Abstract
OBJECTIVE: The purpose of this article is to analyze the expression of Glut-1 and HK-II, the association between their expression and 18F-FDG accumulation in pancreatic cancer. METHODS: Fifty patients with histologically proven pancreatic cancer were included in this preliminary study, all of whom received 18F-FDG PET/CT performance before surgery. Immunohistochemical staining of tumor tissue and adjacent normal tissue was performed for Glut-1 and HK-II. By combining proportions and intensity of immunochemical staining, we obtained the modified immunohistological scores for Glut-1 and HK-II respectively. The relationship between expression of Glut-1, HK-II and series of parameters was analyzed, i.e. clinicopathological characteristics, prognosis of patients and SUVmax of PET-CT. RESULTS: Compared with normal tissue, the Glut-1 and HK-II expression in pancreatic cancer tissue was significantly increased (P < .001). There was no correlation between expression of Glut-1, HK-II and age, gender, tumor size, tumor location, tumor histological type, tumor differentiation, the nerve infiltration, vascular invasion, local infiltration, lymph node metastasis or tumor staging in pancreatic cancer (P > .05). During the follow-up period, the survival curves of low Glut-1 group and high Glut-1 group were statistically different (P = .049). Multivariate analysis (Cox regression) revealed that Glut-1 expression was not associated with mortality (P > .05). No statistical difference was found in the survival curves of negative HK-II group and positive HK-II group (P = .545). There was no correlation between 18F-FDG uptake and expression of Glut-1 and HK-II(P > .05). CONCLUSION: The Glut-1 and HK-II expression in pancreatic cancer tissue was significantly increased. There was no correlation between expression of Glut-1, HK-II and clinicopathological characteristics, prognosis and 18F-FDG uptake.
Introduction
Pancreatic cancer is considered to be one of the most aggressive human cancers, and the prognosis is poor. The 5-year survival rate is less than5% [1], [2]. The main reasons of high mortality lay in difficult early-diagnosis and lack of special treatment. Therefore, understanding the molecular biology of pancreatic cancer is important for early diagnosis and treatment, and may eventually provide new therapeutic targets for pancreatic cancer.
The cancer cells typically depend more on aerobic glycolysis (a persistently high rate of glucose conversion into lactate even under normoxic condition). This increased glycolysis, which accompanied by accelerated glucose uptake, is known as the Warburg effect, after German biochemist Otto Warburg [3], who first described the phenomenon in 1920s. The Warburg effect is a universal property of cancers [4], [5] and a distinctive metabolic characteristic of malignancies that distinguishes them from normal cells. It is reported that glucose uptakes increase in 60–90% of malignant tumors (including pancreatic cancer). This particular way of energy supply makes it possible to kill tumor cells specially. Therapeutic strategies based on glucose metabolism become the hotspots in tumor studies.
The exact mechanism for the accelerated glucose use seen in tumors is not clear. It is mentioned that the increased glucose uptakes mainly lay in two factors: transmembrane transport mediated by specific glucose transporters (Gluts) [6], [7] and increased concentrations of hexokinases (HKs) [8], [9]. As major subtypes of each family, glucose transporter protein, type 1 (Glut-1) and glucose phosphorylation enzyme type II (Hexokinase II, or HK-II) have become the focus of tumor research, and may provide new targets for tumor therapy.
The use of 18F-FDG is based on the increase glucose metabolism of malignant cells, in which 18F-FDG, an analogue of glucose, is absorbed, phosphorylated, and trapped in the cytosol of the cells. 18F-FDG PET/CT has been widely used not only for detecting and staging pancreatic tumors but also for monitoring therapy response [6], [10]. The exact mechanism of 18F-FDG accumulation in pancreatic cancer has not been fully elucidated. Recent years, More and more studies have evaluated the relationship between temporal changes in 18F-FDG uptake and expression of Glut-1 or HK-II [11], [12]. It is indicated that the high expression of Glut-1 is an important factor for 18F-FDG in malignant tumors [13], [14]. Whereas, in different malignant tumors, the correlations between 18F-FDG uptake and expression of Glut-1 or HK-II were different.
But as far as immunohistochemical study of Glut-1 and HK-II in human pancreatic cancer is concerned, only a few cases have been reported. In this study, we examined the immunohistochemical expression of Glut-1 and HK-II in the resected pancreatic tumor. The relationships between expression of Glut-1 or HK-II and clinicopathological characteristics, prognosis of pancreatic cancer patients were analyzed to explore new methods for diagnose and treatment. The correlations between 18F-FDG accumulations and expression of Glut-1 or HK-II were analyzed in order to elucidate the mechanism of 18F-FDG accumulation in pancreatic cancer.
Materials and Methods
Materials
This study consisted of 50 patients who had an pancreatic operation between June 2011 and December 2013. All patients underwent 18F-FDG PET/CT imaging before operation. Final diagnoses were confirmed histopathologically in resected specimens obtained by operation of all patients. None of them received any previous chemotherapeutic or radiotherapeutic treatments. All patients have full clinical data. There were 33 men and 17 women with ages ranging from 43 years to 80 years (median 64.4 years). Tumor staging was based on the TNM system proposed by AJCC (American Joint Committee on Cancer Staging):8 cases in StageI,36 cases in StageII,5 cases in StageIII and 1 case in StageIV. The study was performed with institutional review board approval. An informed consent was obtained from all the patients who participated in this study.
The survival datas of patients were obtained by telephone contact or direct home visit. Survival was determined from the date of surgery until death of patients. Death from a cause other than cancer relapse or survival until the end of observation period (December1, 2013) was considered a censoring event or patient death. The follow-up time was 6 to 35 months (the median follow-up time was 17 months). We lost track of 5 patients during the observation period, and the follow-up rate was 90%. Patient information and tumor characteristics are summarized in Table 1.
Table 1.
Patient Characteristics
| Patient Characteristics | Number |
|---|---|
| Age | |
| ≥60 | 36 |
| <60 | 14 |
| Gender | |
| Male | 34 |
| Female | 16 |
| Tumor size | |
| ≤4 cm | 33 |
| >4 cm | 17 |
| Tumor location | |
| Head of pancreas | 37 |
| Body and tail of pancreas | 12 |
| Both | 1 |
| Tumor histological type | |
| Other types | 12 |
| Ductal adenocarcinoma | 38 |
| Tumor differentiation | |
| II, III, III | 25 |
| I,II | 21 |
| Nerve infiltration | |
| Present | 24 |
| Absent | 26 |
| Vascular invasion | |
| Present | 19 |
| Absent | 31 |
| Local infiltration | |
| Present | 37 |
| Absent | 13 |
| Lymph node metastasis | |
| Present | 24 |
| Absent | 26 |
| Tumor staging | |
| I-IIA | 24 |
| IIB-IV | 26 |
Methods
PET/CT study
The patients fasted for at least six hours before the 18F-FDG injection. Serum levels of glucose were monitored immediately before the 18F-FDG injection. 18F-FDG (approximately 7.4 MBq/kg) was administered intravenously. Simultaneous emission-transmission PET/CT scans were performed one hour after 18F-FDG injection with a dedicated scanner (Siemens Biograph HD 64).
PET images were compared with the corresponding CT images. 18F-FDG accumulation was analyzed semiquantitatively by calculating the standardized uptake value (SUV) in the regions of interest (ROI) placed over the suspected lesions on one-hour images after injection of 18F-FDG. The ROI placed over the tumor was 10 × 10 mm (independent of tumor size) and was placed in tumor areas that showed the highest 18F-FDG activity. The transaxial slice with the highest radioactivity concentration within the tumor was identified, and the decay-corrected maximal count in the tumor area was divided by the injected dose of 18F-FDG normalized for body weight to calculate the maximum standardized uptake value (SUVmax).
Immunohistochemical staining
The expression of Glut-1 and HK-II was detected by immunohistochemistry (enhance labeled polymer system, ELPS) in 50 cases of pancreatic cancer tissue and 50 cases of corresponding adjacent tissue. The resected specimens were fixed in 10% formalin. The immunoperoxidase procedure(avidin-biotin-complex method) was performed on the paraffin embedded sections to allow the detection of Glut-1 and HK-II expression. Antigen retrieval was performed by heating the deparaffinized and rehydrated sections in 10 mmol/l citrate buffer for five minutes. The polyclonal rabbit anti-human Glut-1 antibody (DAKO Cytomation A/S, Copenhagen, Denmark) and monoclonal mouse anti-human HK-II antibody(Abcam ab104836, Cambridge, MA) were used as the primary antibodies at a dilution of 1:100 and 1:200 respectively.
Immunohistochemical analysis for anti-Glut-1 antibody and anti- HK-II antibody was independently performed by three well-experienced physicians who were unaware of patients' datas. A random selection of ten photographic cuts was examined in each paraffin tissue. According to the criteria of Higashi et al [15], the staining intensity was classified as 0, 1, 2 or 3 points for no staining, weak, moderate and strong intensity, respectively. Moreover, The percentage of positive cells was rated as: 1 point, 0%–10% positive cells; 2 points, 11–50% positive cells; 3 points, 51%–75% positive cells; 4 points, >75% positive cells. The expression levels of Glut-1 and HK-II were assessed semiquantitatively using the product of these scores (intensity × % positive): 0–3 points, negative(−),4–5 points, weakly positive(+), 6–8 points, positive (++), 9–12 points, strongly positive (+++) [16]. For statistical reasons, tumors were classified into two groups: (−),negative; (+), (++) and (+++), positive. In survival analysis, tumors were classified into two groups according to the Glut-1 expression: (−), (+) and (++), low reactivity group, (+++)high reactivity group.
Statistical Analysis
Statistical analysis was performed using the SPSS 19.0 statistical software package. The quantitative data was expressed as mean ± standard deviation. The difference between groups of continuous data was identified by Student's t-test. Categorical variables were assessed by χ2 or Fisher's exact test. Spearman's rank correlation coefficient test was carried out for testing the association between ordinal variables. Survival curves were calculated using the Kaplan–Meier method and compared by the log-rank test. The Cox proportional hazards regression model was used for multivariate analyses after univariate analysis defined relevant prognostic variables. Significance was presumed at P < .05.
Results
Immunohistochemical Findings
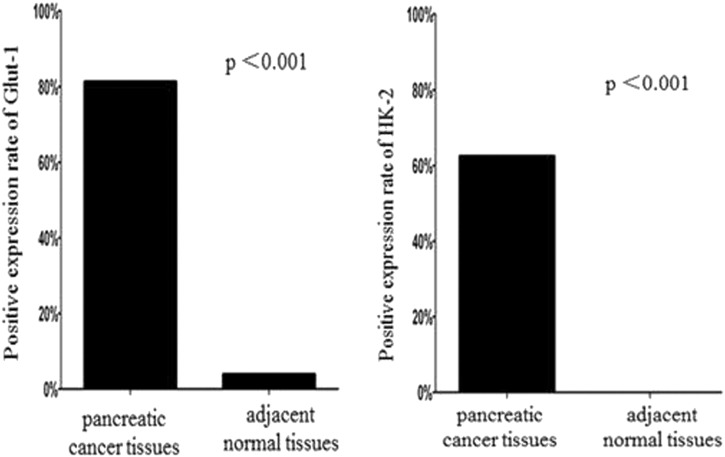
Glut-1 expression in pancreatic tumors occured mainly in the cytoplasm and in the plasma membrane of tumor cells (Figure 1). In the examined patients, pancreatic cancer cells showed different expression of Glut-1: negative in 9 cases,weakly positive in 3cases,positive in 11cases and strongly positive in 25cases. In adjacent normal tissue, negative, weakly positive, positive and strongly positive expression was detected in 48, 2, 0 and 0 cases respectively. The protein expression of Glut-1 in tumor tissue was 81.3%, being significantly higher than that in adjacent normal tissue(4%, P < .001) (Figure 2).
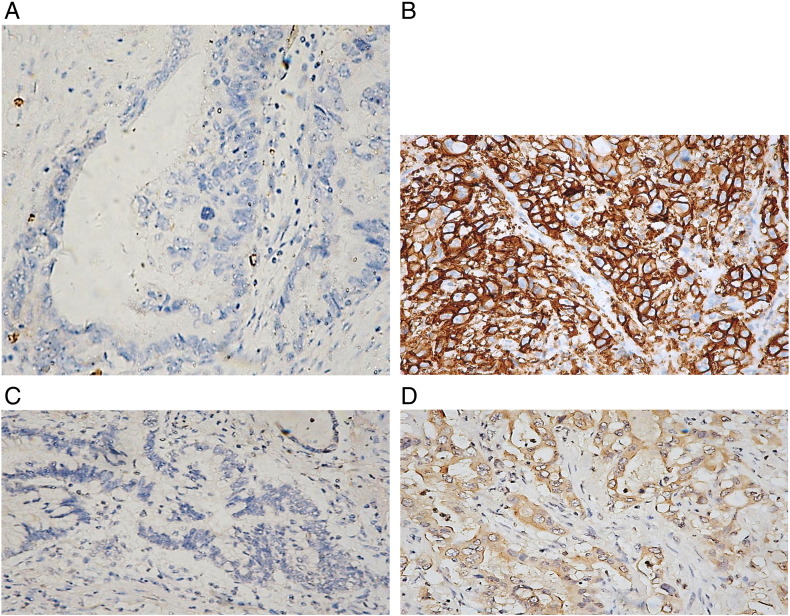
Figure 1.
A: Negative expression of Glut-1 in adjacent normal tissue.
B: Positive expression of Glut-1 in pancreatic cancer.C: Negative expression of HK-II in adjacent normal tissue.D: Positive expression of HK-II in pancreatic cancer.
Figure 2.
The positive expression rate of Glut-1 and HK-IIIn pancreatic cancer tissue and adjacent normal tissue.
HK-II was mainly expressed in the cytoplasm of tumor cells (Figure 1). As well as Glut-1 staining, pancreatic cancer cells showed different expression of HK-II ranged from negative in 18 cases,weakly positive in 3 cases,positive in18 cases,to strongly positive in 9 cases. The expression of HK-II was 62.5% in tumor cells. There were 50, 0, 0 and 0 cases of adjacent normal tissue showing negative, weakly positive, positive and strongly positive HK-II expression. That is to say, there was no HK-II expression in adjacent normal tissue. The HK-II expression between two groups was statistically different (62.5% vs 0%, P < 0. 001) (Figure 2).
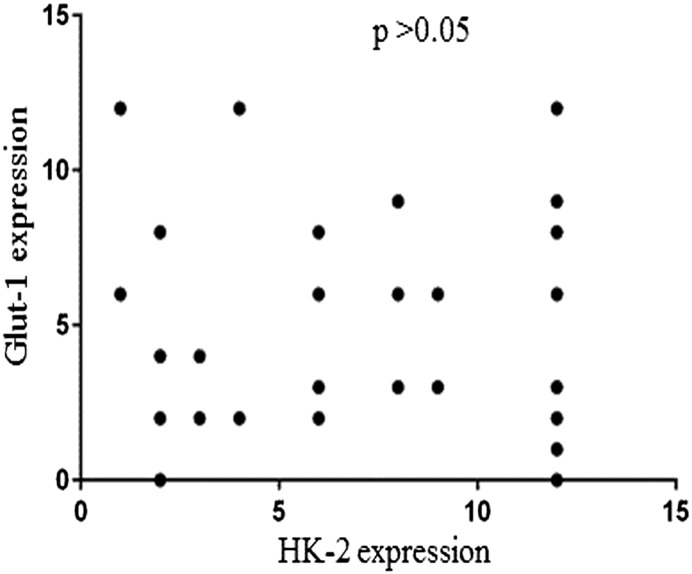
Figure 3 showed the comparative analysis of immunohistochemical staining results using anti-Glut-1 and anti-HK-II antibody. The Glut-1 expression had no correlation with the HK- 2 expression (P > .05).
Figure 3.
The correlation between Glut-1 expression and HK-2 expression.
3.2. Correlations Between Glut-1 or HK-II Expression and Clinicopathological Parameters and Patient Survival
There was no correlation between Glut-1 expression and age, gender, tumor size, tumor location, tumor histological type, tumor differentiation, the nerve infiltration, vascular invasion, local infiltration, lymph node metastasis or tumor staging (P > .05) (Table 2). Meanwhile, the HK-II expression had no correlation with age, gender, tumor size, tumor location, tumor histological type, tumor differentiation, the nerve infiltration, vascular invasion, local infiltration, lymph node metastasis or tumor staging in pancreatic cancer (P > .05) (Table 3).
Table 2.
Associations Between Glut-1 Levels and Clinicopathological Variables in Pancreatic Cancer Patients
| Patient Characteristics | Negative (−) | Positive (+ ~ +++) | P-Value |
|---|---|---|---|
| Age | >.05 | ||
| ≤60 | 6 | 28 | |
| >60 | 3 | 11 | |
| Gender | >.05 | ||
| Male | 6 | 26 | |
| Female | 2 | 13 | |
| Tumor size | .460 | ||
| ≤4 cm | 7 | 24 | |
| >4 cm | 2 | 15 | |
| Tumor location | .178 | ||
| Head of pancreas | 4 | 30 | |
| Body and tail of pancreas | 4 | 8 | |
| Tumor histological type | >.05 | ||
| Other types | 2 | 9 | |
| Ductal adenocarcinoma | 7 | 30 | |
| Tumor differentiation | >.05 | ||
| II, III, III | 4 | 20 | |
| I,II | 4 | 16 | |
| Nerve infiltration | .466 | ||
| Present | 3 | 20 | |
| Absent | 6 | 19 | |
| Vascular invasion | .451 | ||
| Present | 5 | 14 | |
| Absent | 4 | 25 | |
| Local infiltration | .199 | ||
| Present | 5 | 31 | |
| Absent | 4 | 8 | |
| Lymph node metastasis | .279 | ||
| Present | 6 | 17 | |
| Absent | 3 | 22 | |
| Tumor staging | .235 | ||
| I-IIA | 2 | 20 | |
| IIB-IV | 6 | 19 |
Table 3.
Associations Between HK-II Levels and Clinicopathological Variables in Pancreatic Cancer Patients
| Patient Characteristics | Negative (−) | Positive (+ ~ +++) | Pvalue |
|---|---|---|---|
| Age | .870 | ||
| ≥60 | 13 | 21 | |
| <60 | 5 | 9 | |
| Gender | .252 | ||
| Male | 13 | 18 | |
| Female | 4 | 12 | |
| Tumor size | .527 | ||
| ≤4 cm | 11 | 21 | |
| >4 cm | 7 | 9 | |
| Tumor location | .737 | ||
| Head of pancreas | 12 | 22 | |
| Body and tail of pancreas | 5 | 7 | |
| Tumor histological type | >.05 | ||
| Other types | 4 | 7 | |
| Ductal adenocarcinoma | 14 | 23 | |
| Tumor differentiation | >.05 | ||
| II, III, III | 6 | 17 | |
| I, II | 3 | 18 | |
| Nerve infiltration | .881 | ||
| Present | 7 | 14 | |
| Absent | 10 | 16 | |
| Vascular invasion | .441 | ||
| Present | 8 | 10 | |
| Absent | 10 | 20 | |
| Local infiltration | 0.513 | ||
| Present | 12 | 23 | |
| Absent | 6 | 7 | |
| Lymph node metastasis | .551 | ||
| Present | 8 | 16 | |
| Absent | 10 | 14 | |
| Tumor staging | .805 | ||
| I-IIA | 9 | 13 | |
| IIB-IV | 9 | 17 |
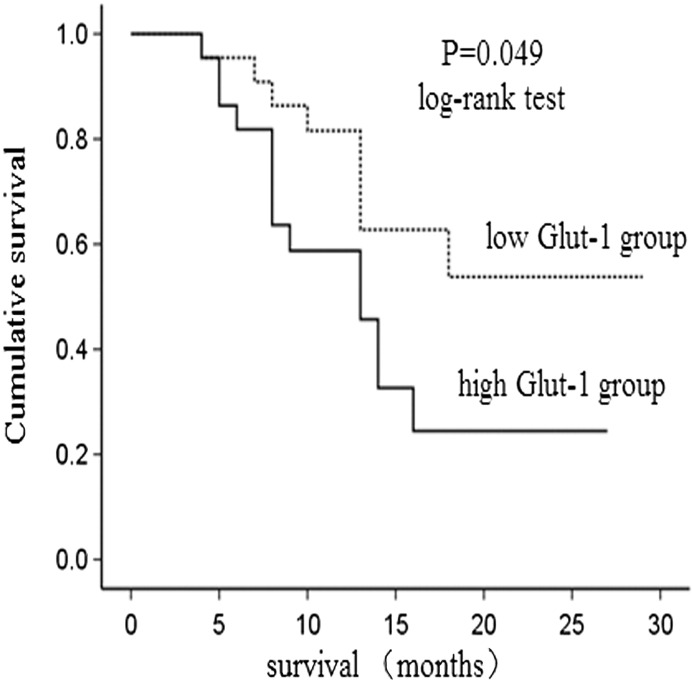
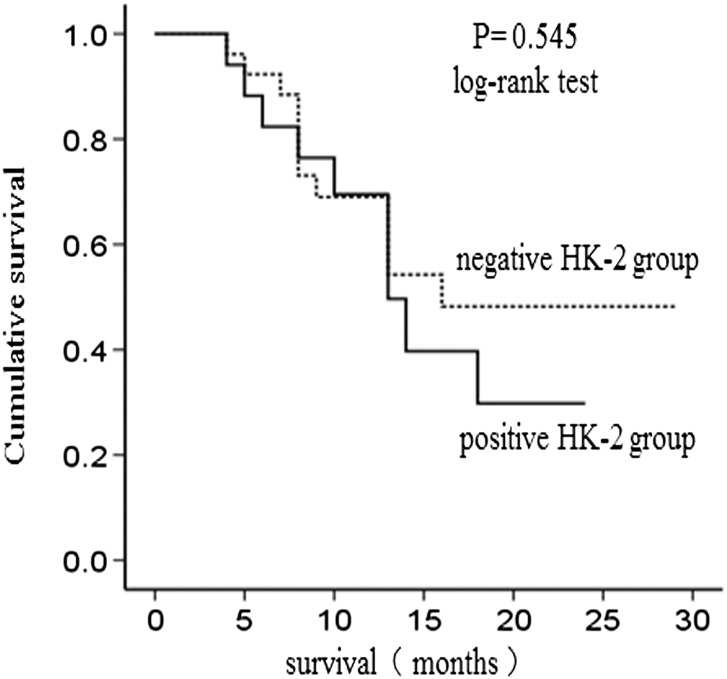
During the follow-up period, the survival curve of low Glut-1 group and high Glut-1 group was statistically different (P = .049) ( Figure 4). No statistical difference was found in the survival curves of negative HK-II group and positive HK-II group (P = .545) (Figure 5). Univariate analysis revealed that higher expression of Glut-1 was correlated with poor prognosis. In addition, we evaluated the impact of clinicopathological features on overall survival. As a result, large tumor size, present of lymph node metastasis, advanced tumor staging significantly predicted patients' survival, whereas age, gender, tumor location, tumor histological type, tumor differentiation, nerve infiltration, vascular invasion and local infiltration had no predictive value. All factors that were significant for predicting overall survival by univariate analysis were included in the multivariate Cox regression analysis, which revealed that Glut-1 was not associated with mortality (P > .05) ( Table 4).
Figure 4.
The survival analysis in Glut-1 low group and Glut-1 high group.
Figure 5.
The survival analysis in HK-2 negative group and HK-2 posotive group.
Table 4.
Univariate and Multivariate Analysis of Overall Survival of Patients with Pancreatic Cancer
| Univariate Analysis | Multivariate Analysis | |
|---|---|---|
| Variables | P value | P value |
| Age | >0.05 | |
| Gender | >0.05 | |
| Tumor size | 0.041* | >0.05 |
| Tumor location | >0.05 | |
| Tumor histological type | >0.05 | |
| Tumor differentiation | >0.05 | |
| Nerve infiltration | >0.05 | |
| Local infiltration | >0.05 | |
| Vascular invasion | 0.053 | |
| Lymph node metastasis | 0.003* | >0.05 |
| Tumor staging | 0.006* | >0.05 |
| Glut-1 | 0.049* | >0.05 |
| HK-II | 0.545 |
Relationships Between 18F-FDG Accumulation and Expression Levels of Glut-1 and HK-II
In the first place, difference analysis was carried out on our data. The mean value of SUVmax in Glut-1 negative group was 5.0 ± 2.8, and the mean value of SUVmax in Glut-1 positive group was 5.5 ± 3.0, the SUVmax increased as the grade of expression increased from negative to positive, but there was no significant difference between them in this study (P = .661). The mean value of SUVmax in HK-II negative and positive group was 5.7 ± 2.6, 5.4 ± 3.1 respectively. No statistical difference was found between them (P = .664) (Table 5).
Table 5.
Relationship Between SUVmax and the Expression of Glut-1 or HK-II in Patients of Pancreatic Cancer. There was no Significant Difference in SUVmax Between Tumors That Exhibited Negative and Positive Glut-1 Expression (P > .05). The Same was True for Tumors That Exhibited Negative and Positive HK-II Expression. (P > .05)
| Patients of Pancreatic Cancer | SUVmax | P Value (t-Test) |
|---|---|---|
| Glut-1 negative group | 5.0 ± 2.8 | 0.661 |
| Glut-1 positive group | 5.5 ± 3.0 | |
| HK-2 negative group | 5.7 ± 2.6 | 0.664 |
| HK-2 negative group | 5.4 ± 3.1 |
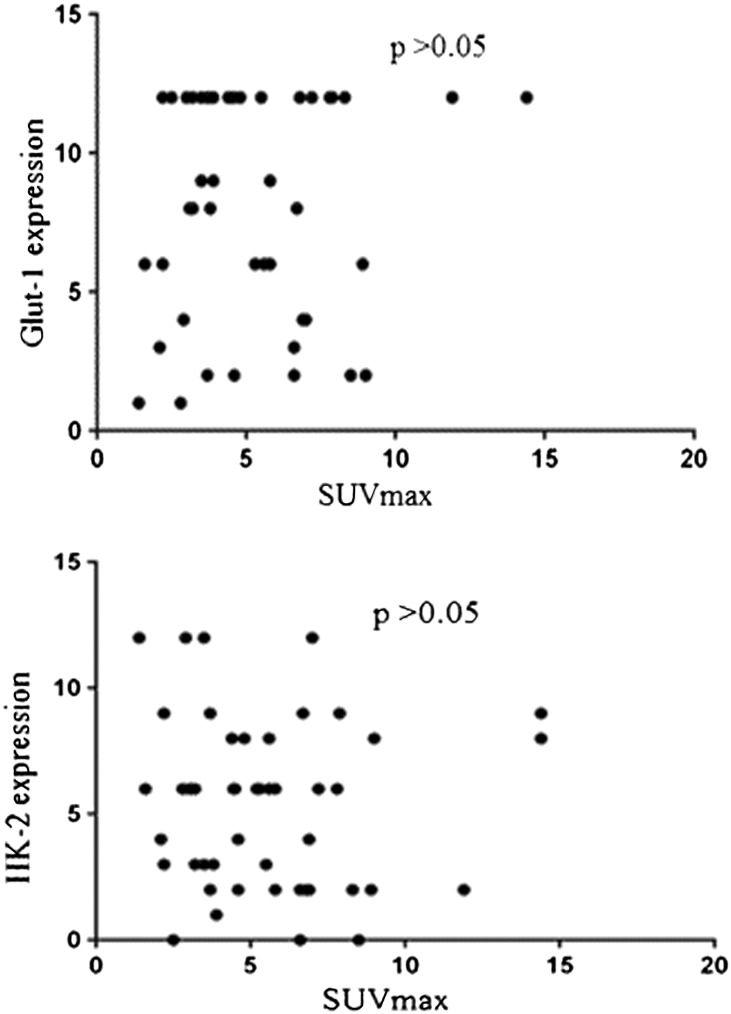
In the second place, correlation analysis was done on our data. Picture 8 showed the comparative analysis between immunohistochemical findings (Glut-1 expression and HK-IIexpression) and SUVmax. 18F-FDG accumulation had no correlation with the expression levels of Glut-1or HK-II (P > .05) (Figure 6).
Figure 6.
The correlation between immunohistochemical findings(Glut-1 expression and HK-IIexpression) and SUVmax.
Discussion
The tumor tissue have accelerated glycolysis under both anaerobic and aerobic conditions [17]. The increased expression and activity of Gluts and HKs are considered to be the priority for sufficient energy supply in tumor cells [18].
Fourteen subtypes of human facilitative glucose transporters have been described. Glut-1 is the most ubiquitously distributed subtype. It has been shown to overexpress in many tumor tissue, such as: colorectal cancer [19], lung cancer [20], thyroid carcinoma [21], ovarian cancer [22], breast cancer [23], prostate cancer [24], gastric cancer [25] and musculoskeletal tumors. In tumor tissue, The glucose uptake through plasma membrane is mentioned to be the rate-limiting step of glucose metabolism [26].
It is reported that Glut-1 is overexpressed in pancreatic cancer [27] and in pancreatic cancer cell lines [28]. Higashi T et al [15] got the same conclusion in one immunohistochemical study about Glut-1. Such overexpression may reflect enhanced glucose consumption. The Glut-1 expression could be regulated by many factors. It is rapidly induced experimentally by glucose, hypoxia ischemia [29], cAMP or VEGF. These same factors have been demonstrated to be alkaline fibroblast growth factor (FGF) [30], tumor necrosis factor alpha (TNF-α) and so on. Our study indicated that the Glut-1 expression in pancreatic tumor was significantly higher than that in normal tissue(P < .001).
Among the four HK isoenzymes (type 1–4), HK −2 is suggested to be the main subtype in regulating glucose metabolism in cancer cells [31], [32], [33]. Warburg discovered that the liver cancer cells had accelerated glycolysis, accompanied by enhanced HK activity [3]. The subsequent studies demonstrated that HK-II proteins were overexpressed in cancer cells of primary breast cancer, liver cancer, gastric cancer [34], colon cancer [35], lung cancer, cervical cancer [36] and musculoskeletal tumors [37]. Up to now, few researchers have focused on the HK-II expression in pancreatic cancer at home and abroad.
Our study firstly showed that the HK-II expression in pancreatic tumors was obviously higher than that in adjacent normal tissue (62.5% vs0%, P < .001). There was no expression of HK-II in normal pancreatic tissue. The result can be explained as follows: first, the enhanced expression and activity of relevant enzymes, which act as decomposing HK-II, eventually lead in the rapid degradation of HK-II; second, the transcription and translation of the corresponding HK-II gene is weakened, causing the low expression of HK-II protein. Further studies are needed to elucidate the exact mechanism.
Tumor cells or transformed cells are known to have enhanced anaerobic glycolysis as a whole, which requires both Gluts and HKs overexpression, although the relative importance (as the rate-limiting step) of the two factors is still controversial. Among the family of Gluts and HKs, Glut-1 and HK-II were selected because each is known as a major subtype of each family for various cancer cells. Higashi et al [38] observed that there was a close correlation between Glut-1 and HK-II expression in one immunohistochemical study of twenty-one pancreatic cancer patients (P = .0022). The 2 immune molecules were known to promote the glycolysis of pancreatic tumors cooperatively. In the present study, we accelerated sample amounts, examined the expression of Glut-1 and HK-II by analyzing the staining intensity and percentage of positively stained cells, and obtained the opposite conclusion. In other words, the Glut-1 expression had no relationship with the HK-II expression (P > .05). More studies on large populations are necessary to elucidate their correlations.
The relationship between Glut-1 expression and clinialpathological characteristics has been controversial in tumors. In esophageal cancer, Glut-1 expression has been correlated to pathological grade, invasion and lymphatic metastasis of tumors [39]. In breast cancer, a positive correlation was found between Glut-1 expression and pathological grade [40]. In non- small cell lung cancer (NSCLC) [41] and colorectal cancer [42], Glut-1 expression has been associated with invasion and tumor stage. However, in nasopharyngeal cancer [43], Glut-1 expression had no association with ages, tumor size, tumor location, histological type or lymphatic metastasis. In order to determine whether up-regulated Glut-1 had influence on patients of pancreatic cancer or not, we further investigated its correlation with clinicopathological parameters as well as its impact on prognosis. It was demonstrated that there was no correlation among Glut-1 expression and tumor size, tumor location, tumor differentiation, lymph node metastasis, vascular invasion, the nerve infiltration, local infiltration, tumor histological type or tumor staging in pancreatic cancer. It is generally accepted that the Glut-1 expression may indicate a poor prognosis [19], [44]. Nevertheless, one previous research has demonstrated no association of Glut-1 expression and survival in pancreatic cancer [27]. Lyshchik A et al [45] discovered the same conclusion. In our research, we did not find a correlation of Glut-1 expression to survival. Although the overexpressed Glut-1 may enhance the transporting of glucose across plasma membrane, eventually, accelerating the rates of glycolysis, it can't provide sufficient energy for the proliferation, invasion and metastasis of pancreatic cancer cells. Whether other less commonly expressed Gluts contribute to the glycolysis in this model was not specifically addressed here.
More and more attention was drawn to the correlation between HK-II expression and clinialpathological characteristics of tumors. The HK-II expression appeared to be associated with carcinogenesis in an early research about cervical cancer [36]. Rho et al. concluded that the increased expression of HK-II in gastric carcinoma is closely related to tumor invasion [34]. But up to now, no study was carried out on the connection between HK-II expression and clinialpathological characteristics of pancreatic cancer. Our study firstly discovered that HK-II expression had no correlation with the clinicopathological features of patients. Furthermore, it is indicated that HK-II expression was not associated with patient survival, in contrary to the conclusion in Lyshchik A's study [45]. Therefore, it is suggested that HK-II may not be useful in predicting survival and further studies with larger number of patients are required to determine the clinical significance of HK-II in pancreatic cancer. The HK protein is the regulator of glycolysis and aerobic oxidation [46], maintaining a proper ratio between the two in the normal tissue. However, in tumors, the HK-II proteins were expressed in high levels, overcharging the balance. The HK-II expression may provide energy for the proliferation and development of cancer cells, but this protein was not the determinant of energy supply in pancreatic cancer.
So far, the exact mechanism of 18F-FDG accumulation in pancreatic cancer is not elucidated. Three steps are required for FDG accumulation in cancer cells: (1) facilitated diffusion through Gluts; (2) subsequent phosphorylation by HK isoforms producing FDG-6-phosphate; (3) decreased dephosphorylation. It is believed that the dephosphorylation process is negligible and the 18F-FDG-6-P is neither transported out of cells nor subjected to glycolytic breakdown; it is metabolically trapped inside cells. Thus, 18F-FDG accumulation depends basically on the rate of transport through the cell membrane and the activity of hexokinases [47], [48].
Recent years, more and more studies have focused on the expression of Glut-1 and HK-II to define the role of these proteins in the regulation of 18F-FDG accumulation. The two immune molecules play different roles in different tumor tissue. In some tumor tissue, the FDG accumulation had correlation with both Glut-1 and HK-II expression. In other cancer lesions, the FDG accumulation had no correlation with Glut-1 expression or HK-II expression. However, it was also reported that the FDG accumulation had connection with each of the two immune molecules.
In one study consisted of 21 pancreatic cancer patients, Higashi et al [38] indicated that Glut-1 and HK-II expression was not strongly related to 18F-FDG uptake (P = .055, P = .1852). The same conclusion was detected on our research although using different immunohistochemical analysis methods and with a large number of patients. Some early investigators indicated that other Gluts and HKs may mediate glucose uptake in certain tumors [38], [49], [50], [51]. Glut-3 and Glut-4 were overexpressed and might serve in 18F-FDG uptake in gastrointestinal cancer [52]. In addition, the Glut-3 expression had been demonstrated to have considerable impact on 18F-FDG uptake in head and neck cancer [53], lymphoma [54],thyroid cancer [55] and malignant melanoma [11]. So it can be deduced that the Glut-1 and HK-IIexpression was not the main factor in 18F-FDG accumulation of pancreatic cancer, maybe there are other subtypes which contribute to a high rate of entry of the FDG into the tumor cells, such as: Glut-2, Glut-3, Glut-4, HK-1 and so on.
There were a few limitations in our study. A small number of patients were enrolled in this study. In addition, subtypes other than Glut-1 and HK-II were not included. Therefore, further studies, on large populations and with more subtypes of Gluts and HKs, are necessary to elucidate the role of these immune molecules and the molecular mechanism underlying the 18F-FDG uptake.
Conclusions
Our datas show that Glut-1 and HK-II proteins are over-expressed in pancreatic cancer, but they have no correlation with clinicopathological features, survival and 18F-FDG uptake. We guess that maybe there are other subtypes which contribute to a high rate of entry of the FDG into pancreatic cancer cells.
Acknowledgements
The authors are grateful to the technologists from the Department of Pathology for their incredible help in preparing the specimens. The authors would like to thank all the staffs of PET center for their valuable help during the research. We also thank Professor Yihui Guan for his valuable suggestions and editorial contributions.
Footnotes
Funding: This work was supported by Shanghai Municipal Health Bureau[grant numbers 20124011].
Contributor Information
Hai-Jing Yang, Email: yhjlqelfe@126.com.
Wei-Jia Xu, Email: wj.xu1204@gmail.com.
Liang Zhong, Email: zhongniping@163.com.
References
- 1.Gudjonsson B. Pancreatic cancer: survival, errorsand evidence[J] Eur J Gastroenterol Hepatol. 2009;21:1379–1382. doi: 10.1097/MEG.0b013e328323aab7. [DOI] [PubMed] [Google Scholar]
- 2.Tempero M.A., Arnoletti J.P., Behrman S., Ben-Josef E., Benson A.B., Berlin J.D., Cameron J.L., Casper E.S., Cohen S.J., Duff M. Pancreatic adenocarcinoma[J] J Natl Compr Canc Netw. 2010;8:972–1017. doi: 10.6004/jnccn.2010.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburg O. On the origin of cancer cells[J] Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 4.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis[J] Nat Rev Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 5.Garber K. Energy deregulation: licensing tumors to grow[J] Science. 2006;312(5777):1158–1159. doi: 10.1126/science.312.5777.1158. [DOI] [PubMed] [Google Scholar]
- 6.Higashi T., Tamaki N., Torizuka T., Nakamoto Y., Sakahara H., Kimura T., Honda T., Inokuma T., Katsushima S., Ohshio G. FDG uptake,GLUT-1 glucose transporter and cellularity in human pancreatic tumors[J] J Nucl Med. 1998;39:1727–1735. [PubMed] [Google Scholar]
- 7.Chung J.K., Lee Y.J., Kim C., Choi S.R., Kim M., Lee K., Jeong J.M., Lee D.S., Jang J.J., Lee M.C. Mechanisms related to 18F- fluorodeoxyglucose uptake of human colon cancers transplanted in nude mice[J] J Nucl Med. 1999;40:339–346. [PubMed] [Google Scholar]
- 8.Rempel A., Bannasch P., Mayer D. Differences in expression and intracellular distribution of hexokinase isoenzymes in rat liver cells of different transformation stages[J] Biochim Biophys Acta. 1994;1219:660–668. doi: 10.1016/0167-4781(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 9.Mathupala S.P., Rempel A., Pedersen P.L. Glucose catabolism in cancer cells. Isolation, sequence, and activity of the promoter for type II hexokinase[J] J Biol Chem. 1995;270:16918–16925. doi: 10.1074/jbc.270.28.16918. [DOI] [PubMed] [Google Scholar]
- 10.Maemura K., Takao S., Shinchi H. Role of positron emission tomography in decisions on treatment strategies for pancreatic cancer[J] J Hepatobiliary Pancreat Surg. 2006;13(5):435–441. doi: 10.1007/s00534-006-1102-8. [DOI] [PubMed] [Google Scholar]
- 11.Park S.G., Lee J.H., Lee W.A., Han K.M. Biologic correlation between glucose transporters, hexokinase-II, Ki-67 and FDG uptake in malignant melanoma[J] Nucl Med Biol. 2012;39(8):1167–1172. doi: 10.1016/j.nucmedbio.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Nakajo M., Kajiya Y., Tani A., Yoneda S., Shirahama H., Higashi M., Nakajo M. 18FDG PET for grading malignancy in thymic epithelial tumors: significant differences in 18FDG uptake and expression of glucose transporter-1 and hexokinase II between low and high-risk tumors: preliminary study[J] Eur J Radiol. 2012;81(1):146–151. doi: 10.1016/j.ejrad.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Zhao S., Kuge Y., Tsukamoto E., Mochizuki T., Kato T., Hikosaka K., Nakada K., Hosokawa M., Kohanawa M., Tamaki N. Fluorodeoxyglucose uptake and glucose transporter expression in experimental inflammatory lesions and malignant tumours: effects of insulin and glucose loading[J] Nucl Med Commun. 2002;23:545–550. doi: 10.1097/00006231-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Mochizuki T., Tsukamoto E., Kuge Y., Kanegae K., Zhao S., Hikosaka K., Hosokawa M., Kohanawa M., Tamaki N. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models[J] J Nucl Med. 2001;42:1551–1555. [PubMed] [Google Scholar]
- 15.Higashi T., Tamaki N., Honda T., Torizuka T., Kimura T., Inokuma T., Ohshio G., Hosotani R., Imamura M., Konishi J. Expression of glucose transporters in human pancreatic tumors compared with increased FDG accumulation in PET study[J] J Nucl Med. 1997;38:1337–1344. [PubMed] [Google Scholar]
- 16.Zhao S., Kuge Y., Mochizuki T., Takahashi T., Nakada K., Sato M., Takei T., Tamaki N. Biologic correlates of intratumoral heterogeneity in 18F-FDG distribution with regional expression of glucose transporters and hexokinase-II in experimental tumor. J Nucl Med. 2005 Apr;46(4):675–682. [PubMed] [Google Scholar]
- 17.Lu H., Forbes R.A., Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis[J] J Biol Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen P.L., Mathupala S., Rempel A., Geschwind J.F., Ko Y.H. Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention[J] Biochim Biophys Acta. 2002;1555:14–20. doi: 10.1016/s0005-2728(02)00248-7. [DOI] [PubMed] [Google Scholar]
- 19.Haber R.S., Rathan A., Weiser K.R., Pritsker A., Itzkowitz S.H., Bodian C., Slater G., Weiss A., Burstein D.E. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis[J] Cancer. 1998;83:34–40. doi: 10.1002/(sici)1097-0142(19980701)83:1<34::aid-cncr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Ito T., Noguchi Y., Satoh S., Hayashi H., Inayama Y., Kitamura H. Expression of facilitative glucose transporter isoforms in lung carcinomas: its relation to histologic type, differentiation grade, and tumor stage[J] Mod Pathol. 1998;11:437–443. [PubMed] [Google Scholar]
- 21.Haber R.S., Weiser K.R., Pritzker A., Reder I., Burstein D.E. GLUT1 glucose transporter expression in benign and malignant thyroid nodules[J] Thyroid. 1997;7:363–367. doi: 10.1089/thy.1997.7.363. [DOI] [PubMed] [Google Scholar]
- 22.Kalir T., Wang B.Y., Goldfischer M., Haber R.S., Reder I., Demopoulos R., Cohen C.J., Burstein D.E. Immunohistochemical staining of GLUT1 in benign, borderline, and malignant ovarian epithelia[J] Cancer. 2002;94:1078–1082. [PubMed] [Google Scholar]
- 23.Younes M., Brown R.W., Mody D.R., Fernandez L., Laucirica R. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers[J] Anticancer Res. 1995;15:2895–2898. [PubMed] [Google Scholar]
- 24.Chandler J.D., Williams E.D., Slavin J.L., Best J.D., Rogers S. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma[J] Cancer. 2003;97:2035–2042. doi: 10.1002/cncr.11293. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura T., Kusakabe T., Sugino T., Watanabe K., Fukuda T., Nashimoto A., Honma K., Suzuki T. Expression of glucose transporter-1in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival[J] Cancer. 2001;92:634–641. doi: 10.1002/1097-0142(20010801)92:3<634::aid-cncr1364>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 26.Waki A., Kato H., Yano R., Sadato N., Yokoyama A., Ishii Y., Yonekura Y., Fujibayashi Y. The importance of glucose transport activity as the rate-limiting step of 2-deoxyglucose uptake in tumor cells in vitro[J] Nucl Med Biol. 1998;25:593–597. doi: 10.1016/s0969-8051(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 27.Sun H.C., Qiu Z.J., Liu J., Sun J., Jiang T., Huang K.J., Yao M., Huang C. Expression of hypoxia-inducible factor-1 alpha and associated proteins in pancreatic ductal adenocarcinoma and their impact on prognosis[J] Int J Oncol. 2007;30(6):1359–1367. [PubMed] [Google Scholar]
- 28.Yamamoto T., Seino Y., Fukumoto H., Koh G., Yano H., Inagaki N., Yamada Y., Inoue K., Manabe T., Imura H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990;170:223–230. doi: 10.1016/0006-291x(90)91263-r. [DOI] [PubMed] [Google Scholar]
- 29.Camps M., Vilaro S., Tester X., Palacín M., Zorzano A. High and polarized expression of GLUT1 glucose transporters in epithelial cells from mammary gland: acute downregulation of GLUT1 carriers by weaning[J] Endocrinology. 1994;134:924–934. doi: 10.1210/endo.134.2.8299587. [DOI] [PubMed] [Google Scholar]
- 30.Rollins B.J., Morrison E.D., Usher P., Flier J.S. Platelet-derived growth factor regulates glucose transporter expression[J] J Biol Chem. 1988;263:16523–16526. [PubMed] [Google Scholar]
- 31.Robey R.B., Hay N. Mitochondrial hexokinases: guardians of the mitochondria[J] Cell Cycle. 2005;4(5):654–658. doi: 10.4161/cc.4.5.1678. [DOI] [PubMed] [Google Scholar]
- 32.Mathupala S.P., Ko Y.H., Pedersen P.L. Hexokinase II: cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria[J] Oncogene. 2006;25(34):4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen P.L. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’most common phenotypes, the "Warburg Effect", i.e., elevated glycolysis in the presence of oxygen[J] J Bioenerg Biomembr. 2007;39(3):211–222. doi: 10.1007/s10863-007-9094-x. [DOI] [PubMed] [Google Scholar]
- 34.Rho M., Kim J., Jee C.D., Lee Y.M., Lee H.E., Kim M.A., Lee H.S., Kim W.H. Expression of type 2 hexokinase and mitochondria-related genes in gastric carcinoma tissue and cell lines[J] Anticancer Res. 2007;27(1A):251–258. [PubMed] [Google Scholar]
- 35.Burt B.M., Humm J.L., Kooby D.A., Squire O.D., Mastorides S., Larson S.M., Fong Y. Using positron emission tomography with 18F-FDG to predict tumor behavior in experimental colorectal cancer[J] Neoplasia. 2001;3(3):189–195. doi: 10.1038/sj.neo.7900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng G.Q., Yang Y., Zhong C.G., Yin H.L., Hu G.H., Tian Y. Study of association between expression of hOGG1, VDAC1, HK-II and cervical carcinoma. J Exp Clin Cancer Res. 2010;29:129. doi: 10.1186/1756-9966-29-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamada K., Tomita Y., Qiu Y., Zhang B., Ueda T., Myoui A., Higuchi I., Yoshikawa H., Aozasa K., Hatazawa J. 8F-FDG-PET of musculoskeletal tumors: a correlation with the expression of glucose transporter 1 and hexokinase II[J] Ann Nucl Med. 2008;22(8):699–705. doi: 10.1007/s12149-008-0173-9. [DOI] [PubMed] [Google Scholar]
- 38.Higashi T., Saga T., Nakamoto Y., Ishimori T., Mamede M.H., Wada M., Doi R., Hosotani R., Imamura M., Konishi J. Relationship between retention index in dual-phase 18-F-FDG PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer[J] J Nucl Med. 2002;43(2):173–180. [PubMed] [Google Scholar]
- 39.Kato H., Takita J., Miyazaki T., Nakajima M., Fukai Y., Masuda N., Fukuchi M., Manda R., Ojima H., Tsukada K. Glut-1 glucose transporter expression in esophageal squamous cell carcinoma is associated with tumor aggressiveness[J] Anticancer Res. 2002;22(5):2635–2639. [PubMed] [Google Scholar]
- 40.Alo P.L., Visca P., Botti C., Galati G.M., Sebastiani V., Andreano T., Di Tondo U., Pizer E.S. Immunohistochemical expression of human erythro- cyte glucose transporter and fatty acid synthase in infiltrating breast carcinomas and adjacent typical/atypical hyperplastic or normal breast tissue[J] Am J Clin Pathol. 2001;116(1):129–134. doi: 10.1309/5Y2L-CDCK-YB55-KDK6. [DOI] [PubMed] [Google Scholar]
- 41.Brown R.S., Leung J.Y., Kison P.V., Zasadny K.R., Flint A., Wahl R.L. Glucose transporters and FDG uptake in untreated primary human non-small cell lung cancer[J] J Nucl Med. 1999;40:556–565. [PubMed] [Google Scholar]
- 42.Younes M., Lechago L.V., Lechago J. Overexpression of the human erythrocyte glucose transporter occurs as a late event in human colorectal carcinogenesis and is associated with an increased incidence of lymph node metastases[J] Clin Cancer Res. 1996;2:1151–1154. [PubMed] [Google Scholar]
- 43.Mineta H., Miura K., Takeboyoshi S., Misawa K., Araki K., Misawa Y., Ueda Y. Prognostic value of glucose transporter 1 expression in patients with hypopharyngeal carcinoma[J] Anticancer Res. 2002;22:3489–3494. [PubMed] [Google Scholar]
- 44.Cooper R., Sarioglu S., Sokmen S., Füzün M., Küpelioğlu A., Valentine H., Görken I.B., Airley R., West C. Glucose transporter-1(Glut-1): a potential marker of prognosis in rectal carcinoma[J] Br J Cancer. 2003;89:870–876. doi: 10.1038/sj.bjc.6601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyshchik A., Higashi T., Hara T., Nakamoto Y., Fujimoto K., Doi R., Imamura M., Saga T., Togashi K. Expression of glucose transporter-1, hexokinase-II, proliferating cell nuclear antigen and survival of patients with pancreatic cancer[J] Cancer Invest. 2007;25(3):154–162. doi: 10.1080/07357900701208931. [DOI] [PubMed] [Google Scholar]
- 46.Wilson J.E. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function[J] J Exp Biol. 2003;206(Pt 12):2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 47.Haberkorn U., Ziegler S.I., Oberdorfer F., Trojan H., Haag D., Peschke P., Berger M.R., Altmann A., van Kaick G. FDG uptake, tumor proliferation and expression of glycolysis associated genes in animal tumor models[J] Nucl Med Biol. 1994;21:827–834. doi: 10.1016/0969-8051(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 48.Golshani-Hebroni S.G., Bessman S.P. Hexokinase binding to mitochondria: a basis for proliferative energy metabolism[J] J Bioenerg Biomembr. 1997;29:331–338. doi: 10.1023/a:1022442629543. [DOI] [PubMed] [Google Scholar]
- 49.Charnley N., Airley R., Du Plessis D., West C., Brock C., Barnett C., Matthews J., Symonds K., Bottomly M., Swindell R. No relationship between 18F- fluorodeoxyglucose positron emission tomography and expression of Glut-1 and Glut-3 and hexokinase I and II in high-grade glioma[J] Oncol Rep. 2008;20(3):537–542. [PubMed] [Google Scholar]
- 50.De Geus-Oei L.F., van Krieken J.H., Aliredjo R.P., Krabbe P.F., Frielink C., Verhagen A.F., Boerman O.C., Oyen W.J. Biological correlates of FDG uptake in non-small cell lung cancer[J] Lung Cancer. 2007;55(1):79–87. doi: 10.1016/j.lungcan.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 51.Godoy A., Ulloa V., Rodríguez F., Reinicke K., Yañez A.J., García Mde L., Medina R.A., Carrasco M., Barberis S., Castro T. Differential subcellular distribution of glucose transporters GlUT1-6 and GLUT9 in human cancer: ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissue[J] J Cell Physiol. 2006;207(3):614–627. doi: 10.1002/jcp.20606. [DOI] [PubMed] [Google Scholar]
- 52.Noguchi Y., Yoshikawa T., Doi C., Makino T., Matsumoto A., Sato S., Ito T. Expression of glucose transporters effects of insulin resistance 1 and 4 in human gastric cancer [abstract] J Jpn Surg Soc. 1995;96:822. [PubMed] [Google Scholar]
- 53.Tian M., Zhang H., Nakasone Y., Mogi K., Endo K. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study[J] Eur J Nucl Med Mol Imaging. 2004;31(1):5–12. doi: 10.1007/s00259-003-1316-9. [DOI] [PubMed] [Google Scholar]
- 54.Shim H.K., Lee W.W., Park S.Y., Kim H., Kim S.E. Relationship between FDG uptake and expressions of glucose transporter type 1, type 3, and hexokinase-II in Reed- Sternberg cells of Hodgkin lymphoma[J] Oncol Res. 2009;17(7):331–337. doi: 10.3727/096504009787721177. [DOI] [PubMed] [Google Scholar]
- 55.Kaida H., Hiromatsu Y., Kurata S., Kawahara A., Hattori S., Taira T., Kobayashi M., Uchida M., Yamada K., Mihashi H. Relationship between clinicopathological factors and fluorine-18-fluorodeoxyglucose uptake in patients with papillary thyroid cancer[J] Nucl Med Commun. 2011;32(8):690–698. doi: 10.1097/MNM.0b013e32834754f1. [DOI] [PubMed] [Google Scholar]