Introduction
Coagulase-negative staphylococci (CoNS), once an umbrella term for normal skin microbiota, are increasingly implicated in hospital-acquired infections. Quick, easy, and accurate speciation of CoNS via matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) was recently validated.1 Integrating this technology into hospital laboratories has allowed multiple members of the genus to surface as potential human pathogens.1 We report a case of Staphylococcus simulans causing a skin and soft tissue infection to alert dermatologists to this emerging CoNS pathogen.
Case report
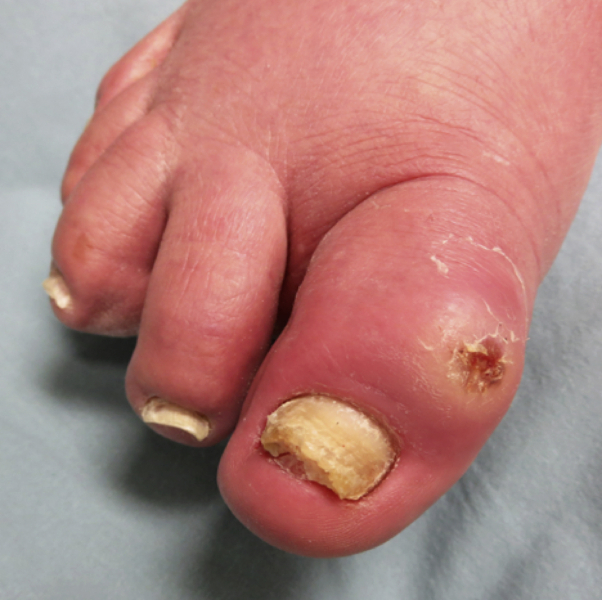
A retired farmer, in his 80s, with osteoarthritis and bilateral knee replacements presented for evaluation of right toe swelling of 5 months' duration. Gout and other crystalline arthropathies were ruled out at initial examination. He was treated previously with 10-day courses of clindamycin and ciprofloxacin with short-lived, mild improvement. Physical examination found violaceous erythema of the dorsal right great toe with an overlying 2-cm soft, mildly fluctuant nodule and a central 5-mm superficial erosion (Fig 1). Two 4-mm punch biopsies were performed: one for histopathology and one for tissue culture. Histopathology findings were remarkable for superficial ulceration with overlying fibrinopurulent debris and numerous bacterial cocci. Tissue culture from the biopsy grew oxacillin-susceptible S simulans identified with MALDI-TOF-MS. Magnetic resonance imaging excluded osteomyelitis, and serum uric acid level was normal. He was initially treated with cephalexin with minimal improvement after 7 days and no improvement after 14 days of doxycycline. Culture and sensitivities grew 2 strains of S simulans, one of which was sensitive and one that was resistant to tetracycline. The patient ultimately had complete resolution after 28 days of trimethoprim-sulfamethoxazole. This drug was chosen based on the results of the MALDI-TOF-MS speciation and susceptibilities of both strains, which were sensitive to trimethoprim-sulfamethoxazole.
Fig 1.
Clinical picture of S simulans infection. Violaceous erythema of the dorsal right great toe with an overlying mildly fluctuant nodule and central superficial erosion.
Discussion
S simulans is a CoNS and a well-established animal pathogen affecting cows, sheep, goats, and horses. It is commonly implicated in bovine mastitis.2 Reports of S simulans as the sole pathogen in human infections are rare; however, we hypothesize that our patient may have acquired infection from his farm and regular handling of animals, an identifiable risk factor. S simulans has also been implicated in osteoarticular infections, native valve endocarditis, and diabetic osteitis,3, 4, 5 with diabetes and prosthetic joints identified as additional risk factors.
Previously classified as rare opportunistic agents, Staphylococcus lugdunensis and Staphylococcus schleiferi are additional CoNS with emerging pathogenicity.6 Limited data exist explaining how these species are gaining pathogenicity, but shared virulence factors with Staphylococcus aureus have been documented in infectious animal isolates, including staphylococcal enterotoxins, tissue necrosis cytotoxin Panton-Valentine leukocidin (pvl), and the methicillin-resistance gene, mecA (Table I).2, 6 Importantly, mecA-positive CoNS are able to horizontally transfer their genes within the staphylococcal genus with the potential to give rise to new methicillin-resistant strains.2 Theoretically, with horizontal gene transfer possible, staphylococcal strains could acquire the ability to generate superantigens and cytotoxins, amplifying their virulence. Of particular dermatologic importance is the presence of pvl-producing S simulans, as pvl-positive strains of S aureus have been preferentially linked to furuncles, cutaneous abscesses, and severe skin necrosis.7 These virulence factors are not routinely tested for in human cultures nor reported in our patient.
Table I.
| Staphylococcal species | Virulence factors |
|---|---|
| S aureus | eta, etb, seg, she, sei, se1j, se1k, se1l, se1m, se1n, se1o, se1p, se1r, ses, set, se1u, se1v, tsst-1, mecA, pvl |
| Staphylococcus epidermidis | seh, sej, mecA |
| Staphylococcus haemolyticus | seh, sej, mecA, pvl |
| Staphylococcus hominis | seh, sej |
| S lugdunensis | seh |
| S schleiferi | seh, sej, mecA |
| Staphylococcus saprophyticus | seh, sej |
| S simulans | seh, sej, mecA, pvl |
| Staphylococcus xylosus | seh, mecA |
eta, Exfoliative toxin a; etb, exfoliative toxin b; se, staphylococcal enterotoxinlike toxin; tsst, toxic shock syndrome toxin-1.
Consistent with this case, CoNS cases can be difficult to treat because of multidrug resistance and varying susceptibilities among strains.2 This finding further highlights the need for precise speciation and sensitivity detection with a low threshold for treatment alteration when clinical improvement does not occur. Cultured CoNS strains are often not speciated, making it difficult to monitor for antibiotic resistance and impossible to identify horizontal gene transfer of virulence factors.6
Adequate speciation and susceptibility determination in skin and soft tissue infections is increasingly important. Dermatologists should be aware of S simulans and other CoNS species as potential pathogens and understand the origin of these newly named bacteria as MALDI-TOF-MS technology becomes more widely available.
Footnotes
Dr Tschetter is currently affiliated with the Department of Dermatology, University of Minnesota, Minneapolis.
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Carpaij N., Willems R.J., Bonten M.J. Comparison of the identification of coagulase-negative staphylococci by matrix-assisted laser desorption ionization time-of-flight mass spectrometry and tuf sequencing. Eur J Clin Microbiol Infect Dis. 2011;30:1169–1172. doi: 10.1007/s10096-011-1204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unal N., Cinar O.D. Detection of stapylococcal enterotoxin, methicillin-resistant and Panton-Valentine leukocidin genes in coagulase-negative staphylococci isolated from cows and ewes with subclinical mastitis. Trop Anim Health Prod. 2012;44:369–375. doi: 10.1007/s11250-011-0032-x. [DOI] [PubMed] [Google Scholar]
- 3.Mallet M., Loiez C., Melliez H., Yazdanpanah Y., Senneville E., Lemaire X. Staphylococcus simulans as an authentic pathogenic agent of osteoarticular infections. Infection. 2011;39:473–476. doi: 10.1007/s15010-011-0173-x. [DOI] [PubMed] [Google Scholar]
- 4.Vallianou N., Evangelopoulos A., Makri P. Vertebral osteomyelitis and native valve endocarditis due to Staphylococcus simulans: a case report. J Med Case Rep. 2008;2:183. doi: 10.1186/1752-1947-2-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Désidéri-Vaillant C., Nédelec Y., Guichon J.M. Staphylococcus simulans osteitis in a diabetic patient. Diabetes Metab. 2011;37:560–562. doi: 10.1016/j.diabet.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Davis M.F., Cain C.L., Brazil A.M. Two coagulase-negative staphylococci emerging as potential zoonotic pathogens: wolves in sheep's clothing? Front Microbiol. 2013;4:123. doi: 10.3389/fmicb.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lina G., Piemont Y., Godail-Gamot F. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]