Abstract
Tumor cells harbor genetic alterations that promote a continuous and elevated production of reactive oxygen species. Whereas such oxidative stress conditions would be harmful to normal cells, they facilitate tumor growth in multiple ways by causing DNA damage and genomic instability, and ultimately, by reprogramming cancer cell metabolism. This review outlines the metabolic-dependent mechanisms that tumors engage in when faced with oxidative stress conditions that are critical for cancer progression by producing redox cofactors. In particular, we describe how the mitochondria has a key role in regulating the interplay between redox homeostasis and metabolism within tumor cells. Last, we will discuss the potential therapeutic use of agents that directly or indirectly block metabolism.
Facts
Deregulated redox homeostasis is a hallmark of cancer cells
Increased ROS levels are able to promote tumor growth and malignant progression
Increase antioxidant ability in malignant cells is a common feature
Alteration of specific metabolic pathways in tumors is frequently found
Tumors can be sensitized to chemotherapy and other antitumor treatment by disabling antioxidant defenses (NADPH and GSH) through metabolic inhibition
Open Questions
What are the redox-sensitive transducers that specifically promote signaling events in cancer cells?
As metabolism can support the intracellular redox homeostasis by NADPH and GSH synthesis, what are the cancer-specific pathways/alterations that can be selectively targeted for therapeutic purposes?
To what extent can the inhibition of antioxidant mechanisms be used to potentially enhance chemo/radiotherapy without inducing side toxicity on normal cells?
Would it be possible to generate animal models that allow real-time detection of metabolic/redox intermediates with high spatial and temporal resolution during cancer progression?
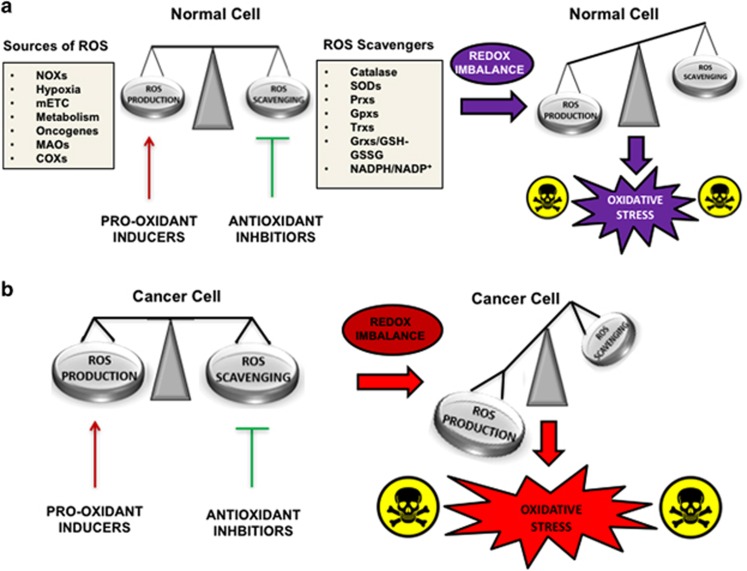
Cancer is one of the leading causes of death worldwide. Despite extensive research and considerable efforts for developing targeted therapies, many tumors are still characterized by poor prognosis and high mortality. For this reason, novel strategies to improve the outcome of patients suffering from aggressive or therapy-resistant malignancies are critically needed. Recent evidences indicate that altered redox balance and deregulated redox signaling, which are two common hallmarks of tumors, can be strongly implicated in malignant progression and resistance to treatment. It has been long postulated that cancer cells exhibit persistently high reactive oxygen species (ROS) levels as a consequence of genetic, metabolic and microenvironment-associated alterations. These are then compensated by an increased antioxidant ability from these cancer cells.1 Although seemingly paradoxical, this pro-oxidant shift can promote tumor growth by inducing DNA damage and genomic instability,2 which then activate an inflammatory response,3 stabilizing the hypoxia inducible factor-14 and thus reprogramming metabolism.5, 6 Due to the selective pressure induced by sustained ROS production, cancer cells have developed an efficient mechanism of ROS detoxification that presents a selective advantage over and upholds its survival under pro-oxidizing conditions. Therefore, the dependency of cancer cells from their antioxidant systems represents a specific vulnerability that must be exploited to induce targeted cell death. This can be achieved by increasing oxidative stress above the toxicity threshold, sparing normal cells, which are characterized by having lower intracellular ROS levels (Figure 1).7 Due to their dualistic nature, ROS can act as ‘good' and ‘bad' molecules, and regulate cellular physiology or induce cytotoxicity depending on the magnitude, duration and site of their generation. Hence, strategies aimed at altering redox signaling events in tumor cells and intend to disable key antioxidant systems in the presence of ROS inducers might represent promising new anticancer treatments.8 Other research looks to the intimate connection between cellular metabolism and redox homeostasis. Their reciprocal relationship is used by cancer cells to generate building blocks for cellular growth or antioxidant power to prevent oxidative damage. By redirecting energetic substrates and metabolic intermediates into the biochemical pathways that generate key antioxidant molecules, malignant cells can directly support the mechanisms of ROS detoxification.9, 10, 11 Therapeutic manipulations aimed at disrupting this functional crosstalk or elevating the burden of oxidative stress in the presence of selective metabolic inhibitors might induce synthetic lethality or sensitize cancer cells in common therapies8, 10, 12
Figure 1.
ROS sources and scavengers in the control of redox homeostasis in normal and cancer cells. (a) Normal cells keep constant ROS production and elimination to maintain a favorable redox balance. Disruption of redox homeostasis by co-treatment with ROS inducers and antioxidant inhibitors induces oxidative stress and variable levels of cell death. (b) Cancer cells exhibit higher steady-state levels of ROS counterbalanced by increased antioxidant capacity. The combined use of pro-oxidizing treatment and antioxidant inhibition is expected to cause severe oxidative stress and severe cytotoxicity
This review focuses on the adaptive mechanisms that tumors use to face oxidative stress conditions. We will discuss the role of ROS in regulating metabolism and progression in cancer cells. Last, we cover potential therapeutic usage of agents that directly or indirectly alter the tumor redox balance.
ROS Homeostasis and Redox Cofactors in Normal and Tumor Cells
Redox homeostasis is an essential requisite for aerobic organisms. They are dependent on the balance between the rate and the magnitude of oxidant production and their elimination over time. ROS are short-lived molecules with unpaired electrons deriving from partially reduced molecular oxygen that are perpetually generated, transformed and eliminated in a variety of cellular processes including metabolism, proliferation, differentiation, immune system regulation and vascular remodeling. These oxygen-containing derivatives are comprised of free radicals such as the superoxide anion (O2−·) or the hydroxyl radical (OH•) as well as non-radical molecules including hypochlorous acid and hydrogen peroxide (H2O2).13, 14 Both exogenous and endogenous sources of ROS production have been extensively described over the past decade.15 The most biologically relevant are represented by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, professional enzymes that catalyze the production of O2−· or H2O2 using NADPH as a reductant16 and the mitochondrial electron transport chain (mETC), wherein mainly complexes I and II generate O2− through univalent reduction of molecular oxygen as a consequence of electron leakage during mitochondrial respiration using nicotinamide adenine dinucleotide (reduced form) (NADH) and FADH.17, 18
To keep a steady-state control over ROS production–detoxification and prevent the harmful effects, aerobic organisms have evolved a complex array of defensive systems. These systems comprise scavenging enzymes and several endogenous or dietary-assumed antioxidant agents that limit ROS accumulation. The most relevant antioxidant enzymes include (i) superoxide dismutases (SODs) that convert superoxide (O2.−) to less reactive H2O2, (ii) catalase that reduces H2O2 to water and molecular oxygen and (iii) glutathione peroxidases that eliminate H2O2 using reducing power derived from glutathione. Other important defensive mechanisms and mediators of redox signaling are represented by the peroxiredoxin, the thioredoxin (TRX) and the glutathione/glutaredoxin systems.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 Due to intrinsic differences in the half-life, stability, chemical reactivity, cellular context, site and source of their generation (exogenous or endogenous), ROS can interact and modify different classes of biological macromolecules including DNA, lipids and proteins.31, 32, 33, 34, 35, 36, 37 The tight regulation of ROS production and detoxification over time and space represents the basis for the maintenance of an appropriate redox homeostasis and redox signaling events. Disruption of redox circuitries that control the turnover of ROS and the related redox signaling events has a profound impact on cellular physiology and in turn may lead to aberrant signaling, unrestrained accumulation of toxic byproducts, oxidative damage and cytotoxicity.38 Although low levels of ROS are believed to regulate redox signaling events, high doses are regarded as being responsible for cell toxicity.31, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 It is also well accepted that the efficacy of many anticancer therapies, including chemotherapeutics and radiotherapy, largely depends on their ability to induce ROS accumulation and evoke cell toxicity and death.14, 50, 51, 52, 53, 54, 55, 56 The high levels of oxidative stress normally associated with malignant progression represent tumor-specific alteration that makes cancer cells vulnerable to further elevation of ROS and strongly dependent on their antioxidant defenses. Both extrinsic and intrinsic factors contribute to generate a persistent amount of high ROS levels in tumors. To prevent excessive oxidative stress and promote redox signaling, tumor cells strategically adjust multiple antioxidant enzymes and make extensive use of their metabolic pathways to provide an adequate supply of antioxidant molecules (such as reduced glutathione (GSH) and NADPH).1 On the basis of these observations, disabling the intrinsic antioxidant mechanisms by promoting ROS production has been conducted in several studies.57, 58, 59, 60, 61, 62, 63 Still, these studies highlight the growing interest in the scientific community towards therapeutic strategies that are aimed at disrupting the redox homeostasis of malignant cells. To identify new strategies and define redox regulation and ROS levels in the context of tumor progression, several laboratories have found success with new approaches on the basis of the metabolic blockade as anticancer treatment (Table 1). Such treatments not only impact tumor growth by starving the cell from specific metabolic pathways but also by changing the redox state within the tumor cell. Laboratories are achieving these with encouraging results trying to understand which metabolic pathway is directly related to redox homeostasis and how it achieves ROS production, or an antioxidant response, in cancer cells.
Table 1. Metabolic blockade-based anticancer treatments and their effect on metabolism or redox balance.
| Name | Type of tumor | Mechanism of action | Impact on redox and metabolism | Clinical stage | References |
|---|---|---|---|---|---|
| Etomoxir | Glioblastoma; leukemia | Inhibition of CPT1 | ROS elevation through NADPH and ATP depletion | Preclinical | 89 |
| 6-aminonicotinamide | Prostate cancer; head and neck carcinoma | Inhibition of 6-phosphogluconate dehydrogenase | ROS increase through NADPH and GSH decrease | Approved | 94, 97 |
| Buthionine sulfoximine | Breast cancer; acute limphoblastic leukemia; multiple myeloma | Inhibition of GSH neosynthesis mediated by glutamate cysteine ligase | ROS increase due to GSH depletion | Approved | 163, 168, 169 |
| Bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide; compound 968 | B-cell lymphoma; acute myeloid leukemia; pancreatic cancer | Inhibition of glutaminase enzymes | Decreased intracellular GSH content and enhanced sensitivity to pro-oxidants | Approved | 108,109,110 |
| Arsenic trioxide | Small cell lung cancer; hepatocellular carcinoma; acute promyelocytic leukemia | Inhibition of mitochondrial respiration; cross-linking of thiols in redox-sensitive cysteines of GSH and antioxidant enzymes | Increased susceptibility to oxidative stress due to GSH oxidation and inactivation of Trx1, Trx2, Prx3 and Gpx2; decrease in ATP synthesis due to factor B inhibition | FDA approved | 22, 161, 163, 164 |
| Anthracyclines (doxorubicin, daunorubicin) | Colon carcinoma; breast cancer; neuroblastoma | Redox cycling and S-glutathionylation of mETC proteins; alteration of iron homeostasis | Increased ROS production due to Fenton's reaction and inhibition of the mETC complexes | FDA approved | 95 |
| Cisplatin | Non-small lung cancer; ovarian cancer; | Interference with the mETC activity; activation of the NADPH oxidases | Induction of intracellular and mitochondrial ROS production leading to lipid peroxidation, DNA damage and Ca2+ influx | FDA approved | 48,49,50,164 |
| Menadione | Pancreatic carcinoma; lung cancer | Triggering of redox cycling reactions; arylation of cellular thiols provoking GSH depletion | Increased ROS levels due to redox cycling; increased susceptibility to oxidation due to GSH decrease | Phase II | 165 |
| Metformin; phenformin | Melanoma; breast cancer; non-small lung cancer | Interference with mETC activity | Increased mitochondrial ROS production | Phase 0–II | 148, 149,150,153,154,155,156,157,158 |
Abbreviation: CPT1, carnitine palmitoyltransferase-1
Metabolic Pathways Involved in ROS Homeostasis in Cancer Cells
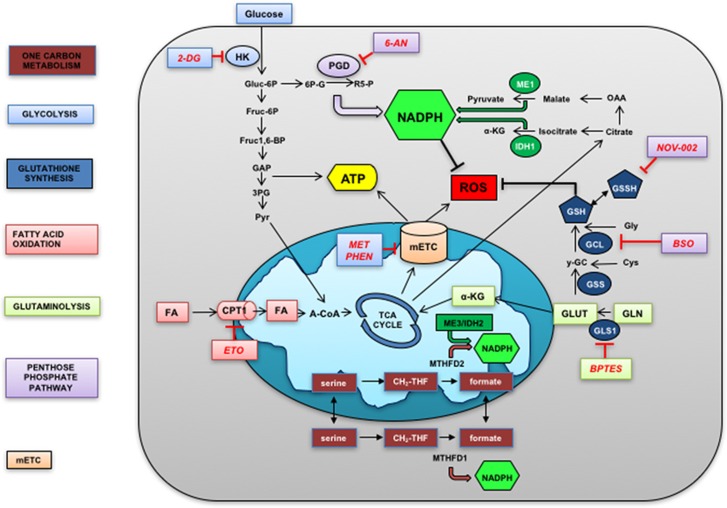
A growing body of evidence indicates that the malignant progression of tumors is characterized by the occurrence of multiple alterations where specific metabolic pathways are linked to the synthesis of essential building blocks (e.g., amino acids, lipids and nucleotides) fostering their uncontrolled growth. However, it is well recognized that part of the energetic substrates involved in these pathways can be also redirected into specific metabolic routes to generate not only antioxidant molecules (NADPH and GSH) but also redox cofactors (i.e., NADH and FADH) that can be readily used to maintain or restore an adequate redox homeostasis.64, 65, 66, 67 Increased attention has been dedicated to the intimate connection and reciprocal crosstalk between metabolism and redox balance of cancer cells, with a particular emphasis on the role of glycolysis, glutaminolysis, fatty acid oxidation (FAO), one-carbon metabolism and the pentose phosphate pathway (PPP).68, 69, 70 For this reason, it is important to analyze more in detail the major metabolic pathways that mainly control the redox homeostasis of cancer cells (Figure 2).
Figure 2.
Cellular metabolic pathway involved in redox homeostasis. Schematic representation of central metabolic pathways described in the text and involved in redox homeostasis. Metabolic pathway in the cytosol and mitochondria are represented. Metabolites in lowercase, enzymes in uppercase and inhibitors in red. Color code indicates metabolic pathways. FA fatty acids; HK, hexokinases; ROS, reactive oxygen species; PGD, phosphogluconate dehydrogenase; ME1, malic enzyme; a-KG, alpha ketoglutarate
Glycolysis
Glycolysis is an essential pathway occurring in the cytosol of mammalian cells through which glucose is transformed to pyruvate. Glucose is taken from the extracellular space by specific transporters (i.e., glucose transporters). Glucose is then converted to glucose-6-phosphate by hexokinase enzymes and enters into a series of ten enzyme-catalyzed reactions culminating in the generation of pyruvate, adenosine tris-phosphate (ATP) and reduced cofactors in the form of NADH.71 As already observed by Otto Warburg in the 1924, tumor cells exhibit a prevalent use of the glycolytic pathway regardless the presence of sufficient oxygen tension, a phenomenon known as Warburg effect.64 Several studies indicate that the pro-glycolytic shift caused by oncogene activation and loss of tumor suppressors represents a selective advantage for tumors by providing essential precursors for building the macromolecules required to sustain growth and proliferation.72, 73 As a matter of fact, therapeutic modulation of glucose metabolism and transport has been widely utilized as an effective anticancer strategy.74, 75, 76, 77, 78, 79, 80 It is now understood that glucose metabolism has an essential role in the control of redox homeostasis in tumors, as glycolytic intermediates can be shuttled into the metabolic pathways that directly or indirectly contribute to generate reducing equivalents, mainly PPP-derived NADPH or glutaminolysis-derived GSH. In this regard, a recent study showed that cancer cells exposed to glucose deprivation increase glucose metabolism to restrict the burden of ROS and prevent hydroperoxide-induced cell death.81 Also, inhibition of lactate dehydrogenase-A through the small-molecule FX11 impaired the malignant progression of lymphoma and pancreatic cancer xenografts by decreasing the intracellular ATP levels and inducing oxidative stress.82 Late, the inhibition of glycolysis and the PPP combined with the disruption of TRX system has proven to represent a successful strategy in selectively increase cytotoxicity in pancreatic and breast cancer cells but not in normal counterparts.83 These results suggest that a combined approach might be a better strategy in targeting malignant tumors when limited efficacy is observed with single agents.
Fatty acid oxidation
The FAO is composed of a cyclical series of controlled oxidations that occur in the mitochondria of mammalian cells, through which long- and short-chain fatty acids are shortened, generating NADH, FADH2 (flavin adenine dinucleotide (reduced form) and acetyl-CoA to support biosynthetic pathways and produce ATP. However, in cancer cells, a consistent fraction of the acetyl-CoA enters into the tricarboxylic acid cycle (TCA) cycle and generates citrate, which is therefore exported into the cytosol and funneled into metabolic reactions catalyzed by the malic enzyme (ME) and the isocitrate dehydrogenase 1 (IDH1), that ultimately produce large amounts of NADPH.84 The importance of FAO for NADPH homeostasis and redox balance of cancer cells prevent cell death during loss of matrix adhesion9 and metabolic stress conditions through the modulation of the liver kinase B1 (LKB1)/AMP kinase axis.85 Overexpression of the key FAO regulators, such as the carnitine palmitoyltransferase-1,86 occurs in both solid tumors and leukemia cells,87 whereas its pharmacological inhibition by etomoxir was found to impair NADPH production and promote oxidative stress-induced cell death in human glioblastoma cells associated with profound ATP depletion88 and to strengthen the pro-apoptotic effect of cytotoxic agents in human leukemia cells.89 Given its importance in many types of tumor, targeting the FAO represent a promising novel strategy to disrupt the redox homeostasis of malignant cells and interfere with biosynthetic or bioenergetics processes that regulate cancer cell survival triggering either apoptosis-dependent or -independent cell death.
Pentose phosphate pathway
The PPP is a major catabolic pathway of glucose through which cancer cells produce large amounts of ribose-5 phosphate, a precursor of nucleotide synthesis and NADPH, a key molecule that is used to drive anabolic processes and to detoxify harmful ROS.90 Activation of the PPP represents a key hallmark of many tumors where this metabolic pathway is found at the crossroad between glycolytic activity, unrestricted proliferation and scavenging of excessive ROS.91 The transcriptional regulation of glucose-6-phosphate dehydrogenase (G6PD), the rate-limiting enzyme of the PPP, by TAp73 and TAp63α was recently described in U2OS osteosarcoma cells, wherein its overexpression enhanced the PPP-dependent production of NADPH.92, 93 Additional mechanisms of G6PD regulation might directly depend on the availability of glucose: glucose funneling into the oxidative branch of the PPP directly controls the redox homeostasis of human clear cell carcinoma cells.94 This latest evidence underlines the importance of the PPP in the regulation of tumor cell survival and therapeutic resistance. In this respect, overexpression of G6PD promote doxorubicin resistance through increased GSH content and multidrug resistance-mediated efflux in HT29 colon carcinoma cells.95 In another study, the inactivation of the oncoprotein mucin1 C-terminal subunit restored the sensitivity of multiple myeloma cells to bortezomib, preventing the TIGAR-dependent glucose entry into the PPP and inducing massive ROS accumulation due to GSH depletion.96 Simultaneous inhibition of glycolysis and PPP through 2-deoxy-d-glucose and 6-aminonicotinamide, respectively, induced oxidative stress and sensitized malignant human cancer cell lines to radiotherapy presumably through the induction of multiple cell death modalities including apoptosis, necrosis and mitotic catastrophe.97 The functional inactivation of rate-limiting enzymes of the PPP or the hindrance of glucose funneling into the G6PD-dependent reactions could represent a promising strategy in overcoming intrinsic or acquired resistance to conventional chemo/radiotherapy in both solid and hematologic tumors.
Glutaminolysis
Glutamine is a non-essential amino acid that has a key role in tumor metabolism, serving as a source of carbon and nitrogen for biosynthetic processes, an intermediate for energy production and a precursor for glutathione synthesis.98 Increased glutamine catabolism is a common hallmark of tumor metabolism reprogramming through which cancer cells support cell proliferation, signal transduction and redox homeostasis.99 It is generally assumed that the expression levels of certain oncogenes (i.e., Ras and Myc) or tumor suppressors (i.e., p53) can strongly influence the extent of glutamine utilization and the metabolic profile of different tumors.5, 100, 101, 102 However, emerging aspects of glutamine metabolism concern the potential mechanisms through which glutamine utilization can regulate the redox balance of malignant cells.68, 103 Indeed, it is increasingly recognized that glutaminase enzymes directly contribute to glutathione synthesis converting glutamine into glutamate and promoting the uptake of cysteine through the Slc7a11 exchanger.104 Similarly, metabolic intermediates such as citrate can be diverted from the TCA cycle and exported into the cytosol, where ME or IDH1 use them to generate reducing power in the form of NADPH.84 This strategy helps tumors to keep the glutathione pool in a reduced state and support the TRX system.105, 106 Additional mechanisms of redox modulation have been reported, wherein the mitochondrial enzyme glutamate dehydrogenase 1, by controlling the intracellular fumarate levels, positively regulates the enzymatic activity of the antioxidant enzyme glutathione peroxidase (Gpx).11 Given the paramount importance of glutamine metabolism in tumor progression and redox control, interfering with its function might represent an attractive anticancer strategy.107 With this respect, the glutaminase enzymes (GLS) as master regulators of glutamine metabolism have been the focus of recent anticancer research. Pharmacological inhibition of GLS1 with bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES) impaired the proliferation of P493 B-cell lymphoma (BCL) cells inducing DNA fragmentation and apoptotic cell death, whereas the genetic silencing of GLS1 prolonged the survival of mice with Myc-induced hepatocellular carcinoma and markedly impaired the growth of P493BCL xenograft.108 Similarly, BPTES-induced GLS1 inhibition selectively suppressed the growth of glioma cells with the R132D mutation in the IDH1 isoform109 and blocked the proliferation of primary acute myeloid leukemia (AML) cells with mutations in the IDH1/2 enzymes.110 By comparing different AML cell lines, Goto et al.111 have reported that glutaminolysis inhibition was associated to the depletion of the intracellular GSH content and subsequent ROS generation, particularly in HL-60 cells characterized by glutamine addiction. In a former study, glutamine deprivation also decrease the GSH levels of neuroblastoma cells, leading to altered redox balance, impaired cell proliferation and increased chemosensitivity to the alkylating agent L-PAM.112 Finally, the combined inhibition of GLS1 and heat-shock protein 90 induced synthetic lethality in cancer cells and mouse embryonic fibroblasts with activating alterations of the mTORC1 pathway through increased ER stress and disruption of the redox balance caused by GSH depletion.83 Taken together, these studies underline the importance of glutamine in the maintenance of redox balance, cell growth and cell survival of both solid and hematologic tumors, setting the rational for therapeutic approaches aimed at the manipulation of glutamine metabolism to block malignancy.
The serine–glycine one-carbon metabolism
The serine–glycine one-carbon metabolism (SGOC) is a complex network of biochemical reactions that integrate inputs from amino acids and glucose derivatives (mainly serine and glycine) and generates multiple outputs as carbon units (tetrahydrofolate (THF) and its derivate) that serve different cellular functions. The redistribution of these carbon units from serine and glycine rely on three pathways: the folate cycle, the methionine cycle and the trans-sulfuration pathway.113 Folate, a vitamin B derivative, is reduced to THF by a series of metabolic reactions and converted into methylenetetrahydrofolate by serine hydroxymethyl transferase (SHMT). This product is either converted to F-THF or reduced by methylenetetrahydrofolate reductase to methylenetetrahydrofolate, whose demethylation completes the folate cycle. The carbon units therefore enter into the methionine cycle with the generation of S-adenosylmethionine by the methionine adenyltransferase, with further conversion by S-adenosyl homocysteine hydrolase into homocysteine.114 The last modular component of the one-carbon metabolism, the trans-sulfuration pathway, is functionally connected to the methionine cycle through the homocysteine, whose condensation with serine by cystathionine synthase generates cystathione, further metabolized to alpha-ketobutyrate and cysteine by cystathione lyase. The cysteine can therefore be diverted into GSH synthesis.113 For long time, SGOC has been associated with cancer cell due to its importance for the regulation of nucleic acid, lipids and protein synthesis of proliferating cells. More recent evidence indicates that this pathway is also crucial for redox balance.115, 116 With this respect, the mitochondria have been shown to have a prominent role.117 Indeed, despite THF-derived carbon units are primarily used for nucleotide synthesis in the cytosol, new methods for tracing NAPDH compartmentalization indicate that serine is predominantly utilized in the mitochondria of mammalian cells to generate NADPH.67, 118 An observation that was further substantiated by a recent study showing that both glycine and serine catabolism were responsible of NADPH production in the mitochondria of HEK-293 and MDA-MB468 cell lines.67 Interestingly, Myc-transformed cells subdued to hypoxia strongly upregulated the expression of the mitochondrial SHMT2, responsible for an abundant NADPH production. Conversely, knockdown of SHMT2 impaired antioxidant ability and increased cell death under hypoxia, but by a not yet known mechanism.119 Also, the antioxidant transcription factor Nrf2 can regulate the expression of key one-carbon metabolism enzymes, including 3-phosphoglycerate dehydrogenase, phosphoserine aminotransferase 1 and methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) in human non-small cell lung cancer (NSCLC) cells, supporting nucleotide and glutathione synthesis.120 Despite the fact that further studies will be necessary to assess potential therapeutic benefit of this approach, novel and selective inhibitors of SHMT2 and MTHFD2 enzymes might represent a promising anticancer strategy against hypoxic tumors characterized by otherwise limited tractability.117 Remarkably, the use of antifolate such as methotrexate and pemetrexed, which are known inhibitors of active SHMTD, still represents a cornerstone of antineoplastic therapy against solid and hematologic tumors including breast cancer, bladder cancer, acute lymphoblastic leukemia and lymphomas.121 Also, in therapy-resistant tumors, downstream pathways of one-carbon metabolism have been successfully targeted with agents that interfere with the nucleotide synthesis such as 5-FU for advanced colorectal cancer or gemcitabine for pancreatic cancer.113 Given the increased interest in metabolic alterations of cancer cells and the intimate connection between SGOC pathways and redox homeostasis of human tumors, key nodes in the one-carbon metabolism might represent a valid therapeutic target at the crossroad between the regulation of cancer growth and antioxidant capacity.
Mitochondria: The Perfect Location to Target Redox Homeostasis and Metabolic Pathways
One emerging aspect in the study of molecular mechanisms controlling redox balance and metabolism in mammalian cells regards the existence of a clear compartmentalization of specific biochemical reactions in different cell organelles. It is increasingly known that mitochondria are key organelles for the regulation of redox signaling and redox homeostasis of normal and cancer cells (Figure 1). By integrating metabolic, bioenergetics and redox cues, the mitochondrial network acts as a central hub that directly or indirectly controls a wide number of cellular processes including proliferation, ATP synthesis and cell death.122 By hosting multiple redox-active complexes and metabolic enzymes that generate superoxide anion, the mitochondria represent a major source of endogenous ROS production. The most well-characterized site is represented by the mETC, through which the electrons from reduced metabolic intermediates (e.g., NADH and FADH2) are transferred to the molecular oxygen. During the electrons flow, depending on the mitochondrial membrane potential status and the oxygen availability, semiquinone radicals can be generated at the level of complexes I, II and III, promoting the univalent reduction of oxygen into superoxide.123 Other sources of mitochondrial-dependent superoxide production include the 2-oxoglutarate dehydrogenase, the pyruvate dehydrogenase in the mitochondrial matrix, the mitochondrial glycerol-3 phosphate dehydrogenase and the electron transfer flavoprotein-ubiquinone oxidoreductase mitochondrial system (flavoprotein-ubiquinone oxidoreductase) located in the inner membrane.38 The generated superoxide can then leave the mitochondrial district through different ways. One well-established route is through its conversion into H2O2 by SOD2, whereas the second mechanism, still a matter of intense debate, postulates its direct diffusion into the cytosol through the voltage-dependent anion channel (VDAC),124, 125 wherein is spontaneously or enzymatically transformed into H2O2 by SOD1. In this way, the mitochondria produce picomolar to nanomolar amounts of peroxide that then leave the site of generation and promote retrograde signaling to the nucleus or regulate activity, localization and stability of redox-sensitive target proteins that are located in the cytosol.126 On the basis of their central role in redox control and metabolism, the mitochondria represent attractive targets for anticancer therapy. Several studies have investigated the effect of mitochondrial-targeted antioxidants and their impact in tumor biology.127 MitoQ is an orally active antioxidant that not only mimics the role of the endogenous mitochondrial antioxidant coenzyme Q10 (CoQ10) but also substantially augments the antioxidant capacity of CoQ10 in a mitochondrial membrane potential-dependent manner.128 MitoQ has been found to kill breast cancer cells and unhealthy mammary cells, supporting a role for MitoQ and similar compounds to be further evaluated for novel anticancer activity.129 Among other aspects, the relative contribution of different metabolic pathways involved in NADPH generation (e.g., ME1, IDH1, PPP and glutaminolysis) has received significant attention1, 102 with particular emphasis on the role of one-carbon metabolism.113 With this respect, mitochondrial NADPH mainly derives from serine catabolism regulated by SHMT2 and MTHFD2, two mitochondrial-specific enzymes that are frequently overexpressed in cancer cells acting as important regulators of tumor redox homeostasis, but absent or underrepresented in normal tissues.67, 130 Genetic depletion of SHMT2 altered the redox balance of Myc-transformed tumors during hypoxia and induced significant cytotoxicity,119 whereas knockdown of MTHFD2 in overexpressing breast cancer tumors has been shown to impair cell migration and invasion, and to sensitize malignant cells to methotrexate by inducing caspase 3/7-independent cell death.131 Taken together, these observations imply that targeting multiple mitochondrial functions with single agents or in combination with inhibitors of different metabolic pathways might represent a promising approach to improve the efficacy of conventional chemotherapeutics, in particular in those tumors wherein the apoptotic machinery is not functional.
Despite the fact that mitochondrial dysfunction has long been considered a metabolic hallmark of cancer cells, studies have also indicated that tumor cells not only have functional mitochondria but also that their activity is essential for tumorigenesis.127 By regulating the generation of ROS, ATP and other metabolites driving bioenergetic and biosynthetic processes, mitochondria have a key role in cancer progression.132, 133 Oncogenic activation of MYC and KRAS promote increased glucose utilization and addiction in different types of tumors,134 while also enhancing the mitochondrial-dependent biosynthesis of macromolecules through increased ATP levels and TCA cycle intermediates.64, 135 To replenish the TCA cycle substrates and fuel their uncontrolled growth, cancer cells utilize specific mechanisms of anaplerosis on the basis of the oxidation of glutamine to α-ketoglutarate and its conversion to oxaloacetate.136 As a consequence of oxidative metabolism, physiological amounts of ROS are produced at the level of the mETC, inducing the pro-tumoral activation of redox-sensitive pathways.31 To prevent ROS-induced toxicity, cancer cells redirect the metabolic intermediates coming from glutamine and one-carbon metabolism into alternative pathways that generate NADPH and GSH, antioxidants molecules readily used by several ROS-scavenging enzymes.67, 118 Interestingly, tumors with impaired TCA cycle activity (or mutations in the mETC complexes) and that rely on glycolysis for ATP synthesis, shift to reductive glutaminolysis to mediate biosynthetic processes and cell survival.137, 138 Such tumor cells depend on mitochondrial-derived ROS to promote cell proliferation and metastasis formation.139,140,141 In contrast to what is generally assumed, many tumors still use mitochondria for ATP production, despite the Warburg effect should provide sufficient amounts of ATP for their biological needs.142 Indeed, poorly vascularized and other subsets of tumors growing under limited glucose conditions heavily rely on oxidative phosphorylation for ATP synthesis,87, 143, 144 a weakness that might be targeted with drugs that limit glucose utilization and block mitochondrial bioenergetics.145, 146 With this respect, two anti-diabetic biguanides, such as metformin and phenformin, show promising antitumoral effects.147 Epidemiological studies have shown that metformin decreases the incidence of cancer and prolongs the survival rate of patients with solid tumors.148, 149 More studies have reported also that the in vivo antitumorigenic effects of metformin depended on the inhibition of the mETC complex-I activity and the decrease of circulating glucose and insulin levels.150,151,152 Metformin was also able to impair the growth of NSCLCs by blocking the activation of the Akt/mammalian target of rapamycin (mTOR) pathway and by potentiating the pro-apoptotic efficacy of ionizing radiation.153 Also, metformin selectively killed breast cancer stem cells through profound depletion of triphosphate nucleotides presumably reflecting a major impairment of the energetic metabolism.154 Therefore, another biguanide, called phenformin, has emerged as a potential anticancer agent due to its liposolubility, higher affinity for mETC complex-I and stronger antineoplastic activity.155 Phenformin enhanced the efficacy of the BRAF inhibitor PLX4270 both in vitro and in genetic models of melanoma driven by BRAFV600E mutations triggering apoptotic cell death upon inhibition of the mTOR pathway.156 Phenformin also induced selective apoptosis in a subset of NSCLCs with loss of the tumor suppressor LKB1 and oncogenic mutations in K-ras lowering the ATP levels and eliciting the activation of caspase 3.157 Despite the encouraging results, it should also be mentioned that the use of phenformin has been associated with lactic acidosis, an important pitfall that might limit its clinical applicability.158 For this reason, novel compounds that target the mETC complex-I (i.e., VLX600) or alternative strategies based on the translation of specific mitochondrial proteins (i.e., tigecycline) have been designed and currently successfully employed in preclinical studies.77, 159 Although the potential utility of these compounds still needs to be validated in long-term clinical trials, encouraging evidences suggest that targeting the bioenergetics function of the mitochondria might represent a valid therapeutic option for cancer treatment.
Conclusions and Perspective
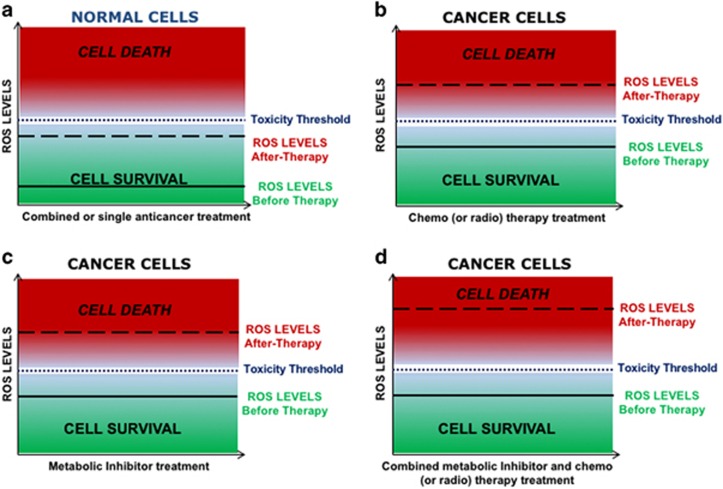
In light of recent research, the inhibition of metabolic pathways or ROS-scavenging mechanisms, followed by the administration of pro-oxidizing agents (i.e. chemo-radiotherapy), represents a promising therapeutic option for tumors characterized by resistance to treatment (Figure 3). With this respect, it is well documented that the efficacy of many anticancer therapies, including chemo- and radiotherapy, largely depends on the extent of the evoked ROS production.14 In many studies, either metabolic inhibition or the blockade of certain antioxidant systems was shown to strongly sensitize cancer cells to apoptotic cell death induced by further elevation of ROS levels.160, 161, 162, 163, 164 However, due to the undesired side effects including cardio-toxicity and nephrotoxicity, extensive research has been pursued to identify novel ROS modulators with a safer therapeutic profile. With this respect, another class of quinone-based compounds, including menadione and other vitamin K3 derivatives, are emerging as promising anticancer agents.165 Remarkably, this combinatorial approach has the ability to engage multiple cell death modalities, not limited by the activation of the intrinsic and extrinsic apoptotic pathways, and therefore might be useful to overcome the mechanisms of therapy resistance due to the overexpression of anti-apoptotic proteins or compromised activation of the caspase cascades. With this respect, the induction of ferroptosis, an iron- and ROS-dependent form of non-apoptotic cell death characterized by altered mitochondrial morphology, is receiving increasing attention for its intimate connection with cellular metabolism and redox balance.166, 167 Indeed, large B lymphomas and renal cell carcinomas with Ras mutations were found to be particularly susceptible to ferroptosis upon BSO-mediated GSH depletion, decreased Gpx4 activity and accumulation of lipid peroxidation.168, 169 In another study erastin, a potent inhibitor of Xc− cystine importer was shown to induce ER stress and trigger ferroptosis in different cancer cell lines.170
Figure 3.
Strategies to manipulate ROS levels as anticancer therapy. Effect of different therapeutic manipulations on the intracellular ROS levels and relative toxicity in both normal and cancer cells. (a) Normal cells treated with conventional chemo/radiotherapy, metabolic inhibitors or combined therapy show a slight increase in cell death. On the contrary treatment of cancer cells with (b) chemo/radiotherapy or (c) metabolic inhibitors elevates the rate of cell death compared with normal cells due to higher basal levels of ROS. When combined approaches on the basis of the use of metabolic inhibitors and conventional therapy (d) or other ROS-inducing agents can synergistically eradicate a larger proportion of cancer cells with marginal impact on normal cells, by elevating the intracellular ROS levels far above the toxicity threshold
In conclusion, it is becoming clear that redox signaling events require the simultaneous regulation of sources, transducers and scavengers in a precise spatial and temporal framework, whose alteration may disrupt key redox nodes and promote aberrant signaling. The loss of control over specific redox circuitries is likely to represent a tumor-specific alteration that may trigger unrestricted proliferation and malignant progression. Moreover, metabolomics studies associated to computational models on the basis of the integrative bioinformatics are becoming increasingly accessible and will surely guarantee a rapid progress in the field of redox biology, in particular addressing how metabolism and its subcellular compartmentalization can influence ROS signaling and redox cofactors. We are now approaching a new era wherein ROS biology and their effects in the physiopathology of cancer may be dissected with unprecedented detail, bringing potential therapeutic benefits derived from selective manipulations of cancer redox balance to be uncovered, paving the way to novel and exciting investigations in the fight against cancer.
Acknowledgments
We thank members of the Santoro lab for critical reading of the manuscript and Ellen Jane Corcoran for editorial and language assistance. This work is supported by the following grants: ERC-CoG 647057, Ministero della Salute RF-2011-02348194 and AIRC MFAG 8911 to MMS.
Glossary
- AML
acute myeloid leukemia
- ATP
adenosine tris-phosphate
- BCL
B-cell lymphoma
- BPTES
bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide
- FADH2
flavin adenine dinucleotide (reduced form)
- FAO
fatty acid oxidation
- G6PD
glucose-6-phosphate dehydrogenase
- GDH1
glutamate dehydrogenase 1
- GLS1
glutaminase 1
- GSH
reduced glutathione
- H2O2
hydrogen peroxide
- IDH1,2
isocitrate dehydrogenase 1,2
- LDH
lactate dehydrogenase
- ME
malic enzyme
- mETC
mitochondrial electron transport chain
- MTHFD2
methylenetetrahydrofolate dehydrogenase 2
- MTOR
mammalian target of rapamycin
- NADH
nicotinamide adenine dinucleotide (reduced form)
- NADPH
nicotinamide adenine dinucleotide phosphate
- NSCLC
non-small cell lung cancer cell
- PPP
penthose phosphate pathway
- ROS
reactive oxygen species
- SGOC
serine–glycine one-carbon metabolism
- SHMT2
serine hydroxymethyl transferase 2
- THF
tetrahydrofolate
- TRX
thioredoxin
The authors declare no conflict of interest.
Footnotes
Edited by A Finazzi-Agrò
References
- Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 2013; 12: 931–947. [DOI] [PubMed] [Google Scholar]
- Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell 2002; 9: 1031–1044. [DOI] [PubMed] [Google Scholar]
- Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med 2011; 208: 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 2007; 12: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009; 458: 762–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 2011; 334: 1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasauer A, Chandel NS. Targeting antioxidants for cancer therapy. Biochem Pharmacol 2014; 92: 90–101. [DOI] [PubMed] [Google Scholar]
- Harris IS, Brugge JS. Cancer: the enemy of my enemy is my friend. Nature 2015; 527: 170–171. [DOI] [PubMed] [Google Scholar]
- Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 2009; 461: 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011; 475: 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Li D, Alesi GN, Fan J, Kang HB, Lu Z et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell 2015; 27: 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris-Pages M, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Metastasis and oxidative stress: are antioxidants a metabolic driver of progression? Cell Metab 2015; 22: 956–958. [DOI] [PubMed] [Google Scholar]
- Lo Conte M, Lin J, Wilson MA, Carroll KS. A chemical approach for the detection of protein sulfinylation. ACS Chem Biol 2015; 10: 1825–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda G, Isvoranu G, Comanescu MV, Manea A, Debelec Butuner B, Korkmaz KS. The redox biology network in cancer pathophysiology and therapeutics. Redox Biol 2015; 5: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panieri E, Santoro MM. ROS signaling and redox biology in endothelial cells. Cell Mol Life Sci 2015; 72: 3281–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004; 4: 181–189. [DOI] [PubMed] [Google Scholar]
- Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci USA 2006; 103: 7607–7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan CL, Orr AL, Perevoshchikova IV, Treberg JR, Ackrell BA, Brand MD. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J Biol Chem 2012; 287: 27255–27264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med 2014; 66: 75–87. [DOI] [PubMed] [Google Scholar]
- Perkins A, Nelson KJ, Parsonage D, Poole LB, Karplus PA. Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem Sci 2015; 40: 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanschmann EM, Godoy JR, Berndt C, Hudemann C, Lillig CH. Thioredoxins, glutaredoxins, and peroxiredoxins—molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid Redox Signal 2013; 19: 1539–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Chew EH, Holmgren A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci USA 2007; 104: 12288–12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Zhang H, Zhang X, Lu J, Holmgren A. Thioredoxin 1 is inactivated due to oxidation induced by peroxiredoxin under oxidative stress and reactivated by the glutaredoxin system. J Biol Chem 2013; 288: 32241–32247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigam L, Jensen LD, Poschmann G, Nystrom S, Bannenberg S, Dreij K et al. Glutaredoxin regulates vascular development by reversible glutathionylation of sirtuin 1. Proc Natl Acad Sci USA 2013; 110: 20057–20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Du Y, Zhang X, Lu J, Holmgren A. Glutaredoxin 2 reduces both thioredoxin 2 and thioredoxin 1 and protects cells from apoptosis induced by auranofin and 4-hydroxynonenal. Antioxid Redox Signal 2014; 21: 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin AV, Pace PE, Behring JB, Paton LN, Soethoudt M, Bachschmid MM et al. Glutathionylation of the active site cysteines of peroxiredoxin 2 and recycling by glutaredoxin. J Biol Chem 2016; 291: 3053–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfs F, Huber M, Gruber F, Bohm F, Pfister HJ, Bochkov VN et al. Dual role of the antioxidant enzyme peroxiredoxin 6 in skin carcinogenesis. Cancer Res 2013; 73: 3460–3469. [DOI] [PubMed] [Google Scholar]
- Chen JW, Dodia C, Feinstein SI, Jain MK, Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem 2000; 275: 28421–28427. [DOI] [PubMed] [Google Scholar]
- Adimora NJ, Jones DP, Kemp ML. A model of redox kinetics implicates the thiol proteome in cellular hydrogen peroxide responses. Antioxid Redox Signal 2010; 13: 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AM, Brown JD, Taylor SR, Rand JD, Morgan BA, Veal EA. Inactivation of a peroxiredoxin by hydrogen peroxide is critical for thioredoxin-mediated repair of oxidized proteins and cell survival. Mol Cell 2012; 45: 398–408. [DOI] [PubMed] [Google Scholar]
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24: R453–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mehdi AB, Pastukh VM, Swiger BM, Reed DJ, Patel MR, Bardwell GC et al. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signal 2012; 5ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ 2009; 16: 1040–1052. [DOI] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem 2002; 277: 20336–20342. [DOI] [PubMed] [Google Scholar]
- Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem 1997; 272: 217–221. [PubMed] [Google Scholar]
- Wu Q, Ni X. ROS-mediated DNA methylation pattern alterations in carcinogenesis. Curr Drug Targets 2015; 16: 13–19. [DOI] [PubMed] [Google Scholar]
- Bielski BH, Arudi RL, Sutherland MW. A study of the reactivity of HO2/O2- with unsaturated fatty acids. J Biol Chem 1983; 258: 4759–4761. [PubMed] [Google Scholar]
- Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 2014; 15: 411–421. [DOI] [PubMed] [Google Scholar]
- Martin KR, Barrett JC. Reactive oxygen species as double-edged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Hum Exp Toxicol 2002; 21: 71–75. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M, Watanabe S, Satooka H. Involvement of aquaporin-3 in epidermal growth factor receptor signaling via hydrogen peroxide transport in cancer cells. Biochem Biophys Res Commun 2016; 471: 603–609. [DOI] [PubMed] [Google Scholar]
- Choi J, Park SJ, Jo EJ, Lee HY, Hong S, Kim SJ et al. Hydrogen peroxide inhibits transforming growth factor-beta1-induced cell cycle arrest by promoting Smad3 linker phosphorylation through activation of Akt-ERK1/2-linked signaling pathway. Biochem Biophys Res Commun 2013; 435: 634–639. [DOI] [PubMed] [Google Scholar]
- Juarez JC, Manuia M, Burnett ME, Betancourt O, Boivin B, Shaw DE et al. Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc Natl Acad Sci USA 2008; 105: 7147–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluckova K, Sticha M, Cerny J, Mracek T, Dong L, Drahota Z et al. Ubiquinone-binding site mutagenesis reveals the role of mitochondrial complex II in cell death initiation. Cell Death Dis 2015; 6: e1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk B, Fulda S. Reactive oxygen species regulate Smac mimetic/TNFalpha-induced necroptotic signaling and cell death. Oncogene 2015; 34: 5796–5806. [DOI] [PubMed] [Google Scholar]
- Shi YL, Feng S, Chen W, Hua ZC, Bian JJ, Yin W. Mitochondrial inhibitor sensitizes non-small-cell lung carcinoma cells to TRAIL-induced apoptosis by reactive oxygen species and Bcl-X(L)/p53-mediated amplification mechanisms. Cell Death Dis 2014; 5: e1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer A, Ferro M, Tillian HM, Tatzber F, Zollner H, Schauenstein E et al. Effect of oxidative stress by iron on 4-hydroxynonenal formation and proliferative activity in hepatomas of different degrees of differentiation. Free Radic Biol Med 1997; 23: 26–33. [DOI] [PubMed] [Google Scholar]
- Cerbone A, Toaldo C, Laurora S, Briatore F, Pizzimenti S, Dianzani MU et al. 4-Hydroxynonenal and PPARgamma ligands affect proliferation, differentiation, and apoptosis in colon cancer cells. Free Radic Biol Med 2007; 42: 1661–1670. [DOI] [PubMed] [Google Scholar]
- Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PloS ONE 2013; 8: e81162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Terazawa R, Kojima K, Nakane K, Deguchi T, Ando M et al. Cisplatin induces production of reactive oxygen species via NADPH oxidase activation in human prostate cancer cells. Free Radic Res 2011; 45: 1033–1039. [DOI] [PubMed] [Google Scholar]
- Roh JL, Park JY, Kim EH, Jang HJ, Kwon M. Activation of mitochondrial oxidation by PDK2 inhibition reverses cisplatin resistance in head and neck cancer. Cancer Lett 2016; 371: 20–29. [DOI] [PubMed] [Google Scholar]
- Yang YJ, Baek JY, Goo J, Shin Y, Park JK, Jang JY et al. Effective killing of cancer cells through ros-mediated mechanisms by AMRI-59 targeting peroxiredoxin I. Antioxid Redox Signal 2015; 24: 453–469. [DOI] [PubMed] [Google Scholar]
- Wason MS, Colon J, Das S, Seal S, Turkson J, Zhao J et al. Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomedicine 2013; 9: 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alajez NM, Shi W, Hui AB, Yue S, Ng R, Lo KW et al. Targeted depletion of BMI1 sensitizes tumor cells to P53-mediated apoptosis in response to radiation therapy. Cell Death Differ 2009; 16: 1469–1479. [DOI] [PubMed] [Google Scholar]
- Singh A, Bodas M, Wakabayashi N, Bunz F, Biswal S. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid Redox Signal 2010; 13: 1627–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Kang MJ, Cho YM. Low production of reactive oxygen species and high DNA repair: mechanism of radioresistance of prostate cancer stem cells. Anticancer Res 2013; 33: 4469–4474. [PubMed] [Google Scholar]
- Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA et al. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci USA 2011; 108: 1433–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs-Tarlovsky V. Role of antioxidants in cancer therapy. Nutrition 2013; 29: 15–21. [DOI] [PubMed] [Google Scholar]
- Leon-Gonzalez AJ, Auger C, Schini-Kerth VB. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem Pharmacol 2015; 98: 371–380. [DOI] [PubMed] [Google Scholar]
- Filomeno M, Bosetti C, Bidoli E, Levi F, Serraino D, Montella M et al. Mediterranean diet and risk of endometrial cancer: a pooled analysis of three Italian case-control studies. Br J Cancer 2015; 112: 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Stantic M, Zobalova R, Bezawork-Geleta A, Stapelberg M, Stursa J et al. Mitochondrially targeted vitamin E succinate efficiently kills breast tumour-initiating cells in a complex II-dependent manner. BMC Cancer 2015; 15: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris IS, Treloar AE, Inoue S, Sasaki M, Gorrini C, Lee KC et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015; 27: 211–222. [DOI] [PubMed] [Google Scholar]
- Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011; 475: 231–234. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shaw AT, Winslow MM, Magendantz M, Ouyang C, Dowdle J, Subramanian A et al. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc Natl Acad Sci USA 2011; 108: 8773–8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV. Links between metabolism and cancer. Genes Dev 2012; 26: 877–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz RM, Lombo F, Mayo JC. Radical decisions in cancer: redox control of cell growth and death. Cancers 2012; 4: 442–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015; 527: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014; 510: 298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberghina L, Gaglio D. Redox control of glutamine utilization in cancer. Cell Death Dis 2014; 5: e1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Du W, Wu M. Regulation of the pentose phosphate pathway in cancer. Protein Cell 2014; 5: 592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and cancer. Cell Metab 2013; 18: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011; 11: 85–95. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 2010; 330: 1340–1344. [DOI] [PubMed] [Google Scholar]
- Zhang D, Li J, Wang F, Hu J, Wang S, Sun Y. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett 2014; 355: 176–183. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell 2008; 13: 472–482. [DOI] [PubMed] [Google Scholar]
- Zhai X, Yang Y, Wan J, Zhu R, Wu Y. Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncology Rep 2013; 30: 2983–2991. [DOI] [PubMed] [Google Scholar]
- Zhang X, Fryknas M, Hernlund E, Fayad W, De Milito A, Olofsson MH et al. Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironments. Nat Commun 2014; 5: 3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A et al. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther 2008; 7: 110–120. [DOI] [PubMed] [Google Scholar]
- Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene 2011; 30: 4297–4306. [DOI] [PubMed] [Google Scholar]
- Godoy A, Ulloa V, Rodriguez F, Reinicke K, Yanez AJ, Garcia Mde L et al. Differential subcellular distribution of glucose transporters GLUT1-6 and GLUT9 in human cancer: ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J Cell Physiol 2006; 207: 614–627. [DOI] [PubMed] [Google Scholar]
- Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J 2009; 418: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA 2010; 107: 2037–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Csibi A, Yang S, Hoffman GR, Li C, Zhang E et al. Synthetic lethality of combined glutaminase and Hsp90 inhibition in mTORC1-driven tumor cells. Proc Natl Acad Sci USA 2015; 112: E21–E29. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer 2013; 13: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012; 485: 661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg K, Yao Y, Reilly PT, Kannan K, Kiarash R, Mason J et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev 2011; 25: 1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell 2012; 22: 547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta 2011; 1807: 726–734. [DOI] [PubMed] [Google Scholar]
- Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest 2010; 120: 142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med 2012; 53: 421–436. [DOI] [PubMed] [Google Scholar]
- Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci 2014; 39: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Jiang P, Mancuso A, Stonestrom A, Brewer MD, Minn AJ et al. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nat Cell Biol 2013; 15: 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessandro A, Amelio I, Berkers CR, Antonov A, Vousden KH, Melino G et al. Metabolic effect of TAp63alpha: enhanced glycolysis and pentose phosphate pathway, resulting in increased antioxidant defense. Oncotarget 2014; 5: 7722–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarelli G, Galleggiante V, Rutigliano M, Sanguedolce F, Cagiano S, Bufo P et al. Metabolomic profile of glycolysis and the pentose phosphate pathway identifies the central role of glucose-6-phosphate dehydrogenase in clear cell-renal cell carcinoma. Oncotarget 2015; 6: 13371–13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimeni M, Voena C, Kopecka J, Riganti C, Pescarmona G, Bosia A et al. Modulation of doxorubicin resistance by the glucose-6-phosphate dehydrogenase activity. Biochem J 2011; 439: 141–149. [DOI] [PubMed] [Google Scholar]
- Yin L, Kufe T, Avigan D, Kufe D. Targeting MUC1-C is synergistic with bortezomib in downregulating TIGAR and inducing ROS-mediated myeloma cell death. Blood 2014; 123: 2997–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma PK, Bhardwaj R, Dwarakanath BS, Varshney R. Metabolic oxidative stress induced by a combination of 2-DG and 6-AN enhances radiation damage selectively in malignant cells via non-coordinated expression of antioxidant enzymes. Cancer Lett 2010; 295: 154–166. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez MV, Sanchez-Jimenez F, Alonso FJ, Segura JA, Marquez J, Medina MA. Glutamine, glucose and other fuels for cancer. Curr Pharm Des 2014; 20: 2557–2579. [DOI] [PubMed] [Google Scholar]
- Daye D, Wellen KE. Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Semin Cell Dev Biol 2012; 23: 362–369. [DOI] [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA 2008; 105: 18782–18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetinbas N, Daugaard M, Mullen AR, Hajee S, Rotblat B, Lopez A et al. Loss of the tumor suppressor Hace1 leads to ROS-dependent glutamine addiction. Oncogene 2014; 34: 4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013; 496: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab 2012; 15: 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 1999; 274: 11455–11458. [DOI] [PubMed] [Google Scholar]
- Mates JM, Segura JA, Martin-Rufian M, Campos-Sandoval JA, Alonso FJ, Marquez J. Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer. Curr Mol Med 2013; 13: 514–534. [DOI] [PubMed] [Google Scholar]
- Lyssiotis CA, Son J, Cantley LC, Kimmelman AC. Pancreatic cancers rely on a novel glutamine metabolism pathway to maintain redox balance. Cell Cycle 2013; 12: 1987–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest 2013; 123: 3678–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Stine ZE, Xia J, Lu Y, O'Connor RS, Altman BJ et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest 2015; 125: 2293–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MJ, Bennett BD, Joshi AD, Gao P, Thomas AG, Ferraris DV et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res 2010; 70: 8981–8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadi A, Jun SA, Tsukamoto T, Fathi AT, Minden MD, Dang CV. Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations. Exp Hematol 2014; 42: 247–251. [DOI] [PubMed] [Google Scholar]
- Goto M, Miwa H, Shikami M, Tsunekawa-Imai N, Suganuma K, Mizuno S et al. Importance of glutamine metabolism in leukemia cells by energy production through TCA cycle and by redox homeostasis. Cancer Invest 2014; 32: 241–247. [DOI] [PubMed] [Google Scholar]
- Izaki S, Goto H, Yokota S. Increased chemosensitivity and elevated reactive oxygen species are mediated by glutathione reduction in glutamine deprived neuroblastoma cells. J Cancer Res Clin Oncol 2008; 134: 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 2013; 13: 572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ. Serine metabolism: some tumors take the road less traveled. Cell Metab 2011; 14: 285–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amelio I, Cutruzzola F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem Sci 2014; 39: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014; 515: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Reyes I, Chandel NS. Mitochondrial one-carbon metabolism maintains redox balance during hypoxia. Cancer Discov 2014; 4: 1371–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CA, Parker SJ, Fiske BP, McCloskey D, Gui DY, Green CR et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Molecular cell 2014; 55: 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Fan J, Venneti S, Wan YW, Pawel BR, Zhang J et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov 2014; 4: 1406–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Chen PH, Mullarky E, Sudderth JA, Hu Z, Wu D et al. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat Genet 2015; 47: 1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daidone F, Florio R, Rinaldo S, Contestabile R, di Salvo ML, Cutruzzola F et al. In silico and in vitro validation of serine hydroxymethyltransferase as a chemotherapeutic target of the antifolate drug pemetrexed. Eur J Med Chem 2011; 46: 1616–1621. [DOI] [PubMed] [Google Scholar]
- Handy DE, Loscalzo J. Redox regulation of mitochondrial function. Antioxid Redox Signal 2012; 16: 1323–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by mitochondrial oxidants. J Biol Chem 2012; 287: 4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem 2003; 278: 5557–5563. [DOI] [PubMed] [Google Scholar]
- Lustgarten MS, Bhattacharya A, Muller FL, Jang YC, Shimizu T, Shirasawa T et al. Complex I generated, mitochondrial matrix-directed superoxide is released from the mitochondria through voltage dependent anion channels. Biochem Biophys Res Commun 2012; 422: 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid Redox Signal 2012; 16: 476–495. [DOI] [PubMed] [Google Scholar]
- Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA 2010; 107: 8788–8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann NY Acad Sci 2010; 1201: 96–103. [DOI] [PubMed] [Google Scholar]
- Rao VA, Klein SR, Bonar SJ, Zielonka J, Mizuno N, Dickey JS et al. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. J Biol Chem 2010; 285: 34447–34459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson R, Jain M, Madhusudhan N, Sheppard NG, Strittmatter L, Kampf C et al. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun 2014; 5: 3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen L, Ketola K, Makela R, Mpindi JP, Viitala M, Kallioniemi O et al. High-throughput RNAi screening for novel modulators of vimentin expression identifies MTHFD2 as a regulator of breast cancer cell migration and invasion. Oncotarget 2013; 4: 48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma LK, Fang H, Liu J, Vartak R, Deng J, Bai Y. Mitochondrial respiratory complex I dysfunction promotes tumorigenesis through ROS alteration and AKT activation. Hum Mol Genet 2011; 20: 4605–4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab 2014; 2: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012; 24: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 2012; 21: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Cheng T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010; 29: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 2012; 481: 385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2012; 481: 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo DK, Green PD, Santos JH, D'Souza AD, Walther Z, Martin WD et al. Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APC(Min/+) mice. Am J Pathol 2012; 180: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 2008; 320: 661–664. [DOI] [PubMed] [Google Scholar]
- Porporato PE, Payen VL, Perez-Escuredo J, De Saedeleer CJ, Danhier P, Copetti T et al. A mitochondrial switch promotes tumor metastasis. Cell Rep 2014; 8: 754–766. [DOI] [PubMed] [Google Scholar]
- Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T et al. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Syst Biol 2013; 9: 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq R, Shoag J, Andreu-Perez P, Yokoyama S, Edelman H, Rowe GC et al. Oncogenic BRAF regulates oxidative metabolism via PGC1alpha and MITF. Cancer Cell 2013; 23: 302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Lim JH, Chim H, Bhalla K, Girnun G, Pierce K et al. PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell 2013; 23: 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Zielonka J, Dranka BP, McAllister D, Mackinnon AC Jr., Joseph J et al. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res 2012; 72: 2634–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 2008; 14: 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. Overcoming drug development bottlenecks with repurposing: repurposing biguanides to target energy metabolism for cancer treatment. Nat Med 2014; 20: 591–593. [DOI] [PubMed] [Google Scholar]
- Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen—induced lung tumorigenesis. Cancer Prev Res (Phila) 2010; 3: 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. J Mol Endocrinol 2012; 48: R31–R43. [DOI] [PubMed] [Google Scholar]
- Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 2014; 3: e02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsoy K, Sabatini DM, Possemato R. Untuning the tumor metabolic machine: Targeting cancer metabolism: a bedside lesson. Nat Med 2012; 18: 1022–1023. [DOI] [PubMed] [Google Scholar]
- Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 2012; 12: 159–169. [DOI] [PubMed] [Google Scholar]
- Storozhuk Y, Hopmans SN, Sanli T, Barron C, Tsiani E, Cutz JC et al. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer 2013; 108: 2021–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzer A, German NJ, Gonzalez-Herrera KN, Asara JM, Haigis MC, Struhl K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proc Natl Acad Sci USA 2014; 111: 10574–10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard MV, Murray KE, Coates PJ, Wullschleger S, Bray SE, Kernohan NM et al. Phenformin as prophylaxis and therapy in breast cancer xenografts. Br J Cancer 2012; 106: 1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Ito K, Perez-Lorenzo R, Del Guzzo C, Lee JH, Shen CH et al. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc Natl Acad Sci USA 2013; 110: 18226–18231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell 2013; 23: 143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimognari FL, Pastorelli R, Incalzi RA. Phenformin-induced lactic acidosis in an older diabetic patient: a recurrent drama (phenformin and lactic acidosis). Diabetes Care 2006; 29: 950–951. [DOI] [PubMed] [Google Scholar]
- Skrtic M, Sriskanthadevan S, Jhas B, Gebbia M, Wang X, Wang Z et al. Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell 2011; 20: 674–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj R, Sharma PK, Jadon SP, Varshney R. A combination of 2-deoxy-D-glucose and 6-aminonicotinamide induces cell cycle arrest and apoptosis selectively in irradiated human malignant cells. Tumour Biol 2012; 33: 1021–1030. [DOI] [PubMed] [Google Scholar]
- Alarifi S, Ali D, Alkahtani S, Siddiqui MA, Ali BA. Arsenic trioxide-mediated oxidative stress and genotoxicity in human hepatocellular carcinoma cells. Onco Targets Ther 2013; 6: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanot M, Boivin A, Malesys C, Beuve M, Colliaux A, Foray N et al. Glutathione depletion and carbon ion radiation potentiate clustered DNA lesions, cell death and prevent chromosomal changes in cancer cells progeny. PloS ONE 2012; 7: e44367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla S, Gordon LI, David K, Prachand S, Singh AT, Yang S et al. Glutathione depletion enhances arsenic trioxide-induced apoptosis in lymphoma cells through mitochondrial-independent mechanisms. Br J Haematol 2010; 150: 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Liu TW, Chen LT, Shiah HS, Wu CM, Cheng YT et al. Combination of arsenic trioxide and BCNU synergistically triggers redox-mediated autophagic cell death in human solid tumors. Free Radic Biol Med 2011; 51: 2195–2209. [DOI] [PubMed] [Google Scholar]
- Verrax J, Beck R, Dejeans N, Glorieux C, Sid B, Pedrosa RC et al. Redox-active quinones and ascorbate: an innovative cancer therapy that exploits the vulnerability of cancer cells to oxidative stress. Anticancer Agents Med Chem 2011; 11: 213–221. [DOI] [PubMed] [Google Scholar]
- Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 2015; 59: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X et al. Ferroptosis: process and function. Cell Death Differ 2016; 23: 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014; 156: 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol 2016; 26: 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 2014; 3: e02523. [DOI] [PMC free article] [PubMed] [Google Scholar]