. 1996).
Material and methods
Mesenchymal stem cells isolation
The mononuclear cells were isolated from 20 ml peripheral blood by Ficoll density gradient. The resulting cell pellet was cultured in DMEM-F12 medium containing 10 % fetal bovine serum (FBS), 100 units/ml Penicillin-Streptomycin and 2 mM L-Glutamate (purchased from GIBCO Company) [Medium A] on uncoated 12.5cm2 flasks (Guangzhou Jet Bio-Filtration Co., Ltd). The first day of culturing cell pellet derived from peripheral blood was defined as day 1 (D1). The cultures were incubated in a humidified atmosphere at 37 °C under 5 % CO2. After 48 h, the non-adherent cells were discarded. The remaining adherent cells were cultivated in a fresh medium A.
Flow cytometry analysis
To determine the phenotypic characterization of adherent cells, the flow cytometry analysis was performed on day 6. Briefly, the cells were harvested with trypsin. Then, the cells were centrifuged at 450 g for 5 min. The cells were suspended in DPBS and incubated with the following antibodies: FITC conjugated CD45 (leukocyte marker), PE conjugated CD14 (monocytic marker), FITC conjugated CD44, PE conjugated CD105 and PE conjugated CD73 for 30 min in the dark. The CD73, CD105 and CD44 were used as MSC surface markers. Negative control staining was performed with IgG1- FITC and IgG1-PE. All of the antibodies were prepared by BD Biosciences. Then, the cells were analyzed by CyFlow Space cytometer (partec, Germany) using FloMax software.
Treatment with noggin
Within 4 days (D6), the adherent cells reached confluence at approximately 80 %. Then, the cells were cultured in Medium A supplemented with 0.1 mM NEAA (GIBCO), 2 % B27 supplement (GIBCO), 1 % N2 supplement (GIBCO), 50 ng/ml Noggin (R&D Systems), 10 ng/ml recombinant human basic fibroblast growth factor (bFGF) (R&D Systems) and 20 ng/ml epidermal growth factor (EGF) (R&D Systems) [Medium B]. Thereafter, the growth factors (Noggin, bFGF and EGF) were added to the medium every day. After three days (D9), the medium B was removed and the cells were maintained in medium C (medium B without bFGF and EGF) for 5 days (D14). The medium was changed daily. For the determination of optimal Noggin concentration, three independent cultures were treated with Medium C containing different concentrations of Noggin as followed: N50 (50 ng/ml Noggin), N75 (75 ng/ml Noggin) and N100 (100 ng/ml Noggin). Untreated PB-MSCs were used as the control group. These cells were cultured in Medium A for 14 days. Thereafter, six and three independent PB-MSCs cultures were examined with conditions similar to N50 and N75, respectively.
RNA extraction and cDNA synthesis
Total RNA was extracted from the control group as well as treated PB-MSCs using Total RNA purification kit (Jena Bioscience, Germany) according to the manufacturer’s instructions. The RNA extraction from all cultures was performed at D14 for matching of results. The genomic DNA contamination was eliminated by DNase I (Fermentas, Waltham, MA, USA) treatment. Absence of contaminating DNA was confirmed using 1 % agarose gel electrophoresis. Then, cDNA was synthesized in a total volume 20 μl using random dART RT kit (EURx Ltd., Poland). The cDNA synthesis reaction was incubated at 25 °C for 10 min, 50 °C for 60 min followed by enzyme inactivation at 85 °C for 5 min.
Nestin mRNA expression analysis
The quantitative RT-PCR was performed using 2x RealQ Master Mix (Ampliqon). The reaction condition was carried out at 95 °C for 10 min, then 40 cycling of 95 °C for 30s, 60 °C for 30s, and 72 °C for 30s. Each reaction was performed in duplicate. The primer sequences were as followed: Nestin - ATCGCTCAGGTCCTGGAA and AAGCTGAGGGAAGTCTTGGA (Liu et al. 2006), HSP90AB1-GGAAGTGCACCATGGAGAGGA and GCGAATCTTGTCCAAGGCATCAG. For all reactions, the melting curve was depicted to verify the specificity of amplified products and the size of reaction product was also determined by 3 % agarose gel electrophoresis.
Neuronal genes expression analysis
Real time PCR was also carried out to quantify the expression level of neuronal markers NFM, beta-tubulin III, MAP2 and NSE as well as neuronal specific genes GABRA3, SLITRK4 and MECP2. The PCR cycling conditions were performed as previously described for Nestin. Primer sequences are available upon request.
Statistical analysis
The expression of each gene of interest was normalized to the housekeeping gene HSP90AB1. Relative expression level was estimated by the pfaffl method (Pfaffl 2001). Social Science Statistics website (http://www.socscistatistics.com/tests/studentttest/Default2.aspx) was used to calculate t-test. P < 0.05 was considered statistically significant. The Mean and standard error (SE) were determined using the EasyCalculation.com website (https://www.easycalculation.com/statistics/standard-deviation.php).
Results
Morphological characterization
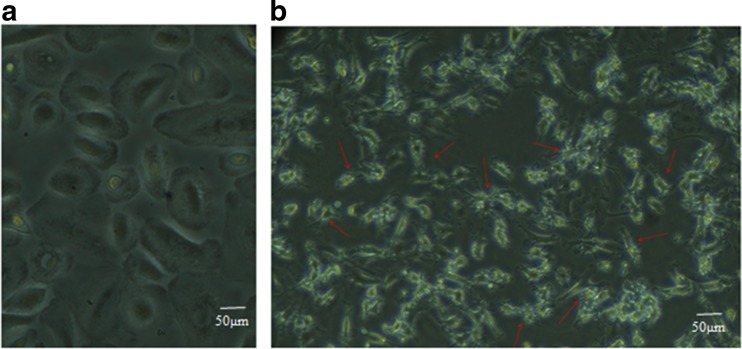
The morphological changes of treated PB-MSCs were observed by inverted microscope and compared with untreated cells. After 6 days of culture, the PB-MSCs reached 70–80 % confluence. These cells had a fibroblast-like morphology before Noggin treatment. The untreated PB-MSCs were flat and wide over time. PB-MSCs were exhibited multipolar processes and bright cell bodies after 8 days of treatment (D14). As represented in Fig. 1, the morphology of treated cells was very similar to glial cells (oligodendrocytes or astrocytes).
Fig. 1.
Morphological appearance of PB-MSCs. a The untreated cells were flat and wide over time. b PB-MSCs showed the multipolar processes and bright cell bodies after treatment with Noggin
Cell surface marker analysis of PB-MSCs by flow cytometry
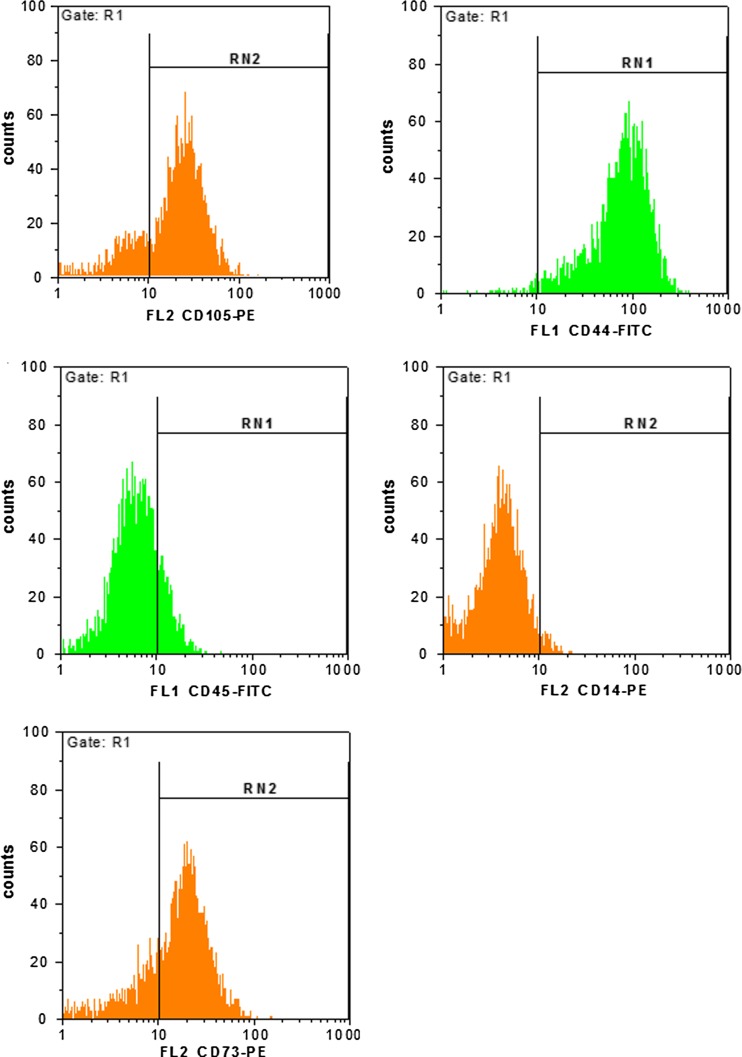
Flow cytometry analysis of surface markers expression indicated that the high expression of markers CD105, CD44 and CD73 was consistent with the profile of mesenchymal stem cells. CD14 and CD45 were not detected, suggesting that these cells had a non-hematopoietic origin (Fig. 2).
Fig. 2.
Flow cytometry analysis of CD105, CD44, CD73, CD14 and CD45 markers on PB-MSCs. Representative histogram plots showed the percentage of positive cells for respective antibodies, demonstrating them as mesenchymal stem cells (MSCs)
Effect of noggin concentration on nestin expression
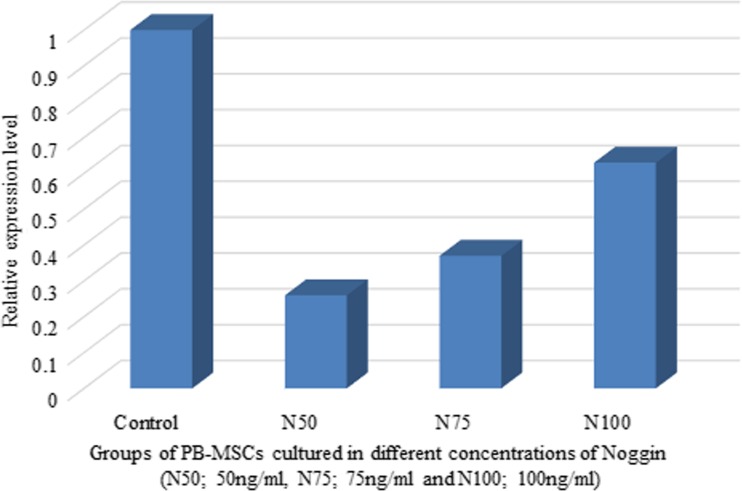
To understand the effect of Noggin concentration on Nestin expression, PB-MSCs were cultured in medium C containing different concentrations of Noggin, as described in Materials and Methods. qPCR analysis of PB-MSCs cultured in different concentrations of Noggin demonstrated that Nestin expression was inversely dependent on the concentration of Noggin. As observed in Fig. 3, culturing PB-MSCs in media containing Noggin was accompanied by down regulation of Nestin. However, the lowest expression of Nestin was observed in PBMSCs treated with 50 ng/ml Noggin. Based on the obtained results in Fig. 3, the following experiments were performed at concentrations 50 ng/ml and 75 ng/ml of Noggin in six and three different cell cultures, respectively. The results of relative expression analysis showed that Nestin expression significantly decreased in PB-MSCs following Noggin treatment (p value = 0.022 and 0.006 for N50 and N75; Fig. 4 and Table 1). The obtained findings suggested that the inhibition of BMP signaling by Noggin may support differentiation of PB-MSCs through down regulation of Nestin expression.
Fig. 3.
Nestin expression analysis in PB-MSCs treated with different concentratons of Noggin (N50; 50 ng/ml; N75; 75 ng/ml and N100; 100 ng/ml). Untreated PB-MSCs were used as control. The X-axis and Y-axis represented cell cultures ID and Relative expression level of Nestin in treated PB-MSCs as compared with untreated cells (control), respectively. PB-MSCs treated with 50 ng/ml (N50) and 75 ng/ml (N75) Noggin were associated with a significant reduction in the Nestin expression
Fig. 4.
Nestin expression level in six cultures of PB-MSCs after treatment with 50 ng/ml Noggin (N = 6, Mean = 0.3, SD = 0.32). The X-axis and Y-axis represented cell cultures ID and relative expression level of Nestin in treated PB-MSCs as compared with untreated cells (control), respectively (T-value = −2.7, p value = 0.022, student’s t-test). Numbers I, II, III, IV, V and VI represented the independent cell cultures of treated PBMSCs
Table 1.
The statistical analysis of expression data obtained from three independent PB-MSCs cultures treated with 75 ng/ml Noggin
| Neuronal markers | T-test | P value | Mean (M) | SE |
|---|---|---|---|---|
| Nestin | −5.14 | 0.006* | 0.04 | 0.029 |
| Beta tubulin III | −1.17 | 0.305 | 0.61 | 0.196 |
| MAP2 | −3.95 | 0.016* | 0.21 | 0.04 |
| NFM | 1.83 | 0.141 | 2.49 | 0.867 |
| NSE | 0.59 | 0.581 | 2.3 | 1.717 |
SE Standard Error
*The result is significant at p < 0.05
Expression of neuronal markers in noggin-treated PB-MSCs
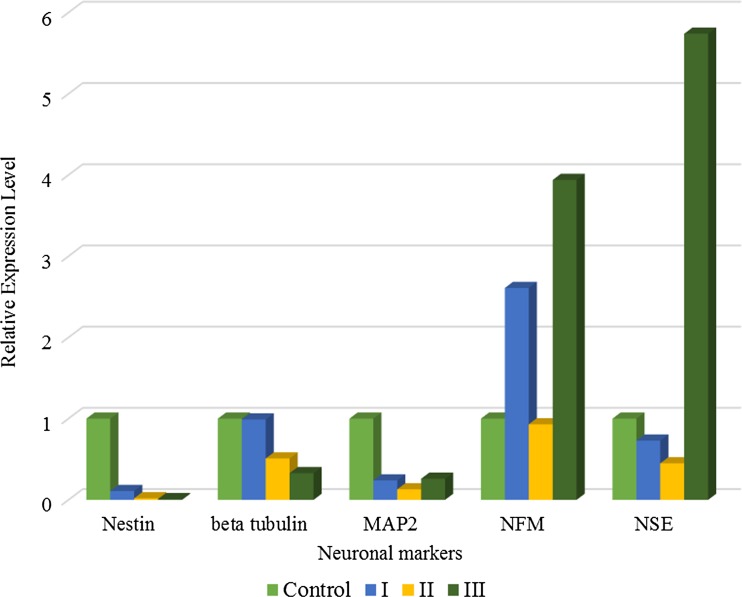
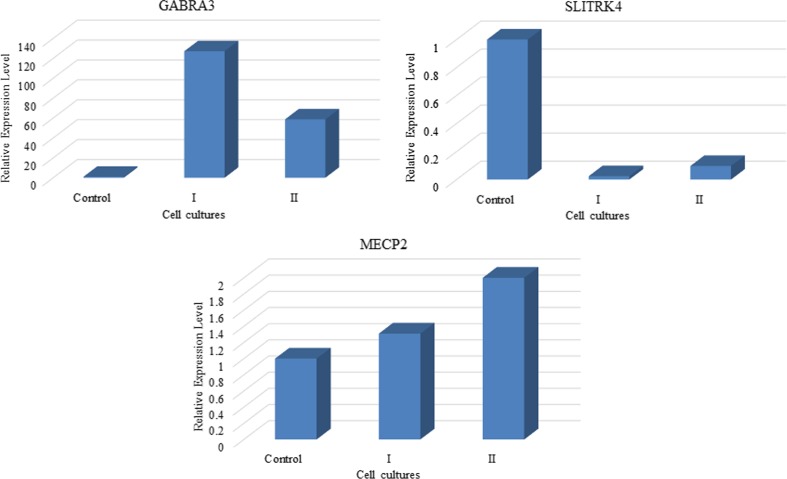
To examine whether Noggin can modulate the neuronal differentiation of PB-MSCs, the expression of neuronal markers was studied using qPCR at three independent cell cultures treated with 75 ng/ml Noggin. The results showed that Nestin, beta tubulin III and MAP2 were decreased in PB-MSCs treated with 75 ng/ml Noggin (Fig. 5 and Table 1). The NFM and NSE expression pattern showed the conflicting results in different cell cultures. The expressions of SLITRK4, MECP2 and GABRA3 were also evaluated in two Noggin-treated PB-MSCs cultures (75 ng/ml). Figure 6 showed that the treatment was accompanied by decreased expression of SLITRK4. In contrast, the GABRA3 and MECP2 expression levels increased in PB-MSCs following the treatment with Noggin.
Fig. 5.
The expression of neuronal markers including Nestin, beta tubulin III, MAP2, NFM and NSE in PBMSCs cultures (N = 3). The X-axis and Y-axis represented neuronal markers and Relative expression level of each marker in treated PB-MSCs as compared with untreated cells (control), respectively. The mean (M) and standard error (SE) of each neuronal marker were shown in Table 1. Numbers I, II and III represented the independent cell cultures of treated PBMSCs. Untreated PB-MSCs were used as control
Fig. 6.
Results of SLITRK4, GABRA3 and MECP2 expression analysis in two independent Noggin-treated PB-MSCs (N = 2). The X-axis and Y-axis represented cell cultures ID and relative expression level of neuron-specific genes in treated PB-MSCs as compared with untreated cells (control), respectively. The expression of GABRA3 and MECP2 increased in PBMSCs after treatment. In contrast to GABRA3 and MECP2, treatment with Noggin decreased SLITRK4 expression in PBMSCs as compared with untreated cells. Numbers I and II represented the independent cell cultures of treated PBMSCs. Untreated PB-MSCs were used as control
Discussion
In recent years, numerous signaling pathways were known to induce proliferation and differentiation of mesenchymal stem cells into adipocytes and osteocytes (James 2013; Longobardi et al. 2006). Different studies indicated that several members of the BMP signaling pathway involved in the differentiation of MSCs into osteocytes, adipocytes and chondrocytes (Kang et al. 2009; Cheng et al. 2001; Majumdar et al. 2001; Lou et al. 1999). Besides, it has been widely known that EGF and bFGF display a crucial role in promoting survival and proliferation of MSCs (Fan et al. 2007; Solchaga et al. 2005; Benavente et al. 2003). Also, high levels of bFGF expression were associated with normal development of the nervous system (Dono et al. 1998). In the present study, the floating cells with round shape were observed 2 days after the addition of bFGF and EGF. They were reminiscent of neural stem cells (NSCs). These observations provided evidence suggesting an important role for bFGF and EGF in stimulation of PB-MSCs proliferation.
Co-culture of neural cells and MSCs derived from bone marrow has been demonstrated to be accompanied by enhanced Nestin expression (Aizman et al. 2013). Some studies indicated that MSCs can have the stimulating effects on differentiation of neural precursors (Robinson et al. 2011; Bai et al. 2007; Kang et al. 2003). Furthermore, it has been revealed that culture of MSCs on plates coated with Extracellular matrix (ECM) could provide more desirable conditions for the growth of Nestin-positive cells (Aizman et al. 2013). In this study, the effects of these conditions were excluded by culturing the MSCs derived from peripheral blood on uncoated plates.
Noggin is a well-characterized antagonist of BMP members including BMP2 and BMP4 (Fig. 7) (Zimmerman et al. 1996; Gerrard et al. 2005). BMP4 has been found to display an important role in regulating mesodermal differentiation (Kawasaki et al. 2000). The results obtained from this study indicated that blocking BMP signaling through Noggin, diminished Nesting expression in PB-MSCs. These results suggested that the suppression of mesodermal differentiation was associated with decreased Nestin expression in MSCs derived from peripheral blood. In the previous study conducted by Fazeli et al. (2015), the authors observed that the expression of Nestin was diminished during differentiation of PB-MSCs into neuronal cells. However, their study was performed on only three independent cultures. Although the authors observed down regulation of Nestin expression in the previous study, there was no statistically significant difference in Nestin expression between Noggin-treated PB-MSCs and untreated cells (Fazeli et al. 2015). In the present study, the inhibition of BMP signaling was studied on Nestin expression in six independent cultures. The expression analysis showed a statistically significant difference between treated PB-MSCs and untreated cells.
Fig. 7.
In silico analysis of Noggin interactions with other proteins. Noggin has been known to inhibit BMP signaling through binding to several BMPs. The protein-protein interactions of Noggin were searched in STRING database (http://string-db.org/). The results obtained from STRING predicted the interaction of Noggin with proteins BMP-2, BMP-4, BMP-5, BMP-6 and BMP-7
Tian et al. (2003) demonstrated that Noggin can inhibit the BMP2 activity (Tian et al. 2003). In the other study, we also evaluated the expression of BMP2 in PB-MSCs following treatment with medium C containing Noggin. The obtained results indicated that there was a decrease in BMP2 expression after cultivating PB-MSCs with 50 ng/ml Noggin (Fazeli et al., submitted; data has been unpublished]. In total, blocking BMP signaling suggested to be accompanied by a significant decrease in Nestin expression.
The findings were inconsistent with the results obtained by Aizman et al. (Aizman et al. 2013). They showed that co-culture of MSCs and neuronal cells in the presence of Noggin caused an increased expression of Nestin (Aizman et al. 2013). The results of this study differed from Aizman et al’s study in some aspects: a) The origin of MSCs (peripheral blood versus bone marrow, b) MSCs in the study done by Aizman et al. were cultured along with neuronal cells on ECM coated plates. These conditions can support Nestin expression. In this study, MSCs were cultured on uncoated plates. Therefore, Nestin expression in our treated cells was not dependent on ECM. It appears that the treatment of MSCs with Noggin in the presence of ECM can support the enhanced Nestin expression (Aizman et al. 2013).
In the present study, the role of Noggin concentration was assessed on Nestin expression in PB-MSCs. Nestin expression was compared with PB-MSCs treated with different concentrations of Noggin (50 ng/ml; N50, 75 ng/ml; N75 and 100 ng/ml; N100). As observed in Fig. 3, the concentrations of 50 ng/ml and 75 ng/ml Noggin led to a substantial reduction in Nestin expression. Although the concentration of 100 ng/ml was accompanied by a decline in Nestin expression, these treated cells showed Nestin expression higher than the cells treated with 50 ng/ml or 75 ng/ml Noggin. It appears that increasing Noggin concentration from 50 ng/ml to 100 ng/ml had a positive impact on treated MSCs and was associated with the increased expression of Nestin. However, PB-MSCs treated with 100 ng/ml Noggin were morphologically distinct from the cultures treated with concentration of 50 ng/ml or 75 ng/ml Noggin.
Nestin has been known to be expressed at the early stage of neural differentiation (Tropepe et al. 2001). Moreover, some studies indicated that Nestin expression in MSCs did not show significant changes during osteogenic differentiation (Wong et al. 2014). In this study, Nestin expression was detected in untreated PB-MSCs. A significant decrease was also observed in Nestin expression in PB-MSCs under the conditions that BMP signaling was blocked (Fig. 4). Therefore, the obtained results suggested that the blockade of BMP signaling in PB-MSCs was not accompanied by differentiation of these cells into osteogenic lineage. The reduction of Nestin expression and Noggin-treated cells morphology suggested that these cells might begin to differentiate into neural lineage.
The neuronal markers expression of beta tubulin III, MAP2, NFM and NSE was evaluated in PB-MSCs treated with 75 ng/ml Noggin (Fig. 5). Their expression analysis showed conflicting results. Therefore, the expression of neuron-specific genes GABRA3, SLITRK4 and MECP2 was examined. The expression of MECP2 was found to be increased in PB-MSCs after treatment with 75 ng/ml Noggin. The obtained results were consistent with the previous studies, indicating that the neuronal maturation was accompanied with the overexpression of MECP2 (Jung et al. 2003; Mullaney et al. 2004).
The previous studies revealed that decreased expression of SLITRK4 supported the increase in neurite length (Marteyn et al. 2011). In this study, the SLITRK4 expression was diminished in the PB-MSCs after treatment, suggesting the differentiation of PB-MSCs into neuronal cells. Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the brain of adult mammals, functioning by binding with specific receptors. The studies have showed that GABA receptors are highly expressed in the central nervous system (CNS) (Sieghart 1995). GABRA3 along with other subunits of GABAA receptors formed the functional chloride channels, mediating the inhibitory synaptic transmission in the mature central nervous system (CNS). However, the studies showed that GABA receptors display a major role in the proliferation, migration and differentiation of neural cells during CNS development (Meier et al. 2003). Results of this study showed that GABRA3 was significantly overexpressed in the PB-MSCs treated with 75 ng/ml Noggin, confirming neural differentiation. In fact, the expression analysis of neuron-specific genes demonstrated the differentiation of PBMCs into neural lineage cells.
There is very little data on signaling pathways regulating Nestin expression. In the present study, the blockade of BMP signaling was investigated on Nestin expression in the mesenchymal stem cells for the first time. The results indicated that Noggin as antagonist of BMP signaling diminished Nestin expression, indicating that the BMP signaling may be necessary for the maintenance of Nestin expression in PB-MSCs.
Compliance with ethical standards
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This study was supported by grant no. 6235 from Shahid Beheshti University of Medical Sciences.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Contributor Information
Mir Davood Omrani, Email: davood_omrani@sbmu.ac.ir, Email: davood_omrani@yahoo.co.uk.
Sayyed Mohammad Hossein Ghaderian, Phone: +982122567222, Email: sghaderian@yahoo.co.uk.
References
- About I, Laurent-Maquin D, Lendahl U, Mitsiadis TA. Nestin expression in embryonic and adult human teeth under normal and pathological conditions. Am J Pathol. 2000;157(1):287–295. doi: 10.1016/S0002-9440(10)64539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizman I, McGrogan M, Case CC. Quantitative microplate assay for studying mesenchymal stromal cell-induced neuropoiesis. Stem Cells Transl Med. 2013;2(3):223–232. doi: 10.5966/sctm.2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Caplan A, Lennon D, Miller RH. Human mesenchymal stem cells signals regulate neural stem cell fate. Neurochem Res. 2007;32(2):353–362. doi: 10.1007/s11064-006-9212-x. [DOI] [PubMed] [Google Scholar]
- Benavente CA, Sierralta WD, Conget PA, Minguell JJ. Subcellular distribution and mitogenic effect of basic fibroblast growth factor in mesenchymal uncommitted stem cells. Growth Factors. 2003;21(2):87–94. doi: 10.1080/08977190310001613789. [DOI] [PubMed] [Google Scholar]
- Blunk T, Sieminski AL, Appel B, Croft C, Courter DL, Chieh JJ, Goepferich A, Khurana JS, Gooch KJ. Bone morphogenetic protein 9: a potent modulator of cartilage development in vitro. Growth Factors. 2003;21(2):71–77. doi: 10.1080/0897719031000148822. [DOI] [PubMed] [Google Scholar]
- Chen G, Deng C, Li YP. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SL, Lou J, Wright NM, Lai CF, Avioli LV, Riew KD. In vitro and in vivo induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene. Calcif Tissue Int. 2001;68(2):87–94. doi: 10.1007/BF02678146. [DOI] [PubMed] [Google Scholar]
- Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- Chou YH, Khuon S, Herrmann H, Goldman RD. Nestin promotes the phosphorylation-dependent disassembly of vimentin intermediate filaments during mitosis. Mol Biol Cell. 2003;14(4):1468–1478. doi: 10.1091/mbc.E02-08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dono R, Texido G, Dussel R, Ehmke H, Zeller R. Impaired cerebral cortex development and blood pressure regulation in FGF-2-deficient mice. EMBO J. 1998;17(15):4213–4225. doi: 10.1093/emboj/17.15.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25(5):1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- Fazeli Z, Ghaderian SMH, Rajabibazl M, Salami S, Vazifeh Shiran N, Omrani MD (2015) Expression pattern of neuronal markers in PB-MSCs treated by growth factors noggin, bFGF and EGF. IJMCM 4(4):209–217 [PMC free article] [PubMed]
- Foudah D, Redondo J, Caldara C, Carini F, Tredici G, Miloso M. Human mesenchymal stem cells express neuronal markers after osteogenic and adipogenic differentiation. Cell Mol Biol Lett. 2013;18(2):163–186. doi: 10.2478/s11658-013-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzerro E, Smerdel-Ramoya A, Zanotti S, Stadmeyer L, Durant D, Economides AN, Canalis E. Conditional deletion of gremlin causes a transient increase in bone formation and bone mass. J Biol Chem. 2007;282(43):31549–31557. doi: 10.1074/jbc.M701317200. [DOI] [PubMed] [Google Scholar]
- Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005;23(9):1234–1241. doi: 10.1634/stemcells.2005-0110. [DOI] [PubMed] [Google Scholar]
- Gilyarov AV. Nestin in central nervous system cells. Neurosci Behav Physiol. 2008;38(2):165–169. doi: 10.1007/s11055-008-0025-z. [DOI] [PubMed] [Google Scholar]
- Heino TJ, Hentunen TA. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr Stem Cell Res Ther. 2008;3(2):131–145. doi: 10.2174/157488808784223032. [DOI] [PubMed] [Google Scholar]
- Hendrickson ML, Rao AJ, Demerdash ON, Kalil RE. Expression of nestin by neural cells in the adult rat and human brain. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0018535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6(4):432–438. doi: 10.1016/S0959-437X(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2009;106(31):12670–12675. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275(13):9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- James AW. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica (Cairo) 2013;2013:684736. doi: 10.1155/2013/684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung BP, Jugloff DG, Zhang G, Logan R, Brown S, Eubanks JH. The expression of methyl CpG binding factor MeCP2 correlates with cellular differentiation in the developing rat brain and in cultured cells. J Neurobiol. 2003;55(1):86–96. doi: 10.1002/neu.10201. [DOI] [PubMed] [Google Scholar]
- Kang SK, Jun ES, Bae YC, Jung JS. Interactions between human adipose stromal cells and mouse neural stem cells in vitro. Brain Res Dev Brain Res. 2003;145(1):141–149. doi: 10.1016/S0165-3806(03)00224-4. [DOI] [PubMed] [Google Scholar]
- Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, Chen J, Bi Y, He BC, Park JK, Jiang W, Tang Y, Huang J, Su Y, Zhu GH, He Y, Yin H, Hu Z, Wang Y, Chen L, Zuo GW, Pan X, Shen J, Vokes T, Reid RR, Haydon RC, Luu HH, He TC. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18(4):545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28(1):31–40. doi: 10.1016/S0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Krupkova O, Jr, Loja T, Zambo I, Veselska R. Nestin expression in human tumors and tumor cell lines. Neoplasma. 2010;57(4):291–298. doi: 10.4149/neo_2010_04_291. [DOI] [PubMed] [Google Scholar]
- Langenfeld E, Deen M, Zachariah E, Langenfeld J. Small molecule antagonist of the bone morphogenetic protein type I receptors suppresses growth and expression of Id1 and Id3 in lung cancer cells expressing Oct4 or nestin. Mol Cancer. 2013;12(1):129. doi: 10.1186/1476-4598-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112(12):3491–3501. doi: 10.1002/jcb.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res. 2006;21(4):626–636. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- Lou J, Xu F, Merkel K, Manske P. Gene therapy: adenovirus-mediated human bone morphogenetic protein-2 gene transfer induces mesenchymal progenitor cell proliferation and differentiation in vitro and bone formation in vivo. J Orthop Res. 1999;17(1):43–50. doi: 10.1002/jor.1100170108. [DOI] [PubMed] [Google Scholar]
- Luther G, Wagner ER, Zhu G, Kang Q, Luo Q, Lamplot J, Bi Y, Luo X, Luo J, Teven C, Shi Q, Kim SH, Gao JL, Huang E, Yang K, Rames R, Liu X, Li M, Hu N, Liu H, Su Y, Chen L, He BC, Zuo GW, Deng ZL, Reid RR, Luu HH, Haydon RC, He TC. BMP-9 induced osteogenic differentiation of mesenchymal stem cells: molecular mechanism and therapeutic potential. Curr Gene Ther. 2011;11(3):229–240. doi: 10.2174/156652311795684777. [DOI] [PubMed] [Google Scholar]
- Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, Sharff KA, Montag AG, Haydon RC, He TC. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25(5):665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189(3):275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- Marteyn A, Maury Y, Gauthier MM, Lecuyer C, Vernet R, Denis JA, Pietu G, Peschanski M, Martinat C. Mutant human embryonic stem cells reveal neurite and synapse formation defects in type 1 myotonic dystrophy. Cell Stem Cell. 2011;8(4):434–444. doi: 10.1016/j.stem.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Meier J, Akyeli J, Kirischuk S, Grantyn R. GABA(a) receptor activity and PKC control inhibitory synaptogenesis in CNS tissue slices. Mol Cell Neurosci. 2003;23(4):600–613. doi: 10.1016/S1044-7431(03)00079-4. [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina Y, Starbuck MW, Gentile MA, Fukuda T, Kasparcova V, Seedor JG, Hanks MC, Amling M, Pinero GJ, Harada S, Behringer RR. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J Biol Chem. 2004;279(26):27560–27566. doi: 10.1074/jbc.M404222200. [DOI] [PubMed] [Google Scholar]
- Morizane A, Doi D, Kikuchi T, Nishimura K, Takahashi J. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J Neurosci Res. 2011;89(2):117–126. doi: 10.1002/jnr.22547. [DOI] [PubMed] [Google Scholar]
- Mullaney BC, Johnston MV, Blue ME. Developmental expression of methyl-CpG binding protein 2 is dynamically regulated in the rodent brain. Neuroscience. 2004;123(4):939–949. doi: 10.1016/j.neuroscience.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112(2):295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Murai J, Yoshikawa H, Tsumaki N. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res. 2006;21(7):1022–1033. doi: 10.1359/jbmr.060411. [DOI] [PubMed] [Google Scholar]
- Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22(3):377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- Park D, Xiang AP, Mao FF, Zhang L, Di CG, Liu XM, Shao Y, Ma BF, Lee JH, Ha KS, Walton N, Lahn BT. Nestin is required for the proper self-renewal of neural stem cells. Stem Cells. 2010;28(12):2162–2171. doi: 10.1002/stem.541. [DOI] [PubMed] [Google Scholar]
- Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward-van Oostwaard D, Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117(Pt 7):1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9) doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AP, Foraker JE, Ylostalo J, Prockop DJ. Human stem/progenitor cells from bone marrow enhance glial differentiation of rat neural stem cells: a role for transforming growth factor β and notch signaling. Stem Cells Dev. 2011;20(2):289–300. doi: 10.1089/scd.2009.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlgren CM, Mikhailov A, Hellman J, Chou YH, Lendahl U, Goldman RD, Eriksson JE. Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J Biol Chem. 2001;276(19):16456–16463. doi: 10.1074/jbc.M009669200. [DOI] [PubMed] [Google Scholar]
- Shih AH, Holland EC. Notch signaling enhances nestin expression in gliomas. Neoplasia. 2006;8(12):1072–1082. doi: 10.1593/neo.06526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47(2):181–234. [PubMed] [Google Scholar]
- Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203(2):398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- Stockhausen MT, Kristoffersen K, Poulsen HS. The functional role of notch signaling in human gliomas. Neuro-Oncology. 2010;12(2):199–211. doi: 10.1093/neuonc/nop022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera-Adame D, Gupta A, Kurtovic S, Chaiboonma KL, Arumugaswami V, Dafoe DC. Bone morphogenetic protein-2/−4 upregulation promoted by endothelial cells in coculture enhances mouse embryoid body differentiation. Stem Cells Dev. 2013;22(24):3252–3260. doi: 10.1089/scd.2013.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Fang C, Dicesare PE. Effects of bone morphogenetic protein-2 on cartilage oligomeric matrix protein expression in chondrocytes. Beijing Da Xue Xue Bao. 2003;35(3):317–320. [PubMed] [Google Scholar]
- Tondreau T, Lagneaux L, Dejeneffe M, Massy M, Mortier C, Delforge A, Bron D. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72(7):319–326. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- Torres J, Prieto J, Durupt FC, Broad S, Watt FM. Efficient differentiation of embryonic stem cells into mesodermal precursors by BMP, retinoic acid and notch signaling. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0036405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30(1):65–78. doi: 10.1016/S0896-6273(01)00263-X. [DOI] [PubMed] [Google Scholar]
- Varga AC, Wrana JL. The disparate role of BMP in stem cell biology. Oncogene. 2005;24(37):5713–5721. doi: 10.1038/sj.onc.1208919. [DOI] [PubMed] [Google Scholar]
- Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, Idowu O, Li M, Shen C, Hu A, Haydon RC, Kang R, Mok J, Lee MJ, Luu HL, Shi LL. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wislet-Gendebien S, Leprince P, Moonen G, Rogister B. Regulation of neural markers nestin and GFAP expression by cultivated bone marrow stromal cells. J Cell Sci. 2003;116(Pt 16):3295–3302. doi: 10.1242/jcs.00639. [DOI] [PubMed] [Google Scholar]
- Wong A, Ghassemi E, Yellowley CE. Nestin expression in mesenchymal stromal cells: regulation by hypoxia and osteogenesis. BMC Vet Res. 2014;10(1):173. doi: 10.1186/s12917-014-0173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li L. BMP signaling and stem cell regulation. Dev Biol. 2005;284(1):1–11. doi: 10.1016/j.ydbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhou J, Fang Z, Jiang M, Chen X. Noggin versus basic fibroblast growth factor on the differentiation of human embryonic stem cells. Neural Regen Res. 2013;8(23):2171–2177. doi: 10.3969/j.issn.1673-5374.2013.23.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86(4):599–606. doi: 10.1016/S0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]