Abstract
Background
Although studies have examined the association between dietary magnesium intake and health outcome, the results are inconclusive. Here, we conducted a dose–response meta-analysis of prospective cohort studies in order to investigate the correlation between magnesium intake and the risk of cardiovascular disease (CVD), type 2 diabetes (T2D), and all-cause mortality.
Methods
PubMed, EMBASE, and Web of Science were searched for articles that contained risk estimates for the outcomes of interest and were published through May 31, 2016. The pooled results were analyzed using a random-effects model.
Results
Forty prospective cohort studies totaling more than 1 million participants were included in the analysis. During the follow-up periods (ranging from 4 to 30 years), 7678 cases of CVD, 6845 cases of coronary heart disease (CHD), 701 cases of heart failure, 14,755 cases of stroke, 26,299 cases of T2D, and 10,983 deaths were reported. No significant association was observed between increasing dietary magnesium intake (per 100 mg/day increment) and the risk of total CVD (RR: 0.99; 95% CI, 0.88–1.10) or CHD (RR: 0.92; 95% CI, 0.85–1.01). However, the same incremental increase in magnesium intake was associated with a 22% reduction in the risk of heart failure (RR: 0.78; 95% CI, 0.69–0.89) and a 7% reduction in the risk of stroke (RR: 0.93; 95% CI, 0.89–0.97). Moreover, the summary relative risks of T2D and mortality per 100 mg/day increment in magnesium intake were 0.81 (95% CI, 0.77–0.86) and 0.90 (95% CI, 0.81–0.99), respectively.
Conclusions
Increasing dietary magnesium intake is associated with a reduced risk of stroke, heart failure, diabetes, and all-cause mortality, but not CHD or total CVD. These findings support the notion that increasing dietary magnesium might provide health benefits.
Electronic supplementary material
The online version of this article (doi:10.1186/s12916-016-0742-z) contains supplementary material, which is available to authorized users.
Keywords: Magnesium, Cardiovascular disease, Type 2 diabetes, All-cause mortality, Meta-analysis
Background
Magnesium is the eighth most common element in our planet’s crust and a biologically active mineral essential for life. All cells require magnesium, which acts as a critical cofactor for hundreds of enzymes involved in glucose metabolism, protein production, and nucleic acid synthesis [1]. Due to the daily loss of magnesium in feces, urine, and sweat [2], humans require magnesium intake (for example, by consuming magnesium-rich foods such as whole grains, green leafy vegetables, and nuts) in order to maintain normal magnesium levels [3, 4].
Despite the availability of many magnesium-rich foods, magnesium deficiency (i.e., hypomagnesemia, which is defined as a serum magnesium concentration < 0.74 mmol/L) is relatively common, with an estimated prevalence of 2.5–15% in the general population [5, 6]. The primary cause of hypomagnesemia is often insufficient dietary intake. Dietary magnesium is absorbed primarily by the small intestine via passive paracellular transport, which is driven by an electrochemical gradient and solvent drag [7, 8]. Interestingly, the intestinal absorption of magnesium is not directly proportional to magnesium intake, but depends primarily on the body’s magnesium status [9]. Peacock et al. once reported a correlation coefficient of 0.053 between baseline dietary and serum magnesium in a cohort study [10]. Even in developed countries such as the United States, many adults fail to meet the recommended daily intake of magnesium [11], despite the fact that epidemiology studies indicate that low levels of serum magnesium can increase the risk of a wide range of diseases, including chronic obstructive pulmonary disease [12], metabolic syndrome [13], type 2 diabetes (T2D) [14], Alzheimer’s disease [15], and cardiovascular disease (CVD) [16]. Therefore, understanding the relationship between dietary magnesium intake and overall health outcome is important for guiding public awareness and establishing clear dietary guidelines, thereby reducing the risk of magnesium deficiency-related diseases.
Previous meta-analyses suggest that the consumption of magnesium is associated with a reduced incidence of CVD and diabetes [17–19]. In recent years, a growing number of well-designed population-based studies focused on the relationship between magnesium and the risk of CVD, diabetes, and all-cause mortality, providing results that might be of great importance with respect to public health issues. However, these studies varied with respect to sample size, magnesium intake, and other characteristics, thereby contributing to inconsistencies within the literature [20, 21]. Therefore, we performed a comprehensive meta-analysis of all published prospective cohort studies in order to quantify the dose–response relationship between dietary magnesium intake and the risk of CVD, T2D, and all-cause mortality.
Methods
This meta-analysis was designed, implemented, analyzed, and reported in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) protocol [22].
Search strategy
We systematically searched the databases PubMed, Embase, and Web of Science for prospective cohort studies published through May 31, 2016. The following keywords were used in the literature search: “magnesium” AND (“cardiovascular disease” OR “coronary heart disease” OR “myocardial infarction” OR “heart failure” OR “stroke” OR “cerebrovascular disease” OR “ischemic heart disease” OR “diabetes” OR “mortality” OR “death”) AND (“cohort” OR “prospective” OR “follow-up” OR “longitudinal” OR “population”). Our search was restricted to studies conducted in humans, and no restriction was imposed with respect to the language of the publications. The references cited within the retrieved relevant articles were also reviewed in order to identify additional studies.
Study selection
Studies that satisfied the following four criteria were included in our meta-analysis: (1) prospective study design; (2) the exposure of interest was dietary intake of magnesium; (3) the outcome was CVD (including coronary heart disease/ischemic heart disease, and/or stroke), type 2 diabetes, and/or all-cause mortality; and (4) the authors reported risk estimates with 95% confidence intervals (95% CI). We excluded reviews, meta-analyses, retrospective studies, and published letters that lacked sufficient data. To ensure the correct identification of eligible studies, we used a two-step selection process [23]. First, two independent investigators (authors XF and KW) conducted an initial screening of all titles and/or abstracts; the full text of each potentially relevant article was then evaluated.
Data extraction
Data were extracted using a standardized data collection form. Two investigators (authors XF and KW) independently extracted detailed information from each included article. Any discrepancies were resolved through group discussion with a third investigator (author FW). We extracted the following information: the first author of the publication, the year of publication, study location, study name (where applicable), the duration of follow-up, sex, sample size (the number of cases and/or participants), the method used to assess dietary intake (food-frequency questionnaire, 24-h recall, or other), the categories of magnesium intake, and the corresponding risk estimates with 95% CIs. We extracted the risk estimates with the most adjustment.
Quality assessment was performed in accordance with the Newcastle-Ottawa scale for non-randomized studies [24]. This scale assigns a maximum of 9 points to each study as follows: 4 points for the selection of participants and measurement of exposure, 2 points for comparability, and 3 points for assessment of outcomes and adequate follow-up. We regarded scores of 0–3, 4–6, and 7–9 as reflecting low, moderate, and high quality, respectively.
Statistical analysis
Relative risk (RR) with the 95% CI was used as the common measure of association across studies; the hazard ratio and incidence rate ratio were considered to approximate RR. Studies that stratified the data by sex and/or stroke subgroup were treated as two separate reports. We used a random-effects model to calculate the summarized RRs and their corresponding 95% CIs for comparison between the highest and lowest levels of magnesium intake [25].
Due to the relatively wide range of definitions for the exposure categories in the included articles, we performed a dose-response analysis based on a 100 mg/day increase in magnesium intake, using the method recommended by Greenland and Longnecker and the publicly available Stata code written by Orsini et al. [26, 27]. The categories of magnesium intake, distributions of cases and person-years, and RRs and 95% CIs were extracted. If the number of cases and/or person-years was not available, variance-weighted least squares regression was used to achieve the pooled risk estimate [28, 29]. If neither median nor mean values were reported, we used the categorical midpoint. If the highest or lowest category was open-ended, the midpoint of the category was estimated by assuming that the width of the category was the same as the next adjacent category. In addition, we evaluated the non-linear association between dietary magnesium intake and risk of outcomes using restricted cubic splines, with three knots at the 10th, 50th, and 90th percentiles of the distribution [30]. A P value for curve linearity or non-linearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to zero.
Heterogeneity among the studies was estimated using the I 2 statistic, with values of 25%, 50%, and 75% representing low, moderate, and high degrees of heterogeneity, respectively [31, 32]. To explore the significance of the difference in RRs and the possible influence of residual confounding factors, we performed subgroup analyses and a meta-regression analysis on possible sources of heterogeneity, including sex, geographic location, and stroke subtype [33].
Publication bias was evaluated using contour-enhanced funnel plots, Egger’s linear regression test, and Begg’s rank correlation test, with significance set to P < 0.10 [34–36]. All statistical analyses were performed using Stata version 12. Except where noted otherwise, differences with a P value < 0.05 were considered significant.
Results
Study characteristics
Figure 1 shows the study selection process and the results of our literature search. Using our search strategy (see Methods), we identified 1128 articles from PubMed, 2221 articles from Embase, and 2031 articles from Web of Science. After duplicates and studies that did not meet the inclusion criteria were excluded, 40 publications comprising 70 studies were ultimately included in our main analysis [20, 21, 37–74]. Because some studies contributed to more than one outcome, and because results stratified by sex and subtypes were treated separately, a total of 70 reports and/or datasets were included in the final meta-analysis. These prospective studies were published from 1999 through 2016, and the follow-up periods ranged from 4 to 30 years. Twenty-two studies were conducted in the United States, six in China, five in Japan, two in Sweden, and one each in the United Kingdom, Spain, Australia, Finland, and Germany. In total, we identified 7678 cases of CVD; 6845 cases of coronary heart disease (CHD); 701 cases of heart failure; 14,755 cases of stroke; 26,299 cases of T2D, and 10,983 cases of all-cause mortality. Dietary magnesium intake was assessed using a validated food frequency questionnaire in all studies except one. Study quality scores ranged from 7 to 9; the mean quality score was 8.2 (Additional file 1: Table S1). Detailed characteristics regarding the studies included in our analysis are summarized in Table 1.
Fig. 1.
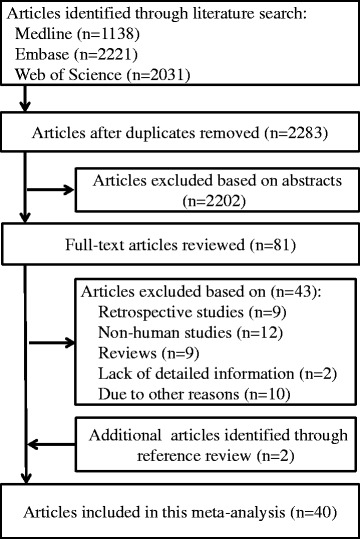
Flow-chart depicting the literature search and selection strategy
Table 1.
Characteristics of the included prospective cohort studies
| Author, year | Location | Study name | Sex | Age range (years) | Follow-up (years) | Cases (cohort size) | Dietary assessment | Quality score |
|---|---|---|---|---|---|---|---|---|
| Adebamowo et al., 2015 [20] | US | Nurses’ Health Study (NHS) | F | 30–55 in NHS I; 25–42 in NHS II | 30 in NHS I; 22 in NHS II | 3780 stroke cases (86,149 in NHS I; 94,715 in NHS II) | Validated FFQ | 8 |
| Adebamowo et al., 2015 [37] | US | Health Professionals Follow-up Study (HPFS) | M | 40–75 | 24 | 1547 stroke cases (42,669) | Validated FFQ | 9 |
| Al-Delaimy et al., 2004 [38] | US | HPFS | M | 40–75 | 12 | 1449 CHD cases (39,633) | Validated FFQ | 9 |
| Ascherio et al., 1998 [39] | US | HPFS | M | 40–75 | 8 | 328 stroke cases (43,738) | Validated FFQ | 9 |
| Bain et al., 2015 [40] | UK | European Prospective Investigation into Cancer (EPIC)-Norfolk cohort | M/F | 40–75 | 5.8 | 928 stroke cases (25,639) | 7-day dietary recall | 9 |
| Chiuve et al., 2011 [41] | US | NHS | F | 30–55 | 26 | 505 sudden cardiac deaths (88,375) | Validated FFQ | 9 |
| Chiuve et al., 2013 [42] | US | NHS | F | 30–55 | 28 | 3614 CHD cases (86,323) | Validated FFQ | 8 |
| Dai et al., 2013 [21] | China | Shanghai Women’s Health Study (SWHS) and Shanghai Men’s Health Study (SMHS) | M/F | 40–70 in SWHS; 40–74 in SMHS | NA | 6224 total deaths, 1947 CVD deaths, 906 CHD deaths, 1041 stroke deaths (136,442) | Validated FFQ | 9 |
| de Oliveira Otto et al., 2012 [43] | US | Multi-Ethnic Study of Atherosclerosis. Participants (MESA) | M/F | 45–84 | 6.2 | 279 CVD case, 399 T2D cases (6814) | Validated FFQ | 7 |
| Guasch-Ferré et al., 2014 [44] | Spain | Prevención con Dieta Mediterránea (PREDIMED) | M/F | 55–80 | 4.8 | 323 total deaths, 81 CVD deaths, 277 CVD cases (7216) | Validated FFQ | 7 |
| Hata et al., 2013 [45] | Japan | The Hisayama Study | M/F | 40–79 | 15.6 | 417 T2D cases (1999) | Validated FFQ | 9 |
| Hodge et al., 2004 [46] | Australia | Melbourne Collaborative Cohort Study | M/F | 40–69 | 4 | 365 T2D cases (31,641) | Validated FFQ | 8 |
| Hopping et al., 2010 [47] | Hawaii, US | Multiethnic Cohort (MEC) | M/F | 45–75 | 14 | 8587 T2D cases (75,512) | Validated FFQ | 8 |
| Hruby et al., 2014 [48] | US | Framingham Heart Study (FHS) Offspring cohort | M/F | 26–81 | 7 | 179 T2D cases (2582) | Validated FFQ | 7 |
| Huang et al., 2015 [49] | Taiwan, China | Nutrition and Health Survey in Taiwan | M/F | 65–97 | 9 | 475 total deaths, 124 CVD deaths, 231 diabetes cases (1400) | 24-h dietary recall and validated FFQ | 7 |
| Iso et al., 1999 [50] | US | NHS | F | 34–59 | 14 | 690 stroke cases (85,764) | Validated FFQ | 8 |
| Kaluza et al., 2010 [51] | Sweden | Cohort of Swedish Men | M | 45–79 | 10 | 2358 total deaths, 819 CVD deaths (23,366) | Validated FFQ | 8 |
| Kao et al., 1999 [52] | US | Atherosclerosis Risk in Communities (ARIC) Study | M/F | 45–64 | 6 | 1106 T2D cases (12,128) | 12-h dietary recall | 8 |
| Kim et al., 2010 [53] | US | Coronary Artery Risk Development in Young Adults Study | M/F | 18–30 | 20 | 330 diabetes cases (4497) | Validated FFQ | 9 |
| Kirii et al., 2010 [54] | Japan | Japan Collaborative Cohort Study (JACC) | M/F | 40–65 | 5 | 459 diabetes cases (11,592) | Validated FFQ | 7 |
| Konishi et al., 2015 [55] | Japan | The Takayama Study | M/F | >35 | 10 | 438 diabetes case (13,525) | Validated FFQ | 8 |
| Larsson et al., 2008 [56] | Finland | Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study | M | 50-69 | 13.6 | 2702 stroke cases (26,556) | Validated FFQ | 9 |
| Larsson et al., 2011 [57] | Sweden | Swedish Mammography Cohort | F | 49-83 | 10.4 | 1680 stroke cases (34,670) | Validated FFQ | 7 |
| Levitan et al., 2013 [58] | US | Women’s Health Initiative | F | 50-79 | 4.6 | 1433 total deaths (161,808) | Validated FFQ | 7 |
| Liao et al., 1998 [59] | US | ARIC | M/F | 45-64 | 4-7 | 319 CHD cases (13,922) | Validated FFQ | 8 |
| Lin et al., 2013 [60] | Taiwan, China | Cardiovascular Disease Risk Factor Two-township Study (CVDFACTS) | M/F | >18 | 12 | 123 stroke cases (2061) | Validated FFQ | 8 |
| Lopez-Ridaura et al., 2004 [61] | US | NHS and HPFS | M/F | 30-55 in NHS; 40-75 in HPFS | 18 in NHS; 12 in HPFS | 4085 T2D cases in NHS (85,060); 1333 T2D cases in HPFS (42,872) | Validated FFQ | 9 |
| Meyer et al., 2000 [62] | US | Iowa Women’s Health Study | F | 55-69 | 6 | 1141 T2D cases (35,988) | Validated FFQ | 9 |
| Nanri et al., 2010 [63] | Japan | Japan Public Health Center-based Prospective Study | M/F | 45-75 | 5 | 1114 T2D cases (59,791) | Validated FFQ | 8 |
| Ohira et al., 2009 [64] | US | ARIC | M/F | 45-64 | 15 | 577 ischemic stroke cases (14,221) | Validated FFQ | 9 |
| Schulze et al., 2007 [65] | Germany | EPIC-Potsdam study | M/F | 35-65 | 7 | 844 T2D cases (25,067) | Validated FFQ | 9 |
| Song et al., 2004 [66] | US | Women’s Health Study (WHS) | F | >45 | 6 | 918 T2D cases (39,345) | Validated FFQ | 9 |
| Song et al., 2005 [67] | US | WHS | F | >45 | 10 | 1037 CVD cases (39,876) | Validated FFQ | 9 |
| Tao et al., 2016 [68] | US | Western New York Exposures and Breast Cancer Study | F | 35-79 | 7.3 | 170 all-cause deaths (1170) | Validated FFQ | 8 |
| Taveira et al., 2016 [69] | US | Jackson Heart Study | M/F | 55-74 | 5 | 270 heart failure hospitalizations (4916) | Validated FFQ | 9 |
| van Dam et al., 2006 [70] | US | Black Women’s Health Study | F | 21-69 | 8 | 1964 T2D cases (41,186) | Validated FFQ | 9 |
| Villegas et al., 2009 [71] | China | Shanghai Women’s Health Study | F | 40-70 | 6.9 | 2270 T2D cases (64,191) | Validated FFQ | 9 |
| Weng et al., 2008 [72] | Taiwan, China | CVDFACTS | M/F | >40 | 10.6 | 132 ischemic stroke (1772) | Validated FFQ | 8 |
| Weng et al., 2012 [73] | Taiwan, China | CVDFACTS | M/F | >30 | 4.6 | 141 T2D cases (1604) | Validated FFQ | 7 |
| Zhang et al., 2012 [74] | Japan | JACC | M/F | 40-79 | 14.7 | 2690 CVD deaths, 1227 stroke deaths, 557 CHD deaths, 431 heart failure deaths (58,615) | Validated FFQ | 8 |
FFQ Food-frequency questionnaire, NA Not available, T2D type 2 diabetes, CVD cardiovascular disease, CHD coronary heart disease
Dietary magnesium intake and the risk of CVD
Ten independent reports from eight studies investigated the association between dietary magnesium intake and the risk of CVD. The pooled results suggest that magnesium intake is not significantly associated with CVD, which was suggested both by the highest category versus lowest category (RR: 0.95; 95% CI, 0.85–1.07) and by the per 100 mg/day increase (RR: 0.99, 95% CI, 0.88–1.10) (Fig. 2); in addition, we found evidence of between-study heterogeneity (I 2 = 45.7% and 69.7%, respectively). We also found no evidence of a non-linear association between dietary magnesium and CVD risk (P = 0.097 for non-linearity; see Fig. 6a).
Fig. 2.
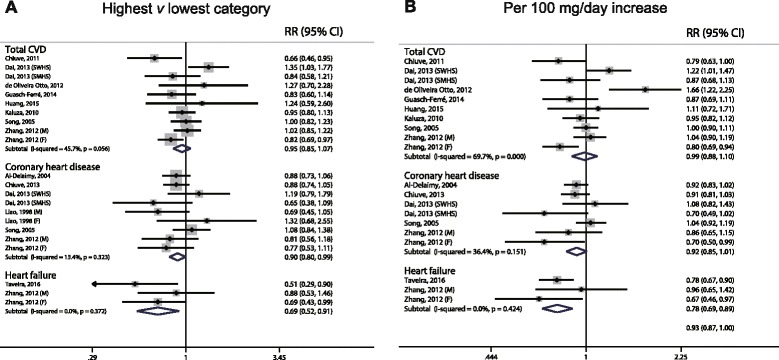
Forest plots of total cardiovascular disease, coronary heart disease, and heart failure for the highest versus lowest categories of dietary magnesium intake (a) and per 100 mg/day increase in dietary magnesium intake (b).
Fig. 6.
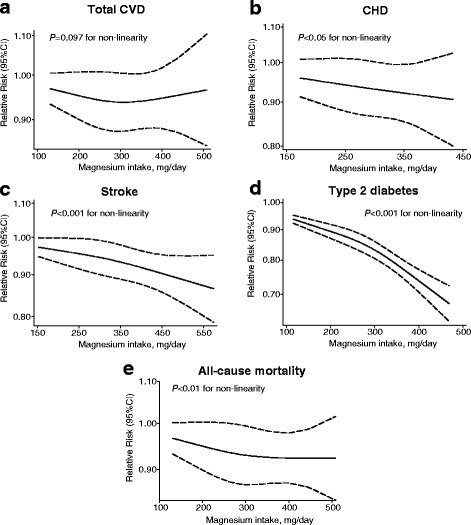
Dose–response analyses of the non-linear association between dietary magnesium intake and the risk of total cardiovascular disease (a), coronary heart disease (b), stroke (c), type 2 diabetes (d), and all-cause mortality (e)
Nine reports from six studies were included in our analysis of the association between magnesium intake and CHD. We found that the highest category of dietary magnesium was associated with a 10% lower risk of CHD compared to the lowest category of dietary magnesium (RR: 0.90; 95% CI, 0.80–0.99; Fig. 2), with low heterogeneity (I 2 = 13.4%). One study was not included in the dose–response analysis because it did not report specific data regarding the level of magnesium intake [59]. When we pooled the data from the remaining five studies, we found that a 100 mg/day increase in dietary magnesium intake was not significantly associated with CHD risk (RR: 0.92; 95% CI, 0.85–1.01; Fig. 2). However, a significant non-linear association was observed using the restricted cubic splines model (P < 0.05 for non-linearity; Fig. 6b).
At the time of our latest search, only three published datasets from two independent cohorts reported the association between magnesium intake and heart failure. We pooled these results and found strong inverse correlations for both the highest category versus lowest category (RR: 0.69; 95% CI, 0.52–0.91) and for a per 100 mg/day increase (RR: 0.78, 95% CI, 0.69–0.89), with no heterogeneity. Because of the limited number of datasets, non-linear association was not investigated in this study.
Dietary magnesium intake and the risk of stroke
Fourteen prospective cohort studies estimated the risk of total stroke between the highest and lowest levels of magnesium intake; these results suggest a significant inverse correlation (RR: 0.88; 95% CI, 0.82–0.95; Fig. 3), with low heterogeneity (I 2 = 22.2%). After we excluded one study that was ineligible due to a lack of detailed magnesium categories [60], the dose–response analysis revealed that for each 100 mg/day increase in magnesium intake, the risk of stroke decreased by 7% (RR: 0.93; 95% CI, 0.89–0.97; Fig. 3), with low heterogeneity (I 2 = 23.8%). Accordingly, we also found a significant non-linear association (P < 0.001 for non-linearity; Fig. 6C).
Fig. 3.
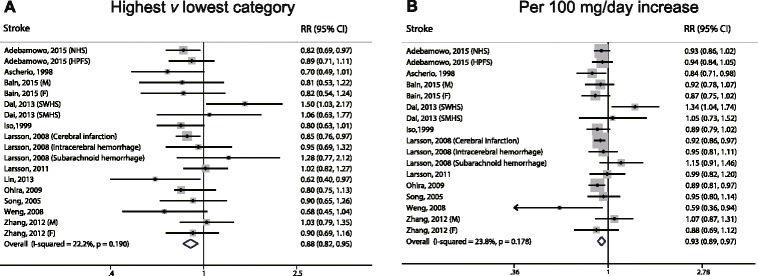
Forest plots of stroke for the highest versus lowest categories of dietary magnesium intake (a) and per 100 mg/day increase in dietary magnesium intake (b)
Dietary magnesium intake and the risk of type 2 diabetes
The multivariable-adjusted RRs of T2D are shown in Fig. 4. When we compared the highest category of magnesium intake with the lowest category of magnesium intake, the pooled RR of T2D was 0.74 (95% CI, 0.69–0.80), with moderate heterogeneity (I 2 = 49.8%). When we examined the risk associated with a 100 mg/day increase in magnesium intake, the pooled RR was 0.81 (95% CI, 0.77–0.86), with relatively high heterogeneity (I 2 = 60.3%). We also found a significant non-linear dose–response relationship between magnesium intake and T2D (P < 0.001 for non-linearity; Fig. 6d).
Fig. 4.
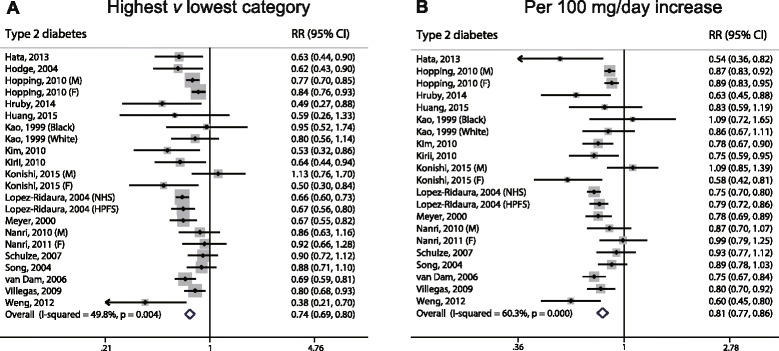
Forest plots of type 2 diabetes for the highest versus lowest categories of dietary magnesium intake (a) and per 100 mg/day increase of dietary magnesium intake (b)
Dietary magnesium intake and all-cause mortality
With respect to all-cause mortality, the association with dietary magnesium intake was not statistically significant between the highest and lowest intake categories (RR: 0.88; 95% CI, 0.76–1.01; Fig. 5), with moderately high heterogeneity (I 2 = 62.4%). However, a dose–response analysis revealed that each 100 mg/day increase in dietary magnesium intake was associated with a 10% lower risk of mortality (RR: 0.90; 95% CI, 0.81–0.99; Fig. 5), with moderately high heterogeneity (I 2 = 62.3%). A cubic spline model revealed an inverse non-linear correlation between dietary magnesium intake and the risk of all-cause mortality (P < 0.01 for non-linearity; Fig. 6e).
Fig. 5.
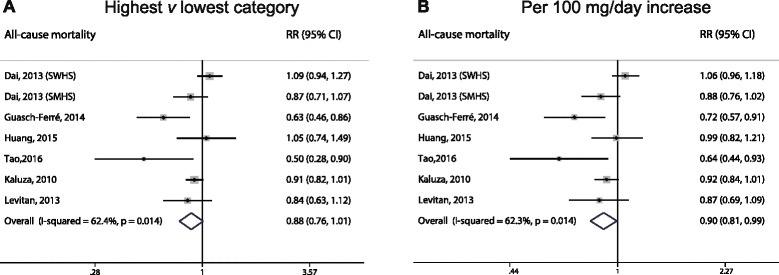
Forest plots of all-cause mortality for the highest versus lowest categories of dietary magnesium intake (a) and per 100 mg/day increase in dietary magnesium intake (b)
Subgroup analyses
Next, we performed subgroup analyses (with sex and study location as subgroups) in order to examine the stability of the primary results; these results are summarized in Table 2. The associations between dietary magnesium intake and the risks of CVD, T2D, and all-cause mortality were similar between our main analyses and our subgroup analyses, and meta-regression did not reveal any substantial change in the pooled relative risks. However, the inverse correlation remained significant only between increased magnesium intake and stroke incidence (RR: 0.92; 95% CI, 0.89–0.95), but not between increased magnesium intake and mortality (RR: 1.07; 95% CI, 0.90–1.28; P = 0.028 for meta-regression), and no study heterogeneity was observed (I 2 = 0%).
Table 2.
Subgroup analyses (per 100 mg/day increase)
| N | RR (95% CI) | I 2 (%) | P value | |
|---|---|---|---|---|
| Total cardiovascular disease | ||||
| Sex | ||||
| Male | 3 | 0.98 (0.89–1.08) | 0.0 | 0.959 |
| Female | 4 | 0.94 (0.73–1.13) | 79.9 | |
| Case | ||||
| Mortality | 8 | 0.93 (0.82–1.05) | 63.9 | 0.255 |
| Incidence | 3 | 1.10 (0.82–1.48) | 82.7 | |
| Location | ||||
| United States | 3 | 1.07 (0.78–1.48) | 86.2 | 0.606 |
| Europe | 2 | 0.93 (0.81–1.05) | 0.0 | |
| Asia | 5 | 0.99 (0.83–1.17) | 70.8 | |
| Coronary heart disease | ||||
| Sex | ||||
| Male | 3 | 0.89 (0.81–0.99) | 3.5 | 0.409 |
| Female | 4 | 0.95 (0.83–1.09) | 51.8 | |
| Case | ||||
| Mortality | 5 | 0.81 (0.69–0.95) | 36.3 | 0.105 |
| Incidence | 3 | 0.97 (0.90–1.05) | 12.7 | |
| Location | ||||
| United States | 3 | 0.95 (0.88–1.03) | 27.6 | 0.439 |
| Asia | 4 | 0.84 (0.68–1.03) | 42 | |
| Stroke | ||||
| Subtype | ||||
| Ischemic | 10 | 0.93 (0.88–0.98) | 28.9 | 0.285 |
| Hemorrhagic | 9 | 0.97 (0.88–1.07) | 29.0 | |
| Sex | ||||
| Male | 8 | 0.93 (0.89–0.98) | 0.9 | 0.884 |
| Female | 7 | 0.94 (0.87–1.02) | 37.2 | |
| Case | ||||
| Mortality | 4 | 1.07 (0.90–1.28) | 45.0 | 0.028 |
| Incidence | 13 | 0.92 (0.89–0.95) | 0.0 | |
| Location | ||||
| United states | 6 | 0.91 (0.87–0.95) | 0.0 | 0.140 |
| Europe | 6 | 0.93 (0.89–0.98) | 0.0 | |
| Asia | 5 | 1.00 (0.80–1.23) | 63.5 | |
| Type 2 diabetes | ||||
| Sex | ||||
| Male | 5 | 0.85 (0.79–0.93) | 48.3 | 0.381 |
| Female | 9 | 0.81 (0.75–0.87) | 65.8 | |
| Location | ||||
| United States | 11 | 0.81 (0.77–0.86) | 63.2 | 0.968 |
| Asia | 9 | 0.79 (0.69–0.90) | 62.4 | |
| All-cause mortality | ||||
| Sex | ||||
| Male | 2 | 0.91 (0.84–0.98) | 0.0 | 0.745 |
| Female | 3 | 0.88 (0.68–1.14) | 62.5 | |
| Location | ||||
| United States | 2 | 0.77 (0.58–1.04) | 46.9 | 0.134 |
| Europe | 2 | 0.83 (0.66–1.06) | 72.6 | |
| Asia | 3 | 0.98 (0.87–1.11) | 51.5 | |
RR relative risk, 95% CI 95 confidence interval (lower limit–upper limit)
Publication bias
Visual inspection of funnel plots revealed no significant publication bias (Additional file 1: Figure S1 and Figure S2). In addition, both Egger’s linear regression test and Begg’s rank correlation test revealed little evidence of publication bias with respect to magnesium intake in relation to the risk of CVD, CHD, stroke, diabetes, or all-cause mortality (Additional file 1: Table S2). The assessment of publication bias was based on the fully adjusted model.
Discussion
This systemic meta-analysis was based on 40 prospective cohort studies, with more than 1 million participants and 67,261 cases in nine countries. Thus, this meta-analysis provides the most up-to-date epidemiological evidence supporting the putative protective effect of magnesium intake against stroke, heart failure, diabetes, and all-cause mortality. A dose–response analysis revealed that a 100 mg/day increase in dietary magnesium intake is significantly associated with a 7%, 22%, 19%, and 10% decrease in the risk of stroke, heart failure, type 2 diabetes, and all-cause mortality, respectively. However, no clear association was found between magnesium intake and the risk of coronary heart disease or total cardiovascular disease, which may have been due – at least in part – to the relatively limited number of studies included in our analysis [75]. Further subgroup analyses did not reveal any significant effect of geographic location or sex on the respective correlations between magnesium intake and disease risk.
Magnesium plays an important role in maintaining human health. A typical adult contains a total of approximately 22–26 grams of magnesium, with 60% in skeletal tissue, and 39% and 1% located in intra- and extracellular regions, respectively [76]. Magnesium is essential to all living organisms, as it controls the function of many crucial enzymes, including those that utilize or synthesize ATP [77]. The recommended dietary allowance of magnesium is 350 mg/day for an average male adult and 300 mg/day for an average adult female, with an additional 150 mg/day during pregnancy and lactation [2]. However, despite these clearly established recommendations, magnesium deficiency remains a global public health problem. For example, diet surveys conducted in both Europe and the United States revealed that the daily intake of magnesium is generally lower than the recommended amounts [77]. Moreover, a major cause of magnesium deficiency can be attributed to an improperly balanced diet and/or impaired intestinal absorption [5, 78].
Compared to oral supplements and intravenous infusion, increasing magnesium intake through diet may only moderately increase one’s magnesium levels; however, increasing dietary magnesium intake is both safe and effective. For example, green leafy vegetables such as spinach provide magnesium through an abundance of chlorophyll molecules. Spices, nuts, beans, cocoa, and whole grains are also rich sources of magnesium [2]. Importantly, although these foods contain relatively high levels of magnesium, the daily requirement for magnesium is difficult to achieve through a single serving of any one food item. Therefore, consuming a wide variety of magnesium-rich foods will help ensure adequate daily intake of magnesium. Here, we focused our analysis on the association between dietary magnesium intake and the incidence of highly prevalent chronic diseases and all-cause mortality.
Larsson et al. [79] previously reported that dietary magnesium intake is inversely correlated with the risk of stroke, and many observational studies have confirmed that magnesium intake affects the risk of cardiovascular disease. However, these studies may have limited implications due to the lack of a non-linear analysis. In our study, we quantitatively investigated the associations between dietary magnesium intake and specific cardiovascular risks, including total CVD, CHD, heart failure, and stroke, by performing a dose–response meta-analysis. Notably, this is the first meta-analysis to investigate the effect of dietary magnesium intake on the risk of heart failure. Recently, Simental-Mendia et al. [80] systematically reviewed all published clinical trials and found beneficial effects of using oral magnesium supplements for 4 months or longer; specifically, insulin sensitivity and glucose control were improved in both diabetic and non-diabetic subjects [80]. Based on their analysis, we updated the most recent meta-analysis by Dong et al., who studied the effect of dietary magnesium on the risk of diabetes [19]. To the best of our knowledge, our study is the first quantitative meta-analysis to investigate the dose–response relationship between dietary magnesium intake and all-cause mortality.
Given the lack of large, randomized intervention trials to increase magnesium intake in order to prevent CVD and/or T2D, our comprehensive analysis of the most up-to-date prospective studies provides strong epidemiological evidence of the effect of dietary magnesium on CVD, T2D, and all-cause mortality. The strength of our meta-analysis lies in three aspects. First, we included all available prospective cohort studies with high quality scores, large sample sizes, and long-term follow-up data. According to the Newcastle-Ottawa scale, the average quality of the included studies was high. Second, the statistical power of our quantitative assessment was greatly increased by the large number of well-recorded cases. Finally, in addition to comparing the highest and lowest categories of magnesium intake, we also performed both linear and non-linear dose–response analyses.
On the other hand, our study has several limitations that warrant discussion. First, given the observational nature of the included studies, we cannot exclude the possibility of residual confounding, even in the fully adjusted models. Although a wide range of potential confounders, including demographics and lifestyle factors, were adjusted for in the primary studies, dietary factors may not have been considered to a sufficient degree. Therefore, we cannot exclude the possibility that other nutrients and/or dietary components correlated with dietary magnesium may have been responsible, either partially or entirely, for the observed associations. Moreover, the majority of dietary data were collected using a food frequency questionnaire; although such a questionnaire can adequately characterize dietary patterns, it can be limited in terms of describing the intake of individual nutrients. For example, Ward et al. [81] compared the data obtained from a food frequency questionnaire with diet diary data in a nested case-control study and found that food frequency questionnaires may not sufficiently capture heterogeneity within a single population; however, they can be appropriate in pooled analyses in which a wider range of intakes are collated [81]. Moreover, measurement error might occur in dietary assessment, which would likely bias true associations towards a null association [82]. In addition, some participants may have changed their diet but may not have updated their information during the follow-up period. A second limitation is that we may have overlooked some studies and/or missed unpublished reports, although every effort was made to contact authors in order to obtain unpublished risk estimates. Third, significant heterogeneity may exist with respect to our meta-analyses between magnesium intake and the relative risks of total CVD, diabetes, and/or all-cause mortality. Although we performed extensive subgroup analyses using meta-regression, the sources of heterogeneity remain unclear.
Conclusions
In conclusion, we observed significant inverse correlations between dietary magnesium intake and the risk of stroke, heart failure, diabetes, and all-cause mortality; in contrast, we found no correlation between dietary magnesium intake and the risk of coronary heart disease or total cardiovascular disease. Our findings underscore the notion that increasing the consumption of magnesium-rich foods may be beneficial to overall health. In the future, large prospective randomized controlled trials should help identify the putative causal role that magnesium plays in reducing the incidence of these diseases.
Acknowledgments
Funding
This study was supported by research grants from the National Natural Science Foundation of China (31530034, 31225013, and 31330036 to FW; 31570791 and 91542205 to JM; 31401016 to LZ) and the Zhejiang Provincial Natural Science Foundation (LZ15H160002 to JM).
Authors’ contributions
XF, JM, and FW contributed to the conception and design of the study. XF and KW searched the databases and checked the results in accordance with the inclusion and exclusion criteria. JW, XH, and LZ conducted the quality assessment of the included studies. XF analyzed the data and wrote the initial draft of the paper. MI, ZP, YL, and YX contributed to discussion. FW and JM reviewed and edited the manuscript. All authors contributed to the writing, reviewing, and revising of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable, as the study is a review of published research and no primary data was collected.
Additional file
Quality assessment and references of all included prospective cohort studies. Table S2. Publication bias measured by Begg’s and Egger’s test. Figure S1. Funnel plots for studies of the association between the highest vs. lowest category in dietary magnesium intake and risk of total CVD (A), CHD (B), stroke (C), type 2 diabetes (D), and all-cause mortality (E). Figure S2. Funnel plots for studies of the association between per 100 mg/day increase in dietary magnesium intake and risk of total CVD (A), CHD (B), stroke (C), type 2 diabetes (D), and all-cause mortality (E). (PDF 1552 kb)
Contributor Information
Xuexian Fang, Email: xuexianfang@zju.edu.cn.
Kai Wang, Email: wmzcmxxy@163.com.
Dan Han, Email: danhan@zju.edu.cn.
Xuyan He, Email: hxy900612@163.com.
Jiayu Wei, Email: weijiayu@zju.edu.cn.
Lu Zhao, Email: lzhao@zju.edu.cn.
Mustapha Umar Imam, Email: mustyimam@gmail.com.
Zhiguang Ping, Email: ping_zhg@163.com.
Yusheng Li, Email: yushengli1970@163.com.
Yuming Xu, Email: 1390371125@126.com.
Junxia Min, Email: junxiamin@zju.edu.cn.
Fudi Wang, Email: fwang@zju.edu.cn, Email: fudiwang.lab@gmail.com.
References
- 1.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95(1):1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 2.Wester PO. Magnesium. Am J Clin Nutr. 1987;45(5 Suppl):1305–12. doi: 10.1093/ajcn/45.5.1305. [DOI] [PubMed] [Google Scholar]
- 3.Belin RJ, He K. Magnesium physiology and pathogenic mechanisms that contribute to the development of the metabolic syndrome. Magnes Res. 2007;20(2):107–29. [PubMed] [Google Scholar]
- 4.Song Y, Liu S. Magnesium for cardiovascular health: time for intervention. Am J Clin Nutr. 2012;95(2):269–70. doi: 10.3945/ajcn.111.031104. [DOI] [PubMed] [Google Scholar]
- 5.Ayuk J, Gittoes NJ. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. 2014;51(Pt 2):179–88. doi: 10.1177/0004563213517628. [DOI] [PubMed] [Google Scholar]
- 6.Whang R, Ryder KW. Frequency of hypomagnesemia and hypermagnesemia. Requested vs routine. JAMA. 1990;263(22):3063–4. doi: 10.1001/jama.1990.03440220087036. [DOI] [PubMed] [Google Scholar]
- 7.Aliaga IL, Miller DL, Wilson HD, Schedl HP. Effects of resection on absorption and secretion of divalent cations by small intestine of rat. Am J Clin Nutr. 1990;52(5):867–71. doi: 10.1093/ajcn/52.5.867. [DOI] [PubMed] [Google Scholar]
- 8.Graham LA, Caesar JJ, Burgen AS. Gastrointestinal absorption and excretion of Mg 28 in man. Metabolism. 1960;9:646–59. [PubMed] [Google Scholar]
- 9.de Baaij JH, Hoenderop JG, Bindels RJ. Regulation of magnesium balance: lessons learned from human genetic disease. Clin Kidney J. 2012;5(Suppl 1):i15–24. [DOI] [PMC free article] [PubMed]
- 10.Peacock JM, Folsom AR, Arnett DK, Eckfeldt JH, Szklo M. Relationship of serum and dietary magnesium to incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 1999;9(3):159–65. doi: 10.1016/S1047-2797(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;133(9):2879–82. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt SP, Khandelwal P, Nanda S, Stoltzfus JC, Fioravanti GT. Serum magnesium is an independent predictor of frequent readmissions due to acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2008;102(7):999–1003. doi: 10.1016/j.rmed.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Sarrafzadegan N, Khosravi-Boroujeni H, Lotfizadeh M, Pourmogaddas A, Salehi-Abargouei A. Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrition. 2016;32(4):409–17. doi: 10.1016/j.nut.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Chambers EC, Heshka S, Gallagher D, Wang J, Pi-Sunyer FX, Pierson RN., Jr Serum magnesium and type-2 diabetes in African Americans and Hispanics: a New York cohort. J Am Coll Nutr. 2006;25(6):509–13. doi: 10.1080/07315724.2006.10719566. [DOI] [PubMed] [Google Scholar]
- 15.Cilliler AE, Ozturk S, Ozbakir S. Serum magnesium level and clinical deterioration in Alzheimer's disease. Gerontology. 2007;53(6):419–22. doi: 10.1159/000110873. [DOI] [PubMed] [Google Scholar]
- 16.Reffelmann T, Ittermann T, Dorr M, Volzke H, Reinthaler M, Petersmann A, Felix SB. Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis. 2011;219(1):280–4. doi: 10.1016/j.atherosclerosis.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 17.Qu X, Jin F, Hao Y, Li H, Tang T, Wang H, Yan W, Dai K. Magnesium and the risk of cardiovascular events: a meta-analysis of prospective cohort studies. PLoS One. 2013;8(3):e57720. doi: 10.1371/journal.pone.0057720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Gobbo LC, Imamura F, Wu JH, de Oliveira Otto MC, Chiuve SE, Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2013;98(1):160–73. doi: 10.3945/ajcn.112.053132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong JY, Xun P, He K, Qin LQ. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care. 2011;34(9):2116–22. doi: 10.2337/dc11-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adebamowo SN, Spiegelman D, Willett WC, Rexrode KM. Association between intakes of magnesium, potassium, and calcium and risk of stroke: 2 cohorts of US women and updated meta-analyses. Am J Clin Nutr. 2015;101(6):1269–77. doi: 10.3945/ajcn.114.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai Q, Shu XO, Deng X, Xiang YB, Li H, Yang G, Shrubsole MJ, Ji B, Cai H, Chow WH et al. Modifying effect of calcium/magnesium intake ratio and mortality: a population-based cohort study. BMJ Open. 2013;3(2). [DOI] [PMC free article] [PubMed]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose–response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–45. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 27.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata Journal. 2006;6(1):40–57. [Google Scholar]
- 28.Larsson SC, Giovannucci E, Wolk A. Folate and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2007;99(1):64–76. doi: 10.1093/jnci/djk006. [DOI] [PubMed] [Google Scholar]
- 29.Wang ZM, Zhou B, Wang YS, Gong QY, Wang QM, Yan JJ, Gao W, Wang LS. Black and green tea consumption and the risk of coronary artery disease: a meta-analysis. Am J Clin Nutr. 2011;93(3):506–15. doi: 10.3945/ajcn.110.005363. [DOI] [PubMed] [Google Scholar]
- 30.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80(15):1198–202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31(29):3805–20. doi: 10.1002/sim.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 34.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–6. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 37.Adebamowo SN, Spiegelman D, Flint AJ, Willett WC, Rexrode KM. Intakes of magnesium, potassium, and calcium and the risk of stroke among men. Int J Stroke. 2015;10(7):1093–100. doi: 10.1111/ijs.12516. [DOI] [PubMed] [Google Scholar]
- 38.Al-Delaimy WK, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Magnesium intake and risk of coronary heart disease among men. J Am Coll Nutr. 2004;23(1):63–70. doi: 10.1080/07315724.2004.10719344. [DOI] [PubMed] [Google Scholar]
- 39.Ascherio A, Rimm EB, Hernan MA, Giovannucci EL, Kawachi I, Stampfer MJ, Willett WC. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation. 1998;98(12):1198–204. doi: 10.1161/01.CIR.98.12.1198. [DOI] [PubMed] [Google Scholar]
- 40.Bain LK, Myint PK, Jennings A, Lentjes MA, Luben RN, Khaw KT, Wareham NJ, Welch AA. The relationship between dietary magnesium intake, stroke and its major risk factors, blood pressure and cholesterol, in the EPIC-Norfolk cohort. Int J Cardiol. 2015;196:108–14. doi: 10.1016/j.ijcard.2015.05.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiuve SE, Korngold EC, Januzzi JL, Jr, Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr. 2011;93(2):253–60. doi: 10.3945/ajcn.110.002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiuve SE, Sun Q, Curhan GC, Taylor EN, Spiegelman D, Willett WC, Manson JE, Rexrode KM, Albert CM. Dietary and plasma magnesium and risk of coronary heart disease among women. J Am Heart Assoc. 2013;2(2):e000114. doi: 10.1161/JAHA.113.000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Oliveira Otto MC, Alonso A, Lee DH, Delclos GL, Bertoni AG, Jiang R, Lima JA, Symanski E, Jacobs DR, Jr, Nettleton JA. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr. 2012;142(3):526–33. doi: 10.3945/jn.111.149781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guasch-Ferre M, Bullo M, Estruch R, Corella D, Martinez-Gonzalez MA, Ros E, Covas M, Aros F, Gomez-Gracia E, Fiol M, et al. Dietary magnesium intake is inversely associated with mortality in adults at high cardiovascular disease risk. J Nutr. 2014;144(1):55–60. doi: 10.3945/jn.113.183012. [DOI] [PubMed] [Google Scholar]
- 45.Hata A, Doi Y, Ninomiya T, Mukai N, Hirakawa Y, Hata J, Ozawa M, Uchida K, Shirota T, Kitazono T, et al. Magnesium intake decreases Type 2 diabetes risk through the improvement of insulin resistance and inflammation: the Hisayama Study. Diabet Med. 2013;30(12):1487–94. doi: 10.1111/dme.12250. [DOI] [PubMed] [Google Scholar]
- 46.Hodge AM, English DR, O'Dea K, Giles GG. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27(11):2701–6. doi: 10.2337/diacare.27.11.2701. [DOI] [PubMed] [Google Scholar]
- 47.Hopping BN, Erber E, Grandinetti A, Verheus M, Kolonel LN, Maskarinec G. Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in Hawaii. J Nutr. 2010;140(1):68–74. doi: 10.3945/jn.109.112441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hruby A, Meigs JB, O'Donnell CJ, Jacques PF, McKeown NM. Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle-aged americans. Diabetes Care. 2014;37(2):419–27. doi: 10.2337/dc13-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang YC, Wahlqvist ML, Kao MD, Wang JL, Lee MS. Optimal Dietary and Plasma Magnesium Statuses Depend on Dietary Quality for a Reduction in the Risk of All-Cause Mortality in Older Adults. Nutrients. 2015;7(7):5664–83. doi: 10.3390/nu7075244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iso H, Stampfer MJ, Manson JE, Rexrode K, Hennekens CH, Colditz GA, Speizer FE, Willett WC. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke. 1999;30(9):1772–9. doi: 10.1161/01.STR.30.9.1772. [DOI] [PubMed] [Google Scholar]
- 51.Kaluza J, Orsini N, Levitan EB, Brzozowska A, Roszkowski W, Wolk A. Dietary calcium and magnesium intake and mortality: a prospective study of men. Am J Epidemiol. 2010;171(7):801–7. doi: 10.1093/aje/kwp467. [DOI] [PubMed] [Google Scholar]
- 52.Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, Brancati FL. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the Atherosclerosis Risk in Communities Study. Arch Intern Med. 1999;159(18):2151–9. doi: 10.1001/archinte.159.18.2151. [DOI] [PubMed] [Google Scholar]
- 53.Kim DJ, Xun P, Liu K, Loria C, Yokota K, Jacobs DR, Jr, He K. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care. 2010;33(12):2604–10. doi: 10.2337/dc10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirii K, Iso H, Date C, Fukui M, Tamakoshi A, Group JS. Magnesium intake and risk of self-reported type 2 diabetes among Japanese. J Am Coll Nutr. 2010;29(2):99–106. doi: 10.1080/07315724.2010.10719822. [DOI] [PubMed] [Google Scholar]
- 55.Konishi K, Wada K, Tamura T, Tsuji M, Kawachi T, Nagata C. Dietary magnesium intake and the risk of diabetes in the Japanese community: results from the Takayama study. Eur J Nutr. 2015. [DOI] [PubMed]
- 56.Larsson SC, Virtanen MJ, Mars M, Mannisto S, Pietinen P, Albanes D, Virtamo J. Magnesium, calcium, potassium, and sodium intakes and risk of stroke in male smokers. Arch Intern Med. 2008;168(5):459–65. doi: 10.1001/archinte.168.5.459. [DOI] [PubMed] [Google Scholar]
- 57.Larsson SC, Virtamo J, Wolk A. Potassium, calcium, and magnesium intakes and risk of stroke in women. Am J Epidemiol. 2011;174(1):35–43. doi: 10.1093/aje/kwr051. [DOI] [PubMed] [Google Scholar]
- 58.Levitan EB, Shikany JM, Ahmed A, Snetselaar LG, Martin LW, Curb JD, Lewis CE. Calcium, magnesium and potassium intake and mortality in women with heart failure: the Women's Health Initiative. Br J Nutr. 2013;110(1):179–85. doi: 10.1017/S0007114512004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 1998;136(3):480–90. doi: 10.1016/S0002-8703(98)70224-8. [DOI] [PubMed] [Google Scholar]
- 60.Lin PH, Yeh WT, Svetkey LP, Chuang SY, Chang YC, Wang C, Pan WH. Dietary intakes consistent with the DASH dietary pattern reduce blood pressure increase with age and risk for stroke in a Chinese population. Asia Pac J Clin Nutr. 2013;22(3):482–91. [PubMed] [Google Scholar]
- 61.Lopez-Ridaura R, Willett WC, Rimm EB, Liu S, Stampfer MJ, Manson JE, Hu FB. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care. 2004;27(1):134–40. doi: 10.2337/diacare.27.1.134. [DOI] [PubMed] [Google Scholar]
- 62.Meyer KA, Kushi LH, Jacobs DR, Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr. 2000;71(4):921–30. doi: 10.1093/ajcn/71.4.921. [DOI] [PubMed] [Google Scholar]
- 63.Nanri A, Mizoue T, Noda M, Takahashi Y, Kirii K, Inoue M, Tsugane S, Japan Public Health Center-based Prospective Study G Magnesium intake and type II diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Eur J Clin Nutr. 2010;64(10):1244–7. doi: 10.1038/ejcn.2010.138. [DOI] [PubMed] [Google Scholar]
- 64.Ohira T, Peacock JM, Iso H, Chambless LE, Rosamond WD, Folsom AR. Serum and dietary magnesium and risk of ischemic stroke: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2009;169(12):1437–44. doi: 10.1093/aje/kwp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med. 2007;167(9):956–65. doi: 10.1001/archinte.167.9.956. [DOI] [PubMed] [Google Scholar]
- 66.Song Y, Manson JE, Buring JE, Liu S. Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care. 2004;27(1):59–65. doi: 10.2337/diacare.27.1.59. [DOI] [PubMed] [Google Scholar]
- 67.Song Y, Manson JE, Cook NR, Albert CM, Buring JE, Liu S. Dietary magnesium intake and risk of cardiovascular disease among women. Am J Cardiol. 2005;96(8):1135–41. doi: 10.1016/j.amjcard.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 68.Tao MH, Dai Q, Millen AE, Nie J, Edge SB, Trevisan M, Shields PG, Freudenheim JL. Associations of intakes of magnesium and calcium and survival among women with breast cancer: results from Western New York Exposures and Breast Cancer (WEB) Study. Am J Cancer Res. 2016;6(1):105–13. [PMC free article] [PubMed] [Google Scholar]
- 69.Taveira TH, Ouellette D, Gulum A, Choudhary G, Eaton CB, Liu S, Wu WC. Relation of Magnesium Intake With Cardiac Function and Heart Failure Hospitalizations in Black Adults: The Jackson Heart Study. Circ Heart Fail. 2016;9(4). [DOI] [PMC free article] [PubMed]
- 70.van Dam RM, Hu FB, Rosenberg L, Krishnan S, Palmer JR. Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care. 2006;29(10):2238–43. doi: 10.2337/dc06-1014. [DOI] [PubMed] [Google Scholar]
- 71.Villegas R, Gao YT, Dai Q, Yang G, Cai H, Li H, Zheng W, Shu XO. Dietary calcium and magnesium intakes and the risk of type 2 diabetes: the Shanghai Women's Health Study. Am J Clin Nutr. 2009;89(4):1059–67. doi: 10.3945/ajcn.2008.27182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weng LC, Yeh WT, Bai CH, Chen HJ, Chuang SY, Chang HY, Lin BF, Chen KJ, Pan WH. Is ischemic stroke risk related to folate status or other nutrients correlated with folate intake? Stroke. 2008;39(12):3152–8. doi: 10.1161/STROKEAHA.108.524934. [DOI] [PubMed] [Google Scholar]
- 73.Weng LC, Lee NJ, Yeh WT, Ho LT, Pan WH. Lower intake of magnesium and dietary fiber increases the incidence of type 2 diabetes in Taiwanese. J Formos Med Assoc. 2012;111(11):651–9. doi: 10.1016/j.jfma.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 74.Zhang W, Iso H, Ohira T, Date C, Tamakoshi A, Group JS. Associations of dietary magnesium intake with mortality from cardiovascular disease: the JACC study. Atherosclerosis. 2012;221(2):587–95. doi: 10.1016/j.atherosclerosis.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 75.Xu T, Sun Y, Xu T, Zhang Y. Magnesium intake and cardiovascular disease mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;167(6):3044–7. doi: 10.1016/j.ijcard.2012.11.090. [DOI] [PubMed] [Google Scholar]
- 76.Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294(1–2):1–26. doi: 10.1016/S0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 77.Grober U, Schmidt J, Kisters K. Magnesium in Prevention and Therapy. Nutrients. 2015;7(9):8199–226. doi: 10.3390/nu7095388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liebscher DH, Liebscher DE. About the misdiagnosis of magnesium deficiency. J Am Coll Nutr. 2004;23(6):730S–1S. doi: 10.1080/07315724.2004.10719416. [DOI] [PubMed] [Google Scholar]
- 79.Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. Am J Clin Nutr. 2012;95(2):362–6. doi: 10.3945/ajcn.111.022376. [DOI] [PubMed] [Google Scholar]
- 80.Simental-Mendia LE, Sahebkar A, Rodriguez-Moran M, Guerrero-Romero F. A systematic review and meta-analysis of randomized controlled trials on the effects of magnesium supplementation on insulin sensitivity and glucose control. Pharmacol Res. 2016;111:272–82. doi: 10.1016/j.phrs.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 81.Ward HA, Keogh R, Lentjes M, Luben RN, Wareham NJ, Khaw KT. Fibre intake in relation to serum total cholesterol levels and CHD risk: a comparison of dietary assessment methods. Eur J Clin Nutr. 2012;66(3):296–304. doi: 10.1038/ejcn.2011.184. [DOI] [PubMed] [Google Scholar]
- 82.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–67. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]


