Natural products produced by members of the phylum Actinobacteria underpin many industrially and medically important compounds; however, the majority of the ~30 biosynthetic pathways harbored by an average species are not expressed in the laboratory. Understanding the diversity of regulatory strategies controlling the expression of these pathways is therefore critical if their biosynthetic potential is to be explored for new drug leads. Our findings reveal that the candicidin cluster-situated regulator FscRI coordinately controls the biosynthesis of both candicidin and antimycin, which is the first observation of cross-regulation of disparate biosynthetic gene clusters specifying unrelated natural products. We anticipate that this will emerge as a major strategy by which members of the phylum Actinobacteria coordinately produce natural products, which will advance our understanding of how the expression of secondary metabolism is controlled and will aid the pursuit of “silent” biosynthetic pathway activation.
KEYWORDS: natural products, regulation of secondary metabolism, secondary metabolism, Streptomyces
ABSTRACT
Streptomyces species produce an incredible array of high-value specialty chemicals and medicinal therapeutics. A single species typically harbors ~30 biosynthetic pathways, but only a few them are expressed in the laboratory; thus, poor understanding of how natural-product biosynthesis is regulated is a major bottleneck in drug discovery. Antimycins are a large family of anticancer compounds widely produced by Streptomyces species, and their regulation is atypical compared to that of most other natural products. Here we demonstrate that antimycin production by Streptomyces albus S4 is regulated by FscRI, a PAS-LuxR family cluster-situated regulator of the polyene antifungal agent candicidin. We report that heterologous production of antimycins by Streptomyces coelicolor is dependent on FscRI and show that FscRI activates the transcription of key biosynthetic genes. We also demonstrate through chromatin immunoprecipitation sequencing that FscRI regulation is direct, and we provide evidence that this regulation strategy is conserved and unique to short-form antimycin gene clusters. Our study provides direct in vivo evidence of the cross-regulation of disparate biosynthetic gene clusters specifying unrelated natural products and expands the paradigmatic understanding of the regulation of secondary metabolism.
IMPORTANCE Natural products produced by members of the phylum Actinobacteria underpin many industrially and medically important compounds; however, the majority of the ~30 biosynthetic pathways harbored by an average species are not expressed in the laboratory. Understanding the diversity of regulatory strategies controlling the expression of these pathways is therefore critical if their biosynthetic potential is to be explored for new drug leads. Our findings reveal that the candicidin cluster-situated regulator FscRI coordinately controls the biosynthesis of both candicidin and antimycin, which is the first observation of cross-regulation of disparate biosynthetic gene clusters specifying unrelated natural products. We anticipate that this will emerge as a major strategy by which members of the phylum Actinobacteria coordinately produce natural products, which will advance our understanding of how the expression of secondary metabolism is controlled and will aid the pursuit of “silent” biosynthetic pathway activation.
INTRODUCTION
Microbial natural products underpin most of the pharmaceuticals in clinical use (1), and filamentous members of the phylum Actinobacteria, such as Streptomyces species, are prolific producers of these diverse small molecules. Streptomyces species typically harbor between 20 and 50 biosynthetic pathways, but only a few them are expressed under common laboratory conditions (2). The biochemical diversity encoded by these silent or unproductive biosynthetic pathways is widely believed to be a tremendous untapped source of new antibacterial agents and other therapeutics. The regulation of natural-product biosynthesis is complex and typically involves pleiotropic global regulators that either directly activate or repress biosynthetic genes or do so via cluster-situated activators or repressors (3). Major roadblocks preventing the exploitation of silent biosynthetic pathways are a lack of insight into their regulation and limited technology for activating their expression. Advances in this area have significant potential to unlock the diversity of natural products for drug discovery.
Antimycin-type depsipeptides are a large class of natural products widely produced by Streptomyces species (see references 4 and 5 for recent reviews). Antimycins are the archetypal members of this family and have been known for more than 65 years (6). They possess a myriad of biological properties, including antifungal, insecticidal, and nematocidal activities, owing to their ability to inhibit mitochondrial cytochrome c reductase (7), and are used commercially as a fish pesticide (brand name, Fintrol) (8). Recently, antimycins were found to be potent and selective inhibitors of the mitochondrial Bcl-2/Bcl-XL-related antiapoptotic proteins that are overproduced by cancer cells and confer resistance to chemotherapeutic agents whose mode of action is activation of apoptosis (9).
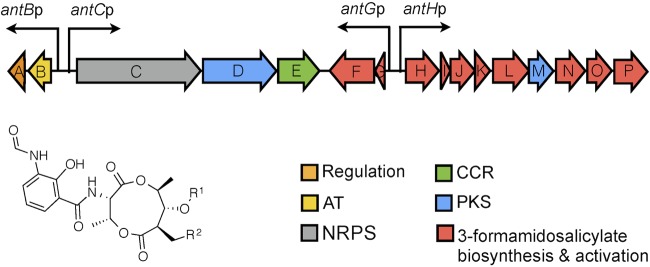
The hybrid nonribosomal peptide synthetase (NRPS)/polyketide synthase (PKS) pathway encoding the biosynthesis of antimycins remained enigmatic until it was elucidated recently in Streptomyces albus S4 (10, 11). The ~25-kb antimycin (ant) biosynthetic gene cluster is composed of 15 genes organized into four polycistronic transcription units, antBA, antCDE, antGF, and antHIJKLMNO (Fig. 1) (12). The antFGHIJKLNO genes specify the biosynthesis of the unusual starter unit 3-formamidosalicyl coenzyme A (CoA) (13–15). AntCD make up the hybrid NRPS/PKS assembly line, while AntE and AntM are crotonyl-CoA reductase and discrete ketoreductase homologs, respectively, and AntB is an acyltransferase responsible for the acyloxyl moiety and the chemical diversity observed at R1 (Fig. 1) (14, 16, 17).
FIG 1 .
Schematic representation of the antimycin biosynthetic gene cluster of S. albus S4. Genes are color coded to indicate their functions. AT, acyltransferase; CCR, crotonyl-CoA carboxylase/reductase. In antimycin A1, R1 is COCH(CH3)CH2CH3 and R2 is (CH2)4CH3. In antimycin A2, R1 is COCH(CH3)2 and R2 is (CH2)4CH3. In antimycin A3, R1 is COCH2CH(CH3)2 and R2 is (CH2)2CH3. In antimycin A4, R1 is COCH(CH3)2 and R2 is (CH2)2CH3.
The ant genes are expressed during vegetative growth and are significantly downregulated during aerial growth, such that the gene cluster is not constitutively active and suggesting that its expression is tightly regulated (12). The ant gene cluster harbors a single cluster-situated regulator, an extracytoplasmic function RNA polymerase sigma (σ) factor named σAntA, which only activates the transcription of operons antGF and antHIJKLMNO, suggesting that the regulator(s) controlling the expression of antBA and antCDE must be encoded at another locus (12). We consistently have been unable to heterologously produce antimycins by using a variety of Streptomyces strains, including S. coelicolor, which had previously been reported to be a suitable host for the expression of this pathway (18). We presumed that this anomaly related to the unknown regulator(s) controlling the expression of antBA and antCDE and sought to identify and characterize the transcription factor(s) in this study.
Here we demonstrate that antimycin production in S. albus S4 is regulated by FscRI, a LuxR family cluster-situated regulator of the polyene antifungal agent candicidin. We report that heterologous production of antimycins by S. coelicolor is dependent on FscRI and show that FscRI activates the expression of antBA and antCDE. We also demonstrate through chromatin immunoprecipitation (ChIP) and sequencing that FscRI regulation is direct and provide evidence that this regulation strategy is conserved and unique to short-form ant gene clusters. Our findings reveal coordinate control of antimycin and candicidin biosynthesis, thereby providing direct in vivo evidence of the cross-regulation of disparate biosynthetic gene clusters specifying unrelated natural products, and expand our paradigmatic understanding of the regulation of secondary metabolism.
RESULTS AND DISCUSSION
Identification of FscRI binding sites associated with antimycin biosynthesis.
We previously characterized σAntA as an activator of antGF and antHIJKLMNO expression and postulated that the regulator(s) governing the expression of the remaining operons (antBA and antCDE) must be encoded elsewhere in the S. albus S4 genome (Fig. 1) (12). Recently, increased antimycin production was observed in a strain of S. albus J1074 engineered to heterologously overproduce PimM (19). PimM is a cluster-situated activator of pimaricin (or natamycin) biosynthesis and belongs to the PAS-LuxR family of transcriptional regulators, which harbor an N-terminal PER-ARNT-SIM) domain that recognizes stimuli such as light, oxygen, redox potential, or other ligands to modulate the activity of a C-terminal helix-turn-helix DNA-binding motif (20–22). Orthologs of PimM also control the production of related polyene antifungal agents, amphotericin (AmphDIV), nystatin (NysRIV), filipin (PteF), and candicidin (FscRI) and a coronafacic-acid-like phytotoxin (CfaR) (23–27). The amino acid sequences of polyene PAS-LuxR regulators are 65 to 94% identical and show functional cross complementarity, a consequence of their nonperfect inverted repeat binding sequence (5′-CTVGGGAWWTCCCBAG-3′) (28). S. albus S4 also produces candicidin (29) and harbors an FscRI ortholog (11); thus, we hypothesized that FscRI was the missing regulator of antimycin biosynthesis.
FscRI was recently characterized in the candicidin producer Streptomyces sp. strain FR-008 and is required for the expression of 16 out of 21 genes within the gene cluster (26). DNA motifs consistent with those recognized by PimM-type regulators were identified upstream of fscA, fscB, and fscD, which each encode a type I PKS, and fscRIV, which is a LAL regulator (a large ATP-binding regulator of the LuxR type) (26). The amino acid sequences of FscRIFR-008 and FscRIS4 are 100% identical, and inspection of the S. albus S4 genome sequence revealed the presence of DNA motifs identical to those upstream of fscA, fscB, fscD, and fscMI in Streptomyces sp. strain FR-008 (Fig. 2). Thus, we used these DNA sequences with the MEME suite (30) to search for similar motifs within the antimycin gene cluster, which resulted in the identification of two putative FscRI binding sites upstream of antBA and one upstream of antCDE (Fig. 2). Taken together, these findings suggest that the cluster-situated regulator of candicidin biosynthesis, FscRI, may directly activate the expression of both antBA and antCDE.
FIG 2 .
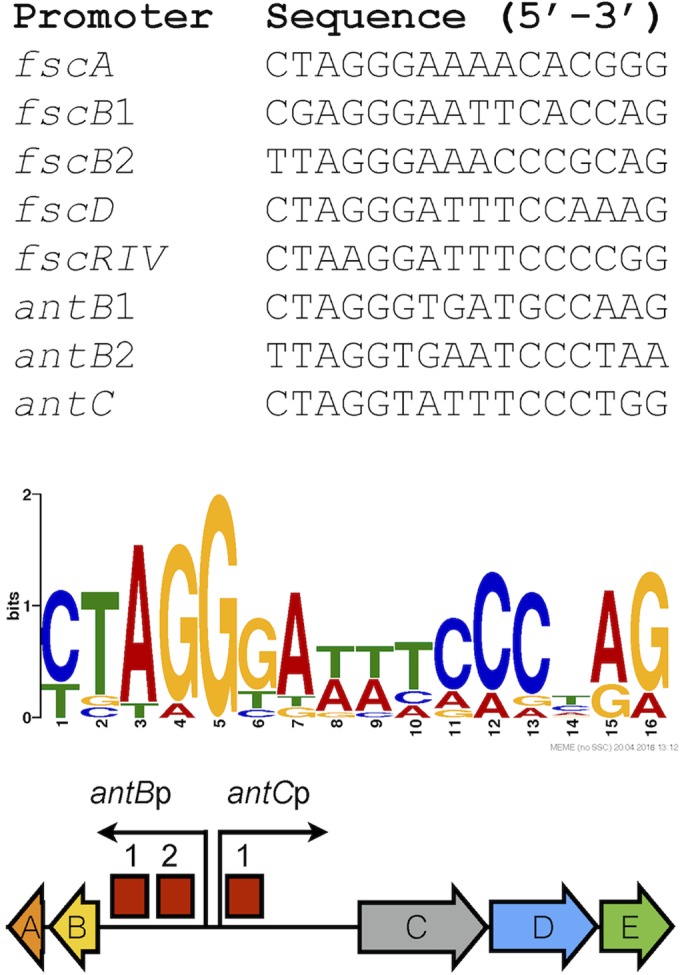
FscRI binding sites within the S. albus S4 antimycin gene cluster. The top panel shows experimentally verified FscRI binding sites upstream of genes within the candicidin biosynthetic gene cluster (fscA, fscB1, fscB2, fscD, and fscMI) and putative FscRI binding sites upstream of antB and antC within the ant biosynthetic gene cluster. The middle panel displays the WebLogo (61) for the verified and putative FscRI binding sites above, and the bottom panel shows the relative locations of FscRI binding sites (as red boxes) upstream of antB and antC.
FscRI is required for antimycin production.
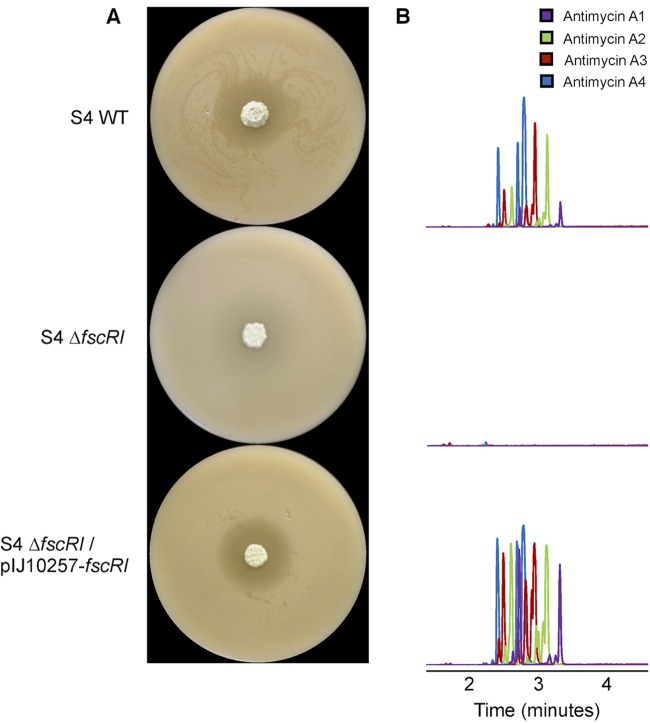
Our bioinformatic analyses led us to hypothesize that FscRIS4 activates the transcription of antBA and antCDE and is thus likely to be required for the production of antimycins. To investigate this possibility, we deleted the fscRI gene by CRISPR/Cas9 editing and tested the resulting mutant (ΔfscRI) against Candida albicans in a bioassay. As predicted, the ΔfscRI mutant strain no longer inhibited the growth of C. albicans, which is consistent with loss of antimycin and candicidin production (Fig. 3) (12). Complementation of this mutant with pIJ10257-fscRI, which contains the fscRI gene under the control of the constitutive ermE* promoter, restored bioactivity against C. albicans to wild-type levels and verified that loss of bioactivity was not due to other mutational events (Fig. 3). Ultrahigh-performance liquid chromatography–high-resolution electrospray ionization–mass spectrometry (LC-HRESI-MS) confirmed that compounds with molecular formulae consistent with antimycins A1 to A4 were only present in chemical extracts prepared from wild-type and ΔfscRI mutant S. albus S4 strains harboring pIJ10257-fscRI but not in those from the ΔfscRI mutant (Fig. 3). Taking these findings together, we conclude that FscRI is required for the production of antimycins and candicidin by S. albus S4. Given the rather flexible and conserved binding site of PAS-LuxR regulators, it is conceivable that orthologs of FscRI could also cross-regulate a gene cluster(s) other than the one in which they are encoded. This is an intriguing possibility that has not been rigorously explored.
FIG 3 .
FscRI is required for the biosynthesis of antimycins by S. albus S4. (A) S. albus S4 strains challenged with C. albicans. The ΔfscRI mutant does not show detectable bioactivity against C. albicans compared to the wild-type (WT) and complemented (ΔfscRI/pIJ10257-fscRI) strains. (B) LC-HRESI-MS analysis of chemical extracts prepared from strains shown in panel A; the extracted ion chromatograms (M+H)+ for antimycins A1 to A4 are shown for each strain.
To our knowledge, cross-regulation of disparate natural-product biosynthetic gene clusters by a cluster-situated regulator has only been demonstrated once previously. In Streptomyces clavuligerus, the cephamycin and clavulanic acid gene clusters comprise a contiguous “supercluster” (31). The biosynthesis of both cephamycin and clavulanic acid is coordinately controlled by CcaR, a SARP (Streptomyces antibiotic regulatory protein)-type activator harbored within the cephamycin gene cluster (32–34). It is interesting that not only are these gene clusters contiguous, but unlike antimycin and candicidin, both molecules are structurally similar and possess complementary biological activities (cephamycin is a β-lactam antibiotic, and clavulanic acid is a β-lactamase inhibitor).
Heterologous production of antimycins by S. coelicolor requires FscRI.
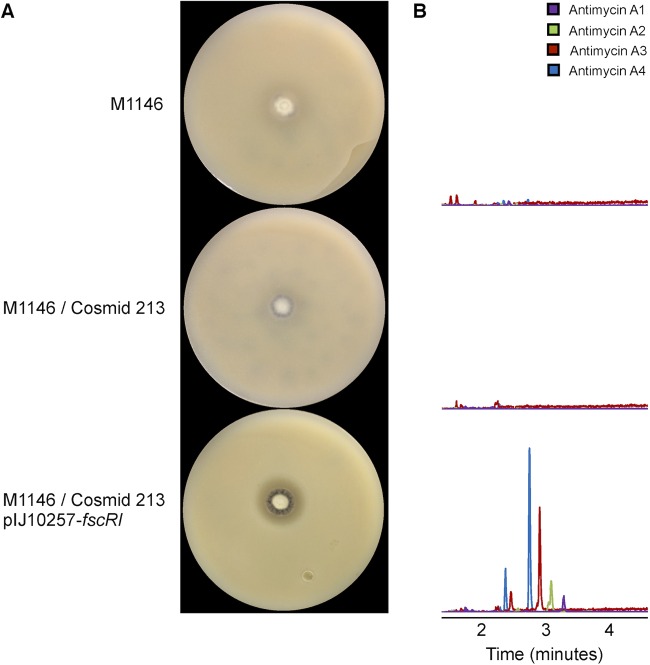
Yan et al. cloned the ant gene cluster from Streptomyces sp. strain NRRL 2288 and heterologously produced antimycins with S. lividans and S. coelicolor M145 (18). To our surprise, even though the ant gene cluster nucleotide sequences of NRRL 2288 and S4 are >97% identical (18), we have consistently been unable to repeat these findings by using both gene clusters and multiple genetic backgrounds, including Streptomyces sp. strain S3, S. lividans 66, and S. coelicolor M145, M1146, M1152, and M1153 (29, 35, 36; R. F. Seipke and M. I. Hutchings, unpublished data). Previously, we presumed that poor availability of one of more biosynthetic precursors (i.e., tryptophan, threonine, pyruvate, and acyl-CoA) precluded the production of antimycins and/or that the pathway was simply not expressed by the heterologous hosts under our growth conditions. But given our observations described above, we hypothesized that S. coelicolor did not produce antimycins because of a lack of FscRI rather than as a result of culture conditions. To test this hypothesis, we introduced cosmid 213, containing the entire S4 ant gene cluster (10), into S. coelicolor M1146 (36) and also introduced pIJ10257-fscRI. The resulting strains were then tested for the ability to inhibit the growth of C. albicans by bioassay. Consistent with our hypothesis, M1146 harboring solely cosmid 213 or pIJ10257-fscRI did not inhibit the growth of C. albicans; however, the cointegrant harboring both cosmid 213 and pIJ10257-fscRI inhibited C. albicans growth (Fig. 4; see Fig. S1 in the supplemental material). We recapitulated this experiment with the ant gene cluster from NRRL 2288 and the pAL2602 cosmid clone generated by Yan et al. (18) and obtained identical results (see Fig. S1). The data set for the heterologous expression of the S4 ant gene cluster was corroborated by LC-HRESI-MS detection of antimycins A1 to A4 in chemical extracts prepared from M1146 harboring cosmid 213 and pIJ10257-fscRI and their absence from M1146 alone or harboring only cosmid 213 (Fig. 4). These results demonstrate that FscRI is required by S. coelicolor for heterologous production of antimycins with two different ant cluster cosmid clones. More importantly, however, our findings suggest that integral components of biosynthetic pathways may be encoded by disparate loci, which is consistent with recent observations that the Pseudonocardia metabolite gerumycin is encoded by two loci separated by over 90 kb (37).
FIG 4 .
Heterologous production of antimycins by S. coelicolor is FscRI dependent. (A) S. coelicolor M1146 strains challenged with C. albicans. Only M1146 harboring both cosmid 213 and pIJ10257-fscRI inhibits the growth of C. albicans compared to M1146 and M1146 harboring cosmid 213. (B) LC-HRESI-MS analysis of chemical extracts prepared from strains shown in panel A; the extracted ion chromatograms (M+H)+ for antimycins A1 to A4 are shown for each strain.
Bioactivity of S. coelicolor M1146 harboring pAL2602 is FscRI dependent. S. coelicolor M1146 harboring both pAL2602 and pIJ10257-fscRI antagonizes the growth of C. albicans, while M1146 harboring only pAL2602 or pIJ10257-fscRI does not. Download Figure S1, TIF file, 2.3 MB (2.3MB, tif) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
FscRI activates the expression of antBA and antCDE.
The observation that FscRI is required for heterologous production of antimycins by S. coelicolor suggests that it activates the expression of both antBA and antCDE. To evaluate this hypothesis, we first engineered cosmid 213 such that antBA and antCDE were expressed from the rpsL(XC) promoter (38) and the ermE* promoter (39), respectively. As we expected, M1146 harboring solely this engineered cosmid displayed an FscRI-independent ability to produce antimycins, which is consistent with bioinformatic data showing the absence of FscRI binding sites elsewhere within the ant gene cluster (Fig. 5). Next, we engineered two more variants of cosmid 213 such that the expression of only antBA or only antCDE was driven by ermEp2, leaving the native FscRI-dependent promoters of antCDE and antBA intact, respectively. The engineered cosmids and either pIJ10257 or pIJ10257-fscRI were mobilized to M1146, and the ability of the resulting strains to produce antimycins was assessed by LC-HRESI-MS. As anticipated, antimycins A1 to A4 were detected only in chemical extracts prepared from M1146 harboring pIJ10257-fscRI and cosmid 213 with either ermEp2-driven antBA or antCDE and not those generated from M1146 harboring just pIJ10257 and cosmid 213 with ermEp2-driven antBA or antCDE (Fig. 5). These data provide in vivo evidence that FscRI may activate the expression of both antBA and antCDE and are consistent with our hypothesis that FscRI acts on these promoters.
FIG 5 .
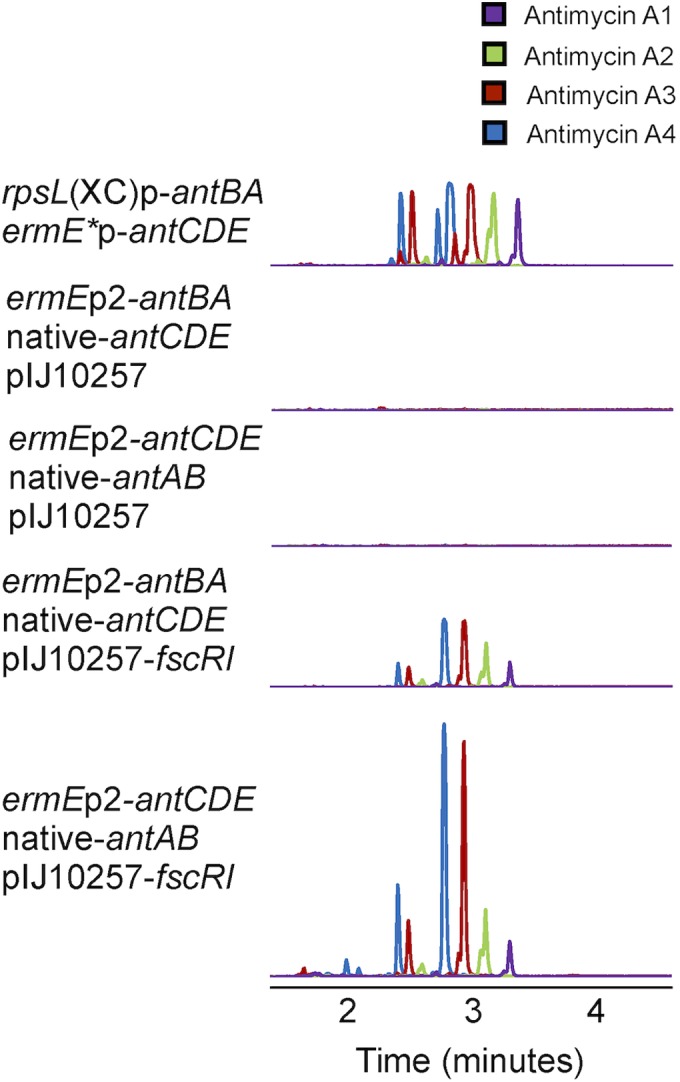
FscRI activates antBA and antCDE expression. LC-HRESI-MS analysis of chemical extracts prepared from S. coelicolor M1146 harboring variants of cosmid 213 engineered as shown here and pIJ10257 or pIJ10257-fscRI as indicated. The extracted ion chromatograms (M+H)+ for antimycins A1 to A4 are shown for each strain.
FscRI directly activates the transcription of antBA and antCDE.
The simplest interpretation of our bioinformatic analysis and heterologous expression data is that FscRI activation of antBA and antCDE is direct. We initially sought to verify this hypothesis by performing electrophoretic mobility shift assays with purified FscRI protein; however, FscRI harboring either an N-terminal or a C-terminal hexahistidine tag was insoluble when overproduced by Escherichia coli (data not shown), which was surprising given than His6-FscRIFR-008 was reportedly soluble (26). Nevertheless, we adopted a ChIP-sequencing approach to determine if the antB and antC promoters were bound by FscRI in vivo. We complemented the ΔfscRI mutant with an N-terminal 3×FLAG-tagged version of FscRI expressed from the ΦC31 integration site. The resulting strain (ΔfscRI/pSETNFLAG-fscRI mutant) inhibited the growth of C. albicans and that of the wild-type strain equally (see Fig. S2 in the supplemental material). ChIP-sequencing was carried out with anti-FLAG antibodies and lysate from the ΔfscRI/pSETNFLAG-fscRI mutant and wild-type strains cultivated in LB, which facilitates the production of both antimycin and candicidin. Immunoprecipitated DNA from two biological replicates of S4 wild-type and ΔfscRI/pSETNFLAG-fscRI and nonimmunoprecipitated chromosomal DNA were sequenced with the Illumina HiSeq3000 platform and processed as described in Materials and Methods. As we anticipated, the numbers of sequencing reads that mapped to the antB and antC promoter regions were enriched for both biological replicates of ΔfscRI/pSETNFLAG-fscRI compared to that of the wild-type mock-immunoprecipitated control (Fig. 6). These data provide definitive evidence that FscRI binds to the antB and antC promoters and likely promotes the transcription of antBA and antCDE (Fig. 6).
FIG 6 .
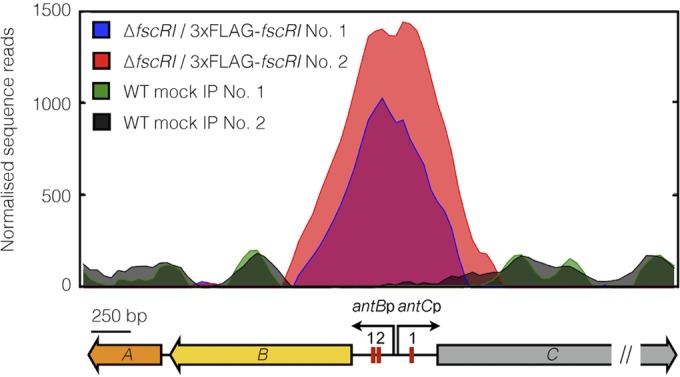
3×FLAG-FscRI binds to the antBA and antCDE promoters in vivo. Shown is a graphic representation of normalized sequence reads mapped to the intergenic region of antB-antC, which is shown at the bottom. The double slash indicates that the sequence window presented does not contain the entire antC coding sequence. The genomic coordinates depicted are nucleotides 16873 to 20973 of contig CADY01000091.1 of the S. albus S4 genome (11). WT, wild type; IP, immunoprecipitation.
Schematic of the pSETNFLAG-fscRI plasmid (left) and antifungal bioactivity of ΔfscRI expressing 3×FLAG-FscRI against C. albicans. aac(3)IV, apramycin resistance cassette; oriT, origin of transfer; attP, ΦC31 attachment site. The sequences of pSETNFLAG and its parent pSET152-ermEp are available at http://www.ryanseipkelab.com/tools.html. Download Figure S2, TIF file, 1.6 MB (1.6MB, tif) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Clustal Ω alignment of the antB-antC intergenic region of S-form ant gene clusters. The putative start codons for antB (bold, red, reverse orientation) and antC (bold, blue, forward orientation) and the three conserved FscRI binding sites are shaded gray. Download Figure S3, TIF file, 1.3 MB (1.3MB, tif) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
FscRI regulation of antimycin biosynthesis is conserved for S-form antimycin gene clusters.
We and others previously identified 14 ant gene clusters that were classified as short-form (S-form, 15 genes), intermediate-form (I-form, 16 genes), and long-form (L-form, 17 genes) clusters (4, 18). There are six taxa, all related to S. albus S4, that encode S-form ant gene clusters: S. albus S4, S. albus J1074, Streptomyces sp. strain SM8, Streptomyces sp. strain NRRL2288, Streptomyces sp. strain LaPpAH-202, and Streptomyces sp. strain CNY228 (40). I-form ant gene clusters are encoded by two species, Streptomyces sp. strain 303MFCol5.2 and Streptomyces sp. strain TOR3209, which lack either antQ or antP, respectively. L-form ant gene clusters are encoded by six taxa, S. ambofaciens ATCC 23877, S. blastmyceticus NBRC 12747, S. gancidicus BKS 13-15, S. griseoflavus Tü4000, S. hygroscopicus subsp. jinggangensis 5008, and S. hygroscopicus subsp. jinggangensis TL01. To determine if FscRI cross-regulation of antimycin biosynthesis is likely to be widespread, we first looked for orthologs of FscRI in genomes of antimycin producers. S. blastmyceticus and Streptomyces sp. strain NRRL2288 were omitted from this analysis because their genome sequences are not available. A tBLASTn search of a local blast database for the deduced amino acid sequence of FscRIS4 revealed that organisms harboring an S-form ant gene cluster also harbor an FscRI ortholog (>99% amino acid sequence identity), whereas the top tBLASTn hits for taxa harboring either an I- or an L-form ant gene cluster displayed a rather low level of shared amino acid identity (36 to 46%), with the exception of one organism, Streptomyces sp. strain TOR3209, which possesses an ortholog of FscRIS4 (79% identical; see Table S3 in the supplemental material). Next, we closely inspected all 14 ant gene clusters for the presence of FscRI DNA-binding motifs, which revealed that only S-form ant gene clusters harbor a motif consistent with that identified in this study, which was somewhat surprising, as we expected Streptomyces sp. strain TOR3209 to also harbor this motif, given that it encodes what appears to be an FscRI ortholog (see Fig. S4 in the supplemental material). Taking these findings together, we conclude that cross-regulation of antimycin biosynthesis by FscRI is likely a conserved regulatory strategy for bacteria that harbor an S-form ant gene cluster but was not a strategy adopted by taxa possessing I-form or L-form variants. The regulation of I-form and L-form ant gene clusters has not yet been investigated, so the regulatory mechanism(s) controlling the expression of antBA and antCDE is unknown; however, bioinformatic analyses suggest that, like S-form gene clusters, the genes encoding the biosynthesis and activation of 3-formamidosalicylate (antGF and antHIJKLMNO) are regulated by σAntA (12).
Schematic of the theophylline riboswitch cassette AprTheo. P1, prime site 1; P2, prime site 2; P3, prime site 3; aprR, apramycin resistance; 6×His, hexahistidine affinity purification tag. The riboswitch is represented by a hairpin. FRT sites are for excision of the resistance marker by the Flp recombinase. The sequence of the plasmid harboring this cassette is available at http://www.ryanseipkelab.com/tools.html. Download Figure S4, TIF file, 0.8 MB (847.4KB, tif) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Antimycin and candicidin do not act synergistically.
It is reasonable to assume that coordinate production of antimycin and candicidin may confer a competitive advantage upon the producer, akin to coordinate control of the β-lactam antibiotic cephamycin and the β-lactamase inhibitor clavulanic acid described above (31–34). One intriguing explanation for this could be that the compounds act synergistically to inhibit the growth of nearby fungi. We therefore used C. albicans to measure the MICs of antimycin (0.125 μg/ml) and candicidin (2 μg/ml) alone, as well as the MICs of pairwise mixtures of these agents, which allowed us to determine the fractional inhibitory concentration (FIC) index (see Materials and Methods). We calculated an FIC index of 2, which indicates that antimycin and candicidin do not interact synergistically or additively but also do not act antagonistically. This was surprising to us, because a slight synergistic effect against the fungus Escovopsis weberi was recently reported (41); however, an FIC index was not calculated, which limits interpretation and comparison of the data. An alternative possibility is that coordinate production of antimycin and candicidin serves to limit the development of resistance to either agent. The target of antimycin is cytochrome c reductase, and resistance can be conferred by a single point mutation (42), whereas development of resistance to candicidin and other polyene antifungal agents relies upon alteration of sterol biosynthesis, which incurs a significant fitness cost (43). It is also possible that coordinate production of these compounds relates to the monomeric precursors utilized by each pathway. For instance, the candicidin gene cluster harbors three genes (pabABC) responsible for the production of p-aminobenzoic acid (PABA) (44). In vitro studies of purified PabC revealed that its PABA synthase activity is inhibited by the aromatic amino acids tyrosine, phenylalanine, and tryptophan (45). It is conceivable that repression of PabC can be alleviated by AntFGHIJKLMNO, which utilize tryptophan to generate the 3-formamidosalicylate starter unit and may underpin the rationale for the coordinate production of antimycin and candicidin.
Model of the regulation of antimycin biosynthesis.
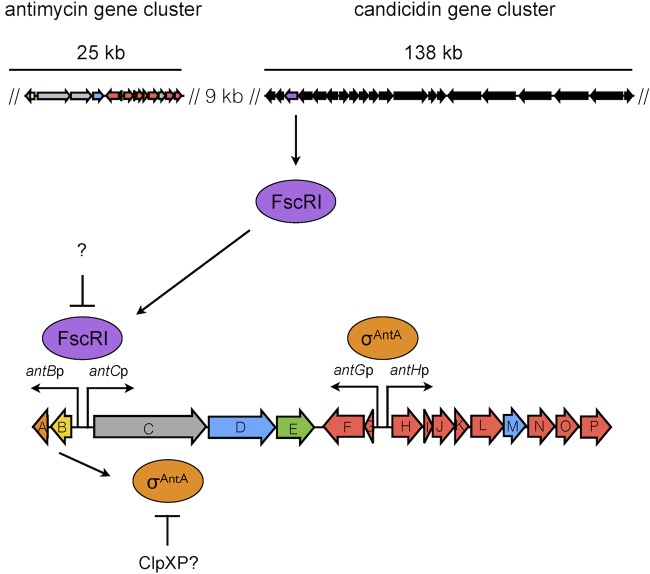
Our model of the regulation of antimycin biosynthesis is depicted in Fig. 7. FscRI activates the expression of antBA and antCDE, which in turn results in the σAntA-mediated expression of antGF and antHIJKLMNO (12). FscRI does not activate its own production; however, the expression of fscRI is regulated by a positive feedback loop where FscRI activates FscRIV, which in turn activates the transcription of fscRI (26). This observation, combined with our findings here and the fact that the ant gene cluster is expressed during vegetative growth and downregulated upon the onset of morphological differentiation (12), suggests that the ligand(s) recognized by the FscRI PAS domain, and perhaps all PAS domains of polyene PAS-LuxR regulators, is available only during vegetative growth. Following the inactivation of FscRI, the cell must have a strategy in place to prevent σAntA from activating its targets. Indeed, in the absence of a cognate anti-sigma factor that would ordinarily perform this task, σAntA seems to have evolved to be a direct substrate for the ClpXP protease (12).
FIG 7 .
Model of regulation of antimycin biosynthesis. The top panel displays the relative locations of the antimycin and candicidin gene clusters in the S. albus S4 chromosome. In the bottom panel, FscRI activates the transcription of antBA and antCDE, which results in production of the core AntC/AntD NRPS/PKS megasynthase and production of the discrete acyltransferase AntB and σAntA, which in turn activates the transcription of the ketoreductase gene antM and nine genes (antFGHIJKLNO) required for the biosynthesis and activation of the 3-formamidosalicylate precursor utilized by AntC. σAntA does not possess a cognate anti-σ factor and instead appears to be inactivated by the ClpXP protease.
As our understanding of the regulation of microbial natural-product biosynthesis increases, we anticipate that cross-regulation by cluster-situated regulatory proteins will emerge as a major strategy by which members of the phylum Actinobacteria coordinately produce selected natural products.
MATERIALS AND METHODS
Growth media, strains, cosmids, plasmids, and other reagents.
E. coli strains were propagated on Lennox agar (LA) or broth (LB) (35), and Streptomyces strains were cultivated with LA, LB, and mannitol-soya flour (MS) agar or broth (35) Culture medium was supplemented with antibiotics as required at the following concentrations: apramycin, 50 μg/ml; carbenicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; hygromycin, 50 μg/ml; kanamycin, 50 μg/ml; nalidixic acid, 25 μg/ml. Streptomyces strains were constructed by cross-genus conjugation with E. coli as previously described (35). Enzymes were purchased from New England BioLabs unless otherwise stated, and oligonucleotides were purchased from Integrated DNA Technologies, Inc. All of the strains, cosmids, and plasmids used in this study are described in Table S1 in the supplemental material, and all of the oligonucleotides and other synthetic DNAs used are provided in Table S2 in the supplemental material.
Bacterial strains, cosmids, fosmids, and plasmids used in this study. Download Table S1, DOCX file, 0.03 MB (32.5KB, docx) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Oligonucleotide primers and other synthetic DNAs used in this study. Download Table S2, DOCX file, 0.1 MB (135.5KB, docx) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
FscRIS4 and putative orthologs encoded by antimycin producers. Download Table S3, DOCX file, 0.1 MB (80.1KB, docx) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Construction of plasmids.
The insert for each plasmid generated in this study was prepared by PCR amplification with Q5 High-Fidelity DNA polymerase and oligonucleotides containing restriction sites. PCR-amplified inserts were restricted and cloned into the relevant plasmids cut with the same enzymes by standard molecular biology procedures. All clones were sequenced to verify the integrity of insert DNA. The restriction sites used for cloning are provided with the plasmid descriptions in Table S1 in the supplemental material.
Design of the apramycin theophylline riboswitch cassette.
A λ Red recombineering template (pUC57-AprTheo) was designed and synthesized by MWG Biotech. The PCR template for recombineering was identical to that of pIJ773 (46), except that one end contained ermEp2 (39) repressed by a theophylline-controlled riboswitch (47). The synthesized cassette also contains an optional hexahistidine tag for knocking in a nickel affinity purification tag at the native locus. A schematic of the AprTheo PCR template is shown in Fig. S4 in the supplemental material, and further details concerning its design, including its DNA sequence, are available from FigShare at https://dx.doi.org/10.6084/m9.figshare.3838032.v1.
Construction of pUC19-promKanprom.
To construct pUC19-promKanprom, the neomycin/kanamycin resistance marker from Supercos1 was PCR amplified with RFS444 and RFS445 and the product was used to replace the apramycin resistance gene and oriT of pIJ773 (46) by recombineering with E. coli GB05-red (48) to result in pIJ773KnFRT. Next, four PCR fragments were produced. (i) RFS406 and RFS407 were used to PCR amplify the kanamycin resistance from pIJ773KnFRT, (ii) RFS658 and RFS659 were used to PCR amplify the rpsL(XC) promoter from pCRISPomyces-2 (38, 49), (iii) RFS667 and RFS668 were used to PCR amplify the ermE* promoter from pSET152-ermEp, and (iv) RFS663 and RFS664 were used to linearize pUC19. The resulting PCR products were restricted with DpnI, gel purified, and assembled with the NEB HiFi DNA Assembly kit. The resulting plasmid, pUC19-promKanprom, contained a kanamycin resistance gene flanked by divergently firing rpsL(XC) and ermE* promoters. A schematic of the promKanprom PCR template is shown in Fig. S5 in the supplemental material, and its DNA sequence is available at http://www.ryanseipkelab.com/tools.html.
Schematic of the promKanprom cassette. P1, prime site (TACGTCTCCGTCGTCTACTC) 1; P2, prime site (CATATGGGGCCTCCTGTTCT); kanR, kanamycin resistance; FRT sites, for excision of the resistance marker by the Flp recombinase. The sequence of the plasmid harboring this cassette is available at http://www.ryanseipkelab.com/tools.html. Download Figure S5, TIF file, 0.8 MB (863.5KB, tif) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Cosmid manipulations.
The AprTheo PCR template described above was used to replace the antB or antC promoter harbored by cosmid 213 (12) by using RFS413 and RFS414 (antBp) or RFS415 and RFS416 (antCp) and the ReDirect PCR targeting system as previously described (46). The apramycin resistance gene and oriT were removed from modified cosmids with the FLP recombinase as previously described (46), resulting in cosmids 213-ABribo-FLP and 213-CDEribo-FLP. Cosmids 213-ABribo-FLP and 213-CDEribo-FLP were moved to E. coli GB05-red (48) and further engineered to harbor the ΦC31 integrase, attP site, and apramycin resistance gene originating from pIJ10702 (also known as pMJCOS1) (50) by RecET recombineering as previously described (48), resulting in cosmids 213-ABribo-FLP-ΦC31 and 213-CDEribo-FLP-ΦC31. Cosmid 213-ΦC31-BC-prom was constructed by replacing the antB-antC intragenic region of cosmid 213-ΦC31 by recombineering with GB05-red and a PCR product generated with pUC19-promKanprom and oligonucleotides RFS654 and RFS657. Thus, the ant gene cluster harbored by this final ΦC31 integrative construct is entirely controlled by divergently firing rpsL(XC) and ermE* promoters.
Deletion of fscRI.
The fscRI gene was deleted by using the pCRISPomyces-2 system described previously (49). First, a single-guide RNA protospacer was generated by annealing oligonucleotides RFS574 and RFS575 and the resulting DNA fragment was cloned into the BbsI site of pCRISPomyces-2 by Golden Gate Assembly. Second, a homology-directed repair template consisting of ~3.8 kb of DNA homologous to the region adjacent to the Cas9-induced double-strand break was generated. The repair template was generated by sequentially cloning a HindIII-SpeI-restricted PCR fragment amplified with RFS521 and RFS522 into pIJ12738 (51) and then cloning a SpeI-KpnI-restricted PCR fragment generated with RFS523 and RFS524. The resulting plasmid (pIJ12738-fscRI-UPDN) was used as a PCR template with RFS572 and RFS573, and the resulting PCR product was restricted with XbaI and cloned into pCRISPomyces-2 containing the protospacer targeting fscRI. The resulting CRISPR/Cas9 editing plasmid, pCRISPomyces-2-fscRI, was mobilized to S4 by conjugal transfer from E. coli ET12567/pUZ8002 as previously described (35). Temperature-sensitive pCRISPomyces-2-fscRI was cured from a single apramycin-resistant transconjugant by passage in LB at 37°C (two rounds) prior to the cultivation of a sporulated lawn on MS agar at 37°C. The resulting spores were serially diluted, and 11 single colonies were replica plated to assess apramycin sensitivity. Five apramycin-sensitive colonies were obtained and subsequently evaluated for the absence of fscRI by polymorphic shift PCR with RFS598 and RFS599. The integrity of the resulting ΔfscRI null mutant was verified by DNA sequencing.
Chemical analysis.
Streptomyces strains were cultured in MS broth (50 ml in a 250-ml flask) while shaking (180 rpm) at 27°C for 7 days. MS broth was supplemented with theophylline (4 mM) from the onset of culturing as required for M1146 strains. Bacterial cells were removed by centrifugation, and metabolites were extracted from the supernatant with a Phenomenex strata-XL C18 (100 μm, 30 mg, 1 ml) solid-phase extraction (SPE) column and a vacuum manifold. The column was first washed with 1 ml of 100% methanol followed by 1 ml of deionized water. The column was then loaded with supernatant (10 ml in total) prior to being washed with 1 ml of deionized water and then washed with 2 ml of 30% methanol. Metabolites were eluted from the SPE column in 100% methanol (0.3 ml). Equal amounts of methanolic extract from two independent replicates of each strain were mixed and centrifuged for 10 min at 16,000 × g just prior to injection in order to remove insoluble material. Only the supernatant (2 μl) was injected into a Bruker MaXis Impact time of flight mass spectrometer and equipped with a Dionex UltiMate 3000 high-performance liquid chromatography apparatus with the same parameters as described previously (15).
Bioassays.
Bioassays were performed essentially as described previously (52), except that 7 instead of 5 ml of soft nutrient agar was used and Streptomyces strains were cultivated at 30°C for 7 (instead of 10) days prior to a challenge with C. albicans CA6 (53). MS agar was supplemented with 2 mM theophylline for M1146 strains as required. Photographs of bioassay plates were taken ~48 h after the challenge.
MIC and FIC index determination.
MIC assays were performed with 96-well flat-bottom microtitration plates in accordance with the Clinical and Laboratory Standards Institute guidelines adapted for C. albicans (54). The FIC index was determined according to reference 55 by evaluating the growth of C. albicans exposed to increasing pairwise concentrations of antimycin (0.0625 to 4 µg/ml) and candicidin (1 to 64 µg/ml). The formula used was FIC index = FIC A + FIC B, where FIC A = MIC of combination/MIC of compound A and FIC B = MIC of combination/MIC of compound B.
Protein expression.
Recombinant His6-FscRI and FscRI-His6 were produced with E. coli Rosetta BL21(DE3) by using pET28a-fscRI and pET30a-fscRI, respectively. Production of His6-FscRI and FscRI-His6 was induced in mid-log phase by the addition of 1.5 mM isopropyl-β-d-thiogalactopyranoside. After 4 h of induction at 28°C, cells were harvested by centrifugation. The cell pellet was resuspended in 1× BugBuster Protein Extraction Reagent (Novagen) diluted with 50 mM Tris-Cl (pH 8.0), 200 mM NaCl, 20 mM imidazole, and 2 U of DNase I and incubated room temperature for 20 min. Insoluble material was removed from the lysate by centrifugation (16,000 × g for 10 min). Soluble and insoluble fractions were visualized with InstantBlue (Expedeon) after 15% SDS-PAGE.
ChIP-sequencing and bioinformatic analyses.
Wild-type or ΔfscRI/pSETNFLAG-fscRI mutant S4 bacteria were cultivated for 2 days in LB while shaking at 200 rpm at 28°C and processed for ChIP-sequencing exactly as described previously, with anti-FLAG M2 agarose beads (Sigma) (56), except that an Active Motif, Inc., EpiShear sonicator (30% amplitude, 30 s on and 30 s off for a total time of 13 min) was used to shear DNA to an average size of approximately 200 to 300 nucleotides. The pure DNA resulting from immunoprecipitates from two biological replicates of wild-type and ΔfscRI/pSETNFLAG-fscRI mutant S4 bacteria, as well as nonimmunoprecipitated chromosomal DNA, were sequenced with the Illumina HiSeq3000 platform with 150-nucleotide paired-end reads by the University of Leeds Next Generation Sequencing Facility at the St. James Teaching Hospital NHS Trust. The forward reads were mapped to the S4 genome with Bowtie 2 version 2.1.0 (57), and the resulting alignments were converted from the .SAM to the .BAM format and sorted according to chromosomal position with SAMtools version 1.1 (58). The aligned and sorted .BAM files for immunoprecipitated samples were converted to the bigWig format and normalized by read count compared to the DNA-only input control by using the default settings of deepTools bamCompare version 2.3.3 (59), with the exception that flag --ratio=subtract was used instead of the default --ratio=log2. This resulted in a single bigWig file for each treatment, which was visualized with Integrated Genomics Viewer version 2.3.78 (60). Plots were generated with the deepTools programs computeMatrix and plotProfile (59) with a bin size of 50 and a custom .BED file specifying the region displayed.
Accession number(s).
The next-generation sequencing data obtained in this study are available under ArrayExpress accession no. E-MTAB-5122. The DNA sequences of tools constructed during this study are available at http://www.ryanseipkelab.com/tools.html.
ACKNOWLEDGMENTS
We thank Wen Liu for kindly providing pAL2602. We also thank Ron Chen and John Munnoch for helpful discussions about ChIP-sequencing, Michael Vockenhuber for theophylline aptamer sequences, and Kenneth McDowall for his helpful insight during this study.
This work was funded by grants from the British Society for Antimicrobial Chemotherapy (GA2014_006P), the Royal Society (RG140011), and the Biotechnology and Biological Sciences Research Council (BB/N007980/1) awarded to R.F.S. and a grant from the Natural Environment Research Council (NE/M001415/1) awarded to P.A.H. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Newman DJ, Cragg GM. 2012. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentley SD, Cerdeño-Tárraga A-M, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang C-H, Kieser T, Larke L, Murphy L, Oliver K, O’Neil S, Rabbinowitsch E, Rajandream M-A, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147. [DOI] [PubMed] [Google Scholar]
- 3.van Wezel GP, McDowall KJ. 2011. The regulation of the secondary metabolism of Streptomyces: new links and experimental advances. Nat Prod Rep 28:1311–1333. doi: 10.1039/c1np00003a. [DOI] [PubMed] [Google Scholar]
- 4.Seipke RF, Hutchings MI. 2013. The regulation and biosynthesis of antimycins. Beilstein J Org Chem 9:2556–2563. doi: 10.3762/bjoc.9.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Zhu X, Kim SJ, Zhang W. 2016. Antimycin-type depsipeptides: discovery, biosynthesis, chemical synthesis, and bioactivities. Nat Prod Rep 33:1146–1165. doi: 10.1039/c6np00004e. [DOI] [PubMed] [Google Scholar]
- 6.Dunshee BR, Leben C, Keitt GW, Strong FM. 1949. The isolation and properties of antimycin A. J Am Chem Soc 71:2436–2437. doi: 10.1021/ja01175a057. [DOI] [Google Scholar]
- 7.Tappel AL. 1960. Inhibition of electron transport by antimycin A, alkyl hydroxyl naphthoquinones and metal coordination compounds. Biochem Pharmacol 3:289–296. doi: 10.1016/0006-2952(60)90094-0. [DOI] [PubMed] [Google Scholar]
- 8.Finlayson BJ, Schnick RA, Cailteux RL, DeMong L, Horton WD, McClay W, Thompson CW. 2002. Assessment of antimycin A use in fisheries and its potential for reregistration. Fisheries 27:10–18. doi:. [DOI] [Google Scholar]
- 9.Tzung SP, Kim KM, Basañez G, Giedt CD, Simon J, Zimmerberg J, Zhang KY, Hockenbery DM. 2001. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol 3:183–191. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- 10.Seipke RF, Barke J, Brearley C, Hill L, Yu DW, Goss RJM, Hutchings MI. 2011. A single Streptomyces symbiont makes multiple antifungals to support the fungus farming ant Acromyrmex octospinosus. PLoS One 6:e22028:e22028–8. doi: 10.1371/journal.pone.0022028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seipke RF, Crossman L, Drou N, Heavens D, Bibb MJ, Caccamo M, Hutchings MI. 2011. Draft genome sequence of Streptomyces strain S4, a symbiont of the leaf-cutting ant Acromyrmex octospinosus. J Bacteriol 193:4270–4271. doi: 10.1128/JB.05275-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seipke RF, Patrick E, Hutchings MI. 2014. Regulation of antimycin biosynthesis by the orphan ECF RNA polymerase sigma factor σAntA. PeerJ 2:e253. doi: 10.7717/peerj.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoenian I, Paetz C, Dickschat JS, Aigle B, Leblond P, Spiteller D. 2012. An unprecedented 1,2-shift in the biosynthesis of the 3-aminosalicylate moiety of antimycins. Chembiochem 13:769–773. doi: 10.1002/cbic.201200033. [DOI] [PubMed] [Google Scholar]
- 14.Sandy M, Rui Z, Gallagher J, Zhang W. 2012. Enzymatic synthesis of dilactone scaffold of antimycins. ACS Chem Biol 7:1956–1961. doi: 10.1021/cb300416w. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Zhu X, Seipke RF, Zhang W. 2015. Biosynthesis of antimycins with a reconstituted 3-formamidosalicylate pharmacophore in Escherichia coli. ACS Synth Biol 4:559–565. doi: 10.1021/sb5003136. [DOI] [PubMed] [Google Scholar]
- 16.Sandy M, Zhu X, Rui Z, Zhang W. 2013. Characterization of AntB, a promiscuous acyltransferase involved in antimycin biosynthesis. Org Lett 15:3396–3399. doi: 10.1021/ol4014365. [DOI] [PubMed] [Google Scholar]
- 17.Yan Y, Chen J, Zhang L, Zheng Q, Han Y, Zhang H, Zhang D, Awakawa T, Abe I, Liu W. 2013. Multiplexing of combinatorial chemistry in antimycin biosynthesis: expansion of molecular diversity and utility. Angew Chem Int Ed Engl 52:12308–12312. doi: 10.1002/anie.201305569. [DOI] [PubMed] [Google Scholar]
- 18.Yan Y, Zhang L, Ito T, Qu X, Asakawa Y, Awakawa T, Abe I, Liu W. 2012. Biosynthetic pathway for high structural diversity of a common dilactone core in antimycin production. Org Lett 14:4142–4145. doi: 10.1021/ol301785x. [DOI] [PubMed] [Google Scholar]
- 19.Olano C, García I, González A, Rodriguez M, Rozas D, Rubio J, Sánchez-Hidalgo M, Braña AF, Méndez C, Salas JA. 2014. Activation and identification of five clusters for secondary metabolites in Streptomyces albus J1074. Microb Biotechnol 7:242–256. doi: 10.1111/1751-7915.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antón N, Santos-Aberturas J, Mendes MV, Guerra SM, Martín JF, Aparicio JF. 2007. PimM, a PAS domain positive regulator of pimaricin biosynthesis in Streptomyces natalensis. Microbiology 153:3174–3183. doi: 10.1099/mic.0.2007/009126-0. [DOI] [PubMed] [Google Scholar]
- 21.Fuqua WC, Winans SC, Greenberg EP. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor BL, Zhulin IB. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev 63:479–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmody M, Byrne B, Murphy B, Breen C, Lynch S, Flood E, Finnan S, Caffrey P. 2004. Analysis and manipulation of amphotericin biosynthetic genes by means of modified phage KC515 transduction techniques. Gene 343:107–115. doi: 10.1016/j.gene.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Sekurova ON, Brautaset T, Sletta H, Borgos SEF, Jakobsen ØM, Ellingsen TE, Strøm AR, Valla S, Zotchev SB. 2004. In vivo analysis of the regulatory genes in the nystatin biosynthetic gene cluster of Streptomyces noursei ATCC 11455 reveals their differential control over antibiotic biosynthesis. J Bacteriol 186:1345–1354. doi: 10.1128/JB.186.5.1345-1354.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vicente CM, Santos-Aberturas J, Payero TD, Barreales EG, de Pedro A, Aparicio JF. 2014. PAS-LuxR transcriptional control of filipin biosynthesis in S. avermitilis. Appl Microbiol Biotechnol 98:9311–9324. doi: 10.1007/s00253-014-5998-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P, Zhao Z, Li H, Chen XL, Deng Z, Bai L, Pang X. 2015. Production of the antibiotic FR-008/candicidin in Streptomyces sp. FR-008 is co-regulated by two regulators, FscRI and FscRIV, from different transcription factor families. Microbiology 161:539–552. doi: 10.1099/mic.0.000033. [DOI] [PubMed] [Google Scholar]
- 27.Cheng Z, Bown L, Tahlan K, Bignell DRD. 2015. Regulation of coronafacoyl phytotoxin production by the PAS-LuxR family regulator CfaR in the common scab pathogen Streptomyces scabies. PLoS One 10:e0122450-17. doi: 10.1371/journal.pone.0122450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos-Aberturas J, Payero TD, Vicente CM, Guerra SM, Cañibano C, Martín JF, Aparicio JF. 2011. Functional conservation of PAS-LuxR transcriptional regulators in polyene macrolide biosynthesis. Metab Eng 13:756–767. doi: 10.1016/j.ymben.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Barke J, Seipke RF, Grüschow S, Heavens D, Drou N, Bibb MJ, Goss RJM, Yu DW, Hutchings MI. 2010. A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant Acromyrmex octospinosus. BMC Biol 8:109. doi: 10.1186/1741-7007-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward JM, Hodgson JE. 1993. The biosynthetic genes for clavulanic acid and cephamycin production occur as a ‘super-cluster’ in three Streptomyces. FEMS Microbiol Lett 110:239–242. doi: 10.1111/j.1574-6968.1993.tb06326.x. [DOI] [PubMed] [Google Scholar]
- 32.Pérez-Llarena FJ, Liras P, Rodríguez-García A, Martín JF. 1997. A regulatory gene (ccaR) required for cephamycin and clavulanic acid production in Streptomyces clavuligerus: amplification results in overproduction of both beta-lactam compounds. J Bacteriol 179:2053–2059. doi: 10.1128/jb.179.6.2053-2059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander DC, Jensen SE. 1998. Investigation of the Streptomyces clavuligerus cephamycin C gene cluster and its regulation by the CcaR protein. J Bacteriol 180:4068–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santamarta I, López-García MT, Kurt A, Nárdiz N, Álvarez-Álvarez R, Pérez-Redondo R, Martín JF, Liras P. 2011. Characterization of DNA-binding sequences for CcaR in the cephamycin-clavulanic acid supercluster of Streptomyces clavuligerus. Mol Microbiol 81:968–981. doi: 10.1111/j.1365-2958.2011.07743.x. [DOI] [PubMed] [Google Scholar]
- 35.Kieser TB, Buttner MJ, Chater MJ, Hopwood KF. 2000, Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom. [Google Scholar]
- 36.Gomez-Escribano JP, Bibb MJ. 2011. Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb Biotechnol 4:207–215. doi: 10.1111/j.1751-7915.2010.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sit CS, Ruzzini AC, Van Arnam EB, Ramadhar TR, Currie CR, Clardy J. 2015. Variable genetic architectures produce virtually identical molecules in bacterial symbionts of fungus-growing ants. Proc Natl Acad Sci USA 112:13150–13154. doi: 10.1073/pnas.1515348112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao Z, Rao G, Li C, Abil Z, Luo Y, Zhao H. 2013. Refactoring the silent spectinabilin gene cluster using a plug-and-play scaffold. ACS Synth Biol 2:662–669. doi: 10.1021/sb400058n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bibb MJ, Janssen GR, Ward JM. 1985. Cloning and analysis of the promoter region of the erythromycin resistance gene (ermE) of Streptomyces erythraeus. Gene 38:215–226. doi: 10.1016/0378-1119(85)90220-3. [DOI] [PubMed] [Google Scholar]
- 40.Seipke RF. 2015. Strain-level diversity of secondary metabolism in Streptomyces albus. PLoS One 10:e0116457:e0116457–14. doi: 10.1371/journal.pone.0116457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoenian I, Spiteller M, Ghaste M, Wirth R, Herz H, Spiteller D. 2011. Chemical basis of the synergism and antagonism in microbial communities in the nests of leaf-cutting ants. Proc Natl Acad Sci USA 108:1955–1960. doi: 10.1073/pnas.1008441108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Rago JP, Colson AM. 1988. Molecular basis for resistance to antimycin and diuron, Q-cycle inhibitors acting at the Qi site in the mitochondrial ubiquinol-cytochrome c reductase in Saccharomyces cerevisiae. J Biol Chem 263:12564–12570. [PubMed] [Google Scholar]
- 43.Vincent BM, Lancaster AK, Scherz-Shouval R, Whitesell L, Lindquist S. 2013. Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS Biol 11:e1001692:e1001692–17. doi: 10.1371/journal.pbio.1001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen S, Huang X, Zhou X, Bai L, He J, Jeong KJ, Lee SY, Deng Z. 2003. Organizational and mutational analysis of a complete FR-008/candicidin gene cluster encoding a structurally related polyene complex. Chem Biol 10:1065–1076. doi: 10.1016/j.chembiol.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Gil JA, Naharro G, Villanueva JR, Martín JF. 1985. Characterization and regulation of p-aminobenzoic acid synthase from Streptomyces griseus. J Gen Microbiol 131:1279–1287. doi: 10.1099/00221287-131-6-1279. [DOI] [PubMed] [Google Scholar]
- 46.Gust B, Challis GL, Fowler K, Kieser T, Chater KF. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci USA 100:1541–1546. doi: 10.1073/pnas.0337542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudolph MM, Vockenhuber MP, Suess B. 2013. Synthetic riboswitches for the conditional control of gene expression in Streptomyces coelicolor. Microbiology 159:1416–1422. doi: 10.1099/mic.0.067322-0. [DOI] [PubMed] [Google Scholar]
- 48.Fu J, Bian X, Hu S, Wang H, Huang F, Seibert PM, Plaza A, Xia L, Müller R, Stewart AF, Zhang Y. 2012. Full-length RecE enhances linear-linear homologous recombination and facilitates direct cloning for bioprospecting. Nat Biotechnol 30:440–446. doi: 10.1038/nbt.2183. [DOI] [PubMed] [Google Scholar]
- 49.Cobb RE, Wang Y, Zhao H. 2015. High-efficiency multiplex genome editing of Streptomyces species using an engineered CRISPR/Cas system. ACS Synth Biol 4:723–728. doi: 10.1021/sb500351f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanai K, Murakami T, Bibb M. 2006. Amplification of the entire kanamycin biosynthetic gene cluster during empirical strain improvement of Streptomyces kanamyceticus. Proc Natl Acad Sci USA 103:9661–9666. doi: 10.1073/pnas.0603251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernández-Martínez LT, Bibb MJ. 2014. Use of the meganuclease I-SceI of Saccharomyces cerevisiae to select for gene deletions in actinomycetes. Sci Rep 4:7100. doi: 10.1038/srep07100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seipke RF, Grüschow S, Goss RJM, Hutchings MI. 2012. Isolating antifungals from fungus-growing ant symbionts using a genome-guided chemistry approach. Methods Enzymol 517:47–70. doi: 10.1016/B978-0-12-404634-4.00003-6. [DOI] [PubMed] [Google Scholar]
- 53.Mattia E, Cassone A. 1979. Inducibility of germ-tube formation in Candida albicans at different phases of yeast growth. J Gen Microbiol 113:439–442. doi: 10.1099/00221287-113-2-439. [DOI] [PubMed] [Google Scholar]
- 54.CLSI 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M07-09, 9th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 55.Liu W, Li LP, Zhang JD, Li Q, Shen H, Chen SM, He LJ, Yan L, Xu GT, An MM, Jiang YY. 2014. Synergistic antifungal effect of glabridin and fluconazole. PLoS One 9:e103442-10. doi: 10.1371/journal.pone.0103442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bush MJ, Bibb MJ, Chandra G, Findlay KC, Buttner MJ. 2013. Genes required for aerial growth, cell division, and chromosome segregation are targets of WhiA before sporulation in Streptomyces venezuelae. mBio 4:e00684-13. doi: 10.1128/mBio.00684-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup . 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramírez F, Dündar F, Diehl S, Grüning BA, Manke T. 2014. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res 42:W187–W191. doi: 10.1093/nar/gku365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. 2011. Integrative genomics viewer. Nat Biotechnol 29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res 14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bioactivity of S. coelicolor M1146 harboring pAL2602 is FscRI dependent. S. coelicolor M1146 harboring both pAL2602 and pIJ10257-fscRI antagonizes the growth of C. albicans, while M1146 harboring only pAL2602 or pIJ10257-fscRI does not. Download Figure S1, TIF file, 2.3 MB (2.3MB, tif) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Schematic of the pSETNFLAG-fscRI plasmid (left) and antifungal bioactivity of ΔfscRI expressing 3×FLAG-FscRI against C. albicans. aac(3)IV, apramycin resistance cassette; oriT, origin of transfer; attP, ΦC31 attachment site. The sequences of pSETNFLAG and its parent pSET152-ermEp are available at http://www.ryanseipkelab.com/tools.html. Download Figure S2, TIF file, 1.6 MB (1.6MB, tif) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Clustal Ω alignment of the antB-antC intergenic region of S-form ant gene clusters. The putative start codons for antB (bold, red, reverse orientation) and antC (bold, blue, forward orientation) and the three conserved FscRI binding sites are shaded gray. Download Figure S3, TIF file, 1.3 MB (1.3MB, tif) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Schematic of the theophylline riboswitch cassette AprTheo. P1, prime site 1; P2, prime site 2; P3, prime site 3; aprR, apramycin resistance; 6×His, hexahistidine affinity purification tag. The riboswitch is represented by a hairpin. FRT sites are for excision of the resistance marker by the Flp recombinase. The sequence of the plasmid harboring this cassette is available at http://www.ryanseipkelab.com/tools.html. Download Figure S4, TIF file, 0.8 MB (847.4KB, tif) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Bacterial strains, cosmids, fosmids, and plasmids used in this study. Download Table S1, DOCX file, 0.03 MB (32.5KB, docx) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Oligonucleotide primers and other synthetic DNAs used in this study. Download Table S2, DOCX file, 0.1 MB (135.5KB, docx) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
FscRIS4 and putative orthologs encoded by antimycin producers. Download Table S3, DOCX file, 0.1 MB (80.1KB, docx) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Schematic of the promKanprom cassette. P1, prime site (TACGTCTCCGTCGTCTACTC) 1; P2, prime site (CATATGGGGCCTCCTGTTCT); kanR, kanamycin resistance; FRT sites, for excision of the resistance marker by the Flp recombinase. The sequence of the plasmid harboring this cassette is available at http://www.ryanseipkelab.com/tools.html. Download Figure S5, TIF file, 0.8 MB (863.5KB, tif) .
Copyright © 2016 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.