Abstract
A recent paper published in Nature reports that the immunosuppressive activity of tumor-associated macrophages is regulated via PI3Kγ signaling. Small-molecule inhibitors targeting PI3Kγ stimulate T cell activity against tumor alone and add additional effects for clinically proven PD1 immunotherapy.
Considerable excitement has recently been generated over the use of immunotherapy to treat cancer. These therapies are based on the rationale that tumors create an immunosuppressive microenvironment, and that removal of this suppression will unleash the immune system against self-derived yet aberrantly growing tumor cells. Thus far the most successful therapies to date are through inhibiting the negative regulators of T cell activity. For example, blocking the immune checkpoints through inhibition of programmed cell death 1 (PD1) or CTLA pathways has shown considerable efficacy in cancers as diverse as melanoma and Hodgkin's lymphoma. However, even with notable successes there are still many cancers that are refractory to such treatments.
The immunosuppressive microenvironment in the tumor tissue is not created solely by tumor cells but it is also mediated by non-tumoral stromal cells, most notably tumor-associated macrophages (TAMs)1. These tumor-reprogramed macrophages are different from those in acute inflammatory responses where they phagocytose antigens, present antigens to and stimulate adaptive immune cells. Instead, TAMs shut down effector T and NK cell activities with soluble immunosuppressive factors and membrane-bound immune checkpoint molecules such as the PD ligand 1 (PDL1)1,2.
Several studies have shown a number of tumor cell-derived regulatory factors that repolarize TAMs3. However, the mechanism by which TAMs integrate external signals and translate them into a transcriptional program within the cells to modulate the immune response is unclear. In a recent study published in Nature, Kaneda et al.4 showed that PI3Kγ acts as a molecular switch turning on immunosuppression while shutting down immune-stimulatory activities. Using both genetic tools and pharmacological inhibitors of PI3Kγ, they showed that in macrophages the lack of PI3Kγ activity induced expression of MHCII and pro-inflammatory cytokines such as IL12 with a concomitant reduction in immunosuppressive molecules exemplified by IL10 and arginase. This shift in transcription program in macrophages upon PI3Kγ deletion/inhibition led to enhanced adaptive immunity, including increased recruitment and cytotoxicity of T cells. This change in the immune environment significantly inhibited the growth and metastasis of tumors in a variety of models.
The authors addressed the question of how PI3Kγ simultaneously inhibits immune-stimulatory inflammation and induces immune suppression and found that this was achieved through regulation of two opposing transcription factors NF-κB and C/EBPβ. On the one hand, genetic deletion of the catalytic subunit p110 of PI3Kγ promoted IκBα degradation and therefore enhanced NF-κB-dependent production of inflammatory cytokines. On the other hand, genetic ablation of p110 reduced transcriptional activity of C/EBP that initiated an immunosuppressive program involving at least arginase 1. Furthermore, this study also showed that the mechanism by which PI3Kγ induces immune suppression does not overlap with that of PD1, as the combinatorial inhibition of both pathways exhibited additive, if not synergistic, effects on tumor suppression. Similar additive effects were also observed with simultaneous T-cell checkpoint molecule inhibition and macrophage targeting using an inhibitor of colony-stimulating factor 1 receptor (CSF1R)5.
These data therefore show increased therapeutic efficacy in mouse models by simultaneously targeting macrophage signaling as well as T cell checkpoints. In this context it is important to know whether re-education of macrophages by restraining them from doing harm while enhancing their immune functions is preferable to their total ablation using approaches such as that described above using anti-CSF1R antibodies. The concept that PI3Kγ inhibition releases NF-κB-dependent inflammatory activation of adaptive immunity supports the possibility of re-education to an anti-tumoral state. Experiments in which tumor cells were co-implanted with wild-type or p110−/− macrophages seem to suggest such a trend, although a direct comparison was not statistically analyzed. However, the comparison of PI3Kγ inhibition with treatment with liposome-encapsulated clodronate that kills most macrophages or a CSF1R inhibitor did not show a difference. In addition to modulating the immune response, TAMs promote tumor growth and progression by various other mechanisms, including induction of tumor invasion of the stromal tissue, intravasation into the blood circulation, blood vessel outgrowth (angiogenesis) and metastatic seeding and survival at distal sites6. It requires further analysis to see whether PI3Kγ regulates any of these activities or is simply limited to immune regulation. Thus, the overall therapeutic benefit of this precision targeting versus more global approaches is still an open question.
This group's previous work also showed that PI3Kγ is required for recruitment of myeloid cells7, but TAM number is unaltered in p110-deleted mice4. The reason for the apparent discrepancy is unclear, but it may involve compensation in the long term and/or local proliferation of tissue-resident macrophages, which have been recently shown to be able to self-maintain locally in the steady state8. In addition, residual myeloid-derived macrophages resulting from p110 deletion may also replicate locally, as shown by others9.
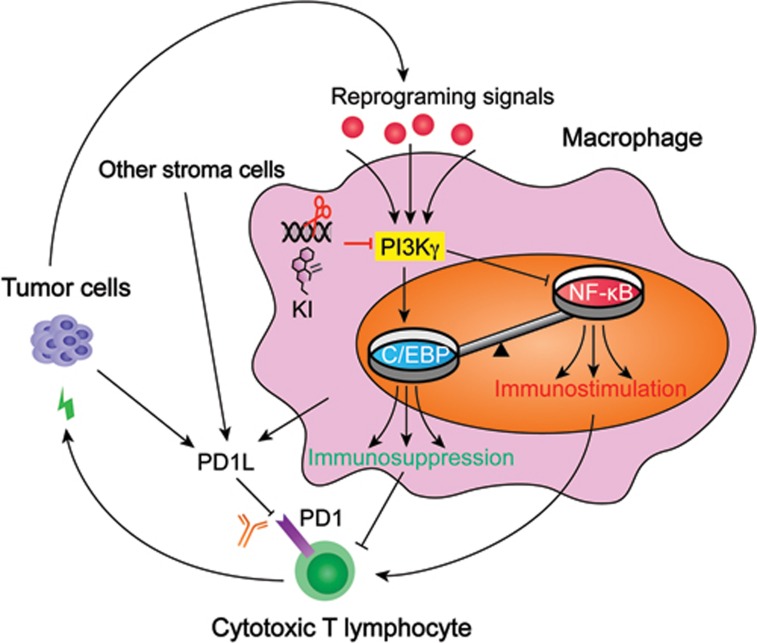
An important question is whether these results obtained in animal models can translate into the clinic. Patients with a low PI3Kγ activity profile have a better survival for lung and head and neck cancers4, suggesting that PI3Kγ inhibition holds promise in cancer treatment. A general question for drug development is the therapeutic window of the drug, i.e., the degree of maintaining therapeutic efficacy without inducing intolerable toxicity. According to their previous study, PI3Kγ appears to be activated by tumor-derived signals and remain at a relatively low level elsewhere7. Thus, PI3Kγ inhibition may target TAMs specifically and leave normal macrophages untouched. Indeed, the data suggest that PI3Kγ is located at a signaling bottleneck, integrating external reprograming signals to control the switch from immunostimulation to immunosuppression (Figure 1). If this is the case in patients, it may be a better choice in combination therapies with checkpoint inhibitors than strategies that do not discriminate different macrophage populations.
Figure 1.
TAMs control the switch of immunostimulation and immunosuppression by PI3Kγ. Tumor cells secrete regulatory molecules to reprogram TAMs to a tumor-promoting phenotype. TAMs integrate these signals through PI3Kγ, which switches on the transcriptional activity of C/EBP while switching off that of NF-κB. Each of the transcription factors regulates a program that respectively inhibits or promotes the immunosurveilance by cytotoxic T lymphocytes (CTLs). Targeting PI3Kγ signaling by genetic ablation (scissors) or kinase inhibitors (KIs) tilt the balance of the immune microenvironment to enhance CTL activities and inhibit tumor growth and metastasis. Tumor cells, TAMs and other stromal cells can inhibit CTLs via the PD1 pathway. Simultaneous targeting of the two pathways has a further elevated effect on the treatment of cancer in mouse models.
References
- Noy R, Pollard JW. Immunity 2014; 41:49–61. [DOI] [PMC free article] [PubMed]
- Ruffell B, Coussens LM. Cancer Cell 2015; 27:462–472. [DOI] [PMC free article] [PubMed]
- de Palma M, Lewis CE. Cancer Cell 2013; 23:277–286. [DOI] [PubMed]
- Kaneda MM, Messer KS, Ralainirina N, et al. Nature 2016 Sep 19. doi:10.1038/nature19834
- Zhu Y, Knolhoff BL, Meyer MA, et al. Cancer Res 2014; 74:5057–5069. [DOI] [PMC free article] [PubMed]
- Qian BZ, Pollard JW. Cell 2010; 141:39–51. [DOI] [PMC free article] [PubMed]
- Schmid MC, Avraamides CJ, Dippold HC, et al. Cancer Cell 2011; 19:715–727. [DOI] [PMC free article] [PubMed]
- Hashimoto D, Chow A, Noizat C, et al. Immunity 2013; 38:792–804. [DOI] [PMC free article] [PubMed]
- Franklin RA, Liao W, Sarkar A, et al. Science 2014; 344:921–925. [DOI] [PMC free article] [PubMed]