Abstract
Vascular endothelial growth factor-A (VEGF-A) is essential for endothelial cell functions associated with angiogenesis. Signal transduction networks initiated by VEGFA/VEGFR2, the most prominent ligand-receptor complex in the VEGF system, leads to endothelial cell proliferation, migration, survival and new vessel formation involved in angiogenesis. Considering its biomedical importance, we have developed the first comprehensive map of endothelial cell-specific signaling events of VEGFA/VEGFR2 system pertaining to angiogenesis. Screening over 20,000 published research articles and following the post-translational modification (PTM) and site specificity of VEGFR2, we have documented 240 proteins and their diverse PTM-dependent reactions involved in VEGFA/VEGFR2 signal transduction. From the ligand-receptor complex, this map has been extended to the level of major transcriptionally regulated genes for which the signaling cascades leading to their transcription factors are reported. We believe that this map would serve as a novel platform for reference, integration, and representation and more significantly, the progressive analysis of dynamic features of VEGF signaling in endothelial cells including their cross-talks with other ligand-receptor systems involved in angiogenesis.
Electronic supplementary material
The online version of this article (doi:10.1007/s12079-016-0352-8) contains supplementary material, which is available to authorized users.
Keywords: KDR, Pathway map, Phosphosite mapping, Vascular endothelial growth factor
Introduction
Angiogenesis, the formation of new blood vessels from pre-existing vasculature, is central to a number of physiological conditions, from embryogenesis to wound healing in adults and is a hallmark of pathological conditions such as tumorigenesis (Folkman 1971; Chatterjee et al. 2013; Shibuya 2014). It is a complex process involving extensive interplay between endothelial cells (EC), soluble factors and extracellular matrix (ECM) components. Under normal physiological conditions ECs are quiescent; on receiving angiogenic signals, ECs undergo activation. Activated ECs release proteases leading to degradation of the underlying basement membrane, migration of ECs into the interstitial space, EC proliferation, cell–cell adhesion, lumen formation, generation of new basement membrane with the recruitment of pericytes, fusion of the newly formed vessels and the initiation of blood flow (Carmeliet and Jain 2011). Deregulation of the balance between pro-angiogenic factors and anti-angiogenic factors can lead to excess angiogenesis or insufficient angiogenesis contributing to pathological conditions (Oklu et al. 2010).
Vascular endothelial growth factor (VEGF) is the principal angiogenic growth factor that modulates angiogenesis through receptor tyrosine kinase VEGF receptors (VEGFRs). Multiple VEGFs (VEGF-A, VEGF-B, VEGF-C and VEGF-D) interact with VEGF receptors such as VEGFR1, VEGFR2 and VEGFR3. Among them, VEGFA/VEGFR-2 signaling appears to mediate cellular responses involved in angiogenesis prominently. VEGFA-165 is notably the most studied among multiple VEGFA isoforms. VEGF participates in the angiogenic response by increasing microvascular permeability, inducing EC proliferation, migration, survival and secretion of matrix metalloproteinases (MMPs) (Lohela et al. 2009). VEGF gradient is recognized by specialized ECs that acquire a specific tip cell phenotype characterized by the formation of numerous filopodia that extends towards the direction in which EC migrates. The ECs that trail tip cells called ‘stalk cells’ are less motile but support sprouting and vessel extension. Tip cells are guided towards avascular regions, where it can sense VEGF using VEGFR2 and VEGFR3 expressed by tip cell filopodia. Tip cell and stalk cell together form a vascular sprout and endothelial tip cells of two sprouts come together to form a new blood vessel (Gerhardt et al. 2003; Benedito et al. 2012).
VEGF binding to VEGFR results in autophosphorylation of specific tyrosine residues in the cytoplasmic domain of VEGFR2. Among them, VEGFR2 pTyr801 also further regulates the phosphorylation of Tyr1054 and Tyr1059 that increases the VEGFR2 kinase activity (Kendall et al. 1999). Phosphorylated VEGFR2 initiates downstream signaling pathways relevant to angiogenesis and produces several cellular responses in ECs including a strong mitogenic signal and survival signal. In contrast, such a strong mitogenic signal is not induced by VEGFA binding to VEGFR1 (Koch and Claesson-Welsh 2012). VEGFA stimulates the activation of diverse signaling proteins in ECs, including non-catalytic region of tyrosine kinase adaptor protein (NCK), Shc-related adaptor protein (SCK), phospholipase C-γ (PLC- γ), protein kinase C (PKC), protein kinase B (PKB/AKT), p38 mitogen-activated protein kinases (p38MAPK), focal adhesion kinase (FAK), Ras-related C3 botulinum toxin substrate (RAC), RHO GTPases, extracellular-signal-regulated kinases (ERK) (Holmes et al. 2007; Koch et al. 2011) and mammalian target of rapamycin (MTOR) (Soumya et al. 2016). The signaling network comprising of synchronously activated signaling modules and their cross talks involving these molecules modulates the process of angiogenesis.
Currently, there are no resources that host a comprehensive VEGF signalling pathway data for visualization and analysis. Although resources such as KEGG, Reactome, NetPath, STKE and BioCarta are known to focus on pathways related to cell signaling and gene regulation, they either lack VEGF signaling pathway or the data available is very generic (Ogata et al. 1999; Nishimura 2004; Joshi-Tope et al. 2005; Schaefer et al. 2009; Kandasamy et al. 2010; Korcsmaros et al. 2010; Chowdhury and Sarkar 2015). For example, VEGF pathway on KEGG contains only 30 proteins representing few activation or inhibition reactions between them without specific post-translational modifications on proteins. With multiple ligands known for VEGFRs, for the first time, we have compiled the best-studied, VEGFA-specific (especially VEGFA-165) VEGFR2 signaling events specifically in endothelial cells. The endothelial VEGFA/VEGFR2 signaling network map represents detailed downstream signaling events and the crosstalk of various signaling pathways modulated in response to VEGFA.
Methods
Manual data mining and curation
Literature survey was carried out using PubMed to retrieve information on proteins associated with VEGFA/VEGFR2 signaling pathway using the keywords ‘Angiogenesis’, ‘Vascular Endothelial Growth Factor’, ‘Vascular Endothelial Growth Factor Receptor’, and their alternative names. The molecular association between the signaling molecules was also retrieved from the EVEX database, a text mining resource that works based on the PubMed abstracts and full texts in PubMed Central. The first report on VEGF appeared in 1989 when Napoleone Ferrara and other Genentech researchers isolated a protein that is mitogenic only to endothelial cells and named it as VEGF (Leung et al. 1989).
We have screened from 83,077 hits, 26,599 articles on VEGF published from the year 1989 to 2016 focusing on VEGF variants associated with VEGFR2. The analysis and the documentation of molecular information were limited to the VEGFA effects on endothelial cells and those studied and reported in non-endothelial cells were excluded in this report. Individual signaling events in VEGFR2 signaling networks leading to cell proliferation, migration and survival were identified, recorded and categorized into protein- protein interactions, enzyme-catalyzed events, activation/inhibition reactions, transport of protein across subcellular compartments, and gene regulation events. We have also mapped the specific site and residue of post-translational modifications of proteins to their current version of Ref Seq protein accessions. All these are manually curated into formatted excel sheets. These categories are in consensus with the NetPath pathway resource (Kandasamy et al. 2010; Raju et al. 2011) for the conversion of pathway data into community data formats such as PSI-MI, BioPAX and SBML by computational biologists (Hucka et al. 2003; Hermjakob et al. 2004; Demir et al. 2010).
Graphical representation of the pathway
For the development of a highly interactive pathway map that reflect a large body of information effectively, it was manual-drawn using the visualization tool named ‘PathVisio’ (http://www.PathVisio.org) that also provides the GPML format of the pathway reactions (van Iersel et al. 2008). Incorporation of such a large body of information was not possible using automated tools such as Cytoscape or VisAnt. The PTMs on VEGFR2 and their specificity to activation of specific proteins or signaling modules were followed. Whenever available, specific VEGFR2-PTM dependent molecular association and enzyme-substrate reactions were captured and represented. A set of criteria to filter high-confidence reactions of VEGFA/ VEGFR2 signaling has been applied (Raju et al. 2011). The signaling events filtered using the criteria employed was selected to represent topology-devised graphical map of the signaling network. The PubMed reference to each of the reactions has also been provided on the edges that connect the molecules in the gpml file.
With a large number of crossing-over edges, the pathway map is made visualizable and user-friendly by introducing color-specific edges for VEGFR2-PTM dependent reactions and also for specific type of reactions as represented in the pathway map legend. Distinct types of edges are also used to distinguish diverse type of reactions (direct interactions, indirect interactions, inhibition, transport reactions, and gene regulation). All the direct interactions (protein-protein interactions, enzyme-substrate reactions, inhibition reactions, gene regulation by specific transcription factors) are represented by solid edges and the indirect reactions (reactions for which intermediate molecules are involved) are represented using dashed lines. The PTM types are also color distinguished. The second messenger small molecules such as Ca2+, arachidonic acid, cyclic GMP (cGMP), phosphatidyl inositol 4, 5-diphosphate (PIP2), inositol-1,4,5-triphosphate (IP3) and diacyl glycerol (DAG) are also incorporated appropriately. A detailed legend is provided for better view of the pathway map.
Results
Data assembly, integration and development of endothelial cell VEGFA/VEGFR2 signaling pathway map
We have identified and documented 240 proteins for which the activity or function was related to VEGFA/VEGFR2 interaction including extracellular and cell surface proteins that modulates VEGFA/VEGFR2 signaling and also VEGFR1 that bind VEGFA and its isoforms (Supplementary Table I). The signaling events involving these proteins were derived from experimentally validated data involving multiple experimental techniques and approaches such as cell based in vitro angiogenesis assays, assays for inhibition/activation of proteins, inhibitor-based studies, small interfering RNA (siRNA) transfection studies, antibody-based identifications of proteins, protein-protein interactions and their PTMs states, mutagenesis studies, and polymerase chain reaction (PCR)-based approaches for gene expression. However, all the reactions share a common factor that they are all induced significantly by VEGFA treatment in endothelial cells with respect to their unstimulated counterpart.
To develop the VEGFA dependent angiogenesis pathway map, we first categorized the cellular events into compartments such as extracellular events, plasma membrane events, cytoplasmic events and nuclear events. Among these, 11 proteins shuttled from plasma membrane to endosome, 15 proteins shuttled from cytoplasm to nucleus and 3 proteins [forkhead transcription factors 1, 3, 4 (FOXO 1, 3 and 4)] shuttled from nucleus to cytoplasm. The intracellular compartmentalization is considered reversible.
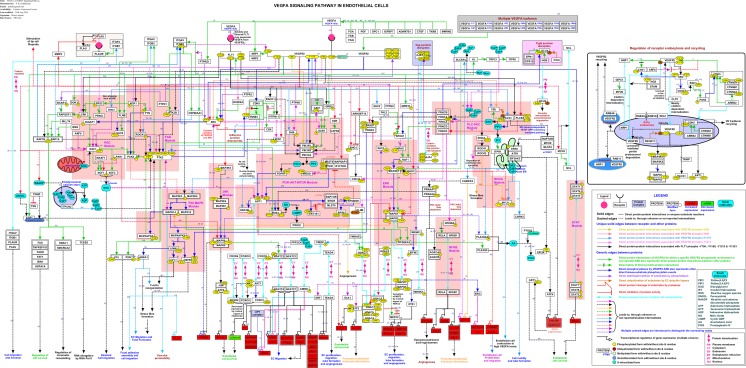
The signaling pathways regulating cell survival, cell migration, cell proliferation, cellular interactions downstream of VEGFA/VEGFR2 signaling relevant to angiogenesis, regulation of VEGFR2, phosphosite specificity of VEGFR2 towards downstream signaling, molecular function-based information and the information on the compartmentalization of proteins were integrated together to develop the complete VEGFA dependent angiogenesis pathway map (Fig. 1). This integration led to the challenges of representation of cross-talks among proteins in the canonical signaling modules such as phosphatidyl inositol-3-kinase (PI3K), AKT, PLC-γ, PKC, PKD, ERK, p38MAPK, Janus kinase (JNK), FAK, RAC, cell division cycle 42 (CDC42), RHO-A/C, signal transducer and activator of transcription (STAT), nuclear factor kappa B (NFKB) and MTOR. Hence, the representation of the pathway map was made using diverse type of edges and colors distinct from the conventional pathway maps to delineate the cross-talks for better visibility. A detailed legend is provided in the pathway map. For increased visibility, accessibility and analysis, the pathway map is also submitted to WikiPathways (http://wikipathways.org/index.php/Pathway:WP3888) that also ensures incorporation of this pathway map data into various analysis tools through GPML and BioPAX level 3 formats.
Fig. 1.
A schematic representation of VEGFA-VEGFR2 signaling pathway VEGFA-VEGFR2 signaling pathway map depicts molecules involved in protein-protein interactions, catalytic reactions, activation/inhibition and translocation events induced by VEGFA towards angiogenesis in endothelial cells. Modification dependency of VEGFA –VEGFR2 signaling is also shown in the pathway map. The references to each of the reactions are provided on the edges of each line that connect the molecules. Color specific events represent various specific reactions. Key signalling modules have been highlighted with colour background. Pathway legend is provided to identify the color code. Protein-protein interactions, post translational modifications (phosphorylation, methylation, nitrosylation, ubiquitination and glutathionylation) and gene regulations on the map are represented by solid edges and indirect reactions are represented using dashed lines
A summary of VEGFA/VEGFR2 signaling pathways in endothelial cells
The individual molecules represented in the pathway could be classified by two approaches based on the involvement of the molecules in various cellular functions critical in angiogenesis. 70 proteins involved in cell proliferation, 95 proteins involved in cell survival, 103 proteins involved in cell migration and 31 proteins involved in receptor endocytosis and recycling were categorized (Supplementary Table II). Second approach of classification of proteins could be based on their molecular functions into receptors, enzymes, and transcription factors. Together, 14 receptors, 96 enzymes and 28 transcription factors were documented in VEGFA/VEGFR2 signaling pathway (Supplementary Table III). The total number of reactions documented for VEGFA/VEGFR2 signaling involved 95 protein-protein interaction, 36 VEGFA induced direct phosphorylation, 5 dephosphorylation, 4 ubiquitination, 1 methylation, 1 glutathionylation and 1 nitrosylation events (Supplementary Table IV).
Autophosphorylation of VEGFR2 on various sites upon VEGFA binding leads to the recruitment of molecules such as SH2 domain containing adaptor protein B (SHB), SRC proto-oncogene (SRC), SH2 domain containing 2 A (TSAD), fibroblast growth factor receptor substrate 2 (FRS2). An important pathway of VEGF regulated proliferation appears to be through p-Tyr 1175-PLCγ-mediated activation of PKC and downstream induction of the ERK and other PKC-dependent pathways (Simons et al. 2016).
VEGFR2 dependent activation of PI3K-AKT signaling regulates cell survival (Jiang and Liu 2009; Koch et al. 2011). Multiple signaling pathways converge on AKT that regulates cell survival, cell proliferation, anti-apoptotic and cell permeability functions. AKT induces NFkB which further activates cyclinD1, cyclooxygenase-2 (COX-2) and c-myc thereby regulating cell proliferation and cell survival. Activation of endothelial nitric oxide synthase (eNOS) by AKT leads to regulation of cell permeability. Downstream activation of MTORC1 by AKT regulates gene expression of VEGF and angiogenesis whereas AKT is regulated at the upstream level by MTORC2 (Karar and Maity 2011). AKT also mediates crosstalk between Wnt signaling and Notch signalling (Yamamizu et al. 2010). Wnt signaling synergizes with Notch by upregulating delta like-4 (Dll4) which further activates AKT thereby inhibiting β-catenin degradation complex (Dejana 2010).
Endothelial cell migration induced by VEGFA results from several signaling pathways downstream of VEGFR2 of which the complementary role of signaling through p38MAPK (actin polymerization) and FAK (focal adhesion turnover) is particularly important in directed migration (Zhao and Guan 2011; Sawada et al. 2015). In response to VEGFA, the increased actin polymerization required to trigger actin based motility involves the recruitment of an adapter protein, NCK and its complexes to VEGFR2. Two converging signaling mechanisms were identified in this context. The first signal that emanates from VEGFR2/NCK is associated with the recruitment of NCK to phospho-Tyr1214 within VEGFR2 and it mediates actin polymerization and formation of stress fibers downstream from sequential activation of CDC42, mitogen activated protein kinase kinase 3 (MKK3) and phosphorylation of heat shock protein 27 (HSP27). The formation of stress fibres by binding of VEGFA to VEGFR2 requires cooperative interaction between VEGFR2 and integrins, especially α5β3 (Lamalice et al. 2007; Koch and Claesson-Welsh 2012).
Directed phosphoproteome of VEGFA/VEGFR2 signaling in endothelial cells
Phosphorylation is an important post-translational modification of proteins. We have included the phosphoproteins in the pathway only for those cases where their activity or modulation of interactions with other proteins is proven. Phosphosite mapping resulted in the documentation of 89 phosphorylation sites (including phosphorylation and dephosphorylation sites) in 53 proteins involved in VEGFA/VEGFR2 signaling pathway, of which 20 were of tyrosine, 21 were of threonine and 48 were of serine specific-sites. Seven phosphosites mapped in VEGFR2 receptor were: Y1054, Y1214, Y801, Y1175, Y951, Y1059 and Y996. Phosphorylation of tyrosine residues of VEGFR2 further transduces the signal to various downstream molecules to regulate events of angiogenesis and their site specificity towards downstream signalling were also documented for representation. Y1214 phosphorylation regulates signaling events involved in cell migration. Y801 phosphorylation regulates cell survival. Y1175 regulates both cell proliferation and migration. Y951 and Y1059 phosphorylation regulates cell survival, cell migration and cell proliferation. Specific function of Y996 phosphorylation was not clear. Phosphorylated Y1054 interacts with integrins and regulates signaling events involved in cell migration.
Context-dependent VEGF signaling
VEGF signaling depends on the activation state of ECs. The changes in oxygen status, metabolite status and inflammatory mediators can be sensed by different sensing mechanisms within the cell which can further modulate VEGF signaling. Such context dependent information can be further integrated into this signaling map.
Inflammation mediating enzyme COX produces prostaglandins which links the inflammatory status of the cell with VEGF signaling. Nutrient status of the cell is linked to VEGF signaling through AMP-activated protein kinase (AMPK) and MTOR. During conditions of energy starvation AMPK is activated which further acts through its downstream effector, MTOR, leading to upregulation of VEGF. FOXO, predominant transcription factors expressed in endothelial cells are involved in linking metabolite status of the cell with VEGF signaling. During hypoxic conditions hypoxia - inducible factor (HIF) promotes VEGF transcription. In addition, hormones [follicle stimulating hormone, progesterone and testosterone] and growth factors other than VEGF regulate angiogenesis concurrent with VEGF signaling. Fibroblast growth factor, epidermal growth factor and placental growth factor mediated signaling pathways occur synchronously with VEGFA signaling in endothelial cells to regulate angiogenesis (Kumar et al. 2014) These multiple signaling pathways converge at signaling molecules AKT and MTOR which further modulate downstream signaling regulating angiogenesis.
Regulation of VEGF dependent angiogenic signaling
There are control systems that regulate VEGFA/VEGFR2 signaling. Plasma membrane levels of VEGFR2 are regulated by endocytosis and secretory transport through the Golgi apparatus. 31 molecules were involved in regulating receptor endocytosis and recycling, which included 11 enzymes and 1 receptor. Binding of VEGFA to the cell surface localized VEGFR2 stimulates trafficking of intracellular VEGFR2 from the Golgi apparatus to the plasma membrane. Golgi localized t-soluble N-ethylmaleimide attachment protein receptor (t-SNARE) protein and syntaxin 6 were reported to regulate cellular VEGFR2 levels and the inhibition of syntaxin 6 reduced VEGF- induced angiogenesis.
Endothelial cell function and blood vessel formation are regulated by recycling of VEGFR2 from endosome to plasma membrane. Quiescent VEGFR2 is efficiently recycled back to the cell surface via Rab4a and Rab11a-positive endosomes depending on the association of VEGFR2 with neuropilin-1 (NRP1). Rab5a regulated VEGFR2 trafficking through early endosomes whereas Rab7a regulated VEGFR2 trafficking out of late endosomes. VE-cadherin also controls VEGFR2 activity by inhibiting VEGFR2 internalization and promoted its inactivation at the cell surface. Protein-protein interactions involving VEGFA and other proteins such as connective tissue growth factor (CTGF/CCN2) and perlecan in the extracellular space and proteins such as heparan sulphate proteoglycan (HSPG), NRP and VEGFR1 on the cell surface can affect the level of VEGFA and its isoforms available for binding to VEGFR2 and subsequent induction of downstream signal transduction pathways. Further, VEGFR1 binding, unlike NRP1 and NRP2 can trigger signals, though weak, converging to VEGFR2 signaling pathway. The regulation of VEGF signaling by integrins and other signaling systems are currently beyond the limit of confinement in this article.
Discussion
Angiogenesis requires the coordinated action of a variety of growth factors, metabolites and cell adhesion molecules in endothelial cells (Kumar et al. 2014; Kitazume et al. 2014; Kunhiraman et al. 2016). VEGF/VEGFR signaling is the well-characterized signaling pathway in angiogenesis. During angiogenesis, VEGFA binds to its cognate receptor, VEGFR-2, and activates multiple downstream pathways via signaling intermediates, such as mitogen activated protein kinases (MAPK), PI3K, AKT, PlC-γ and small GTPases (Shibuya and Claesson-Welsh 2006; Lohela et al. 2009). As a result, VEGF signaling promotes endothelial proliferation, filopodial extension, degradation of the extracellular matrix and angiogenesis. VEGFA/VEGFR2 signaling consists of critical junctions/branching points where various downstream signaling pathways regulating cell migration, proliferation and survival converge/communicate together by regulatory feed forward and feedback loops and is essential for modulating angiogenesis. As it involves a series of connected, but overlapping, signaling events, it becomes clear that a strict temporal and spatial regulation of cell signaling pathways and downstream gene expression are required within a developing vessel for proper regulation. Moreover, there is a great deal of cross-talk of VEGF system with other extrinsic signaling systems that they interact synergistically or antagonistically such as Wnt (Dejana 2010) and Notch (Yamamizu et al. 2010) pathways that act synergistically in modulating angiogenesis. Modulation of angiogenesis signalling cascades by microRNAs is an emerging area in angiogenesis research (Fish et al. 2008; Chen et al. 2016). In order to reduce the complexity of the pathway map, we did not consider the regulatory role of microRNAs in the formation of vasculature in this pathway. It is possible to edit and integrate the pathway modules that lead to the regulation of expression of specific microRNAs and their targets into this pathway map for analysis and representation.
Our current work focused on developing a comprehensive VEGFA/VEGFR2 signaling pathway map in endothelial cells pertaining to angiogenesis. This signaling pathway map was developed based on data, relating mostly to human umbilical vein, retrieved from relevant published research articles considering primarily the endothelial cells, the principal cell involved in angiogenesis. VEGFA-VEGFR2 signaling might exhibit similarity and differences in ECs in different vascular beds. In vascular tissues, angiogenic activity of VEGF is regulated by the expression of VEGF isoforms and their receptors; in avascular tissues, VEGF exists as an inactive form in the extracellular matrix (ECM) through dynamic interaction with components of ECM and/or other factors such as CTGF, which inhibits the angiogenic activity of VEGF (Inoki et al. 2002; Khattab et al. 2015). VEGFA/VEGFR2 signaling shows significant difference between sprouting tip cells and non-sprouting stalk cells apparently through modulation of Notch signalling (Blanco and Gerhardt 2013). VEGFA/VEGFR2 signaling also exhibits difference in the process of arterial–venous endothelial cell differentiation (Zhang et al. 2008). The pathway map is provided in. gpml format so that users can add more entities (such as proteins, mRNA, microRNA and inhibtors) and reactions based on the supporting documents to the map in the future. This also ensures maintaining it up-to-date, integration, analysis and representation of novel data and findings by both biologists and bioinformaticians alike.
The comprehensive signaling pathway map presented in this study will be useful in studying agents targeting angiogenesis associated disorders. In view of its biomedical importance, we anticipate that the availability of a comprehensive pathway map will further accelerate the research on angiogenesis and its associated functions. Thus, the angiogenesis pathway data organized in this study will serve as a template for gene set enrichment and pathway analysis of data. Such a development will increase the chances of identifying the role of angiogenesis signaling under diverse physiological and pathological conditions in humans.
Electronic supplementary material
List of total proteins (with gene name and gene ID) in the VEGFA/VEGFR2 pathway map (XLS 77 kb)
Classification of VEGF AA-VEGF AR2 signaling molecules based on their role in cellular function (XLS 33.5 kb)
Classification of molecules based on cellular functions (DOC 111 kb)
List of molecular associations, catalysis, transport, VEGF regulated phosphosites and indirect interactions in the VEGFA/VEGFR2 pathway map (XLS 80 kb)
Acknowledgment
Financial assistance from Kerala State Council for Science Technology and Environment, Thiruvananthapuram, is gratefully acknowledged.
Abbreviations
- AMPK
AMP-activated protein kinase
- CDC42
Cell division cycle 42
- cGMP
Cyclic GMP
- COX-2
Cyclooxygenase-2
- CTGF/CCN2
Connective tissue growth factor
- DAG
Diacyl glycerol
- Dll4
Delta like-4
- EC
Endothelial cells
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- eNOS
Endothelial nitric oxide synthase
- ERK
Extracellular-signal-regulated kinases
- FAK
Focal adhesion kinase
- FGF
Fibroblast growth factor
- FOXO
Forkhead transcription factors
- FRS2
Fibroblast growth factor receptor substrate 2
- FSH
Follicle stimulating hormone
- HSP27
Heat shock protein 27
- HSPG
Heparan sulphate proteoglycan
- IP3
Inositol-1,4,5-triphosphate
- JNK
Janus kinase
- MKK3
Mitogen activated protein kinase kinase3
- MMPs
Matrix metalo proteases
- MTOR
Mammalian target of rapamycin
- NCK
Non-catalytic region of tyrosine kinase adaptor protein
- NRP
Neuropilin
- p38MAPK
p38 mitogen-activated protein kinases
- PCR
Polymerase chain reaction
- PI3K
Phosphatidyl inositol -3-kinase
- PIP2
Phosphatidyl inositol 4, 5-diphosphate
- PKB
Protein kinase B
- PKC
Protein kinase C
- PLC-γ
Phospholipase C-γ
- PlGF
Placental growth factor
- PTM
Post-translational modification
- RAC
Ras-related C3 botulinum toxin substrate
- SAPK2/p38
Stress-activated protein kinase 2/p38
- SCK
Shc-related adaptor protein
- SHB
SH2 domain containing adaptor protein B
- siRNA
Small interfering RNA
- SRC
SRC proto-oncogene
- t-SNARE
t-soluble N-ethylmaleimide attachment protein receptor
- VEGF-A
Vascular endothelial growth factor-A
- VEGFR1
Vascular endothelial growth factor receptor 1
- VEGFR2
Vascular endothelial growth factor receptor 2
References
- Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- Blanco R, Gerhardt H. VEGF and notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3:a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Heukamp LC, Siobal M, Schöttle J, Wieczorek C, Peifer M, Frasca D, Koker M, König K, Meder L, Rauh D, Buettner R, Wolf J, Brekken RA, Neumaier B, Christofori G, Thomas RK, Ullrich RT. Tumor VEGF: VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J Clin Invest. 2013;123:1732–1740. doi: 10.1172/JCI65385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Li Y, Li Y, Jin L, Su Z, Yu Z, Yang S, Mao X, Lai Y. Tumor suppressive microRNA-429 regulates cellular function by targeting VEGF in clear cell renal cell carcinoma. Mol Med Rep. 2016;13:1361–1366. doi: 10.3892/mmr.2015.4653. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Sarkar RR (2015). Comparison of human cell signaling pathway databases—evolution, drawbacks and challenges. Database (Oxford). 2015:bau126. doi:10.1093/database/bau126 [DOI] [PMC free article] [PubMed]
- Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010;107:943–952. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]
- Demir E, Cary MP, Paley S, Fukuda K, Lemer C, Vastrik I, G W, D’ Eustachio P, Schaefer C, et al. The BioPAX community standard for pathway data sharing. Nat Biotechnol. 2010;28:935–942. doi: 10.1038/nbt.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197108122850711. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermjakob H, Montecchi-Palazzi L, Bader G, Wojcik J, Salwinski L, Ceol A, Moore S, Orchard S, Sarkans U, et al. The HUPO PSI’s molecular interaction format–a community standard for the representation of protein interaction data. Nat Biotechnol. 2004;22:177–183. doi: 10.1038/nbt926. [DOI] [PubMed] [Google Scholar]
- Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19:2003–2012. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Hucka M, Finney A, Sauro HM, Bolouri H, Doyle JC, Kitano H, Arkin AP, Bornstein BJ, Bray D, et al. The systems biology markup language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics. 2003;19:524–531. doi: 10.1093/bioinformatics/btg015. [DOI] [PubMed] [Google Scholar]
- Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Liu LZ. PI3K/PTEN Signaling in Angiogenesis and Tumorigenesis. Adv Cancer Res 102: 19–65. 2009 doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi-Tope G, Gillespie M, Vastrik I, D'Eustachio P, Schmidt E, de Bono B, Jassal B, Gopinath GR, GR W, Matthews L, Lewis S, Birney E, Stein L. Reactome: a knowledgebase of biological pathways. Nucleic Acids Res. 2005;33(Database issue):D428–D432. doi: 10.1093/nar/gki072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K, Mohan SS, Raju R, Keerthikumar S, Kumar GS, Venugopal AK, Telikicherla D, Navarro JD, Mathivanan S, Pecquet C, Gollapudi SK, et al. NetPath: a public resource of curated signal transduction pathways. Genome Biol. 2010;11(1):R3. doi: 10.1186/gb-2010-11-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karar J, Maity A (2011) PI3K/AKT/mTOR Pathway in Angiogenesis. Frontiers in Molecular Neuroscience 4 [DOI] [PMC free article] [PubMed]
- Kendall RL, Rutledge RZ, Mao X, Tebben AJ, Hungate RW, Thomas KA. Vascular endothelial growth factor receptor KDR tyrosine kinase activity is increased by autophosphorylation of two activation loop tyrosine residues. J Biol Chem. 1999;274:6453–6460. doi: 10.1074/jbc.274.10.6453. [DOI] [PubMed] [Google Scholar]
- Khattab HM, Aoyama E, Kubota S, Takigawa M. Physical interaction of CCN2 with diverse growth factors involved in chondrocyte differentiation during endochondral ossification. J Cell Commun Signal. 2015;9:247–254. doi: 10.1007/s12079-015-0290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazume S, Imamaki R, Ogawa K, Taniguchi N. Sweet role of platelet endothelial cell adhesion molecule in understanding angiogenesis. Glycobiology. 2014;24:1260–1264. doi: 10.1093/glycob/cwu094. [DOI] [PubMed] [Google Scholar]
- Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harbor perspectives in medicine. 2012;2(7):a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- Korcsmaros T, Farkas IJ, Szalay MS, Rovó P, Fazekas D, Spiró Z, Böde C, Lenti K, Vellai T, Csermely P. Uniformly curated signaling pathways reveal tissue-specific cross-talks and support drug target discovery. Bioinformatics. 2010;26:2042–2050. doi: 10.1093/bioinformatics/btq310. [DOI] [PubMed] [Google Scholar]
- Kumar VB, Binu S, Soumya SJ, Haritha K, Sudhakaran PR. Regulation of vascular endothelial growth factor by metabolic context of the cell. Glycoconj J. 2014;31:427–434. doi: 10.1007/s10719-014-9547-5. [DOI] [PubMed] [Google Scholar]
- Kunhiraman H, Edatt L, Thekkeveedu S, Poyyakkara A, Raveendran V, Kiran MS, Sudhakaran PR, Kumar SV. 2-Deoxy Glucose Modulates Expression and Biological Activity of VEGF in a SIRT-1 Dependent Mechanism. J Cell Biochem. 2016 doi: 10.1002/jcb.25629. [DOI] [PubMed] [Google Scholar]
- Lamalice L, Le Boeuf FL, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Lohela M, Bry M, Tammela T, Alitalo K (2009) VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol 21:154–165. doi:10.1089/152791601750294344.10.1016/j.ceb.2008.12.012 [DOI] [PubMed]
- Nishimura D (2004). Biotech Software & Internet Report. 2(3): 117-120. doi:
- Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999;27:29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oklu R, Walker TG, Wicky S, Hesketh R. Angiogenesis and current antiangiogenic strategies for the treatment of cancer. J Vasc Interv Radiol. 2010;21:1791–1805. doi: 10.1016/j.jvir.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Raju R, Nanjappa V, Balakrishnan L, Radhakrishnan A, Thomas JK, Sharma J, Tian M, Palapetta SM, Subbannayya T, Sekhar NR, Muthusamy B et al., (2011). NetSlim: high-confidence curated signaling maps. Database (Oxford) 2011:bar032. doi:10.1093/database/bar032 [DOI] [PMC free article] [PubMed]
- Sawada J, Li F, Komatsu M. R-Ras inhibits VEGF-induced p38MAPK activation and HSP27 phosphorylation in endothelial cells. J Vasc Res. 2015;52:347–359. doi: 10.1159/000444526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer CF, Anthony K, Krupa S, Buchoff J, Day M, Hannay T, Buetow KH. PID: the pathway interaction database. Nucleic Acids Res. 2009;37(Database issue):D674–D679. doi: 10.1093/nar/gkn653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M. VEGF-VEGFR Signals in Health and Disease. BiomolTher (Seoul) 2014;22:1–9. doi: 10.4062/biomolther.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016 doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- Soumya SJ, Athira AP, Binu S, Sudhakaran PR (2016). mTOR as a modulator of metabolite sensing relevant to angiogenesis. In: Maiese K (Ed.) Molecules to Medicine with mTOR: Translating Critical Pathways of the Mammalian Target of Rapamycin into Novel Therapeutic Strategies. Elsevier Science & Technology Books. Academic Press, USA. pp. 229–243. doi: 10.1016/B978-0-12-802733-2.00014-1
- van Iersel MP, Kelder T, Pico AR, Hanspers K, Coort S, Conklin BR, Evelo C. Presenting and exploring biological pathways with PathVisio. BMC Bioinforma. 2008;9:399. doi: 10.1186/1471-2105-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamizu K, Matsunaga T, Uosaki H, Fukushima H, Katayama S, Hiraoka-Kanie M, Mitani K, Yamashita JK. Convergence of notch and beta-catenin signaling induces arterial fate in vascular progenitors. J Cell Biol. 2010;189:325–338. doi: 10.1083/jcb.200904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Zhou J, Fan Q, Zheng Z, Zhang F, Liu X, Hu S. Arterial-venous endothelial cell fate is related to vascular endothelial growth factor and Notch status during human bone mesenchymal stem cell differentiation. F EBS Lett. 2008;582:2957–2964. doi: 10.1016/j.febslet.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63:610–615. doi: 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of total proteins (with gene name and gene ID) in the VEGFA/VEGFR2 pathway map (XLS 77 kb)
Classification of VEGF AA-VEGF AR2 signaling molecules based on their role in cellular function (XLS 33.5 kb)
Classification of molecules based on cellular functions (DOC 111 kb)
List of molecular associations, catalysis, transport, VEGF regulated phosphosites and indirect interactions in the VEGFA/VEGFR2 pathway map (XLS 80 kb)