Abstract
AIM
To investigate whether circulating cytokeratin-positive (CK+) cells in the mesenteric blood of resected colorectal specimens are prognostic and correlate with tumor budding.
METHODS
Fifty-six colorectal specimens were collected between 9/2007 and 7/2008. Blood from the mesenteric vein was drawn immediately after receiving the fresh and unfixed specimens in the pathology department. After separation of the mononuclear cells by Ficoll-Hypaque density-gradient centrifugation, cytological smears were immunocytochemically stained for CK18. Tumor budding was evaluated on slides stained for pan-cytokeratin. The identification of ≥ 30 buds/1.3 mm2 was defined as high grade budding.
RESULTS
CK+ cells and clusters were identified in 29 (48%) and 14 (25%) of the samples, respectively. Two cells were identified in one of three non-malignant cases. Clusters were found exclusively in malignant cases. The occurrence of CK+ cells or clusters was not associated with any of the evaluated clinicopathological factors, including surgical technique and tumor budding. Moreover, the occurrence of CK+ cells or clusters had no influence on the cancer-specific survival [75 mo (CI: 61; 88) vs 83 mo (CI: 72; 95) and 80 mo (CI: 63; 98) vs 79 mo (CI: 69; 89), respectively].
CONCLUSION
CK+ cells and showed neither prognostic significance nor an association with tumor budding. It is very likely that CK18-staining is not specific enough to identify the relevant cells.
Keywords: Colorectal cancer, Circulating cells, Tumor budding, Peripheral blood, Survival
Core tip: Blood from the mesenteric vein of 56 colorectal specimens was drawn and evaluated for CK18 positive epithelial cells (CK+). CK+ cells and clusters were identified in a high proportion of cases. However, these cells and clusters were not associated with any of the evaluated clinicopathological factors, including surgical technique and tumor budding. Moreover, the occurrence of CK+ cells or clusters had no influence on the cancer specific survival. Immunocytochemical staining for CK18 does not seem to be a specific marker of mesenteric blood cells for prognostic identification of relevant circulating tumor cells.
INTRODUCTION
Colorectal cancer is a leading cause of cancer-related death, with almost 50000 estimated deaths in the United States in 2016[1]. The prognosis and therapy strongly depend on the UICC tumor stage. Nevertheless, it is well known that a certain proportion of stage I/II cancers develop an aggressive clinical course. However, approximately 40% of stage III cancers show a favorable outcome despite the occurrence of regional lymph node (LN) metastases[2]. Therefore, alternative or additional prognostic factors are necessary to improve both prognostic estimation and therapeutic stratification in colorectal cancer. The National Comprehensive Cancer Network (NCCN) defined risk factors in stage II colorectal cancers that justify the administration of an adjuvant therapy[3]. Several attempts have been made to identify other staging strategies. A very sophisticated approach is the development of multigene assays that could be demonstrated to be prognostic in stage II colorectal cancers[4,5]. However, due to the limited evidence concerning their clinical value, these tests were not recommended by the NCCN. The only molecular feature that garnered a recommendation is the microsatellite instability (MSI) status[3]. Very recently, MSI, which is caused by mismatch repair (MMR) deficiency, was demonstrated to be highly predictive for immunotherapy by PD-1 blockade[6,7] Since 2005, Pagès et al[8] focused on the host’s immune response to the tumor. They developed an immune score based on the densities of CD3+ and CD8+ T-cells and showed that this score is independently prognostic. Currently, a large international multicenter study is ongoing to validate the prognostic role of the immunoscore[9]. A different approach is the detection, quantification and analysis of circulating tumor cells (CTC). These cells circulate in the blood stream or are found in the bone marrow and are believed to be a source of distant metastases. Based on our experiences handling and cannulating fresh colorectal specimens[10,11] for LN isolation, we hypothesized that the detection of epithelial cells in the venous blood of these specimens could be prognostic for the development of hematogenous tumor dissemination and progressive disease. Furthermore, we were interested in whether the occurrence of circulating CK+ cells is associated with tumor budding. Therefore, we collected blood samples from these specimens and evaluated the occurrence of cytokeratin-positive (CK+) cells. In this retrospective study we analyzed the prognostic role of these cells in colorectal cancer.
MATERIALS AND METHODS
Patients
Fifty-six colorectal cancer cases were collected between September 2007 and July 2008. We assumed a strong correlation between the detection of circulating CK+ cells and the occurrence of distant metastases with lethal outcome. An absolute difference concerning lethal outcome of 50% with a power of 0.8 and with Alpha = 0.05 resulted in a calculated sample size of 19 cases in each group (proportions sample size test). Inclusion criteria were proven or suspected cancer, a curative intent and free resection margins. For the survival analysis, only malignant cases with a minimal survival time of 2 mo were included. Follow-up data were provided by the Clinical and Population-Based Cancer Registry of Augsburg. Additional data were acquired from clinical and laboratory information systems. Informed and written consent was obtained from all patients. The study was approved by the ethics committee of the Landesärztekammer Bayern. The study was performed according to the national rules.
Blood sample collection
Immediately after resection, colorectal specimens were delivered fresh to the in-house laboratory of the Institute of Pathology. The specimens were not opened to avoid contamination by epithelial cells from the mucosa. Manual manipulation was reduced to a minimum to reduce the chance of artificial tumor dissemination. After gentle cleaning, the specimens were placed on a clean board and the main vessels were clamped proximally. Then, the ligation or the clip that was placed by the surgeon was withdrawn. The venous vessel was then cannulated with a standard i.v.-catheter (17 Gauge, Braun, Melsungen, Germany). Zero point five milliliter to 8 mL (mean: 3.8 mL; SD: 2.6 mL) of venous blood was drawn using NH4-heparin blood collection tubes (Sarstedt, Nürnbrecht, Germany) (Figure 1). Then, the blood sample was immediately stored until future use.
Figure 1.
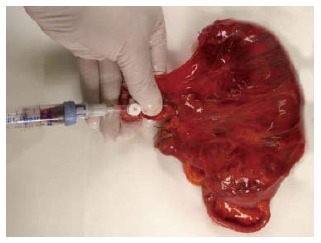
This image illustrates the blood draw from the mesenteric vein. A standard i.v. catheter is used to cannulate the mesenteric vein after removal of the clip.
Blood sample preparation and immunocytochemistry
The protocol for preparing the cytological samples was initially established for the detection of CK+ cells in bone marrow aspirates[12,13]. In brief, the mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation (density, 1.077 g/mole) at 900 × g for 30 min. The cells were then washed and centrifuged at 150 × g for 5 min. Approximately 1 × 106 cells were placed on each glass slide.
To detect epithelial cells within the peripheral blood, a monoclonal antibody against cytokeratin 18 [Clone CK18 (Clone CK2), 1: 100; Chemicon, Hofheim, Germany] was used. The reactions were developed with the alkaline phosphatase anti-alkaline phosphatase technique combined with a new fuchsin stain to indicate antibody binding, as previously described[12,13]. CK+ cells and clusters were counted manually (Figure 2). For that all slides were screened by a very experienced technician. All positive cases were confirmed by a hemato-oncologist (DO). Data concerning interobserver agreement between these two investigators are not available.
Figure 2.
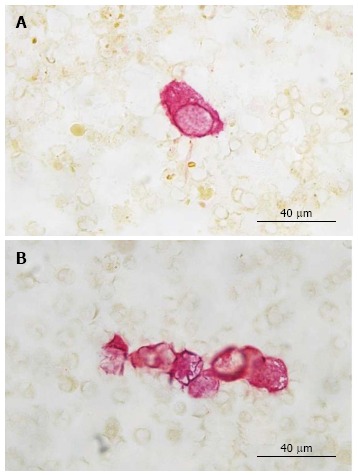
Cytokeratin 18 (Clone cytokeratin 2) cytochemistry. A: A single CK+ cell is shown in this image; B: A CK+ cell cluster is shown in this image. CK+: Cytokeratin-positive.
Histopathological evaluation, immunohistochemistry and tumor budding
Colorectal specimens were macroscopically evaluated after fixing overnight in 10% buffered formalin. LNs were dissected using the methylene-blue method[10,11]; samples from the resection margins, the tumor-region and other conspicuous areas were paraffin-embedded. The slides were stained with hematoxylin and eosin (HE) and evaluated by an experienced pathologist (BM). Based on the HE-morphology, slides were selected for further pan-cytokeratin staining which was performed to enable optimal evaluation of tumor budding. For this evaluation, monoclonal mouse antibody AE1/AE3 was used (dilution 1:50; DAKO). Immunoreactions were developed using a labelled streptavidin-biotin system (DAKO Real detection system). All reactions were performed on a Dako-Autostainer system (DAKO, Glostrup, Denmark).
Tumor budding was evaluated by one pathologist (BM). It was defined as detached single tumor cells or clusters of up to four cells. The cut-off for high-grade budding was adapted from Ueno et al[14] and defined as ≥ 30 buds/20 × magnification (= 1.3 mm²).
Statistical analysis
Metric data were compared using the Mann-Whitney rank sum test. Tabulated data were analyzed with the χ2 test or Fisher’s exact test depending on the expected frequency of the observations. Mean values are given ± 1 standard deviation (SD). Linear regression analysis was performed to calculate correlations between metric data. For the survival analyses, Kaplan-Meier curves were calculated and log-rank tests were performed. ROC analyses were performed to determine the optimized cut-offs. The calculation of the follow-up time was performed according to Schemper and Smith[15]. A P value < 0.05 was considered significant. All calculations were performed using the statistics package SigmaPlot 13.0 (Systat, Richmond, VA, United States). The statistical methods of this study were reviewed by Bruno Märkl.
RESULTS
Patients
Fifty-six patients were consecutively collected within 10 mo between 2007 and 2008. The patient characteristics are summarized in Table 1. The mean and median follow-up times were 74 (95%CI: 68; 79 mo) and 80 mo (CI: 77; 83 mo), respectively.
Table 1.
Clinicopathological data
| Complete collective n = 56 | CK+ cell negative n = 27 | CK+ cell positive n = 29 | P-value | CK+ cell cluster negative n = 42 | CK+ cell cluster positive n = 14 | P-value | |
| Mean age ± SD | 70 ± 13 | 71 ± 11 | 69 ± 11 | 0.844 | 71 ± 12 | 66 ± 13 | 0.167 |
| Gender (M:F) | 1:1.5 | 1:1.7 | 1:1.4 | 1.0 | 1:2 | 1:0.75 | 0.538 |
| Laparoscopic surgery | 15 (27%) | 5 (19%) | 10 (34%) | 10 (24%) | 5 (36%) | ||
| Open surgery | 41 (73%) | 22 (81%) | 19 (66%) | 0.223 | 32 (76%) | 9 (64%) | 0.489 |
| Right colon | 21 (38%) | 10 (37%) | 11 (38%) | 16 (38%) | 5 (36%) | ||
| Left colon | 29 (52%) | 13 (48%) | 16 (55%) | 0.927 | 21 (50%) | 8 (57%) | 0.979 |
| Rectum | 6 (11%) | 4 (15%) | 2 (7%) | 0.4141 | 5 (12%) | 1 (7%) | 1.0a |
| Mean LN count ± SD | 32 ± 19 | 29 ± 16 | 35 ± 21 | 0.219 | 30 ± 16 | 36 ± 25 | 0.961 |
| LN positivity | 20 (36%) | 11 (41%) | 9 (31%) | 0.632 | 16 (38%) | 4 (29%) | 0.747 |
| Low grade | 33 (59%) | 17 (63%) | 22 (76%) | 28 (67%) | 11 (79%) | ||
| High grade | 20 (36%) | 8 (30%) | 6 (21%) | 0.576 | 11 (26%) | 3 (21%) | 0.735 |
| Non-malignant | 3 (5%) | 2 (7%) | 1 (3%) | n.c. | 3 (7%) | 0 (0%) | n.c. |
| pT1/2 | 16 (29%) | 7 (26%) | 9 (31%) | 11 (26%) | 5 (36%) | ||
| pT3/4 | 37 (66%) | 18 (67%) | 19 (66%) | 0.977 | 28 (67%) | 9 (64%) | 0.736 |
| Mean budding ± SD | 21 ± 27 | 20 ± 23 | 22 ± 30 | 0.957 | 19 ± 20 | 21 ± 26 | 0.663 |
| High grade budding | 16 (29%) | 6 (22%) | 10 (34%) | 0.472 | 10 (24%) | 6 (43%) | 0.190 |
| Distant metastases | 11 (20%) | 5 (19%) | 6 (21%) | 1.0 | 8 (19%) | 3 (21%) | 1.0 |
Rectum vs colon. CK+: Cytokeratin positive; SD: Standard deviation; LN: Lymph node; n.c.: Not calculated.
CK+ cells and clusters and their relation to clinicopathological characteristics
CK+ cells were found in 29 (52%) cases with a mean number of 12 ± 14 cells/106 cells. One of these cases was non-malignant with two detected CK+ cells. CK+ cell clusters were detected in 14 (25%) cases. The mean number of clusters in positive cases was 3 ± 3 clusters/106 cells. No clusters were found in non-malignant cases (Figure 2). There was a strong correlation between CK+ cells and clusters (R = 0.727; P < 0.001). Clusters were always accompanied with single CK+ cells.
None of the evaluated clinicopathological features (age, gender, location, LN count, grading, T-stage, metastases) showed an association with the occurrence of CK+ cells or clusters (Table 1). In particular, neither CK+ cells nor CK+ clusters showed an association with tumor budding (R = 0.180; P = 0.185 and R = 0.0637; P = 0.647, respectively). The surgical technique (open vs laparoscopic technique) did not influence the occurrence of CK+ cells or clusters (Table 1).
Survival analysis
Forty-eight cases met the inclusion criteria for the cancer-specific survival (CSS) analysis. The CSS analysis revealed no significant differences between cases with or without CK+ cells or clusters (Figure 3A and B). Despite the lack of significance, the Kaplan-Meier curve for CK+ cells discriminated between CK+ positive and negative cases with mean CSS times of 75 mo (CI: 61; 88) vs 83 mo (CI: 72; 95) (Figure 3A), respectively. The outcome of CK+ cluster positive and negative cases was identical, with mean survival times of 80 mo (CI: 63; 98) vs 79 mo (CI: 69; 89) (Figure 3B), respectively. A non-significant trend towards an adverse outcome was found in cases with high-grade tumor budding, with a mean survival time of 71 mo (CI: 53; 89 mo) vs 83 mo (CI: 73; 93 mo) (P = 0.187, Figure 3C), respectively. ROC analysis identified a certain cut-off that was not positive, i.e., did not reveal a threshold with areas under the curve of 0.51 and 0.55 for CK+ cells and clusters, respectively.
Figure 3.
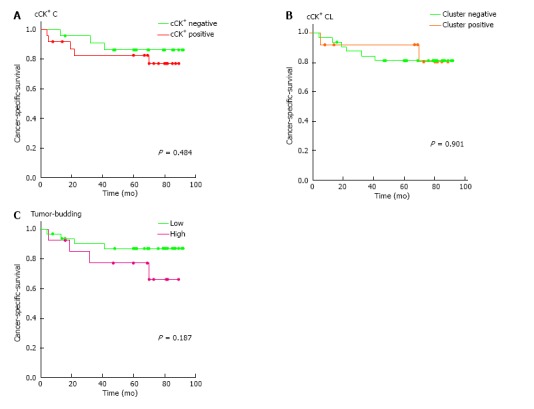
Cancer specific survival. A: Circulating CK+ cell negative vs positive; B: Circulating CK+ cell cluster negative vs positive; C: Tumor budding negative/low grade vs high grade. CK+: Cytokeratin-positive.
DISCUSSION
In this study, we investigated the prognostic role of circulating CK+ cells and clusters obtained from the mesenteric blood of colorectal specimens. It was our hypothesis that the venous blood from these specimens should be enriched in circulating CK+ positive cells originating from the tumor. We used a technique that was well established for the detection of CK+ cells in the bone marrow of breast, prostate, lung and colorectal cancer patients. Using this method, the detection of cytokeratin-positive cells in the bone marrow could be demonstrated to be prognostic[12,13,16,17].
In this study, we found circulating CK+ cells and clusters in the mesenteric blood in a high proportion of cases (52% and 27%, respectively). This positive rate is within the range published in the literature (Table 1). However, it must be noted that only Leather et al[18] used immunocytochemistry to detect circulating epithelial cells in the mesenteric blood. In all other identified studies, molecular or flow cytometry techniques were used[19-35]. By using case numbers, we calculated a mean positivity rate in these studies of 43%. When we restricted this calculation to studies that also included stage IV cases, the mean positivity rate was 55%. We detected 2 CK+ cells/106 cells in one non-malignant case with diverticulitis. The phenomenon of circulating epithelial cells in the blood in the absence of a malignant tumor has been found by other authors. Pantel et al[36] reported the detection of CK+ cells in benign colon diseases using two different commercial tests in 11.3% and 18.9% of cases, respectively. In summary, this indicates that the results generated with our immunocytochemical method are comparable to other techniques and are valid.
Despite using an obviously sensitive method, we could not confirm our hypothesis of circulating epithelial cells in the mesenteric blood being prognostic markers of colorectal cancer that correlate with tumor budding. This study is limited by a relatively small case number (n = 56) and is therefore underpowered to detect effects that are possibly smaller than expected. We presumed that the prognostic effect of CK+ cells was at least as strong as node positivity. Indeed, nodal status revealed a good discrimination with regards to cancer specific survival with a P value of 0.058 (data not shown). The strengths of this study are the long follow-up time and the precise evaluation of histological features including an immunohistochemical tumor budding assessment.
Tumor budding is a well investigated prognostic parameter in gastrointestinal cancers. Despite considerable limitations due to the lack of a generally accepted definition and only moderate interobserver agreement, it has been shown in many studies[37,38]. It is believed to be an expression of the epithelial-mesenchymal transition (EMT), which is an important initial step in cancer progression[39]. None of the studies shown in Table 2 investigated the possible relationship between the phenomenon of tumor cell isolation at the invasion front of colorectal cancers and the occurrence of CTCs in the blood. Moreover, a literature search within the Medline, Embase and Google Scholar databases did not reveal an investigation that addressed this topic. Cao et al[40] postulated in a review that EMT leads to tumor budding and subsequent blood vessel invasion. However, this is not supported by other references. To us, it seemed quite obvious that a correlation between these two factors exists. However, we were not able to confirm this hypothesis. We could not identify a correlation between tumor budding and circulating CK+ cells and could not confirm that a combination of tumor budding and CK+ cells was prognostic. Tumor budding alone discriminated clearly between two prognostic groups (Figure 3C). However, significance was likely not achieved due to the small sample number.
Table 2.
Literature: Circulating tumor cells in the mesenteric blood
| Ref. | n | Year | Method | Material | Stages | %positive | Prognostic relevance |
| Leather et al[18] | 42 | 1993 | ICC | Mesenteric and peripheral blood | I, II, III, IV | 15 | n.a. |
| Nakamori et al[19] | 35 | 1997 | PCR | Mesenteric and peripheral blood | I, II, III, IV | 26 | uv predictive for recurrence |
| Luo et al[20] | 54 | 1999 | PCR | Mesenteric blood | I, II, III, IV | 76 | Predictive for metastases |
| Taniguchi et al[22] | 53 | 2000 | PCR | Mesenteric and peripheral blood | I, II, III | 68 | uv survival |
| Yamaguchi et al[23] | 52 | 2000 | PCR | Mesenteric blood | I, II, III, IV | 44 | mv survival |
| Iinuma et al[21] | 23 | 2000 | MACS | Mesenteric blood | I, II, III, IV | 39 | uv survival |
| Fujita et al[25] | 35 | 2001 | PCR | Mesenteric blood | I, II, III | 29 | uv recurrence/survival |
| Etoh et al[24] | 24 | 2001 | PCR | Mesenteric blood | I, II, III, IV | 29 | uv recurrence/survival |
| Guller et al[26] | 39 | 2002 | PCR | Mesenteric and peripheral blood | I, II, III | 81/282 | 3 |
| Tien et al[27] | 58 | 2002 | PCR | Mesenteric and peripheral blood | II, III, IV | 454 | n.a. |
| Akashi et al[28] | 80 | 2003 | PCR | Mesenteric blood | I, II, III | 44 | uv metastatic disease; mv no |
| Nozawa et al[29] | 41 | 2003 | RTA | Mesenteric and peripheral blood | I, II, III, IV | 37 | uv predictive for metastatic disease |
| Sunouchi et al[30] | 37 | 2003 | PCR | Mesenteric blood | I, II, III, IV | 43 | uv survival |
| Zhang et al[32] | 58 | 2005 | PCR | Bone marrow, portal blood, peripheral blood | I, II, III, IV | 74 | correlation with stage - no outcome analysis |
| Sadahiro et al[31] | 100 | 2005 | PCR | Mesenteric and peripheral blood | I, II, III | 455/486 | no |
| Kanellos et al[34] | 108 | 2006 | PCR | Mesenteric blood | I, II, III | 11 | uv metastatic disease/survival |
| Iinuma et al[33] | 167 | 2006 | PCR | Mesenteric and peripheral blood | I, II, III, IV | 10/347 | mv survival |
| Tseng et al[35] | 135 | 2015 | FACS | Mesenteric | I, II, III | 68 | mv survival |
Blood;
Blood and peritoneal fluid;
No separate evaluation for blood samples;
Multiple measurements;
Mesenteric blood;
Peripheral blood;
Mesenteric. n.a.: Not available; uv: Uni-variable; mv: Multi-variable; ICC: Immunocytochemistry; PCR: Polymerase chain reaction; MACS: Magnetic activated cell sorting; FACS: Fluorescence activated cell sorting.
The data concerning the prognostic significance of CTCs and disseminated tumor cells (DTCs) are conflicting[41]. However, there is growing evidence that CTC/DTCs are of prognostic significance. Two commercial tests based on immunomagnetic separation targeting EpCAM (BerEp4) are currently available. They have proven to be prognostic, particularly in the metastatic stage of different cancers including colorectal cancer[42,43]. Two meta-analyses addressed this topic. Katsuno et al[44] restricted their analysis to molecularly detected CTCs in mesenteric blood and included 9 studies. They found a favorable outcome in patients negative for CTCs [hazard ratio (HR) 0.4-0.08][44]. Rahbari et al[45] included 36 studies with a total 3094 patients. They also identified a prognostic effect of CTCs. However, stratification according to the sampling compartment revealed that CTCs of peripheral blood were prognostic but those of the mesenteric bone marrow blood were not[45]. Similarly, our study found that the identification of CK+ cells or clusters had no prognostic effect. In addition, the approach using ROC analyses to identify a certain cut-off of cells which might be prognostic failed.
CTCs seem to comprise different cell types of neoplastic and non-neoplastic origin. Moreover, it is very likely that cells derived from cancer have different potential to escape from immunogenic destruction and to establish tumor growth at a distant site. Depending on the compartment, cells may undergo a change in their phenotype[40,41,46]. As mentioned before, EMT is a hallmark process in cancer progression and is associated with impaired outcome[46,47]. Cells undergoing EMT lose their epithelial phenotype and gain mesenchymal features. The use of methods optimized for the detection of epithelial cells is prone to fail in the detection of all CTCs. Moreover, these methods may fail to detect the most relevant cells[48]. Currently, the most interesting cells in this context are cells with stem-cell features. The realization of a fast, exact and cost effective technical method to detect these cells is likely the most promising approach.
In this study, we hypothesized that the immunocytochemical detection of CK+ cell in the mesenteric blood of colorectal cancer specimens correlates with tumor budding and could serve as an easy to determine prognostic factor. Drawing the blood after resection would avoid delay and additional risk during the operation. None of these hypotheses could be confirmed in our study. Given the current literature, peripheral blood and not mesenteric blood is the optimal material for the detection of CTCs. More sophisticated techniques including molecular approaches are relatively expensive and their availability is limited. Nevertheless, they have the potential to detect exactly the cells which are most likely to be relevant to the clinical course of the disease. Immunocytochemical detection seems to be less specific and is not favorable.
ACKNOWLEDGMENTS
The authors are thankful to Carmen Sailer for her excellent technical assistance.
COMMENTS
Background
Colorectal cancer is one of the most common cancers in men and women. Its prognosis depends mainly on the (UICC-) tumor stage. However, it is also known that certain proportion of cancers with otherwise favorable features and low stages show an aggressive clinical course while locally advanced cancers so not relapse. It is accepted that the detection of circulating tumor cells has the potential to improve the prognosis estimation not only in colorectal cancer.
Research frontiers
The main topic in the research field of circulating tumor cells is the influence of the different compartments (peripheral blood, mesenteric blood or bone marrow) on the clinical significance of the detected cells. Other important questions are the methods for the assessment and the type of cells (e.g., stem cells) which are most informative to predict the outcome.
Innovations and breakthroughs
The innovation of this study is the evaluation of the blood draw from resected specimens. A direct correlation with tumor budding as a source for the circulating tumor cells is also a new approach.
Applications
Because the authors’ hypotheses could not be confirmed, the main conclusions are that mesenteric blood is probably not the best compartment for the identification of the relevant cells and more sophisticated methods may be superior over immunocytochemistry. Molecular techniques are more sensitive in detecting cells with a high potential to serve as the origin for distant metastases.
Terminology
Circulating tumor cells are cells that lost its cohesion to the primary tumor mass and achieved access to the vascular system including the bone marrow.
Peer-review
This is an interesting manuscript which appears to add to the existing body of literature around this subject. The design is clear.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Ethikkomission der Landesärztekammer Bayern.
Informed consent statement: All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All authors declare that no conflicting interests (including but not limited to commercial, personal, political, intellectual or religious interests) exist.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at bruno.maerkl@klinikum-augsburg.de. Participants consent was not obtained but the presented data are anonymized and risk of identification is low.
Manuscript source: Invited manuscript
Specialty Type: Oncology
Country of Origin: Germany
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: August 5, 2016
First decision: September 2, 2016
Article in press: October 19, 2016
P- Reviewer: Horne J, Lewitowicz P, Shimada Y S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
References
- 1.Society AC. Atlanta: American Cancer Society; 2016. Cancer Facts & Figures 2016. [Google Scholar]
- 2.The National Cancer Registration Service EO. Bowel cancer survival statistics. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/survival#one.
- 3.NCCN. USA: NCCN; 2015. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Colon Cancer (Version 2.2015) [Google Scholar]
- 4.Kopetz S, Tabernero J, Rosenberg R, Jiang ZQ, Moreno V, Bachleitner-Hofmann T, Lanza G, Stork-Sloots L, Maru D, Simon I, et al. Genomic classifier ColoPrint predicts recurrence in stage II colorectal cancer patients more accurately than clinical factors. Oncologist. 2015;20:127–133. doi: 10.1634/theoncologist.2014-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray RG, Quirke P, Handley K, Lopatin M, Magill L, Baehner FL, Beaumont C, Clark-Langone KM, Yoshizawa CN, Lee M, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol. 2011;29:4611–4619. doi: 10.1200/JCO.2010.32.8732. [DOI] [PubMed] [Google Scholar]
- 6.Diaz LA, Le DT. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;373:1979. doi: 10.1056/NEJMc1510353. [DOI] [PubMed] [Google Scholar]
- 7.Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res. 2016;22:813–820. doi: 10.1158/1078-0432.CCR-15-1678. [DOI] [PubMed] [Google Scholar]
- 8.Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 9.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerwel TG, Spatz J, Anthuber M, Wünsch K, Arnholdt H, Märkl B. Injecting methylene blue into the inferior mesenteric artery assures an adequate lymph node harvest and eliminates pathologist variability in nodal staging for rectal cancer. Dis Colon Rectum. 2009;52:935–941. doi: 10.1007/DCR.0b013e31819f28c9. [DOI] [PubMed] [Google Scholar]
- 11.Märkl B, Kerwel TG, Jähnig HG, Oruzio D, Arnholdt HM, Schöler C, Anthuber M, Spatz H. Methylene blue-assisted lymph node dissection in colon specimens: a prospective, randomized study. Am J Clin Pathol. 2008;130:913–919. doi: 10.1309/AJCPVAPB5APABJNX. [DOI] [PubMed] [Google Scholar]
- 12.Braun S, Pantel K, Müller P, Janni W, Hepp F, Kentenich CR, Gastroph S, Wischnik A, Dimpfl T, Kindermann G, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med. 2000;342:525–533. doi: 10.1056/NEJM200002243420801. [DOI] [PubMed] [Google Scholar]
- 13.Pantel K, Schlimok G, Angstwurm M, Weckermann D, Schmaus W, Gath H, Passlick B, Izbicki JR, Riethmüller G. Methodological analysis of immunocytochemical screening for disseminated epithelial tumor cells in bone marrow. J Hematother. 1994;3:165–173. doi: 10.1089/scd.1.1994.3.165. [DOI] [PubMed] [Google Scholar]
- 14.Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. 2002;40:127–132. doi: 10.1046/j.1365-2559.2002.01324.x. [DOI] [PubMed] [Google Scholar]
- 15.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 16.Lindemann F, Schlimok G, Dirschedl P, Witte J, Riethmüller G. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet. 1992;340:685–689. doi: 10.1016/0140-6736(92)92230-d. [DOI] [PubMed] [Google Scholar]
- 17.Weckermann D, Polzer B, Ragg T, Blana A, Schlimok G, Arnholdt H, Bertz S, Harzmann R, Klein CA. Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J Clin Oncol. 2009;27:1549–1556. doi: 10.1200/JCO.2008.17.0563. [DOI] [PubMed] [Google Scholar]
- 18.Leather AJ, Gallegos NC, Kocjan G, Savage F, Smales CS, Hu W, Boulos PB, Northover JM, Phillips RK. Detection and enumeration of circulating tumour cells in colorectal cancer. Br J Surg. 1993;80:777–780. doi: 10.1002/bjs.1800800643. [DOI] [PubMed] [Google Scholar]
- 19.Nakamori S, Kameyama M, Furukawa H, Takeda O, Sugai S, Imaoka S, Nakamura Y. Genetic detection of colorectal cancer cells in circulation and lymph nodes. Dis Colon Rectum. 1997;40:S29–S36. doi: 10.1007/BF02062017. [DOI] [PubMed] [Google Scholar]
- 20.Luo C, Li S. [The detection and its clinical significance of cancer cells in portal vein blood of patients with colorectal carcinoma] Zhonghua Waike Zazhi. 1999;37:214–215. [PubMed] [Google Scholar]
- 21.Iinuma H, Okinaga K, Adachi M, Suda K, Sekine T, Sakagawa K, Baba Y, Tamura J, Kumagai H, Ida A. Detection of tumor cells in blood using CD45 magnetic cell separation followed by nested mutant allele-specific amplification of p53 and K-ras genes in patients with colorectal cancer. Int J Cancer. 2000;89:337–344. doi: 10.1002/1097-0215(20000720)89:4<337::aid-ijc4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi T, Makino M, Suzuki K, Kaibara N. Prognostic significance of reverse transcriptase-polymerase chain reaction measurement of carcinoembryonic antigen mRNA levels in tumor drainage blood and peripheral blood of patients with colorectal carcinoma. Cancer. 2000;89:970–976. [PubMed] [Google Scholar]
- 23.Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000;232:58–65. doi: 10.1097/00000658-200007000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etoh T, Ueo H, Inoue H, Sato K, Utsunomiya T, Barnard GF, Kitano S, Mori M. Clinical significance of K-Ras mutations in intraoperative tumor drainage blood from patients with colorectal carcinoma. Ann Surg Oncol. 2001;8:407–412. doi: 10.1007/s10434-001-0407-8. [DOI] [PubMed] [Google Scholar]
- 25.Fujita S, Kudo N, Akasu T, Moriya Y. Detection of cytokeratin 19 and 20 mRNA in peripheral and mesenteric blood from colorectal cancer patients and their prognosis. Int J Colorectal Dis. 2001;16:141–146. doi: 10.1007/s003840100286. [DOI] [PubMed] [Google Scholar]
- 26.Guller U, Zajac P, Schnider A, Bösch B, Vorburger S, Zuber M, Spagnoli GC, Oertli D, Maurer R, Metzger U, et al. Disseminated single tumor cells as detected by real-time quantitative polymerase chain reaction represent a prognostic factor in patients undergoing surgery for colorectal cancer. Ann Surg. 2002;236:768–775; discussion 775-776. doi: 10.1097/00000658-200212000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tien YW, Lee PH, Wang SM, Hsu SM, Chang KJ. Simultaneous detection of colonic epithelial cells in portal venous and peripheral blood during colorectal cancer surgery. Dis Colon Rectum. 2002;45:23–29. doi: 10.1007/s10350-004-6109-0. [DOI] [PubMed] [Google Scholar]
- 28.Akashi A, Komuta K, Haraguchi M, Ueda T, Okudaira S, Furui J, Kanematsu T. Carcinoembryonic antigen mRNA in the mesenteric vein is not a predictor of hepatic metastasis in patients with resectable colorectal cancer: a long-term study. Dis Colon Rectum. 2003;46:1653–1658. doi: 10.1007/BF02660771. [DOI] [PubMed] [Google Scholar]
- 29.Nozawa H, Watanabe T, Ohnishi T, Tada T, Tsurita G, Sasaki S, Kitayama J, Nagawa H. Detection of cancer cells in mesenteric vein and peripheral vessels by measuring telomerase activity in patients with colorectal cancer. Surgery. 2003;134:791–798. doi: 10.1016/s0039-6060(03)00382-9. [DOI] [PubMed] [Google Scholar]
- 30.Sunouchi K, Machinami R, Mori M, Namiki K, Hattori S, Murata Y, Tsuchiya T, Mizuno H, Tadokoro M. Clinical impact of carcinoembryonic antigen messenger ribonucleic acid expression in tumor-draining vein blood on postoperative liver metastasis in patients with colorectal carcinoma: a prospective, cohort study. Dis Colon Rectum. 2003;46:467–473. doi: 10.1007/s10350-004-6584-3. [DOI] [PubMed] [Google Scholar]
- 31.Sadahiro S, Suzuki T, Ishikawa K, Saguchi T, Maeda Y, Yasuda S, Makuuchi H, Yurimoto S, Murayama C. Detection of carcinoembryonic antigen messenger RNA-expressing cells in portal and peripheral blood during surgery does not influence relapse in colorectal cancer. Ann Surg Oncol. 2005;12:988–994. doi: 10.1245/ASO.2005.03.565. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XW, Yang HY, Fan P, Yang L, Chen GY. Detection of micrometastasis in peripheral blood by multi-sampling in patients with colorectal cancer. World J Gastroenterol. 2005;11:436–438. doi: 10.3748/wjg.v11.i3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iinuma H, Okinaga K, Egami H, Mimori K, Hayashi N, Nishida K, Adachi M, Mori M, Sasako M. Usefulness and clinical significance of quantitative real-time RT-PCR to detect isolated tumor cells in the peripheral blood and tumor drainage blood of patients with colorectal cancer. Int J Oncol. 2006;28:297–306. [PubMed] [Google Scholar]
- 34.Kanellos I, Zacharakis E, Kanellos D, Pramateftakis MG, Tsahalis T, Altsitsiadis E, Betsis D. Prognostic significance of CEA levels and detection of CEA mRNA in draining venous blood in patients with colorectal cancer. J Surg Oncol. 2006;94:3–8. doi: 10.1002/jso.20549. [DOI] [PubMed] [Google Scholar]
- 35.Tseng JY, Yang CY, Yang SH, Lin JK, Lin CH, Jiang JK. Circulating CD133(+)/ESA(+) cells in colorectal cancer patients. J Surg Res. 2015;199:362–370. doi: 10.1016/j.jss.2015.05.057. [DOI] [PubMed] [Google Scholar]
- 36.Pantel K, Denève E, Nocca D, Coffy A, Vendrell JP, Maudelonde T, Riethdorf S, Alix-Panabières C. Circulating epithelial cells in patients with benign colon diseases. Clin Chem. 2012;58:936–940. doi: 10.1373/clinchem.2011.175570. [DOI] [PubMed] [Google Scholar]
- 37.Puppa G, Senore C, Sheahan K, Vieth M, Lugli A, Zlobec I, Pecori S, Wang LM, Langner C, Mitomi H, et al. Diagnostic reproducibility of tumour budding in colorectal cancer: a multicentre, multinational study using virtual microscopy. Histopathology. 2012;61:562–575. doi: 10.1111/j.1365-2559.2012.04270.x. [DOI] [PubMed] [Google Scholar]
- 38.Märkl B, Arnholdt HM. Prognostic significance of tumor budding in gastrointestinal tumors. Expert Rev Anticancer Ther. 2011;11:1521–1533. doi: 10.1586/era.11.156. [DOI] [PubMed] [Google Scholar]
- 39.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao H, Xu E, Liu H, Wan L, Lai M. Epithelial-mesenchymal transition in colorectal cancer metastasis: A system review. Pathol Res Pract. 2015;211:557–569. doi: 10.1016/j.prp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Hardingham JE, Grover P, Winter M, Hewett PJ, Price TJ, Thierry B. Detection and Clinical Significance of Circulating Tumor Cells in Colorectal Cancer--20 Years of Progress. Mol Med. 2015;21 Suppl 1:S25–S31. doi: 10.2119/molmed.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardingham JE, Kotasek D, Farmer B, Butler RN, Mi JX, Sage RE, Dobrovic A. Immunobead-PCR: a technique for the detection of circulating tumor cells using immunomagnetic beads and the polymerase chain reaction. Cancer Res. 1993;53:3455–3458. [PubMed] [Google Scholar]
- 43.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 44.Katsuno H, Zacharakis E, Aziz O, Rao C, Deeba S, Paraskeva P, Ziprin P, Athanasiou T, Darzi A. Does the presence of circulating tumor cells in the venous drainage of curative colorectal cancer resections determine prognosis? A meta-analysis. Ann Surg Oncol. 2008;15:3083–3091. doi: 10.1245/s10434-008-0131-8. [DOI] [PubMed] [Google Scholar]
- 45.Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M, Weitz J. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138:1714–1726. doi: 10.1053/j.gastro.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Lim SH, Becker TM, Chua W, Ng WL, de Souza P, Spring KJ. Circulating tumour cells and the epithelial mesenchymal transition in colorectal cancer. J Clin Pathol. 2014;67:848–853. doi: 10.1136/jclinpath-2014-202499. [DOI] [PubMed] [Google Scholar]
- 47.Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grover PK, Cummins AG, Price TJ, Roberts-Thomson IC, Hardingham JE. Circulating tumour cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann Oncol. 2014;25:1506–1516. doi: 10.1093/annonc/mdu018. [DOI] [PubMed] [Google Scholar]


