Abstract
Major depressive disorder (MDD) and other mood disorders remain difficult to effectively treat, and innovative interventions and therapeutic targets are needed. Psychological stressors and inappropriate inflammation increase the risk and severity of mood disorders; however, only recently have the importance of sterile inflammatory processes in this effect been revealed. This review will introduce the reader to pathogen vs sterile inflammation, inflammatory receptor–ligand interactions, microbial-associated molecular patterns (MAMPs), pathogen-associated molecular patterns (PAMPs), danger-associated molecular patterns (DAMPs), and the more recent discovery of the role of the inflammasome in peripheral and central nervous system cytokine/chemokine inflammatory responses. The review will focus on current preclinical and clinical evidence that sterile inflammation and inflammasome-dependent signaling may contribute to mood disorders. By understanding these inflammatory signaling processes, new approaches for quieting chronic or inappropriate inflammatory states may be revealed and this could serve as novel pharmacological targets for the treatment of mood disorders.
INTRODUCTION
Inflammatory states in the body and/or central nervous system (CNS) impact a variety of brain functions including mood (Miller and Raison, 2015). There is strong evidence that patients with major depressive disorder (MDD), for example, have elevated levels of inflammatory proteins in the blood, and many inflammatory diseases are associated with increased rates of MDD (Howren et al, 2009; Schiepers et al, 2005). In addition, repeated or severe stressor exposure, in the absence of pathogenic disease, increases affective dysregulation and inflammatory proteins in tissues and blood in humans (Bierhaus et al, 2003; Pace et al, 2006) and animals (Maslanik et al, 2012a, b). The stress-evoked cytokine/chemokine response is an example of sterile inflammation (Fleshner, 2013). Although immunologists have long recognized the adaptive nature of this local tissue response after injury, it has only recently been appreciated that a psychological or systemic stressor in the absence of overt tissue damage can also trigger systemic and CNS sterile inflammation and that in some contexts this can be detrimental to physical and mental health.
This review will introduce the reader to pathogen vs sterile inflammation, inflammatory receptor–ligand interactions, microbial-associated molecular patterns (MAMPs), pathogen-associated molecular patterns (PAMPs), danger-associated molecular patterns (DAMPs), and the more recent discovery of the role of the inflammasome in body and CNS cytokine/chemokine inflammatory responses. The review will focus on current preclinical and clinical evidence that sterile inflammation and inflammasome-dependent signaling may contribute to mood changes. By understanding these inflammatory signaling processes, new approaches for quieting chronic inflammatory states may be revealed, and could serve as novel pharmacological targets for treating mood disorders.
Clinical Evidence Linking Inflammation and Stress-Related Mood Disorders
Compelling clinical research has established that repeated, chronic and excessive stressor exposure increases inflammatory state and is associated with increased risk and severity of a variety of mood disorders (for reviews see Kiecolt-Glaser et al, 2010, 2015; Segerstrom and Miller, 2004; Slavich and Irwin, 2014). For example, exposure to repeated life stressors or laboratory stressors involving social conflict, threat, isolation, and rejection increases inflammatory markers including C-reactive protein (CRP), interleukin-6 (IL-6), interleukin-1β (IL-1β) and soluble tumor necrosis factor-α receptor (TNFsr) in the blood (Aschbacher et al, 2012; Denson et al, 2009; Dickerson et al, 2009; Steptoe et al, 2007) and NF-κB mRNA (a transcription factor for inflammatory proteins) in leukocytes (Murphy et al, 2013; Pace et al, 2012). This elevated inflammatory state has also been associated with increased risk and severity of mood disorders including depression (Aschbacher et al, 2012; Danese et al, 2008; Pace et al, 2012).
An acute excessive stressor or a single major life event can also increase inflammatory markers. For example, Schultze-Florey et al (2012) reported that the death of a spouse increased IL-1β and IL-6 activity in older adults. Interestingly, increases in inflammatory markers following exposure to an acute traumatic event may be predictive of the development, symptom severity, and duration of mood disorders such as depression, anxiety, and post-traumatic stress disorder (PTSD; for a review see Felger et al, 2016). Pervanidou et al (2007), for example, reported that children with higher concentrations of plasma IL-6 following a motor vehicle accident had a greater chance of developing PTSD compared with children who did not have elevated IL-6 after the accident or controls. Furthermore, Michopoulos et al (2015) and Heath et al (2013) reported that CRP concentration was correlated with PTSD symptom severity and duration, such that PTSD patients with high, compared with low, CRP had greater symptom severity and duration (Heath et al, 2013; Michopoulos et al, 2015). There is, therefore, a growing body of clinical studies establishing the association between stressor exposure, inflammation, and mood disorders. This review will focus on exciting advances in our understanding of the nature of signals and cellular sources responsible for stress-evoked increases in inflammatory state and will reveal novel future therapeutic targets to reduce stress-evoked elevation of inflammation and mood disorders.
Pathogen vs Sterile Inflammation
Innate immune cells are the primary effectors of the inflammatory response, although adaptive immune cells such as Th1 cells are important collaborators. These innate immune cells are found throughout the body and CNS and respond to pathogenic challenges, cellular stress, tissue damage, and tissue repair. Receptor–ligand recognition schema is an important primary distinguishing characteristic between adaptive and innate immune cells. Cells of the innate immune system use germline-encoded receptors designed to recognize conserved molecular patterns and have been coined pattern recognition receptors (PRRs). The nature of the molecular patterns capable of binding PRRs is vast and include those commonly associated with microbes in general (MAMPs). MAMPs encompass both pathogenic and commensal/symbiotic microbes. Those patterns associated with microbes that are typically pathogenic to the host have been coined PAMPs. Examples of MAMPs and PAMPs include lipopolysaccharide (LPS, a membrane-integrated component of many Gram-negative pathogenic bacteria) or CpG DNA (a common viral motif). Importantly, a robust inflammatory response is the consequence of PRR-PAMP binding.
Ligands associated with cellular stress and tissue damage have been coined DAMPs. In contrast to PAMPs and MAMPs, which are associated with microbes and viruses, DAMPs are endogenous molecules derived from self and are increased after cellular stress and tissue damage. In most instances these self-molecules only function as DAMPS when they are in the extracellular environment because of stress-evoked release and/or necrotic cell death. The consequence of PRR-DAMP binding is sterile inflammation (Kono and Rock, 2008; Rock et al, 2010). Newly identified potential DAMPs are rapidly emerging in the literature, including extracellular heat-shock protein 72 (Hsp72), uric acid crystals, high-mobility group box 1 (HMGB1), and adenosine triphosphate (ATP), discussed below.
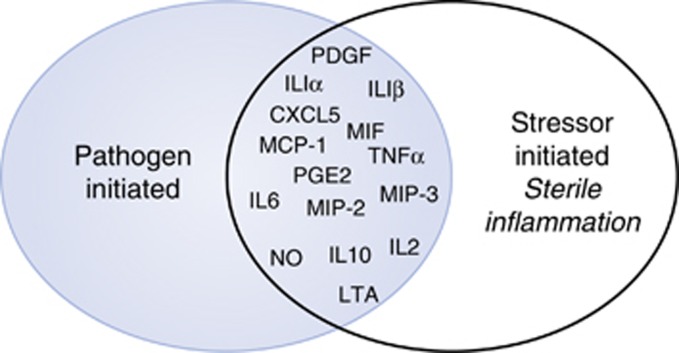
There is considerable overlap in a broad number of inflammatory proteins that are modulated after either LPS (PAMP) or stressor exposure (DAMP) and that are detectable in the blood and peripheral tissues (ie, adipose, liver, spleen, lymph nodes). Figure 1 depicts the overlap of cytokines, chemokines, and anti-microbials (mRNA or protein) after either LPS (Campisi et al, 2003b; He et al, 2014) or a well-established acute stressor (100, 5-s, inescapable tailshocks (Maslanik et al, 2012a, b)). Brain tissue and microglia also respond to PAMPs and DAMPs and increase inflammatory proteins as described below.
Figure 1.
Substantial overlap between pathogen-associated molecular pattern (PAMP) and stress-evoked, likely danger-associated molecular pattern (DAMP), inflammatory protein changes. This schematic is based on results from a series of studies measuring inflammatory proteins in blood and peripheral tissues (spleen, liver) using multiplex enzyme-linked immunosorbent assays (ELISAs) and mRNA using quantitative real-time PCR (qRT-PCR) after lipopolysaccharide (LPS) (PAMP) or tailshock.
Stress, DAMPs, and Sterile Inflammation
Local sterile inflammation after tissue injury and necrotic cell death has an important role in tissue repair (Rock et al, 2011), whereas systemic sterile inflammation or the cytokine storm triggered after major trauma can be lethal (Schneider et al, 2011). Recently, it has been reported that psychological and/or acute intense stressor exposure in the absence of overt tissue damage can evoke a detectable local and systemic sterile inflammatory response and that DAMPs may have a role (Fleshner, 2013; Frank et al, 2015b; Maslanik et al, 2013; Miller et al, 2015).
Fleshner and co-workers reported in a series of studies that rats exposed to uncontrollable tailshock (Campisi and Fleshner, 2003a; Campisi et al, 2012; Maslanik et al, 2013), a predatory cat with no physical contact (Fleshner et al, 2004), or predatory ferret odor (Figure 2), had increased blood levels of several DAMPs (ie, Hsp72, uric scid crystals) and little evidence of cell death (ie, low levels of LDH). It appears therefore that stressor exposure may stimulate DAMP release that is not dependent on necrotic cell death. In support of this idea is the observation that the release of Hsp72 (a possible DAMP) after tailshock is dependent on α-adrenergic, but not β- or glucocorticoid, receptor signaling (Johnson et al, 2005; Johnson and Fleshner, 2006). Tailshock also increases tissue and blood concentrations of many cytokines and chemokines, and both MAMP (derived from the gut bacteria) and DAMP signals are involved (Maslanik et al, 2012b, 2013). Importantly, in this series of studies it was also discovered that some features of the sterile inflammatory response after stressor exposure are dependent on the inflammasome, especially nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain protein 3 (NLRP3) (Maslanik et al, 2013).
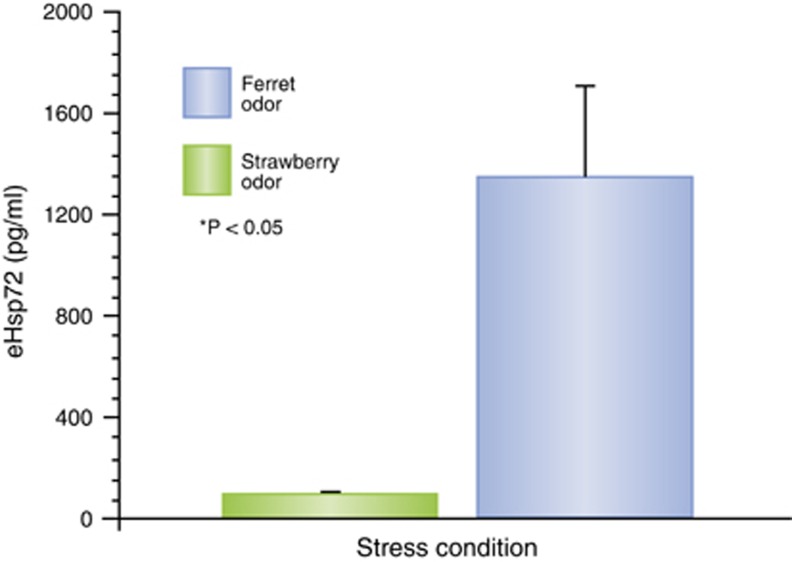
Figure 2.
The previously unpublished results of a study testing the effect of a psychological stressor, predatory order, on danger-associated molecular pattern (DAMPs) in the blood. Adult, male Sprague–Dawley rats (8 per group) had towels placed in their home cages that were saturated either with neutral control odor (strawberry) or a predatory odor (ferret urine) 10 min per day for 7 days. Serum levels of heat-shock protein 72 (Hsp72) were measured using enzyme-linked immunosorbent assay (ELISA).
Inflammasomes are intracellular multiprotein complexes that function as sensors of DAMPs or PAMPs, which leads to the activation of proinflammatory caspases, and the cleavage and release of proinflammatory cytokines (Walsh et al, 2014). Several distinct inflammasomes have been characterized, which generally consist of a cytosolic sensor (eg, a nucleotide binding domain), an adaptor protein (eg, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC)), and an effector caspase, typically caspase-1. Our focus here will be on the NLRP3 inflammasome as it has received the most attention and has an important role in peripheral and CNS inflammatory states stimulated by MAMPs, PAMPs, and/or DAMPs.
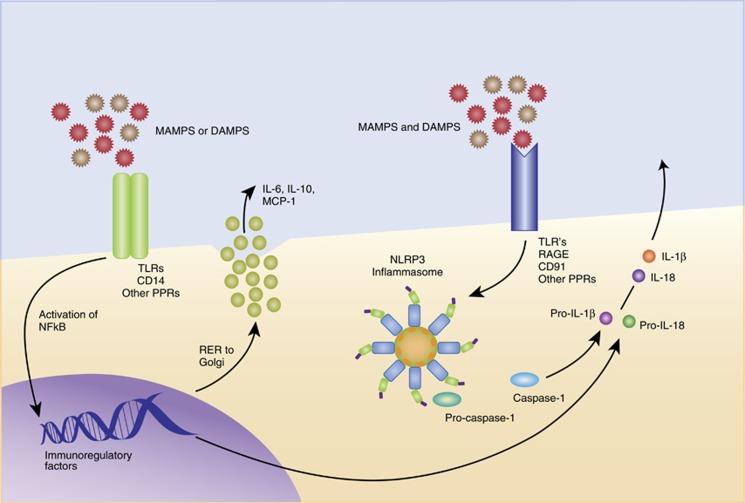
Unlike the activation of other inflammasomes, NLRP3 inflammasome formation and activation requires a two-step process. Figure 3 depicts both single signal (inflammasome-independent) and double signal (inflammasome-dependent) sterile inflammation. For cytokines that are inflammasome independent, the process begins with NF-κB activation after MAMPs or DAMPs binding to TLR-4, CD14, and other potential PRRs. This binding stimulates inflammatory protein gene transcription, translation, protein synthesis, and release. In contrast, inflammasome-dependent cytokine synthesis and release is a two-step process that is initiated after ligation of TLRs and other receptors capable of binding DAMPS and/or MAMPs leading to NLRP3 gene transcription, translation, and protein production (Duewell et al, 2010). Once sufficient NLRP3 protein has been formed and the cell is ‘primed', a second activation signal triggers NLRP3 to interact with the adaptor protein ASC, which then recruits procaspase-1 through its caspase recruitment domain. This assembly of proteins is considered the inflammasome, and once formed triggers the activation/cleavage of procaspase 1. Mature, active caspase-1 then acts to cleave pro-IL-1β into mature IL-1β (and IL-18) protein. The NLRP3 inflammasome is also unusual in that it responds to a very broad range of signals as the second event that produces inflammasome formation/activation (eg, ATP, K+ efflux, β-amyloid, silica, uric acid crystals, ROS; (Elliott and Sutterwala, 2015). In addition, there are several candidate receptors capable of binding DAMPs and MAMPs, including TLRs, RAGE, and CD91 (Asea et al, 2002; Calderwood et al, 2007a, b). This scenario has led to the suggestion that the NLRP3 inflammasome is ‘a common response mechanism to perturbations of homeostasis in the broadest sense' (Bauernfeind et al, 2012).
Figure 3.
Both single signal (inflammasome-independent) and double signal (inflammasome-dependent) sterile inflammation. Inflammasome-dependent cytokine synthesis and release is a two-step process that is initiated after ligation of Toll-like receptor (TLRs) and other receptors capable of binding danger-associated molecular patterns (DAMPs) and/or microbial-associated molecular patterns (MAMPs) leading to NLRP3 (nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain protein 3) gene transcription, translation, and protein production (Duewell et al, 2010). Once sufficient NLRP3 protein has been formed, then a second activation signal is needed that leads NLRP3 to interact with the adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), which then recruits procaspase-1 through its caspase recruitment domain. This assembly of proteins is considered the inflammasome, and once formed triggers the activation/cleavage of procaspase 1. Mature, active caspase-1 then acts to cleave pro-interleukin-1β (IL-1β) into mature IL-1β (and IL-18) protein. The NLRP3 inflammasome responds to a broad range of signals, for example, ATP, K+ efflux, β-amyloid, silica, uric acid crystals, and reactive oxygen species (ROS) (Elliott and Sutterwala, 2015). In addition, there are several candidate receptors capable of binding DAMPs and MAMPs, including TLRs, RAGE, and CD91 (Asea et al, 2002; Calderwood et al, 2007a, b). This figure was adapted from a figure courtesy of Tom Maslanik, PhD/MBA.
The NLRP3 inflammasome has been implicated in a diverse array of sterile inflammatory diseases including ischemia–reperfusion injury, autoinflammatory diseases, type 2 diabetes, gout and pseudogout, obesity, atherosclerosis, and Alzheimer's disease (Leemans et al, 2011), and sterile inflammatory diseases such as these also have a high comorbidity with MDDs (Katon, 2011). In addition, there is evidence of activation of the NLRP3 inflammasome in patients with MDD (Alcocer-Gomez and Cordero, 2014a; Drago et al, 2015). Specifically, patients with depression had increased expression of NLRP3 and caspase-1 in peripheral blood mononuclear cells (Alcocer-Gomez et al (2014b)). Thus, sterile inflammation mediated by DAMPs and the inflammasome and evoked after exposure to psychological stressors may have a role in the pathogenesis of MDDs and other psychopathologies (Alcocer-Gomez and Cordero, 2014a; Kessler, 1997; Miller et al, 2015).
Of note, many of these peripheral inflammatory processes have also been characterized in the CNS of animals exposed to stress or animal models of depression, which we address in the following sections. It is important to note that peripheral inflammatory processes are capable of inducing neuroinflammatory processes through several well-characterized immune-to-brain signaling pathways (for a review see Capuron and Miller, 2011), which include humoral as well as neural pathways. For example, the humoral pathway may involve blood-borne cytokines inducing neuroinflammatory processes in the brain through active cytokine transport across the BBB, entry into the brain at circumventricular organs, in which the BBB is absent or weak, or binding of cognate receptors on brain endothelial cells, which leads to transduction of cytokine signaling into the brain. Alternatively, cytokines as well as PAMPs (eg, LPS) are capable of activating afferent vagal nerve fibers in the periphery, which leads to activation of neural pathways in brain regions implicated in motivation and mood (McCusker and Kelley, 2013). Once a peripheral inflammatory stimulus signals the brain, microglia mediate, in large part, the neuroinflammatory response to that stimulus. Accordingly, the following sections will focus on this pivotal innate immune effector cell of the CNS.
Microglia and Neuroinflammation
Resident microglial cells largely mediate innate immunity in the CNS. Microglia are mononuclear phagocytes that occupy the brain parenchyma and are ontogenetically distinct from other CNS mononuclear phagocytes including meningeal, choroid plexus, and perivascular macrophages, which reside outside the brain parenchyma (Katsumoto et al, 2014). It is important to note that these macrophage subtypes also serve a critical role in the brain's innate immune response and may contribute to the processes under discussion here (Schiltz and Sawchenko, 2003). Unlike other CNS macrophages, in the adult CNS microglia are maintained independent of circulating blood monocytes and are thought to self-renew from progenitor cells in the CNS (Katsumoto et al, 2014). Microglia are normally in a quiescent state, during which their processes sample their immediate environment several times a second (Nimmerjahn et al, 2005).
Microglia perform several critical functions in the CNS including immunosurveillance for pathogens, cellular debris, apoptotic cells, and alterations in neuronal phenotype (Ransohoff and Cardona, 2010). Recent reviews suggest that microglia may enter a spectrum of activation states (Mosser and Edwards, 2008; Rivest, 2009) characterized by varying blends of immunophenotypes and cytokine profiles. Importantly, microglia can enter a state called ‘primed' (Perry et al, 2007). Here, they show phenotypic signs of activation (eg, upregulated MHCII), but do not secrete increased cytokines. However, if stimulated while in this state, microglia produce and release exaggerated quantities of mediators, including IL-1β. This primed activation state is particularly relevant to stress-induced priming of neuroinflammatory processes, which is discussed in detail below. It should be noted that microglia express PPRs such as TLRs, which include TLR2 and TLR4, and TLR ligation potently activates microglia (Aravalli et al, 2007). In addition, several inflammasomes have been characterized in microglia including NLRP1, NLRP3, and NLRC4 (Walsh et al, 2014). Notably, several studies have characterized inflammasomes in neurons (de Rivero Vaccari et al, 2009; Kummer et al, 2007), which are capable of producing IL-1β (de Rivero Vaccari et al, 2009). The NLRP3 inflammasome has been the most studied inflammasome in the CNS (Walsh et al, 2014), and as expanded upon below, has also been the focus of the preponderance of studies into the role of inflammasomes in animal models of depression. Interestingly, recent investigations into the effects of stress on NLRP3 in the CNS suggest that NLRP3 may also be uniquely sensitive to the homeostatic perturbations produced by stress.
Animal Models of Depression: Role of Inflammasomes
A number of studies have demonstrated that IL-1β mediates depressive-like behaviors induced by stress as well as proinflammatory challenges, which of course implicates a mechanistic role for inflammasomes because inflammasomes mediate, in large part, the processing and maturation of IL-1β (Jo et al, 2016). Further, when administered to naive animals, IL-1β is sufficient to induce depressive-like behaviors (Goshen et al, 2009). While these studies implicate inflammasome activation in stress-induced depressive-like behaviors, inflammasomes were, in large part, not directly studied. It is important to emphasize here that stress-induced changes in brain IL-1β do not necessarily reflect inflammasome activation because inflammasomes are not strictly required for the processing of IL-1β into its mature, active form. Of note, several inflammasome-independent or noncanonical pathways have been characterized, which also result in caspase activation and maturation of IL-1β (Netea et al, 2015). Further, inflammasome-dependent activation of IL-1β is cell type specific (Netea et al, 2015). Interestingly, Burm et al (2015) have recently demonstrated that IL-1β activation is not strictly caspase-1 dependent in primary microglia, suggesting that inflammasomes may not be necessary for the processing of IL-1β into its mature form in this CNS innate immune cell. These are important points to consider in studies that examine the role of brain inflammasomes in animal models of depression.
The role of inflammasomes in stress-induced depressive-like behaviors has recently become a subject of study. Pan et al (2014) conducted one of the initial studies on the role of the NLRP3 inflammasome in stress-induced depressive-like behavior. Rats were exposed to 12 weeks of chronic unpredictable stress (CUS). Briefly, CUS typically involves the repetitive and unpredictable presentation of aversive stimuli/conditions, including cage tilting, water and food deprivation, wet bedding, light cycle disruption, strobe lighting, and continuous noise (Willner, 1997). Indeed, this paradigm is highly effective at inducing depressive-like behaviors such as reductions in sucrose preference and juvenile social exploration. Pan et al (2014) found that CUS exposure suppressed sucrose intake relative to unstressed controls, while increasing the levels of IL-1β mRNA and mature IL-1β protein in the prefrontal cortex (PFC). Interestingly, CUS also increased NLRP3 protein and mRNA, and mature caspase-1 protein in the PFC. Moreover, these effects of CUS were significantly blunted by chronic fluoxetine treatment, which was administered during weeks 6–12 of CUS exposure. However, it is important to note that the correlative nature of these findings precludes definitive conclusions regarding a causal role for the NLRP3 inflammasome in CUS-induced depressive-like behaviors as well as neuroinflammatory processes. In addition, much the same changes have been reported in the hippocampus (Deng et al, 2015a; Liu et al, 2015) and PFC (Deng et al, 2015a). Of note, CUS exposure also was found to increase protein levels of the NLRP3 adaptor protein ASC (Deng et al, 2015a, b; Li et al, 2015). As noted above, the current conceptualization of NLRP3 inflammasome activation suggests that both a priming stimulus and a subsequent activating stimulus are required to form and activate the NLRP3 inflammasome. However, it is not clear what mediators play the roles of the NLRP3 priming stimulus and activating stimulus during exposure to CUS. Thus, it is unclear how these effects of CUS on NLRP3, caspase-1, and mature IL-1β fit into our current understanding of the function of NLRP3. Could it be that initial stress exposure primes NLRP3 inflammasome formation to subsequent stress exposure during CUS? One possibility is that the repetitive, unpredictable presentation of heterogeneous stressors during CUS may serve to both prime and activate the NLRP3 inflammasome.
A recent study by Cheng et al (2016) may provide insight into this question. They found that exposure to an initial series of acute footshocks increased NLRP3 as well as mature caspase-1 in the hippocampus. It would seem that IL-1β should also have been increased given the stress-induced increase in mature caspase-1, but it was not. However, if a second series of footshocks was administered 24 h after the initial series of footshocks, IL-1β protein was increased, suggesting that the initial series of shocks had primed the NLRP3 inflammasome. These findings further suggest that a similar priming phenomenon may occur during exposure to CUS. We have also found that exposure to acute stress, in this case inescapable tailshock, increases NLRP3 protein in the hippocampus up to 24 h after stress treatment; however, microglial IL-1β was not increased at that time-point post stress (Weber et al, 2015). Importantly, treatment of hippocampal microglia with the TLR4 agonist LPS resulted in a potentiated IL-1β response in animals exposed to stress 24 h earlier, suggesting that the NLRP3 inflammasome was primed by initial stress exposure. Although these studies only show a correlation between the NLRP3 inflammasome and stress-induced neuroinflammatory processes and depressive-like behaviors, several studies have provided evidence for a causal role for the inflammasome in these processes.
Alcocer-Gomez et al (2015) used NLRP3 knockout (KO) mice to study the role of NLRP3 in mediating the effects of chronic immobilization stress on neuroinflammatory processes and depressive-like behavior. Mice were exposed to a 2-h immobilization stress every day for 30 consecutive days. They found that exposure of wild-type mice to immobilization induced a decrease in sucrose preference and social interaction, and an increase in immobility in the forced swim test. This stress-induced depressive phenotype was completely abrogated in NLRP3 KO mice. Furthermore, KO of the NLRP3 gene prevented the stressor treatment from increasing protein levels of mature IL-1β in the PFC and hippocampus. In addition, Wong et al (2016) recently reported that capsase-1-deficient mice (casp1−/−) were protected from developing anxiety- and depression-like behaviors after chronic restraint stress (Wong et al, 2016). These findings suggest that the NLRP3 inflammasome may have a causal role in stress effects on depressive-like behavior.
A recent investigation by Iwata et al (2015) also found that 21 days of CUS resulted in a depressive behavioral phenotype including decreased sucrose preference, which was abrogated in NLRP3 KO mice. In wild-type mice, they also found that peripheral administration of an IL-1β-blocking antibody ameliorated the behavioral effects of CUS, including decrements in sucrose preference and immobility in the forced swim test. These findings suggest that stress-induced activation of the NLRP3 inflammasome and subsequent maturation and release of IL-1β was necessary for the depressive-like behavioral effects of stress. Iwata et al (2015) also explored the role of ATP, a well-characterized activator of the NLRP3 inflammasome (Elliott and Sutterwala, 2015), in stress-induced activation of NLRP3, IL-1β release, and behavioral effects, which we address in further detail below. Clearly, the studies by Alcocer-Gomez et al (2015) and Iwata et al (2015) provide strong evidence for a casual role for the NLRP3 inflammasome in the effects of stress on depressive-like behavior.
It is remarkable that exposure to an acute or chronic stressor typically results in the induction of neuroinflammation in the absence of infection or pathogen exposure. Thus, consistent with previous work establishing sterile inflammation in the periphery after stressor exposure, these current studies suggest that stressor exposure may also stimulate sterile inflammation in the brain, or neuroinflammation (Chen and Nunez, 2010). As discussed previously, sterile inflammation is driven in large part by DAMPs that are released after physical and psychological stressors (Leemans et al, 2011). Thus, it is reasonable to propose that sterile inflammatory processes have a mediating role in the effects of stress on inflammasome function, neuroinflammation, and the behavioral sequelae of inflammatory processes. Of note, there is some evidence that neuronal damage may occur in major depression (Duman, 2009), suggesting that DAMPs may also have a role in the pathophysiology of depression. Here, we explore several studies that address this notion.
Mechanisms of Stress and NLRP3 Inflammasome Priming/Activation
HMGB1
The DAMP HMGB1 has been implicated in stress-induced priming of NLRP3 and the neuroinflammatory response to a subsequent immune challenge (Frank et al, 2015b). HMGB1 has a pivotal role in the induction of neuroinflammatory processes in neurodegenerative conditions (Fang et al, 2012), cerebral ischemia (Yang et al, 2010), traumatic brain injury (Corps et al, 2015), seizure (Vezzani, 2014), and ethanol-induced neurotoxicity (Zou and Crews, 2014). HMGB1 is a DNA structural protein that has been co-opted by the innate immune system to signal damage during sterile inflammatory conditions (Bianchi, 2007). For example, cellular necrosis is a hallmark of several neuroinflammatory conditions. Here, HMGB1 is passively released into the extracellular space where it signals through TLRs (eg, TLR2/TLR4) to induce NF-κB signaling and proinflammatory cytokine production. HMGB1 can also function as a chemotactic factor and also be actively secreted from innate immune cells (Yang et al, 2013). Of particular relevance here, HMGB1 has been found to prime the NLRP3 inflammasome (Xiang et al, 2011).
We recently found that exposure to an acute stressor (inescapable tailshock) induces HMGB1 in the hippocampus concomitant with increased protein levels of active NF-κB and NLRP3 protein (Weber et al, 2015). A number of studies have demonstrated that prior exposure to acute or chronic stressors potentiates the neuroinflammatory and microglial proinflammatory response to a subsequent immune challenge (Frank et al, 2015b). Interestingly, we found that intracisterna magna administration of an HMGB1 antagonist (box A) before stress exposure blocked the potentiated microglial proinflammatory response to an immune challenge ex vivo. This finding suggests that HMGB1 mediates stress-induced priming of the microglia proinflammatory response.
Consistent with these findings, Cheng et al (2016) also found that exposure to a series of footshocks increased hippocampal protein levels of HMGB1 along with increased active NF-κB, NLRP3, and mature caspase-1 protein (Cheng et al, 2016). As noted earlier, initial stress exposure failed to increase IL-1β protein; however, exposure to second series of footshocks 24 h after the initial series of footshocks resulted in increased hippocampal IL-1β protein and mRNA. Intranasal pre-treatment with HMGB1 siRNA was found to significantly reduce hippocampal protein levels of HMGB1 and blocked stress-induced increases in IL-1β protein, suggesting that HMGB1 mediates the priming effects of stress on IL-1β. Further, Cheng et al (2016) found that the effects of stress on NF-κB, NLRP3, and mature caspase-1 were also blocked in TLR4 KO mice. However, stress effects on HMGB1 were not blocked in TLR4 KO mice.
This set of findings suggests that initial stress exposure induces the release of HMGB1 in the CNS, which then signals through TLR4 to prime the NLRP3 inflammasome. Upon exposure to a subsequent series of footshocks, the IL-1β response was potentiated. Again, a key question here is what signal mediates NLRP3 inflammasome activation upon exposure to a second series of footshocks. It should be noted that exposure to the second series of footshocks resulted in a potentiated HMGB1 response in the hippocampus, suggesting that HMGB1 may serve as both the NLRP3 inflammasome priming stimulus as well as the activating stimulus.
We recently characterized the importance of the redox state of HMGB1 in the priming of the neuroinflammatory and depressive behavioral responses to a subsequent immune challenge (Frank et al, 2015a). HMGB1 can function either as a chemotactic or proinflammatory mediator depending on the redox state of three critical cysteines (Venereau et al, 2012). The disulfide form mediates the proinflammatory effects of HMGB1 by signaling through TLR4, whereas the reduced form mediates the chemotactic properties of HMGB1 by forming a complex with the chemokine CXCL12 and signaling through CXCR4. We found that the disulfide form upregulates NLRP3 expression and potentiates the neuroinflammatory response to a peripheral LPS challenge. In addition, we found that the disulfide form potentiated the LPS-induced reductions in social exploration as measured in the juvenile social investigation test.
Taken together, these findings suggest that HMGB1 mediates stress-induced priming of the neuroinflammatory and behavioral response to subsequent immune challenges and that HMGB1 priming of the NLRP3 inflammasome likely mediates these stress effects on neuroinflammation and behavior.
ATP
ATP is also considered to be a danger signal that is released from stressed or injured cells to signal damage to innate immune cells (Ferrari et al, 2006). Iwata et al (2015) have found that exposure to acute immobilization stress (60 min) resulted in the intrahippocampal release of ATP concomitant with IL-1β protein as well as NLRP3 inflammasome activation. These effects of stress were prevented in a Nlrp3 KO mouse and were blocked by peripheral administration of an antagonist to the P2X7 receptor, which mediates ATP activation of the NLRP3 inflammasome (Jin and Flavell, 2010), suggesting that stress-induced release of ATP mediates NLRP3 inflammasome activation and subsequent release of IL-1β. Further, they found that chronic administration of a P2X7 receptor antagonist during exposure to CUS ameliorated several effects of stress on depressive-like behaviors including the sucrose preference test, the novelty suppressed feeding test, and elevated plus maze. Although the role of ATP and P2X7 in this model is clear, it is not possible to conclude that this signaling is restricted to the brain because both the P2X7 receptor antagonist and the Nlrp3 KO could have impacted sterile inflammation in the periphery as well as the brain (Karmakar et al, 2016).
Importantly, a key question that remains with regard to stress-induced DAMP signaling and neuroinflammation is the mechanism whereby stress exposure induces DAMP release in the CNS. A number of mediators of the stress response, most notably noradrenaline and glucocorticoids, have been found to mediate the effects of stress on neuroinflammatory processes (Frank et al, 2013; Wohleb et al, 2011). However, it is unknown whether these stress mediators induce DAMPs, which may then function as the proximal signals of neuroinflammation.
Future Directions, Clinical Implications, and Potential Therapeutics
It is becoming increasingly accepted that inflammation contributes to an array of CNS disorders including MDD (Howren et al, 2009; Schiepers et al, 2005). In addition, stress is a well-established trigger of affective dysfunction and severe and/or repeated psychological stressor exposure also increases inflammatory proteins in blood and tissues. Growing evidence supports the hypothesis that sterile inflammatory processes likely have a role in stress-induced inflammation (Figure 4). Thus, understanding the mechanisms of sterile inflammation, the unique DAMP/MAMP/PAMP signaling in the periphery and CNS, and the role of inflammasome activation is critical for advancing the development of novel therapeutics to be used in the treatment of affective disorders (Miller et al, 2015). In fact, there are several promising pharmacological candidates in use or in development that specifically target the inflammasome for the treatment of CNS inflammation after brain injury and stroke (de Rivero Vaccari et al, 2016a, b). Neutralizing antibodies targeting NLRP1 was reported to reduce CNS inflammation after stroke in mice (Abulafia et al, 2009). In addition, de Rivero Vaccari et al, 2008, 2016b reported that antibody neutralization of ASC reduced CNS inflammasome activation and inflammation after neural trauma. Although not yet tested in psychiatric patients, these advances could aid the rapid development of therapeutics for MDD.
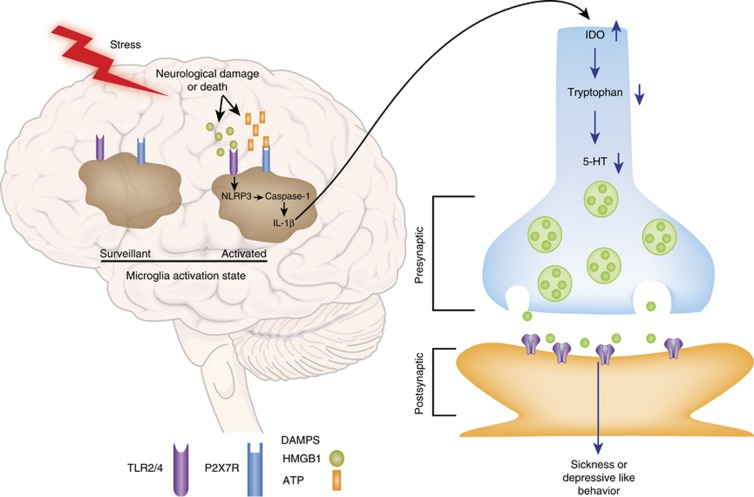
Figure 4.
A model of stress-induced sterile inflammatory processes in the brain and the neural impact of these processes. We propose that exposure to stressors results in the release of danger-associated molecular patterns (DAMPs) within the brain, presumably from damaged or dying neurons. These neuron-derived DAMPs then target their cognate receptors on microglia leading to inflammasome activation and the synthesis and secretion of interleukin-1β (IL-1β). The secreted form of IL-1β may drive the induction of indoleamine 2,3-dioxygenase (IDO), which catabolizes tryptophan into kynurenine and thereby reduces the available pool of tryptophan for serotonin (5-HT) synthesis. Reductions in 5-HT synthesis may then mediate, in part, the effects of stress on sickness behavior.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
Funding: Dr Fleshner's research is currently funded by Mead Johnson Nutrition and the Office of Naval Research. Dr Frank's research is currently funded by the National Institutes of Health. Dr Maier's research is currently funded by the National Institutes of Health and the Department of Defense.
References
- Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD (2009). Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab 29: 534–544. [DOI] [PubMed] [Google Scholar]
- Alcocer-Gomez E, Cordero MD (2014. a). NLRP3 inflammasome: a new target in major depressive disorder. CNS Neurosci Ther 20: 294–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcocer-Gomez E, de Miguel M, Casas-Barquero N, Nunez-Vasco J, Sanchez-Alcazar JA, Fernandez-Rodriguez A, Cordero MD (2014. b). NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav Immun 36: 111–117. [DOI] [PubMed] [Google Scholar]
- Alcocer-Gomez E, Ulecia-Moron C, Marin-Aguilar F, Rybkina T, Casas-Barquero N, Ruiz-Cabello J et al (2015). Stress-induced depressive behaviors require a functional NLRP3 inflammasome. Mol Neurobiol 51: 1–9. [DOI] [PubMed] [Google Scholar]
- Aravalli RN, Peterson PK, Lokensgard JR (2007). Toll-like receptors in defense and damage of the central nervous system. J Neuroimmune Pharmacol 2: 297–312. [DOI] [PubMed] [Google Scholar]
- Aschbacher K, Epel E, Wolkowitz OM, Prather AA, Puterman E, Dhabhar FS (2012). Maintenance of a positive outlook during acute stress protects against pro-inflammatory reactivity and future depressive symptoms. Brain Behav Immun 26: 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE et al (2002). Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277: 15028–15034. [DOI] [PubMed] [Google Scholar]
- Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V (2012). NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol 189: 4175–4181. [DOI] [PubMed] [Google Scholar]
- Bianchi ME (2007). DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81: 1–5. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D et al (2003). A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA 100: 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burm SM, Zuiderwijk-Sick EA, T Jong AE, van der Putten C, Veth J, Kondova I, Bajramovic JJ (2015). Inflammasome-induced IL-1beta secretion in microglia is characterized by delayed kinetics and is only partially dependent on inflammatory caspases. J Neurosci 35: 678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ Jr. (2007. a). Extracellular heat shock proteins in cell signaling and immunity. Ann NY Acad Sci 1113: 28–39. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Theriault J, Gray PJ, Gong J (2007. b). Cell surface receptors for molecular chaperones. Methods 43: 199–206. [DOI] [PubMed] [Google Scholar]
- Campisi J, Fleshner M (2003. a). Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. J Appl Physiol 94: 43–52. [DOI] [PubMed] [Google Scholar]
- Campisi J, Hansen MK, O'Connor KA, Biedenkapp JC, Watkins LR, Maier SF et al (2003. b). Circulating cytokines and endotoxin are not necessary for the activation of the sickness or corticosterone response produced by peripheral E. coli challenge. J Appl Physiol 95: 1873–1882. [DOI] [PubMed] [Google Scholar]
- Campisi J, Sharkey C, Johnson JD, Asea A, Maslanik T, Bernstein-Hanley I et al (2012). Stress-induced facilitation of host response to bacterial challenge in F344 rats is dependent on extracellular heat shock protein 72 and independent of alpha beta T cells. Stress 15: 637–646. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH (2011). Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 130: 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Nunez G (2010). Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Pardo M, Armini RS, Martinez A, Mouhsine H, Zagury JF et al (2016). Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav Immun 53: 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corps KN, Roth TL, McGavern DB (2015). Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol 72: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A (2008). Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry 65: 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Brand F III, Adamczak S, Lee SW, Perez-Barcena J, Wang MY et al (2016. a). Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem 136(Suppl 1): 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Dietrich WD, Keane RW (2016. b). Therapeutics targeting the inflammasome after central nervous system injury. Transl Res 167: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW (2009). Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab 29: 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW (2008). A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci 28: 3404–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XY, Li HY, Chen JJ, Li RP, Qu R, Fu Q et al (2015. a). Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav Brain Res 291: 12–19. [DOI] [PubMed] [Google Scholar]
- Deng XY, Xue JS, Li HY, Ma ZQ, Fu Q, Qu R et al (2015. b). Geraniol produces antidepressant-like effects in a chronic unpredictable mild stress mice model. Physiol Behav 152(Part A): 264–271. [DOI] [PubMed] [Google Scholar]
- Denson TF, Spanovic M, Miller N (2009). Cognitive appraisals and emotions predict cortisol and immune responses: a meta-analysis of acute laboratory social stressors and emotion inductions. Psychol Bull 135: 823–853. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME (2009). Social-evaluative threat and proinflammatory cytokine regulation: an experimental laboratory investigation. Psychol Sci 20: 1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago A, Crisafulli C, Calabro M, Serretti A (2015). Enrichment pathway analysis. The inflammatory genetic background in bipolar disorder. J Affect Disord 179: 88–94. [DOI] [PubMed] [Google Scholar]
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG et al (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464: 1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS (2009). Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness: stress and depression. Dialog Clin Neurosci 11: 239–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott EI, Sutterwala FS (2015). Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev 265: 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Schachner M, Shen YQ (2012). HMGB1 in development and diseases of the central nervous system. Mol Neurobiol 45: 499–506. [DOI] [PubMed] [Google Scholar]
- Felger JC, Haroon E, Miller AH (2016) Inflammation and immune function in post-traumatic stress disorder: mechanisms, consequences and translational implications In: Ressler KJ, Liberzon I(eds) Neurobiology of Posttraumatic Stress Disorder. Oxford Press: New York, NY. [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M et al (2006). The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176: 3877–3883. [DOI] [PubMed] [Google Scholar]
- Fleshner M (2013). Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav Immun 27: 1–7. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Campisi J, Amiri L, Diamond DM (2004). Cat exposure induces both intra- & extra-cellular HSP72: The role of adrenal hormones. Psychoneuroendocrinology 29: 1142–1152. [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF (2013). Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun 33: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Fonken LK, Hershman SA, Watkins LR, Maier SF (2015. a). The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: A role for the NLRP3 inflammasome. Brain Behav Immun 55: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR, Maier SF (2015. b). Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav Immun 48: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Avital A, Kreisel T, Licht T, Segal M, Yirmiya R (2009). Environmental enrichment restores memory functioning in mice with impaired IL-1 signaling via reinstatement of long-term potentiation and spine size enlargement. J Neurosci 29: 3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Narayanan PK, Fort MM (2014). Assessment of the performance of three multiplex array panels for the detection of circulating cytokines and chemokines in naive, LPS, and SEB-treated cynomolgus macaques. Toxicol Pathol 42: 286–292. [DOI] [PubMed] [Google Scholar]
- Heath NM, Chesney SA, Gerhart JI, Goldsmith RE, Luborsky JL, Stevens NR, Hobfoll SE (2013). Interpersonal violence, PTSD, and inflammation: potential psychogenic pathways to higher C-reactive protein levels. Cytokine 63: 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71: 171–186. [DOI] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S et al (2015). Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol Psychiatry 80: 12–22. [DOI] [PubMed] [Google Scholar]
- Jin C, Flavell RA (2010). Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol 30: 628–631. [DOI] [PubMed] [Google Scholar]
- Jo EK, Kim JK, Shin DM, Sasakawa C (2016). Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13: 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Fleshner M (2005). Adrenergic receptors mediate stress-induced elevations in extracellular Hsp72. J Appl Physiol 99: 1789–1795. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Fleshner M (2006). Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol 79: 425–434. [DOI] [PubMed] [Google Scholar]
- Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E (2016). Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1beta secretion in response to ATP. Nat Commun 7: 10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon WJ (2011). Epidemiology and treatment of depression in patients with chronic medical illness. Dialog Clin Neurosci 13: 7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumoto A, Lu H, Miranda AS, Ransohoff RM (2014). Ontogeny and functions of central nervous system macrophages. J Immunol 193: 2615–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC (1997). The effects of stressful life events on depression. Annu Rev Psychol 48: 191–214. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, Fagundes CP (2015). Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry 172: 1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Hantsoo L (2010). Close relationships, inflammation, and health. Neurosci Biobehav Rev 35: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Rock KL (2008). How dying cells alert the immune system to danger. Nat Rev Immunol 8: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F et al (2007). Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem 55: 443–452. [DOI] [PubMed] [Google Scholar]
- Leemans JC, Cassel SL, Sutterwala FS (2011). Sensing damage by the NLRP3 inflammasome. Immunol Rev 243: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Liu Y, Yin S, Lu C, Liu D, Jiang H et al (2015). Long-term effects of early adolescent stress: dysregulation of hypothalamic-pituitary-adrenal axis and central corticotropin releasing factor receptor 1 expression in adult male rats. Behav Brain Res 288: 39–49. [DOI] [PubMed] [Google Scholar]
- Liu B, Xu C, Wu X, Liu F, Du Y, Sun J et al (2015). Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience 294: 193–205. [DOI] [PubMed] [Google Scholar]
- Maslanik T, Bernstein-Hanley I, Helwig B, Fleshner M (2012. a). The impact of acute-stressor exposure on splenic innate immunity: a gene expression analysis. Brain Behav Immun 26: 142–149. [DOI] [PubMed] [Google Scholar]
- Maslanik T, Mahaffey L, Tannura K, Beninson L, Greenwood BN, Fleshner M (2013). The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun 28: 54–62. [DOI] [PubMed] [Google Scholar]
- Maslanik T, Tannura K, Mahaffey L, Loughridge AB, Beninson L, Ursell L et al (2012. b). Commensal bacteria and MAMPs are necessary for stress-induced increases in IL-1beta and IL-18 but not IL-6, IL-10 or MCP-1. PLoS One 7: e50636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker RH, Kelley KW (2013). Immune-neural connections: how the immune system's response to infectious agents influences behavior. J Exp Biol 216(Part 1): 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO et al (2015). Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry 172: 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL (2015). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP (2008). Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ML, Slavich GM, Rohleder N, Miller GE (2013). Targeted rejection triggers differential pro- and anti-inflammatory gene expression in adolescents as a function of social status. Clin Psychol Sci 1: 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA (2015). Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol 33: 49–77. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314–1318. [DOI] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH et al (2006). Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry 163: 1630–1633. [DOI] [PubMed] [Google Scholar]
- Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM (2012). Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun 26: 13–17. [DOI] [PubMed] [Google Scholar]
- Pan Y, Chen XY, Zhang QY, Kong LD (2014). Microglial NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun 41: 90–100. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C (2007). Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 7: 161–167. [DOI] [PubMed] [Google Scholar]
- Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C (2007). Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology 32: 991–999. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE (2010). The myeloid cells of the central nervous system parenchyma. Nature 468: 253–262. [DOI] [PubMed] [Google Scholar]
- Rivest S (2009). Regulation of innate immune responses in the brain. Nat Rev Immunol 9: 429–439. [DOI] [PubMed] [Google Scholar]
- Rock KL, Lai JJ, Kono H (2011). Innate and adaptive immune responses to cell death. Immunol Rev 243: 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Latz E, Ontiveros F, Kono H (2010). The sterile inflammatory response. Annu Rev Immunol 28: 321–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M (2005). Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry 29: 201–217. [DOI] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE (2003). Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci 8: s1321–s1329. [DOI] [PubMed] [Google Scholar]
- Schneider EM, Flacke S, Liu F, Lorenz MR, Schilling P, Nass ME et al (2011). Autophagy and ATP-induced anti-apoptosis in antigen presenting cells (APC) follows the cytokine storm in patients after major trauma. J Cell Commun Signal 5: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze-Florey CR, Martinez-Maza O, Magpantay L, Breen EC, Irwin MR, Gundel H et al (2012). When grief makes you sick: bereavement induced systemic inflammation is a question of genotype. Brain Behav Immun 26: 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE (2004). Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130: 601–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR (2014). From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 140: 774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y (2007). The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun 21: 901–912. [DOI] [PubMed] [Google Scholar]
- Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F et al (2012). Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med 209: 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A (2014). Epilepsy and inflammation in the brain: overview and pathophysiology. Epilepsy Curr 14(Suppl.): 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JG, Muruve DA, Power C (2014). Inflammasomes in the CNS. Nat Rev Neurosci 15: 84–97. [DOI] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF (2015). Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague–Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. J Neurosci 35: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P (1997). Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology 134: 319–329. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT et al (2011). Beta-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 31: 6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J et al (2016). Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry 6: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Shi X, Li Y, Xu J, Yin L, Xiao G (2011). Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J Immunol 187: 4809–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Antoine DJ, Andersson U, Tracey KJ (2013). The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol 93: 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QW, Xiang J, Zhou Y, Zhong Q, Li JC (2010). Targeting HMGB1/TLR4 signaling as a novel approach to treatment of cerebral ischemia. Front Biosci (Schol Ed) 2: 1081–1091. [DOI] [PubMed]
- Zou JY, Crews FT (2014). Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS One 9: e87915. [DOI] [PMC free article] [PubMed] [Google Scholar]