Abstract
The study of inflammation in fear- and anxiety-based disorders has gained interest as growing literature indicates that pro-inflammatory markers can directly modulate affective behavior. Indeed, heightened concentrations of inflammatory signals, including cytokines and C-reactive protein, have been described in posttraumatic stress disorder (PTSD), generalized anxiety disorder (GAD), panic disorder (PD), and phobias (agoraphobia, social phobia, etc.). However, not all reports indicate a positive association between inflammation and fear- and anxiety-based symptoms, suggesting that other factors are important in future assessments of inflammation's role in the maintenance of these disorders (ie, sex, co-morbid conditions, types of trauma exposure, and behavioral sources of inflammation). The most parsimonious explanation of increased inflammation in PTSD, GAD, PD, and phobias is via the activation of the stress response and central and peripheral immune cells to release cytokines. Dysregulation of the stress axis in the face of increased sympathetic tone and decreased parasympathetic activity characteristic of anxiety disorders could further augment inflammation and contribute to increased symptoms by having direct effects on brain regions critical for the regulation of fear and anxiety (such as the prefrontal cortex, insula, amygdala, and hippocampus). Taken together, the available data suggest that targeting inflammation may serve as a potential therapeutic target for treating these fear- and anxiety-based disorders in the future. However, the field must continue to characterize the specific role pro-inflammatory signaling in the maintenance of these unique psychiatric conditions.
INTRODUCTION
Fear- and anxiety-related psychiatric disorders are all associated with exaggerated fear reactions to stimuli specific to each disorder in the absence of any actual danger (Singewald et al, 2015). Indeed, posttraumatic stress disorder (PTSD), generalized anxiety disorder (GAD), panic disorder (PD), and phobias (agoraphobia, social phobia, etc.) are all characterized by pathological fear and/or anxiety (APA, 2014). Furthermore, these fear and anxiety disorders are associated with impaired ability to extinguish learned fear and compromised capacity to learn safety behaviors (Singewald et al, 2015). Together, fear- and anxiety-based psychiatric disorders are at the same time the most prevalent (Kessler et al, 2005) and the most costly of mental health disorders (Gustavsson et al, 2011). As PTSD and other fear related disorders are associated with an array of other adverse mental and physical health outcomes (Boscarino, 2004; Kessler et al, 2005), ongoing translational and clinical research has focused on elucidating the neurobiological substrates underlying these conditions in order to inform the development of treatments and interventions that attenuate and/or prevent their associated adverse outcomes.
One biological process that has been increasingly interrogated over the last decade is the inflammatory system, as it has a clear role in the pathophysiology of chronic mental and physical illness. Immune signaling contributes to the regulation of the hypothalamic-pituitary-adrenal (HPA) axis and other neurobiological processes that modulate affective behavior in the face of stressor exposure (Haroon et al, 2012). Indeed, exposure to traumatic and stressful events (including exposure to fear- and anxiety-provoking stimuli) results in HPA axis reactivity, activation of the immune system, and the release of pro-inflammatory cytokines (reviewed in Haroon et al (2012)). Over time and with continuous exposure to stressors, both HPA and immune function become dysregulated. Although extensive work has been done to characterize the role of endocrine dysfunction in the pathophysiology and maintenance of PTSD (Daskalakis et al, 2013; Hauger et al, 2012; O'Donovan et al, 2013; Yehuda and LeDoux, 2007), our understanding of the role of inflammation in the etiology and maintenance of fear- and anxiety-based disorders remains limited. Thus, in the current review, we will summarize significant findings that indicate that PTSD, and other fear- and anxiety-based disorders, are characterized by increased inflammatory processes associated with greater symptom severity. We focus specifically on alterations in pro- and anti-inflammatory signals, in and ex vivo stimulation of the immune cell responses and distribution, and immune transcription factors, gene expression and methylation in these disorders. We will also discuss the limited data available that suggest anxiety disorders are similarly associated with increased inflammation. For the purposes of this review, we will focus exclusively on anxiety disorders as defined by the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (APA, 2014), namely GAD, PD, and phobias. Finally, we will discuss the neurobiological mechanisms by which heighted inflammation occurs in these fear- and anxiety-related disorders and how inflammatory processes may work to exacerbate severity of these conditions. Understanding the role of inflammation in these highly prevalent and burdensome disorders has important translational and clinical implications, potentially offering new therapeutic targets for treatment upon future investigation.
PTSD and Inflammation
PTSD is a severe and heterogeneous psychiatric condition, often presenting with different re-experiencing, avoidance/numbing, and hyperarousal symptoms following exposure to a life-threatening event that results in psychological trauma (ie, exposure to an event including death or threatened death, actual or threatened serious injury, or actual or threatened sexual violence; Kessler et al, 1995). Underlying alterations in neuroendocrine, psychophysiological, and neurobiological systems have all been implicated in the etiology and maintenance of PTSD (for review see Michopoulos et al (2015b)). Importantly, although many will experience a traumatic event in their lifetime (70% of the general population), only 7.8% of the US population will go on to develop PTSD in the aftermath of trauma (Keane et al, 2009). PTSD is associated with significant co-morbidities including major depression, substance and alcohol abuse, PD, suicide, reduced life expectancy, as well as disability in daily activities, and increased health care utilization (Dedert et al, 2010; Khoury et al, 2010; Norrholm et al, 2011). Adverse physical health co-morbidities are also common in individuals with PTSD, including obesity, diabetes, cardiovascular disease (Boscarino, 2004; Coughlin, 2011; Heppner et al, 2009). These data in parallel with evidence indicating that PTSD is a chronic disorder with dysregulated stress axis function (Michopoulos et al, 2015b), have recently led to a burgeoning attempt to understand how inflammatory processes in the context of trauma exposure and PTSD are altered, and how they might drive changes in neurobiological pathways and affective behavior.
Alterations in basal concentrations of inflammatory signals in PTSD
Exposure to trauma is associated with pro-inflammatory activity (Tursich et al, 2014). Specifically, increased circulating concentrations of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and the acute phase reactant C-reactive protein (CRP) are all significantly associated with trauma exposure as shown in a recent meta-analysis (Tursich et al, 2014). The majority of these studies have specifically assessed the influence of childhood maltreatment and adversity on inflammation in adulthood (Baumeister et al, 2015; Lin et al, 2016). Indeed, individuals who were exposed to childhood maltreatment, as well as those exposed to difficult family and socio-economic circumstances in childhood (Taylor et al, 2006), show heightened levels of CRP in adulthood (Bertone-Johnson et al, 2012; Danese et al, 2007; Lin et al, 2016; Matthews et al, 2014; Rooks et al, 2012; Tietjen et al, 2012). Parental separation in early childhood is also associated with increased CRP in adulthood (Lacey et al, 2013; McDade et al, 2013). Concentrations of IL-6, IL-1β, and TNF-α are elevated with childhood maltreatment (Gouin et al, 2012; Hartwell et al, 2013; Kiecolt-Glaser et al, 2011; Smith et al, 2011; Tietjen et al, 2012). Importantly, exposure to trauma in childhood is associated with increased risk for developing PTSD and other psychiatric conditions (Edwards et al, 2003).
Elevated concentrations of pro-inflammatory markers have been observed in individuals with PTSD (Guo et al, 2012; Hoge et al, 2009; Table 1, A). More specifically, circulating concentrations of IL-1β (Oganesyan et al, 2009; Spivak et al, 1997; Tucker et al, 2004; von Kanel et al, 2007), IL-2 (Guo et al, 2012), and IL-6 (Bersani et al, 2016; Guo et al, 2012; Maes et al, 1999; Newton et al, 2014; Oganesyan et al, 2009) are elevated in PTSD. Central levels of IL-6 in cerebrospinal fluid have also been found to be elevated in PTSD (Baker et al, 2001). Concentrations of TNF-α are also increased in PTSD (Bersani et al, 2016; Oganesyan et al, 2009; Vidovic et al, 2011; von Kanel et al, 2007), and correlate positively with total PTSD symptomology, as well as all three DSM-IV-TR symptom sub-clusters (ie, avoidance, re-experiencing, and hyperarousal (von Kanel et al, 2007)). In addition, peripheral intercellular adhesion molecule-1 (Guo et al, 2012; Hoge et al, 2009; Plantinga et al, 2013) and interferon (INF)-γ (Hoge et al, 2009) are elevated in individuals with PTSD. Increased concentrations of CRP are also seen in individuals with PTSD (Bersani et al, 2016; Heath et al, 2013; Miller et al, 2001; Plantinga et al, 2013). More recently, heightened peripheral CRP concentrations were associated with higher PTSD symptoms and greater odds for a PTSD diagnosis (Michopoulos et al, 2015c). Furthermore, elevated CRP is also associated with impaired inhibition of fear-potentiated startle in the presence of a safety signal, a well-characterized biomarker of PTSD (Jovanovic et al, 2012). Combining concentrations of IL-1β, IL-6, TNF-α, IFN-γ, and CRP into a single pro-inflammatory score also indicated that inflammation is elevated in PTSD (Lindqvist et al, 2014b).
Table 1. Pro- (1A) and Anti- (1B) Immunological Factors Associated with PTSD.
Asterisks denote signals that were significantly different between PTSD cases and healthy controls in systematic meta-analysis conducted by Passos et al (2015).
Although these data together collectively indicate that PTSD is associated with increases in pro-inflammatory markers, there are also data suggesting that there is no relationship between PTSD and heightened inflammation. For instance, studies have described decreased levels of CRP in individuals with PTSD (Sondergaard et al, 2004) or even a lack of association between PTSD and CRP levels (McCanlies et al, 2011; Muhtz et al, 2011; von Kanel et al, 2007), and PTSD and IL-6 (Song et al, 2007b) and IL-2 (Song et al, 2007b; Tucker et al, 2004). This same discrepancy in the literature also surrounds alterations in anti-inflammatory cytokines in individuals with PTSD (Table 1, B). In some studies, concentrations of IL-8 (Jergovic et al, 2015; Song et al, 2007b) and IL-4 (Smith et al, 2011; von Kanel et al, 2007) are lower in individuals with PTSD. IL-4 concentrations have also been correlated negatively with total hyperarousal symptoms (von Kanel et al, 2007). However, there are also reports of increased concentrations of IL-4, IL-8, and IL-10 in those with PTSD (Guo et al, 2012). The inconsistencies between these published reports on PTSD and inflammation may be related to small sample sizes, distinct study and ethnic populations, the presence of uncontrolled confounders (medication usage, presence of infection, co-morbidity with depression, and other chronic illnesses), and the use of different control groups for comparison.
A meta-analysis comparing individuals with PTSD and healthy, non-traumatized controls from 20 independent studies was recently conducted to systematically address whether PTSD is associated with alterations in inflammatory signals discussed above and outlined in Table 1 (Passos et al, 2015). The systematic meta-analysis revealed that levels of IL-1β, IL-6, TNF-α, and IFN-γ are elevated in PTSD (Passos et al, 2015). Analyses also revealed that the duration of illness was associated positively with pro-inflammatory markers (Passos et al, 2015). Furthermore, the authors were able to conduct subgroup meta-analyses to disentangle to influence of possible confounders, such as medication usage and co-morbid major depression. TNF-α concentrations are still significantly associated with PTSD in unmedicated individuals, and TNF-α, IL-1b, and IL-6 levels are still augmented significantly in those with PTSD and no co-morbid depression (Passos et al, 2015). Although this meta-analysis and coincident subgroup analysis move the field forward, future studies and analyses are necessary to determine how other factors (ie, smoking status, alcohol use, obesity, infection, and pulmonary and cardiovascular disease) influence the association between PTSD and inflammation.
Immune challenge response is altered in PTSD
Importantly, changes in circulating inflammatory markers are not the only alterations in inflammatory pathways reported in PTSD. The production of circulating cytokines in response to an immune challenge is also altered in individuals with PTSD such that production of pro-inflammatory markers is increased and production of anti-inflammatory markers is decreased. More specifically, endotoxin-induced increases in IL-6 are heightened in individuals with PTSD (Rohleder et al, 2004). Ex vivo administration of phytohemagglutinin (PHA), a potent inducer of cytokine production from T cells, to peripheral blood mononuclear cells (PBMCs) results in heightened increases in TNF-α and IL-6 secretion in individuals with PTSD compared with traumatized and non-traumatized controls (Gill et al, 2008). In contrast, production of anti-inflammatory IL-4 and the antiviral IFN-γ in blood following PHA stimulation in men with PTSD is attenuated compared with matched controls without PTSD (Kawamura et al, 2001). There has also been one report that increased spontaneous production of IL-1 and TNF-α in PBMCs is present in individuals with PTSD compared with healthy controls (Gola et al, 2013). Finally, cell-mediated immunity, as assessed with a delayed-type hypersensitivity in vivo skin test, has also been shown to be enhanced in individuals with PTSD (Altemus et al, 2003; Masoudzadeh et al, 2012).
PTSD is associated with differential immune cell distribution and function
The above-described perturbations in the production and response of cytokines suggest that the function and distribution of immune cells may be altered in individuals with PTSD. Greater percentages and numbers of lymphocytes (Boscarino and Chang, 1999b; Vidovic et al, 2011), as well as greater T cells and leukocytes (Boscarino and Chang, 1999b) have been associated with the presence of PTSD. Individuals with PTSD also have reduced numbers of naive CD8(+) T lymphocytes and increases in the proportions of CD3(+) central and effector memory T lymphocytes compared with individuals without PTSD (Sommershof et al, 2009). Furthermore, higher levels of CD4 and CD5 expression (a marker of early immune response activation; Lemieux et al, 2008) on T cells is correlated positively with intrusive and negatively with avoidant symptoms in women with PTSD (Lemieux et al, 2008). Individuals with PTSD also exhibit increases in total PBMCs, pro-inflammatory Th1 and Th17 cells, and decreased T-regulatory (T-reg) cells that are correlated with increased peripheral concentrations of IFN-γ and IL-17 (Zhou et al, 2014). Because T-reg cells are critical for containing pro-inflammatory responses and Th1 and Th17 cells activate inflammatory responses (Afzali et al, 2007), these alterations in the composition of T-cell subsets may act in aggregate to direct systemic inflammatory tone into an overdrive state in PTSD (Jergovic et al, 2014). Finally, immunological aging of T-cell phenotypes has also been associated with PTSD (Aiello et al, 2016).
Immune gene transcription, expression, and methylation changes in PTSD
Alterations in the transcriptional patterns of expression for genes involved in inflammatory pathways have also been associated with PTSD (Segman et al, 2005; Yehuda et al, 2009; Zieker et al, 2007). Nuclear factor-κB (NFκB), signal transducer and activator of transcription 5B, and nuclear factor I/A are all critical transcription factors with substantive roles in the activation of cytokine responses to challenge whose activity is increased in the presence of PTSD (Guardado et al, 2016; O'Donovan et al, 2011; Pace et al, 2012; Sarapas et al, 2011). Gene expression of the pro-inflammatory cytokine IL-18 and its receptor IL-18R1 is decreased in individuals with PTSD (Mehta et al, 2011; Segman et al, 2005; Zieker et al, 2007). This decrease in IL-18 gene expression is consistent with epigenetic findings indicating that increased methylation in the promoter region of IL-18 is associated with the development of PTSD in soldiers following military deployment (Rusiecki et al, 2013). Gene expression of IL-16 is also downregulated, whereas expression of the IL-8 receptor is upregulated in chronic PTSD (Zieker et al, 2007). In addition, transcripts of genes encoding enzymes involved in metabolism of reactive oxygen species (ROS) such as GSTM1 and GSTM2 (glutathione S-transferase mu 1 and 2) are altered in chronic PTSD (Neylan et al, 2011) and have been associated with risk for PTSD development in a prospective manner (Glatt et al, 2013; Tylee et al, 2015). Finally, the expression of the gene for thioredexin (TXNRD1), a protein critical for responding to oxidative stress, is elevated in subjects with PTSD compared with traumatized controls (Logue et al, 2015).
The changes in inflammatory gene expression that have been described in individuals with PTSD are coincident with alterations in epigenetic markers, as immune-related methylation profiles are altered in PTSD (Heinzelmann and Gill, 2013). Methylation of mannosidase, alpha class 2C, member 1 (MAN2C1), acid phosphatase 5 (ACP5), and toll-like receptor (TLR) 8, which are genes involved in immune function, was found to be altered in PTSD (Smith et al, 2011; Uddin et al, 2011). Decreased methylation of immune-related genes TLR1 and TLR3 has also been described in individuals with PTSD in a manner related to the severity and burden of the traumatic event (Uddin et al, 2010). This epigenetic variability in immune function in PTSD is associated with exaggerated immune response to a cytomegalovirus challenge (Uddin et al, 2010). Furthermore, a recent analysis of microRNA expression in PBMCs from individuals with PTSD revealed that levels of microRNAs involved in immune signaling pathways are dysregulated (Zhou et al, 2014). Specifically, expression of microRNA-125a (MiR-125a) is downregulated in PTSD (Zhou et al, 2014). MiR-125a typically acts to decrease IFN-γ secretion from PBMCs, suggesting that lower levels of this microRNA in PTSD facilitate augmented IFN-γ levels.
Although a substantial amount of work has been done to characterize immune-related alterations in gene expression and methylation, there is a paucity of studies that address genomic markers that are associated with both heightened inflammation and PTSD severity. Results from a single genome-wide association study of PTSD indicate that PTSD in women is associated with an enrichment of genes involved in inflammatory pathways (Guffanti et al, 2013). Similarly, so far only one study, which we are aware of, has assessed the influence of single-nucleotide polymorphisms (SNPs) within an inflammatory genes that increases risk for inflammation and PTSD (Michopoulos et al, 2015c). This study determined that a single SNP in the CRP gene, rs1130864, was associated with heightened peripheral concentrations of CRP, augmented PTSD symptoms, and increased odds of a PTSD diagnosis in traumatized individuals (Michopoulos et al, 2015c). Overall, the discussed cross-sectional genetic and epigenetic studies indicate that higher inflammation is associated with increased PTSD severity. However, these genomic findings suggest that heightened baseline inflammatory markers due to genetic variability may serve as a biomarker of PTSD vulnerability.
The notion that augmented inflammation prior to trauma exposure increases individual risk for PTSD is supported by more recent prospective studies of PTSD risk. Indeed, higher pre-deployment concentrations of CRP increase post-deployment risk for development of PTSD (Eraly et al, 2014). Elevated IL-6 concentrations immediately following exposure to motor vehicle collision in child trauma survivors are also predictive of elevated risk for PTSD development (Pervanidou et al, 2007). High IL-8 and low transforming growth factor-β (TFG-β, normally involved in immunosuppression) have also been shown to be predictors of PTSD in the acute aftermath of trauma (Cohen et al, 2011). Transcripts of genes involved in ROS metabolism, including GSTM1 and GSTM2, have been associated with risk for PTSD development in a prospective manner (Glatt et al, 2013; Tylee et al, 2015). In addition, enriched expression of genes implicated in innate immunity and INF signaling at baseline (pre-deployment) is associated with increased risk for PTSD development post-deployment (Breen et al, 2015).
Overall, existing findings indicate that PTSD is associated with a pro-inflammatory state. Peripheral levels of cytokines and CRP are elevated in individuals with PTSD. Heightened expression of pro-inflammatory genes and coincident alterations in methylation patterns are also altered in PTSD. However, not all studies addressing this relationship have reported a positive association between increased inflammation and PTSD (McCanlies et al, 2011; Sondergaard et al, 2004; von Kanel et al, 2007). This discrepancy in the literature highlights the importance of other factors that may be influencing the association between inflammation and PTSD, such as type and chronicity of trauma exposure, clinician-administered vs self-reported measures of PTSD diagnosis, control group assessed (healthy vs trauma-exposed), and sociodemographics of individuals studied. The association between PTSD and inflammation is also impacted by other adverse mental and physical health outcomes, including depression (Gill et al, 2010; Maes et al, 1999) and cardiovascular disease (Spitzer et al, 2010; von Kanel et al, 2007), indicating that taking these co-morbid conditions into account when studying the relationship between PTSD and inflammation is critical in future studies. Furthermore, preclinical studies have suggested that stress-induced inflammatory responses can be causally related to hypertension and other cardiovascular risk factors secondary to T-lymphocyte and inflammatory effects on the vascular cellular architecture (Marvar et al, 2012).
Another important consideration is that the majority of studies examining the link between inflammation and PTSD have been cross-sectional in nature, and thus not able to address causation, or whether alterations in immune function precede trauma exposure or develop after trauma with chronic PTSD. Although no data as yet prospectively demonstrate that chronic PTSD results in augmented inflammation, prospective studies have begun to show that higher levels of inflammation prior to trauma exposure increases risk for subsequent development of PTSD following trauma exposure (Breen et al, 2015; Eraly et al, 2014; Glatt et al, 2013; Tylee et al, 2015). Continued accumulation of such prospective data is particularly important as it may aid in the identification of primary, and perhaps secondary prevention strategies to reduce risk for PTSD, or its exacerbation, in populations at high risk of trauma exposure.
Fear- and Anxiety-Based Disorders and Inflammation
The primary anxiety disorders, based on the relatively new DSM-5 nosology of fear- and anxiety-based disorders include GAD (excessive anxiety and worry paired with physical symptoms), PD (recurrent, unexpected panic attacks coupled with fear of future attacks), agoraphobia (intense fear or anxiety triggered by anticipation of exposure to places from which escape would be difficult or help not readily available), social phobia (avoidance of social situations due to fear of negative evaluation), and specific phobia (excessive fear and avoidance of a circumscribed class of objects or contexts). Although each of these anxiety disorders has its own distinct set of diagnostic criteria, the disorders share underlying features of excessive fear and anxiety, and thus may also share neurobiological features. GAD, PD, agoraphobia, social phobia, and specific phobias are often found to be highly co-morbid with each other as well as other psychiatric disorders including depression, PTSD, and substance-use disorders (Conway et al, 2006; Kaufman and Charney, 2000; Regier et al, 1998). These anxiety disorders often emerge early in life and are associated with a long course of illness and significant functional impairment. In contrast to PTSD, the literature on inflammation in relation to anxiety disorders remains extremely limited. Findings are equivocal and significant variability in samples studied as well as the measures used makes adequate comparisons across studies challenging. The majority of published studies has examined individual anxiety disorders and has focused predominantly on GAD, PD, and agoraphobia. Our review of the literature did not identify any studies examining associations between social phobia or specific phobias and inflammation. Therefore, we will provide a brief overview of the findings on altered immune function from studies examining GAD and PD (both with and without agoraphobia), as well as agoraphobia.
Initial evidence indicates that anxiety disorders may be related to heightened pro-inflammatory markers, with one study that used a mixed anxiety disorder patient group finding increased levels of CRP among male anxiety disorder patients compared with controls (Vogelzangs et al, 2013). Other evidence in children with GAD (Copeland et al, 2012) and stable coronary heart disease patients with GAD (Bankier et al, 2008) also found higher rates of CRP in individuals with GAD compared with controls. In contrast, in a sample of patients with agoraphobia, no difference in mean level of CRP compared with controls was found, although agoraphobic patients did show a significant increase in CRP levels over time, whereas controls did not (Wagner et al, 2015). Findings have also been equivocal with regard to peripheral cytokine levels. For example, some studies have found increased circulating concentrations of TNF-α among GAD (Vieira et al, 2010) and PD patients (Hoge et al, 2009), whereas other research has found no difference in TNF-α concentrations between anxiety disorders more generally (Vogelzangs et al, 2013), agoraphobia (Wagner et al, 2015) or PD patients and controls (Brambilla et al, 1999). Similarly, some studies have shown increased levels of IL-1β (Brambilla et al, 1994; Hoge et al, 2009) and IL-6 (Hoge et al, 2009) in PD patients, whereas others have found no difference in IL-1β and IL-6 between PD patients and controls (Rapaport and Stein, 1994; Tukel et al, 2012). Interestingly, lower levels of the pro-inflammatory cytokine IFN-γ have been found in both GAD and PD patients (Tukel et al, 2012; Vieira et al, 2010). Lower circulating concentrations of anti-inflammatory cytokines such as IL-2 and IL-4 have also been described in GAD patients (Vieira et al, 2010). However, IL-4 and IL-2 have also been found to be increased in PD (Hoge et al, 2009; Koh and Lee, 2004; Rapaport and Stein, 1994) or not different from controls in individuals with PD (Tukel et al, 2012).
Looking beyond inflammatory marker levels, evidence with regard to immune function or genetic mechanisms in relation to anxiety disorders remains unclear. Two studies examining immune function using circulating lymphocyte phenotypic markers in PD patients found evidence that individuals with PD may show alterations in circulating lymphocyte profiles or diminished cell activation (Manfro et al, 2000; Rapaport, 1998). Other evidence in a mixed anxiety disorder group found that those with anxiety disorders had lymphocyte and T-cell counts above the average range, as well as highly sensitized T-cell lymphocytes (Boscarino and Chang, 1999b). Similarly, studies of potential genetic and epigenetic alterations of immune function in relation to anxiety disorders are scarce. To our knowledge, only one study has been conducted on immune-related gene expression in GAD. Within a community sample of individuals with and without GAD, Wingo and Gibson (2015) found that only males with GAD showed changes in immune-related gene expression compared with controls (Wingo and Gibson, 2015).
Overall, the existing findings provide some preliminary evidence that GAD and PD in particular may be associated with a pro-inflammatory state, as evidenced by findings that peripheral concentrations of cytokines and CRP are elevated in these disorders. As not all studies addressing this relationship have reported a positive association between increased inflammation and these anxiety disorders, the importance of factors such as population assessed (community vs clinical), clinician-administered vs self-reported measures of anxiety disorder diagnosis, sociodemographic factors of individuals studied (eg, sex), and co-morbid mental and physical health problems must be considered. Looking more generally at anxiety and associations with pro-inflammatory markers in non-patient populations, researchers have found evidence that anxiety is related to higher concentrations of CRP and peripheral cytokines (Brennan et al, 2009; O'Donovan et al, 2010; Pitsavos et al, 2006) and predicts increased inflammatory response following acute stress (Carroll et al, 2011; Moons et al, 2010; Moons and Shields, 2015), demonstrating that anxiety and fear even at non-clinical levels impacts the immune response in important ways. There remains a great deal to understand about the association between anxiety disorders and inflammation, and more research is needed before any clear conclusions can be made.
Mechanisms of Increased Inflammation in Fear- and Anxiety-Based Disorders
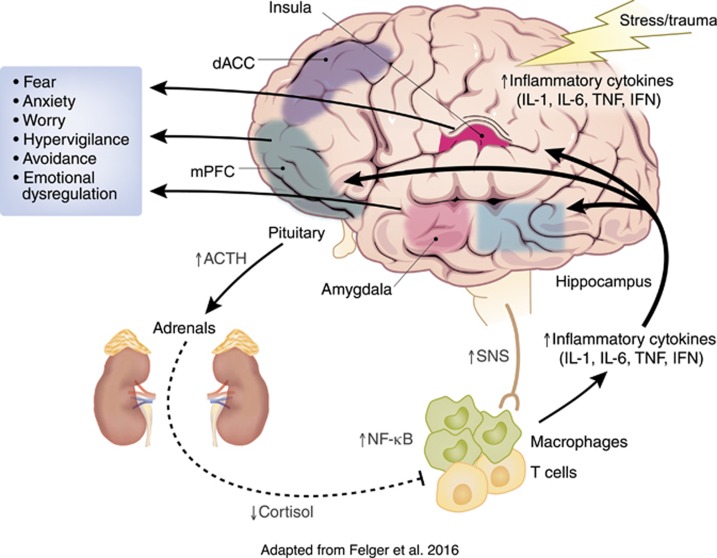
The most parsimonious explanation of increased inflammation in fear- and anxiety-based disorders is via the activation of the stress response (Figure 1). Prolonged exposure to stressful stimuli that elicit fear and anxiety in PTSD, GAD, PD, and phobias activate both central and peripheral immune cells to release cytokines, such as IL-1β (Koo and Duman, 2008; Maier and Watkins, 1998). For example, exposure to the laboratory Trier Social Stress Test activates the peripheral inflammatory response via increased NFκB transcriptional activity that results in increased circulating concentrations of IL-6 (Bierhaus et al, 2003). On a cellular level, the release of danger-associated molecular patterns, such as heat-shock proteins and adenosine triphosphate, in response to stress exposure induces a NLRP3 inflammasome response that leads to the release of IL-1β and other cytokines (Iwata et al, 2013; Maslanik et al, 2013). Although exposure to stressors can lead directly to increased inflammation through these aforementioned processes in the absence of pathogens, activation of the HPA axis and autonomic nervous system also modulate stress-induced inflammatory processes in a reciprocal manner.
Figure 1.
Inflammation in fear- and anxiety-based disorders: mechanisms and consequences. Exposure to trauma and acute stressors in individuals with fear- and anxiety-based may facilitate increased immune activity in both the periphery and the central nervous system (CNS) via stress and trauma effects on neuroendocrine systems and the sympathetic nervous system (SNS). The overactivity of the SNS and decreased activity of the parasympathetic nervous system in fear- and anxiety-based disorders increases the release of pro-inflammatory cytokines. Suppressed ability of glucocorticoids to inhibit inflammatory processes in these chronic stress states also contributes to a pro-inflammatory state that can influence neurotransmitter systems, neurocircuitry, and finally, affective behavior. Cytokines may contribute to the maintenance of fear- and anxiety-based symptoms by affecting the activity and connections of regions of the brain implicated in the etiology of these disorders, including the amygdala, hippocampus, insula, medial prefrontal cortex (mPFC), and the anterior cingulate (ACC). Figure adapted from Felger et al (2016) and reproduced by permission of Oxford University Press (http://global.oup.com/?cc=us).
HPA axis activation and subsequent secretion of glucocorticoids in response to stressor exposure typically acts to prevent pro-inflammatory activity via inhibition of NFκB (Rhen and Cidlowski, 2005). In contrast, increased norepinephrine production following stressor exposure induces NFκB activity that activates the immune system and increases cytokine production (Bierhaus et al, 2003). This sympathetic activation of the innate immune system acts via nerve fibers that innervate lymphoid organs that coordinate the innate immune responses to threat via alterations in adrenergic signaling (Nance and Sanders, 2007; Tan et al, 2007). Interestingly, evidence suggests that activation of the parasympathetic nervous system also can modulate the activity of the immune system via alterations in vagal release of acetylcholine from T cells (Tracey, 2009). For example, motor vagus nerve stimulation attenuates the activation of NFκB following an immune challenge (Tracey, 2009). Taken together, these data suggest that any chronic condition that results in the diminished actions of glucocorticoids, and increased sympathetic and decreased parasympathetic activity should occur in tandem with increased inflammation. PTSD represents exactly such a chronic condition.
HPA axis dysregulation in PTSD facilitates a pro-inflammatory state
Exposure to trauma activates neuroendocrine responses and leads to long-lasting changes in the regulation of the HPA axis that compromise its ability to function appropriately. Decreased basal levels of circulating (Yehuda et al, 2005) and urinary free cortisol (Mason et al, 1986) have been described in individuals with PTSD. However, studies have also found increased or no differences in basal glucocorticoid levels (Meewisse et al, 2007) and diurnal cortisol rhythms in individuals with PTSD (Freidenberg et al, 2010; Maes et al, 1998), suggesting that other factors maybe be contributing to HPA dysregulation in PTSD, such as sex (Freidenberg et al, 2010), type and duration of trauma exposure, and severity of PTSD symptoms (Shea et al, 2005). Regardless of the equivocal nature of findings describing differences in basal cortisol levels in individuals with PTSD, more consistent are the findings that PTSD is associated with enhanced glucocorticoid negative-feedback inhibition of the HPA axis as evidenced by increased suppression of cortisol levels following a dexamethasone-suppression test (Yehuda et al, 1995). Heightened levels of peripheral and central corticotropin-releasing hormone (CRH; Baker et al, 2005; de Kloet et al, 2008) and elevated glucocorticoid receptor (GR) expression levels in lymphocytes (Matic et al, 2013) occur in tandem with enhanced glucocorticoid sensitivity in PTSD.
One critical modulator of HPA axis responsivity and glucocorticoid function is FKBP5, a heat-shock protein 90 co-chaperone that functions to negatively regulate the GR complex by inhibiting ligand binding and nuclear translocation of GR (reviewed in Binder (2009)). The expression of FKBP5 is induced by glucocorticoids, thus forming an ultra-short intracellular negative-feedback loop for GR activity in response to stressor exposure (Vermeer et al, 2003), such that increased expression of FKBP5 following GR activation leads to the subsequent reduction in GR sensitivity (Binder, 2009). The reciprocal association between decreased levels of FKBP5-mRNA and enhanced GR sensitivity is characteristic of PTSD in trauma survivors (Yehuda et al, 2009). Importantly, SNPs in the FKBP5 gene that are associated with higher FKBP5-mRNA induction upon cortisol release (rs1360780, rs9296158, rs3800373, and rs9470080) are also associated with increased PTSD symptom severity in those with high levels of child abuse (Binder et al, 2008). Interestingly, some alleles of these FKBP5-SNPs are associated with enhanced glucocorticoid sensitivity, whereas other alleles of these SNPs are associated with GR resistance in individuals with PTSD (Binder et al, 2008).
Thus, the presence of these FKPB5 alleles can result in low levels of circulating cortisol and/or GR resistance in PTSD can lead to a pro-inflammatory state via decreases in anti-inflammatory GR signaling (Cohen et al, 2012). FKBP5-genotype status and trauma exposure history maybe also lead to increased inflammation, as exposure to childhood trauma is associated with epigenetic de-methylation near the rs1360780 FKBP5-SNP that is associated with GR dysregulation and increased expression of mRNA transcripts involved in T-cell receptor, TFG-β, and inflammatory response signaling pathways (Klengel et al, 2013). Together these data highlight the notion that both trauma exposure and PTSD can facilitate a pro-inflammatory state as described in Table 1. It is important to emphasize that this chronic low-grade inflammation characteristic of PTSD and other fear- and anxiety-based disorders can act to further impair GR signaling in a number of ways (reviewed in Pace et al (2007)). First, expression of GR is increased in whole-cell radioligand binding in vitro studies in response to challenge with pro-inflammatory cytokines (IL-6, TNF-α, and IFN-α; Miller et al, 1999). Similar studies assessing cytosolic radioligand assays find GR expression to be decreased upon treatment with these same pro-inflammatory signals (Miller et al, 1999). Second, TNF-α or IL-1 administration in vitro alters the expression of the two GR isoforms, hGRα (active) and hGRβ (non-active), via NFκB activity (Webster et al, 2001). The proportion of hGRα (active) and hGRβ directly influences the ability of glucocorticoids to activate GR-dependent genes by contributing to glucocorticoid resistance (Lewis-Tuffin and Cidlowski, 2006). Finally, cytokines can impair GR function by disrupting GR translocation and inhibiting downstream GR signaling, including NFκB and mitogen-activated protein kinase cascades (Miller et al, 1999; Pace et al, 2007).
Autonomic nervous system and immune interactions in PTSD
Heightened sympathetic tone in the form of increased catecholamine secretion has been described consistently in individuals with PTSD (Southwick et al, 1999). Peripheral and central concentrations of norepinephrine are augmented in individuals with PTSD at baseline (Delahanty et al, 2005; Geracioti et al, 2001; Southwick et al, 1999) and following exposure to threatening stimuli (Blanchard et al, 1991; Geracioti et al, 2008). This increased norepinephrine production in PTSD in response to stressful or threatening stimuli can induce cytokine release (Bierhaus et al, 2003). The subsequent immune response occurs via NFκB-dependent and -independent mechanisms (Bierhaus et al, 2003; Tan et al, 2007). More specifically, activation of adrenergic-β2 receptors stimulates IL-6 and IL-1β secretion from macrophages and monocytes via NFκB and ERK signaling pathways, respectively (Bierhaus et al, 2003; Tan et al, 2007). Thus, the increased expression and function of adrenergic-β2 receptors that has been described in individuals with PTSD (Gurguis et al, 1999) can also contribute to increased inflammation in those with the disorder (Table 1). Finally, another robust phenotype characteristic of PTSD that may contribute to increased inflammation is decreased parasympathetic drive as evidenced by decreased heart rate variability (HRV; Blechert et al, 2007; Cohen et al, 2000).
HPA, autonomic nervous system, and immune interactions in other fear- and anxiety-based disorders
Hair cortisol levels are elevated in individuals with GAD and PD (Staufenbiel et al, 2013) and increased salivary cortisol concentrations have been associated with late-life GAD (Mantella et al, 2008). Higher cortisol awaking response has been described in individuals with PD with agoraphobia (Vreeburg et al, 2010) and cortisol nonsuppression in response to dexamethasone in those with agoraphobia and PD (Coryell et al, 1989). Although these data suggest that increased HPA axis functioning and GR dysregulation is present in these fear- and anxiety-based disorders, other reports have shown decreased cortisol levels or no differences in HPA axis function in those with GAD, PD, and phobia (Hek et al, 2013; van Veen et al, 2008). For instance, CRH levels in GAD and PD are not different from controls (Fossey et al, 1996; Jolkkonen et al, 1993). One factor that might account for these discrepancies is the onset of anxiety symptoms, as childhood anxiety disorder is associated with decreased basal HPA tone, increased sympathetic and attenuated parasympathetic activity (Dieleman et al, 2015). Increases in sympathetic tone, decreases in parasympathetic tone, and compromised vagal tone have also been described in PD (Blechert et al, 2007; Cohen et al, 2000). Similarly, studies have described decreased HRV in GAD (Thayer et al, 1996), social anxiety (Alvares et al, 2013), and specific phobia (Bornas et al, 2006). A recent meta-analysis concluded that all these anxiety disorders are all associated with reduced HRV (Chalmers et al, 2014). Taken together, the heightened sympathetic tone and reduction in parasympathetic activity, as well as the likely dysregulation of the HPA axis in anxiety disorders could lead to increased inflammation (Table 2) via similar pathways to those discussed above in the context of PTSD. However, future studies are necessary to better describe the effects of HPA axis and autonomic nervous system dysfunction on chronic low-grade inflammation in GAD, PD, social anxiety, and phobias.
Table 2. Immunological Factors Associated with Anxiety Disorders.
| Immune biomarkers | Relationship to anxiety disorders | References |
|---|---|---|
| Interleukin-6 | Increased in PD | Hoge et al (2009) |
| Interleukin-1β | Increased in PD | Brambilla et al (1994) and Hoge et al (2009) |
| Interleukin-2 | Decreased in GAD Increased in PD | Koh and Lee (2004), Rapaport and Stein (1994), and Vieira et al (2010) |
| C-reactive protein | Increased in GAD Increases over time in agoraphobia | Bankier et al (2008), Copeland et al (2012), and Wagner et al (2015) |
| Tumor necrosis factor-α | Increased in GAD and PD Increases over time in agoraphobia | Hoge et al (2009), Vieira et al (2010), and Wagner et al (2015) |
Behavioral sources of inflammation in fear- and anxiety-based disorders
It is critical to keep in mind that there are many behavioral symptoms that also contribute to inflammatory response and functioning that often co-occur with PTSD and other fear- and anxiety-based disorders. These disorders cause intense distress and disrupt daily-life functioning, which in turn impacts general health habits and how individuals take care of themselves. For example, disruptions in regular sleep patterns can have a very detrimental effect on the immune system (Bryant et al, 2004) and persistent problems with sleep (eg, difficulty falling asleep, middle of the night awakenings) are a symptom of both PTSD and GAD. Severe sleep loss has been shown to increase circulating levels of CRP (Meier-Ewert et al, 2004) and IL-6 (Vgontzas et al, 1999). Even mild reductions in sleep quantity can increase inflammation levels (Vgontzas et al, 2004), suggesting that chronic problems with sleep, which is characteristic of PTSD and GAD, could contribute to a pro-inflammatory state.
Patients with PTSD or anxiety disorders may also be less likely to engage in healthy behavior, such as balanced eating and exercise, and more likely to engage in unhealthy behaviors, such as smoking. Indeed, individuals with PTSD and anxiety disorders are more likely to smoke (Fu et al, 2007; Morissette et al, 2007). PTSD and anxiety disorders are also associated with obesity, which may result in part from emotional eating behavior (Scott et al, 2008). Smoking has been linked to a pro-inflammatory state (Frohlich et al, 2003; Jamal et al, 2014). Obesity and high BMI are also associated with increased concentrations of inflammatory markers, such as CRP and IL-6 (Khaodhiar et al, 2004). However, at least with PTSD, increased inflammation levels have remained significant after adjusting for BMI in multiple studies (Heath et al, 2013; McCanlies et al, 2011; Spitzer et al, 2010). This suggests that although health risk factors such as smoking and BMI may contribute to inflammation, the relationship between inflammation and PTSD remains significant beyond these behavioral risk factors. Because of the strong impact of these behavioral symptoms on immune function, it will be important in future research to disentangle the particular effects of such behaviors from the effects of PTSD and anxiety disorders to better understand the unique contribution of fear- and anxiety-based disorders on inflammation.
Consequences of Increased Inflammation in Fear- and Anxiety-Based Disorders
Increased inflammation and cytokine activity in PTSD and other fear- and anxiety-based disorders can lead to an array of other adverse physical health and behavioral outcomes, including cardiovascular disease (CVD), diabetes, chronic fatigue syndrome, fibromyalgia, gastrointestinal disease, musculoskeletal disorders, and autoimmune disorders such as thyroiditis and rheumatoid arthritis, and irritable bowel disease (Boscarino, 2004; O'Donovan et al, 2015b). A pro-inflammatory state may serve as an underlying biological mechanism by which PTSD and other fear- and anxiety-based disorders are highly co-morbid with CVD (Boscarino and Chang, 1999a) and metabolic syndrome (Weiss et al, 2011), as well as other physical illnesses (Boscarino, 2004). Furthermore, low-grade inflammation may also facilitate the expression of behavioral co-morbidities, such as depression, that are characterized by alterations in stress pathways and neurotransmitter systems (Haroon et al, 2012). Although inflammation can similarly facilitate changes in stress and metabolic systems in PTSD that may account for physical health co-morbidities associated with PTSD, those are out of the scope of the current review. We will next consider the effects of inflammation on neurocircuitry involved in the regulation of fear and anxiety that can contribute to the manifestation of fear and anxiety symptoms in PTSD, GAD, and other fear- and anxiety-based disorders.
Amygdala and hippocampus
The role of the amygdala in the etiology and maintenance of PTSD and other fear- and anxiety-based disorders has been very well characterized in translational neuroscience (Etkin and Wager, 2007). Specifically, amygdala activation in response to threatening stimuli is increased in PTSD, GAD, social anxiety disorder, specific phobia, and PD (Fonzo et al, 2015; Killgore et al, 2014; Monk et al, 2008). This heightened amygdala response to stress is associated with increased IL-6 production (Inagaki et al, 2012; Muscatell et al, 2015). Specifically, endotoxin administration in healthy controls increases IL-6 and TNF-α levels (Eisenberger et al, 2010) and amygdala response to socially threatening stimuli (Harrison et al, 2009b; Inagaki et al, 2012). Administration of typhoid vaccination also increases IL-6 response (Harrison et al, 2009a) and amygdala activity (Harrison et al, 2009b). Importantly, these increases in cytokine activity and amygdala responsivity to stress exposure are also associated with greater social disconnection and depressed mood (Muscatell et al, 2015), as well as cognitive disturbance and increased fatigue (Harrison et al, 2009b). Furthermore, this inflammation-induced amygdala response may also contribute to the association between increased CRP levels and heightened psychophysiological hyperarousal in traumatized individuals with PTSD (Michopoulos et al, 2015b).
The hippocampus is another brain structure within the medial temporal lobe whose function, structure, and functional connectivity with other regions is comprised in individuals with PTSD, GAD, and PD (Cui et al, 2016; Fani et al, 2012b; Woon et al, 2010). Hippocampal alterations, including smaller hippocampal volume, are associated with both emotional and cognitive deficits in individuals with PTSD (Bremner, 2006). Inflammatory processes may have a critical role in the etiology of hippocampal atrophy, as translational work in rodent models indicates that cytokines released from microglia inhibit neurogenesis within the dentate gyrus (Ekdahl et al, 2003) and promote neuronal apoptosis (Cunningham et al, 2005). In turn, reduced hippocampal volume is associated with increased inflammation and more severe PTSD symptoms in veterans (O'Donovan et al, 2015a). Cytokine actions can also facilitate cognitive and emotional deficits associated with PTSD, as IL-1β blocks long-term potentiation in the hippocampus, and impairs spatial and contextual memory processing (Cunningham et al, 1996; Yirmiya and Goshen, 2011). Inflammatory challenges increase IL-1 in the medial temporal lobe (Ban et al, 1992) and also facilitate deficits in memory performance and contextual fear conditioning in rodents (Pugh et al, 1998; Yirmiya and Goshen, 2011). Finally, typhoid vaccination in healthy humans results in compromised spatial memory and decreased glucose metabolism in the perirhinal and entorhinal cortex (Harrison et al, 2014).
Medial prefrontal cortex and anterior cingulate
Regions of the medial prefrontal cortex (mPFC), including the rostral anterior cingulate cortex, subgenual ACC (sgACC, Brodmann's Area 25) and medial frontal gyrus, are all heavily connected to the amygdala and hippocampus, and critically involved in emotion regulation, attention bias, and fear extinction in individuals with PTSD and other anxiety disorders (Banich et al, 2009; Cui et al, 2016; Etkin and Wager, 2007; Fani et al, 2012a; Monk et al, 2008). Activation of the ventral mPFC (including the sgACC and the orbitofrontal cortex) due to a grief-elicitation task in women undergoing bereavement stress is associated with increased IL-1β and TNF receptor II (TNF-II; O'Connor et al, 2009). Similarly, activation of the sgACC in response to typhoid vaccination is increased concurrently with mood deterioration (Harrison et al, 2009a). Functional connectivity between the sgACC and the amygdala and mPFC is also reduced upon typhoid vaccination in a manner that was associated with vaccine-induced increases in IL-6 (Harrison et al, 2009a). Similarly, increased CRP and IL-6 is associated with decreased functional connectivity between the ventral mPFC and the striatum (Felger et al, 2015). Finally, exposure to a laboratory stressor that increases IL-6 concentrations peripherally was associated with increased functional connectivity between the dorsomedial PFC and the amygdala in a manner that was correlated with IL-6 (Muscatell et al, 2015).
Another area that has been extensively studied in the context of inflammation effects of neurocircuitry is the dorsal ACC (dACC, Brodmann's Area 24), as it is a target of central cytokine action and has a critical role in detecting and responding to threatening social and physical pain stimuli (Eisenberger and Lieberman, 2004). More specifically, the dACC serves as a stress-response system in both an emotional and physical way, as activation of the dACC leads to downstream stimulation of the autonomic nervous system (Critchley et al, 2005; Matthews et al, 2004). Neuroimaging studies in hepatitis C patients indicate that treatment with INF-α results in increased dACC activity that is correlated with visual–spatial-attention errors (Capuron et al, 2005). Typhoid vaccination also results in heightened activation of the dACC that is concurrent with increased blood floor during a Stroop task (Eisenberger and Lieberman, 2004; Harrison et al, 2009b). Similarly, endotoxin administration in healthy controls results in augmented social pain-related neural activity in the dACC that is associated with increases in IL-6 of females but not males (Eisenberger et al, 2005), corroborating previous reports of sex differences in inflammation in individuals with PTSD (Neylan et al, 2011).
Exaggerated activation of the dACC has been well described in individuals with PTSD (Felmingham et al, 2009; Milad et al, 2009; Pannu Hayes et al, 2009; Shin et al, 2007; Shin et al, 2001) and is associated with increased attention bias to threat (Fani et al, 2012a). Women with PTSD due to interpersonal trauma also show increased dACC activity in response to viewing threatening facing (Eisenberger et al, 2005). Individuals with neuroticism (Eisenberger et al, 2005) and high trait anxiety (Paulus et al, 2004) also show increased dACC activation. Augmented activity of the dACC has also been described as a mediator of hyperarousal symptoms in individuals with PTSD (Hamner et al, 1999), and has been associated with increased familial risk for PTSD development (Shin et al, 2011). Although most studies have focused on the role of mPFC and ACC in the etiology of PTSD, some studies indicate that the same regions are critical to the etiology of PD, GAD, social anxiety disorder, specific phobia, and agoraphobia. A meta-analysis indicates that reduced volume of the ventral ACC and the inferior frontal gyrus is common to anxiety disorders (social anxiety, GAD, PD, agoraphobia, and specific phobia; Shang et al, 2014). Finally, the dACC in the etiology of PD is suggested by data that indicate that surgery damage to the dACC can induce panic attacks (Shinoura et al, 2011). Overall these data indicate that inflammatory processes within the dACC may serve as a potent mechanism by which behavioral alterations may occur in individuals with fear- and anxiety-based disorders.
Insula
The insula is another brain area whose activity is associated with that of the amygdala and critical for the manifestation of emotional distress characteristic of PTSD and anxiety disorders. For instance, women with PTSD show decreased activation of the insula in response to shifts in interceptive responses (Simmons et al, 2009) and anxiety-prone individuals show heightened insula activity in anticipation of aversive visual stimuli (Simmons et al, 2006). Women with PTSD from intimate partner violence show increased activation of the insula and amygdala, along with concurrent decreases in the functional connectivity between the insula, amygdala, and ACC (Fonzo et al, 2010). Increased activity of the insula has also been associated with other anxiety-based disorders (Paulus and Stein, 2006). Specifically, anxious individuals and those with PD (Fonzo et al, 2015) show increased insula activity (Alvarez et al, 2015). Importantly, peripheral inflammatory stimuli are capable of increasing the activity of the insula. Typhoid vaccination and endotoxin administration both increase insula activity (Eisenberger et al, 2009; Harrison et al, 2009b). Endotoxin administration also augmented glucose metabolism in the insula as determined by positron emission tomography neuroimaging (Hannestad et al, 2012).
Possible Mechanism by Which Inflammation Alters Brain and Behavior in Fear- and Anxiety-Based Disorders
It is clear that inflammatory processes influence neurobiological substrates underlying behavior and emotion characteristic of fear- and anxiety-based disorders (Figure 1). One biological mechanism by which inflammation influences neural networks critical for the regulation of emotional behavior and fear processes is by altering central neurotransmitter systems (reviewed in Dunn et al (1999)). For instance, administration of IFN-α for the treatment of hepatitis C increases glutamate to creatinine levels in the dACC that correlate with anhedonia and fatigue (Haroon et al, 2014). Increased CRP in depression is also associated with heightened glutamate levels within the basal ganglia (Haroon et al, 2016). Augmented release of glutamate from astrocytes has been shown to be decrease brain-derived neurotrophic factor and increase excitotoxicity (Hardingham and Bading, 2010).
Cytokines can also lead to excitotoxicity by altering the function of indoleamine 2,3 dioxygenase, the enzyme that acts to convert tryptophan into kynurenine (Schwarcz, 2004). Kynurenine can be broken down by macrophages and microglia to form quinolinic acid, a N-methyl-D-aspartate receptor agonist that can directly simulate and block the re-uptake of glutamate by astrocytes (Tavares et al, 2002). Although alterations in the kynurenine pathway have been described in major depression, there are currently no published accounts of alterations in this system in PTSD and other fear- and anxiety-based disorders. However, injections of TNF-α and IL-6 in the amygdala results in glutamate toxicity that is associated with impaired auditory fear conditioning in rodents (Hao et al, 2014; Jing et al, 2015). It is also important to note that levels of other neurotransmitters are altered in PTSD and other fear- and anxiety-based disorders that maybe related to inflammation-induced exicitotoxicity (Crowley et al, 2016). Specifically, reduced GABA concentrations have been reported within the insula of individuals with PTSD (Rosso et al, 2014) and within the mPFC, ACC, and occipital cortex of those with PD (Goddard et al, 2001; Long et al, 2013), and reductions in GABA signaling can contribute to glutamate excitotoxicity via inflammatory pathways (Crowley et al, 2016).
Treatment Implications and Future Directions
In sum, heightened inflammation and increased cytokine production in the face of HPA axis and autonomic nervous system dysregulation is characteristic of fear- and anxiety-based disorders. Although the majority of work has characterized a pro-inflammatory state in individuals with PTSD, the limited data available regarding the relationship between GAD, PD, phobias, and inflammation also point toward augmented inflammation. It is clear that more studies are necessary in these other anxiety disorders to further delineate the role of inflammation in their pathophysiology, as a state of chronic low-grade inflammation in fear- and anxiety-based disorders may lead directly to alterations in neurobiology critical for the control of emotional behavior and fear regulation that are perturbed in these disorders.
Future studies addressing these gaps in knowledge will also allow for meta-analyses to be conducted within fear- and anxiety-based disorders and across anxiety disorders as a whole. Overall, our non-systematic review is the first to synthesize available data on inflammation across fear- and anxiety-based disorders as defined by the DSM-5 (APA, 2014). The available data indicate a clear role for inflammation in the etiology and maintenance of fear- and anxiety-based disorders, increased inflammation is not specific to these disorders, as other mental health problems, such as depression, are also associated with a pro-inflammatory state (Dowlati et al, 2010). The lack of specificity in the era of the Research Domain Criteria marshaled in by the National Institutes of Mental Health (Cuthbert, 2014) suggests that inflammation may better serve as a biomarker of specific sub-domains of dysfunction (ie, negative- and positive-valence systems).
Regardless of specificity for DSM-based diagnoses, the presence of heightened inflammatory processes in these fear- and anxiety-disorders has important translational and clinical implications by providing the field with a range of new therapeutic targets that must be thoughtfully further investigated (Michopoulos and Jovanovic, 2015a). One possible therapeutic avenue that warrants further investigation is the use angiotensin-converting enzyme inhibitors (ACE-I) and blockers (ARBs) in the treatment of PTSD, as these classes of pharmacological agents are effective in managing cardio-metabolic disease that is highly co-morbid with PTSD. ACE-I/ARBs are typically prescribed to decrease blood pressure and sympathetic activity (Savoia and Schiffrin, 2007). However, these agents are also capable of reducing neuroinflammation (Benicky et al, 2011; Welty et al, 2015), as angiotensin-II activity increases CRP and IL-6 release (Sano et al, 2001; Zhao et al, 2013). The promise of using ACE-I/ARBs to alleviate PTSD symptoms in traumatized individuals is highlighted by evidence indicating that traumatized individuals using ACE-I/ARB medication have decreased odds of PTSD diagnosis and fewer PTSD symptoms compared with traumatized individuals not taking these medications (Khoury et al, 2012). Thus, interventions that attenuate inflammatory processes in fear- and anxiety-based disorders may prove to be effective in mitigating the symptoms of these disorders, as well as decreasing associated adverse cardio-metabolic outcomes.
Although the promise of anti-inflammatory pharmacological agents for the treatment of chronic psychopathology has gained traction based on the work done with regard to depression (Raison et al, 2013; Uher et al, 2014), other forms of intervention could prove efficacious in dampening inflammatory processes in fear- and anxiety-based disorders. Cognitive and behavioral interventions, including prolonged exposure, are effective treatments for fear- and anxiety-based disorders (Butler et al, 2006; Powers et al, 2010), but it still remains unclear whether these treatments also reduce inflammation. Other forms of behavioral interventions that have been used for the treatment of fear- and anxiety-based disorder, such as community-based educational intervention, exercise, yoga, and mediation, have also been associated with decreases in inflammation (Bower and Irwin, 2016; Pace et al, 2009; Villablanca et al, 2015). Finally, it is important to note that dietary intake and the microbiome are potent modulators of inflammatory pathways (Kiecolt-Glaser, 2010; Petra et al, 2015), indicating that adherence to a Mediterranean diet or use of probiotics can also lead to a reduction in inflammation (Dai et al, 2008; Kekkonen et al, 2008).
Empirical evidence is still necessary to determine whether anti-inflammatory treatments are beneficial for all individuals with fear- and anxiety-based disorders. It may be the case that anti-inflammatory interventions are only efficacious in those individuals who show significantly elevated levels of inflammation, similar to what has been described in depression (Raison et al, 2013). Furthermore, acknowledging factors that are associated with systemic inflammation, such as obesity and exposure to childhood adversity, is critical for better informing treatment selection in individuals with fear- and anxiety-based disorders (Kiecolt-Glaser et al, 2015). This more personalized approach to therapeutic intervention may yield better therapeutic results. However, the field must continue to characterize factors that are associated with increased inflammation and adverse mental and physical health outcomes. For example, there is a clear sex difference in the prevalence of PTSD and other fear- and anxiety-based disorders (Kessler et al, 1995; McLean et al, 2011), and yet there is a paucity of data explaining the etiology of this sex difference. Recent work highlights the importance of considering sex as an important biological variable, as endotoxin-induced increases IL-6 and TNF-α are correlated with greater feelings of depressed mood and social disconnection only in women (Moieni et al, 2015). Thus, taking into consideration the whole range of biological and behavioral factors that influence inflammatory processes will ultimately improve the treatment and management of fear- and anxiety-based disorders, as well as better inform therapeutic strategies using anti-inflammatory agents.
FUNDING AND DISCLOSURE
This review was supported in part by the National Institute of Health: HD085850 for V.M., MH102890 for A.B., MH099211 for C.F.G., MH071537 and MH094757 for K.J.R., and MH100122 for T.J. The authors declare no conflict of interest.
Acknowledgments
The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Afzali B, Lombardi G, Lechler RI, Lord GM (2007). The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol 148: 32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello AE, Dowd JB, Jayabalasingham B, Feinstein L, Uddin M, Simanek AM et al (2016). PTSD is associated with an increase in aged T cell phenotypes in adults living in Detroit. Psychoneuroendocrinology 67: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M, Cloitre M, Dhabhar FS (2003). Enhanced cellular immune response in women with PTSD related to childhood abuse. Am J Psychiatry 160: 1705–1707. [DOI] [PubMed] [Google Scholar]
- Alvares GA, Quintana DS, Kemp AH, Van Zwieten A, Balleine BW, Hickie IB et al (2013). Reduced heart rate variability in social anxiety disorder: associations with gender and symptom severity. PloS One 8: e70468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Kirlic N, Misaki M, Bodurka J, Rhudy JL, Paulus MP et al (2015). Increased anterior insula activity in anxious individuals is linked to diminished perceived control. Transl Psychiatry 5: e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) (2014). Diagnostic and Statistical Manual of Mental Disorders. 5th edn. Washington, DC.
- Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, Bednarik L et al (2005). Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. Am J Psychiatry 162: 992–994. [DOI] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA et al (2001). Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation 9: 209–217. [DOI] [PubMed] [Google Scholar]
- Ban E, Haour F, Lenstra R (1992). Brain interleukin 1 gene expression induced by peripheral lipopolysaccharide administration. Cytokine 4: 48–54. [DOI] [PubMed] [Google Scholar]
- Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W (2009). Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav Rev 33: 613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankier B, Barajas J, Martinez-Rumayor A, Januzzi JL (2008). Association between C-reactive protein and generalized anxiety disorder in stable coronary heart disease patients. Eur Heart J 29: 2212–2217. [DOI] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V (2015). Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry 21: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benicky J, Sanchez-Lemus E, Honda M, Pang T, Orecna M, Wang J et al (2011). Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology 36: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersani FS, Wolkowitz OM, Lindqvist D, Yehuda R, Flory J, Bierer LM et al (2016). Global arginine bioavailability, a marker of nitric oxide synthetic capacity, is decreased in PTSD and correlated with symptom severity and markers of inflammation. Brain Behav Immun 52: 153–160. [DOI] [PubMed] [Google Scholar]
- Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW (2012). Inflammation and early-life abuse in women. Am J Prev Med 43: 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D et al (2003). A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA 100: 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB (2009). The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 34 Suppl 1: S186–S195. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB et al (2008). Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama 299: 1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard EB, Kolb LC, Prins A, Gates S, McCoy GC (1991). Changes in plasma norepinephrine to combat-related stimuli among Vietnam veterans with posttraumatic stress disorder. J Nerv Ment Dis 179: 371–373. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH (2007). Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med 69: 935–943. [DOI] [PubMed] [Google Scholar]
- Bornas X, Llabres J, Noguera M, Lopez AM, Gelabert JM, Vila I (2006). Fear induced complexity loss in the electrocardiogram of flight phobics: a multiscale entropy analysis. Biol Psychol 73: 272–279. [DOI] [PubMed] [Google Scholar]
- Boscarino JA (2004). Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci 1032: 141–153. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Chang J (1999. a). Electrocardiogram abnormalities among men with stress-related psychiatric disorders: implications for coronary heart disease and clinical research. Ann Behav Med 21: 227–234. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Chang J (1999. b). Higher abnormal leukocyte and lymphocyte counts 20 years after exposure to severe stress: research and clinical implications. Psychosom Med 61: 378–386. [DOI] [PubMed] [Google Scholar]
- Bower JE, Irwin MR (2016). Mind-body therapies and control of inflammatory biology: a descriptive review. Brain Behav Immun 51: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla F, Bellodi L, Perna G (1999). Plasma levels of tumor necrosis factor-alpha in patients with panic disorder: effect of alprazolam therapy. Psychiatry Res 89: 21–27. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Bellodi L, Perna G, Bertani A, Panerai A, Sacerdote P (1994). Plasma interleukin-1 beta concentrations in panic disorder. Psychiatry Res 54: 135–142. [DOI] [PubMed] [Google Scholar]
- Breen MS, Maihofer AX, Glatt SJ, Tylee DS, Chandler SD, Tsuang MT et al (2015). Gene networks specific for innate immunity define post-traumatic stress disorder. Mol Psychiatry 20: 1538–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD (2006). Traumatic stress: effects on the brain. Dialogues Clin Neurosci 8: 445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AM, Fargnoli JL, Williams CJ, Li T, Willett W, Kawachi I et al (2009). Phobic anxiety is associated with higher serum concentrations of adipokines and cytokines in women with diabetes. Diabetes Care 32: 926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant PA, Trinder J, Curtis N (2004). Sick and tired: Does sleep have a vital role in the immune system? Nat Rev Immunol 4: 457–467. [DOI] [PubMed] [Google Scholar]
- Butler AC, Chapman JE, Forman EM, Beck AT (2006). The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev 26: 17–31. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS et al (2005). Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry 58: 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC et al (2011). Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain Behav Immun 25: 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers JA, Quintana DS, Abbott MJ, Kemp AH (2014). Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiatry 5: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M (2000). Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Res 96: 1–13. [DOI] [PubMed] [Google Scholar]
- Cohen M, Meir T, Klein E, Volpin G, Assaf M, Pollack S (2011). Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents. Int J Psychiatry Med 42: 117–131. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS et al (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA 109: 5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF (2006). Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 67: 247–257. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ (2012). Generalized anxiety and C-reactive protein levels: a prospective, longitudinal analysis. Psychol Med 42: 2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell W, Noyes R Jr., Schlechte J (1989). The significance of HPA axis disturbance in panic disorder. Biol Psychiatry 25: 989–1002. [DOI] [PubMed] [Google Scholar]
- Coughlin SS (2011). Post-traumatic stress disorder and cardiovascular disease. Open Cardiovasc Med J 5: 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ (2005). Anterior cingulate activity during error and autonomic response. NeuroImage 27: 885–895. [DOI] [PubMed] [Google Scholar]
- Crowley T, Cryan JF, Downer EJ, O'Leary OF (2016). Inhibiting neuroinflammation: the role and therapeutic potential of GABA in neuro-immune interactions. Brain Behav Immun 54: 260–277. [DOI] [PubMed] [Google Scholar]
- Cui H, Zhang J, Liu Y, Li Q, Li H, Zhang L et al (2016). Differential alterations of resting-state functional connectivity in generalized anxiety disorder and panic disorder. Hum Brain Mapp 37: 1459–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AJ, Murray CA, O'Neill LA, Lynch MA, O'Connor JJ (1996). Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett 203: 17–20. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH (2005). Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci 25: 9275–9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN (2014). Translating intermediate phenotypes to psychopathology: The NIMH Research Domain Criteria. Psychophysiology 51: 1205–1206. [DOI] [PubMed] [Google Scholar]
- Dai J, Jones DP, Goldberg J, Ziegler TR, Bostick RM, Wilson PW et al (2008). Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr 88: 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R (2007). Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA 104: 1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Lehrner A, Yehuda R (2013). Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol Metab Clin North Am 42: 503–513. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Lentjes EG, Heijnen CJ, Stalla GK et al (2008). Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Prog Brain Res 167: 287–291. [DOI] [PubMed] [Google Scholar]
- Dedert EA, Calhoun PS, Watkins LL, Sherwood A, Beckham JC (2010). Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med 39: 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahanty DL, Nugent NR, Christopher NC, Walsh M (2005). Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology 30: 121–128. [DOI] [PubMed] [Google Scholar]
- Dieleman GC, Huizink AC, Tulen JH, Utens EM, Creemers HE, van der Ende J et al (2015). Alterations in HPA-axis and autonomic nervous system functioning in childhood anxiety disorders point to a chronic stress hypothesis. Psychoneuroendocrinology 51: 135–150. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK et al (2010). A meta-analysis of cytokines in major depression. Biol Psychiatry 67: 446–457. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Wang J, Ando T (1999). Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv Exp Med Biol 461: 117–127. [DOI] [PubMed] [Google Scholar]
- Edwards VJ, Holden GW, Felitti VJ, Anda RF (2003). Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry 160: 1453–1460. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR (2010). Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun 24: 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR (2009). An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. NeuroImage 47: 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD (2004). Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci 8: 294–300. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Satpute AB (2005). Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cogn Affect Behav Neurosci 5: 169–181. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O (2003). Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA 100: 13632–13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A et al (2014). Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry 71: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Jovanovic T, Ely TD, Bradley B, Gutman D, Tone EB et al (2012. a). Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol 90: 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K et al (2012. b). White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology 37: 2740–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X et al (2015). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. (e-pub ahead of print; doi:10.1038/mp.2015.168). [DOI] [PMC free article] [PubMed]
- Felger JC, Haroon E, Miller AH (2016) Inflammation and immune function in PTSD: mechanisms, consequences, and translational implications. In: Liberzon I, Ressler KJ (eds). Neurobiology of PTSD: From Brain to Mind. Oxford University Press: New York, pp 239–263. [Google Scholar]
- Felmingham KL, Williams LM, Kemp AH, Rennie C, Gordon E, Bryant RA (2009). Anterior cingulate activity to salient stimuli is modulated by autonomic arousal in posttraumatic stress disorder. Psychiatry Res 173: 59–62. [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Ramsawh HJ, Flagan TM, Sullivan SG, Letamendi A, Simmons AN et al (2015). Common and disorder-specific neural responses to emotional faces in generalised anxiety, social anxiety and panic disorders. Br J Psychiatry 206: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB (2010). Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry 68: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossey MD, Lydiard RB, Ballenger JC, Laraia MT, Bissette G, Nemeroff CB (1996). Cerebrospinal fluid corticotropin-releasing factor concentrations in patients with anxiety disorders and normal comparison subjects. Biol Psychiatry 39: 703–707. [DOI] [PubMed] [Google Scholar]
- Freidenberg BM, Gusmano R, Hickling EJ, Blanchard EB, Bremner JD, Frye C (2010). Women with PTSD have lower basal salivary cortisol levels later in the day than do men with PTSD: a preliminary study. Physiol Behav 99: 234–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich M, Sund M, Lowel H, Imhof A, Hoffmeister A, Koenig W (2003). Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95). Eur Heart J 24: 1365–1372. [DOI] [PubMed] [Google Scholar]
- Fu SS, McFall M, Saxon AJ, Beckham JC, Carmody TP, Baker DG et al (2007). Post-traumatic stress disorder and smoking: a systematic review. Nicotine Tob Res 9: 1071–1084. [DOI] [PubMed] [Google Scholar]
- Geracioti TD Jr., Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB et al (2001). CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry 158: 1227–1230. [DOI] [PubMed] [Google Scholar]
- Geracioti TD Jr., Baker DG, Kasckow JW, Strawn JR, Jeffrey Mulchahey J, Dashevsky BA et al (2008). Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinology 33: 416–424. [DOI] [PubMed] [Google Scholar]
- Gill J, Luckenbaugh D, Charney D, Vythilingam M (2010). Sustained elevation of serum interleukin-6 and relative insensitivity to hydrocortisone differentiates posttraumatic stress disorder with and without depression. Biol Psychiatry 68: 999–1006. [DOI] [PubMed] [Google Scholar]
- Gill J, Vythilingam M, Page GG (2008). Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J Trauma Stress 21: 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Tylee DS, Chandler SD, Pazol J, Nievergelt CM, Woelk CH et al (2013). Blood-based gene-expression predictors of PTSD risk and resilience among deployed marines: a pilot study. Am J Med Genet B Neuropsychiatr Genet 162B: 313–326. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OA et al (2001). Reductions in occipital cortex GABA levels in panic disorder detected with 1h-magnetic resonance spectroscopy. Arch Gen Psychiatry 58: 556–561. [DOI] [PubMed] [Google Scholar]
- Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M et al (2013). Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry 13: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK (2012). Childhood abuse and inflammatory responses to daily stressors. Ann Behav Med 44: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardado P, Olivera A, Rusch HL, Roy M, Martin C, Lejbman N et al (2016). Altered gene expression of the innate immune, neuroendocrine, and nuclear factor-kappa B (NF-kappaB) systems is associated with posttraumatic stress disorder in military personnel. J Anxiety Disord 38: 9–20. [DOI] [PubMed] [Google Scholar]
- Guffanti G, Galea S, Yan L, Roberts AL, Solovieff N, Aiello AE et al (2013). Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718.1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology 38: 3029–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Liu T, Guo JC, Jiang XL, Chen F, Gao YS (2012). Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac J Trop Med 5: 323–325. [DOI] [PubMed] [Google Scholar]
- Gurguis GN, Andrews R, Antai-Otong D, Vo SP, Blakeley JE, Orsulak PJ et al (1999). Neutrophil beta2-adrenergic receptor coupling efficiency to Gs protein in subjects with post-traumatic stress disorder and normal controls. Psychopharmacology 143: 131–140. [DOI] [PubMed] [Google Scholar]
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E et al (2011). Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21: 718–779. [DOI] [PubMed] [Google Scholar]
- Hamner MB, Lorberbaum JP, George MS (1999). Potential role of the anterior cingulate cortex in PTSD: review and hypothesis. Depress Anxiety 9: 1–14. [PubMed] [Google Scholar]
- Hannestad J, Subramanyam K, Dellagioia N, Planeta-Wilson B, Weinzimmer D, Pittman B et al (2012). Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J Nucl Med 53: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Jing H, Bi Q, Zhang J, Qin L, Yang P (2014). Intra-amygdala microinfusion of IL-6 impairs the auditory fear conditioning of rats via JAK/STAT activation. Behav Brain Res 275: 88–95. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H (2010). Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev 11: 682–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T et al (2016). Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry. (e-pub ahead of print; doi:10.1038/mp.2015.206). [DOI] [PMC free article] [PubMed]
- Haroon E, Raison CL, Miller AH (2012). Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37: 137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Woolwine BJ, Chen X, Pace TW, Parekh S, Spivey JR et al (2014). IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology 39: 1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD (2009. a). Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 66: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ et al (2009. b). Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry 66: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Doeller CF, Voon V, Burgess N, Critchley HD (2014). Peripheral inflammation acutely impairs human spatial memory via actions on medial temporal lobe glucose metabolism. Biol Psychiatry 76: 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell KJ, Moran-Santa Maria MM, Twal WO, Shaftman S, DeSantis SM, McRae-Clark AL et al (2013). Association of elevated cytokines with childhood adversity in a sample of healthy adults. J Psychiatr Res 47: 604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Olivares-Reyes JA, Dautzenberg FM, Lohr JB, Braun S, Oakley RH (2012). Molecular and cell signaling targets for PTSD pathophysiology and pharmacotherapy. Neuropharmacology 62: 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath NM, Chesney SA, Gerhart JI, Goldsmith RE, Luborsky JL, Stevens NR et al (2013). Interpersonal violence, PTSD, and inflammation: potential psychogenic pathways to higher C-reactive protein levels. Cytokine 63: 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzelmann M, Gill J (2013). Epigenetic mechanisms shape the biological response to trauma and risk for PTSD: a critical review. Nurs Res Pract 2013: 417010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hek K, Direk N, Newson RS, Hofman A, Hoogendijk WJ, Mulder CL et al (2013). Anxiety disorders and salivary cortisol levels in older adults: a population-based study. Psychoneuroendocrinology 38: 300–305. [DOI] [PubMed] [Google Scholar]
- Heppner PS, Crawford EF, Haji UA, Afari N, Hauger RL, Dashevsky BA et al (2009). The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Med 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM (2009). Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety 26: 447–455. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI (2012). Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage 59: 3222–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Duman RS (2013). The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun 31: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal O, Aneni EC, Shaharyar S, Ali SS, Parris D, McEvoy JW et al (2014). Cigarette smoking worsens systemic inflammation in persons with metabolic syndrome. Diabetol Metab Syndr 6: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergovic M, Bendelja K, Savic Mlakar A, Vojvoda V, Aberle N, Jovanovic T et al (2015). Circulating levels of hormones, lipids, and immune mediators in post-traumatic stress disorder - a 3-month follow-up study. Front Psychiatry 6: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergovic M, Bendelja K, Vidovic A, Savic A, Vojvoda V, Aberle N et al (2014). Patients with posttraumatic stress disorder exhibit an altered phenotype of regulatory T cells. Allergy Asthma Clin Immunol 10: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Hao Y, Bi Q, Zhang J, Yang P (2015). Intra-amygdala microinjection of TNF-alpha impairs the auditory fear conditioning of rats via glutamate toxicity. Neurosci Res 91: 34–40. [DOI] [PubMed] [Google Scholar]
- Jolkkonen J, Lepola U, Bissette G, Nemeroff C, Riekkinen P (1993). CSF corticotropin-releasing factor is not affected in panic disorder. Biol Psychiatry 33: 136–138. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Charney D (2000). Comorbidity of mood and anxiety disorders. Depress Anxiety 12 Suppl 1: 69–76. [DOI] [PubMed] [Google Scholar]
- Kawamura N, Kim Y, Asukai N (2001). Suppression of cellular immunity in men with a past history of posttraumatic stress disorder. Am J Psychiatry 158: 484–486. [DOI] [PubMed] [Google Scholar]
- Keane TM, Marx BP, Sloan DM (2009) Post-traumatic stress disorder: definition, prevalence, and risk factorsIn:Shiromani PJ, Keane TM, LeDoux JE (eds). Post-Traumatic Stress Disorder: Basic Science and Clinical Practice. Humana Press: New York. pp 1–19. [Google Scholar]
- Kekkonen RA, Lummela N, Karjalainen H, Latvala S, Tynkkynen S, Jarvenpaa S et al (2008). Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J Gastroenterol 14: 2029–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 593–602. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048–1060. [DOI] [PubMed] [Google Scholar]
- Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR (2004). Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. J Parenter Enteral Nutr 28: 410–415. [DOI] [PubMed] [Google Scholar]
- Khoury L, Tang YL, Bradley B, Cubells JF, Ressler KJ (2010). Substance use, childhood traumatic experience, and posttraumatic stress disorder in an urban civilian population. Depress Anxiety 27: 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B et al (2012). The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry 73: 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK (2010). Stress, food, and inflammation: psychoneuroimmunology and nutrition at the cutting edge. Psychosom Med 72: 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, Fagundes CP (2015). Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry 172: 1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R (2011). Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med 73: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Britton JC, Schwab ZJ, Price LM, Weiner MR, Gold AL et al (2014). Cortico-limbic responses to masked affective faces across ptsd, panic disorder, and specific phobia. Depress Anxiety 31: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM et al (2013). Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci 16: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KB, Lee Y (2004). Reduced anxiety level by therapeutic interventions and cell-mediated immunity in panic disorder patients. Psychother Psychosom 73: 286–292. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS (2008). IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA 105: 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey RE, Kumari M, McMunn A (2013). Parental separation in childhood and adult inflammation: the importance of material and psychosocial pathways. Psychoneuroendocrinology 38: 2476–2484. [DOI] [PubMed] [Google Scholar]
- Lemieux A, Coe CL, Carnes M (2008). Symptom severity predicts degree of T cell activation in adult women following childhood maltreatment. Brain Behav Immun 22: 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Cidlowski JA (2006). The physiology of human glucocorticoid receptor beta (hGRbeta) and glucocorticoid resistance. Ann N Y Acad Sci 1069: 1–9. [DOI] [PubMed] [Google Scholar]
- Lin JE, Neylan TC, Epel E, O'Donovan A (2016). Associations of childhood adversity and adulthood trauma with C-reactive protein: a cross-sectional population-based study. Brain Behav Immun 53: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C et al (2014. a). Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun 42: 81–88. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C et al (2014. b). Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun 42: 81–88. [DOI] [PubMed] [Google Scholar]
- Logue MW, Smith AK, Baldwin C, Wolf EJ, Guffanti G, Ratanatharathorn A et al (2015). An analysis of gene expression in PTSD implicates genes involved in the glucocorticoid receptor pathway and neural responses to stress. Psychoneuroendocrinology 57: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Z, Medlock C, Dzemidzic M, Shin YW, Goddard AW, Dydak U (2013). Decreased GABA levels in anterior cingulate cortex/medial prefrontal cortex in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry 44: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Lin A, Bonaccorso S, van Hunsel F, Van Gastel A, Delmeire L et al (1998). Increased 24-hour urinary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiatr Scand 98: 328–335. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R et al (1999). Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry 45: 833–839. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR (1998). Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev 105: 83–107. [DOI] [PubMed] [Google Scholar]
- Manfro GG, Pollack MH, Otto MW, Worthington JJ, Rosenbaum JF, Scott EL et al (2000). Cell-surface expression of L-selectin (CD62L) by blood lymphocytes: correlates with affective parameters and severity of panic disorder. Depress Anxiety 11: 31–37. [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE et al (2008). Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology 33: 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvar PJ, Vinh A, Thabet S, Lob HE, Geem D, Ressler KJ et al (2012). T lymphocytes and vascular inflammation contribute to stress-dependent hypertension. Biol Psychiatry 71: 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslanik T, Mahaffey L, Tannura K, Beninson L, Greenwood BN, Fleshner M (2013). The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun 28: 54–62. [DOI] [PubMed] [Google Scholar]
- Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L (1986). Urinary free-cortisol levels in posttraumatic stress disorder patients. J Nerv Ment Dis 174: 145–149. [DOI] [PubMed] [Google Scholar]
- Masoudzadeh A, Modanloo Kordi M, Ajami A, Azizi A (2012). Evaluation of cortisol level and cell-mediated immunity response changes in individuals with post-traumatic stress disorder as a consequence of war. Med Glas 9: 218–222. [PubMed] [Google Scholar]
- Matic G, Milutinovic DV, Nestorov J, Elakovic I, Jovanovic SM, Perisic T et al (2013). Lymphocyte glucocorticoid receptor expression level and hormone-binding properties differ between war trauma-exposed men with and without PTSD. Prog Neuropsychopharmacol Biol Psychiatry 43: 238–245. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Chang YF, Thurston RC, Bromberger JT (2014). Child abuse is related to inflammation in mid-life women: role of obesity. Brain Behav Immun 36: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE (2004). Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. NeuroImage 22: 1151–1156. [DOI] [PubMed] [Google Scholar]
- McCanlies EC, Araia SK, Joseph PN, Mnatsakanova A, Andrew ME, Burchfiel CM et al (2011). C-reactive protein, interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine 55: 74–78. [DOI] [PubMed] [Google Scholar]
- McDade TW, Hoke M, Borja JB, Adair LS, Kuzawa C (2013). Do environments in infancy moderate the association between stress and inflammation in adulthood? Initial evidence from a birth cohort in the Philippines. Brain Behav Immun 31: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG (2011). Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res 45: 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M (2007). Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry 191: 387–392. [DOI] [PubMed] [Google Scholar]
- Mehta D, Gonik M, Klengel T, Rex-Haffner M, Menke A, Rubel J et al (2011). Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression studies. Arch Gen Psychiatry 68: 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF et al (2004). Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 43: 678–683. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Jovanovic T (2015. a). Chronic inflammation: a new therapeutic target for post-traumatic stress disorder? Lancet Psychiatry 2: 954–955. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Norrholm SD, Jovanovic T (2015. b). Diagnostic biomarkers for posttraumatic stress disorder: promising horizons from translational neuroscience research. Biol Psychiatry 78: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO et al (2015. c). Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry 172: 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB et al (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Pariante CM, Pearce BD (1999). Effects of cytokines on glucocorticoid receptor expression and function. Glucocorticoid resistance and relevance to depression. Adv Exp Med Biol 461: 107–116. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Sutherland AG, Hutchison JD, Alexander DA (2001). C-reactive protein and interleukin 6 receptor in post-traumatic stress disorder: a pilot study. Cytokine 13: 253–255. [DOI] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI (2015). Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology 40: 1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM et al (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry 65: 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons WG, Eisenberger NI, Taylor SE (2010). Anger and fear responses to stress have different biological profiles. Brain Behav Immun 24: 215–219. [DOI] [PubMed] [Google Scholar]
- Moons WG, Shields GS (2015). Anxiety, not anger, induces inflammatory activity: an avoidance/approach model of immune system activation. Emotion 15: 463–476. [DOI] [PubMed] [Google Scholar]
- Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT (2007). Anxiety, anxiety disorders, tobacco use, and nicotine: a critical review of interrelationships. Psychol Bull 133: 245–272. [DOI] [PubMed] [Google Scholar]
- Muhtz C, Godemann K, von Alm C, Wittekind C, Goemann C, Wiedemann K et al (2011). Effects of chronic posttraumatic stress disorder on metabolic risk, quality of life, and stress hormones in aging former refugee children. J Nerv Ment Dis 199: 646–652. [DOI] [PubMed] [Google Scholar]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE et al (2015). Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav Immun 43: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance DM, Sanders VM (2007). Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav Immun 21: 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TL, Fernandez-Botran R, Miller JJ, Burns VE (2014). Interleukin-6 and soluble interleukin-6 receptor levels in posttraumatic stress disorder: associations with lifetime diagnostic status and psychological context. Biol Psychol 99: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylan TC, Sun B, Rempel H, Ross J, Lenoci M, O'Donovan A et al (2011). Suppressed monocyte gene expression profile in men versus women with PTSD. Brain Behav Immun 25: 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B et al (2011). Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry 69: 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MF, Irwin MR, Wellisch DK (2009). When grief heats up: pro-inflammatory cytokines predict regional brain activation. NeuroImage 47: 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Chao LL, Paulson J, Samuelson KW, Shigenaga JK, Grunfeld C et al (2015. a). Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology 51: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K et al (2015. b). Elevated risk for autoimmune disorders in iraq and afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry 77: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O'Farrelly C et al (2010). Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun 24: 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Slavich GM, Epel ES, Neylan TC (2013). Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci Biobehav Rev 37: 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L et al (2011). Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis Markers 30: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oganesyan LP, Mkrtchyan GM, Sukiasyan SH, Boyajyan AS (2009). Classic and alternative complement cascades in post-traumatic stress disorder. Bull Exp Biol Med 148: 859–861. [DOI] [PubMed] [Google Scholar]
- Pace TW, Hu F, Miller AH (2007). Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun 21: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD et al (2009). Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology 34: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM (2012). Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun 26: 13–17. [DOI] [PubMed] [Google Scholar]
- Pannu Hayes J, Labar KS, Petty CM, McCarthy G, Morey RA (2009). Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Res 172: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J et al (2015). Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2: 1002–1012. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Simmons A, Stein MB (2004). Anterior cingulate activation in high trait anxious subjects is related to altered error processing during decision making. Biol Psychiatry 55: 1179–1187. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB (2006). An insular view of anxiety. Biol Psychiatry 60: 383–387. [DOI] [PubMed] [Google Scholar]
- Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C et al (2007). Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology 32: 991–999. [DOI] [PubMed] [Google Scholar]
- Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC (2015). Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther 37: 984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsavos C, Panagiotakos DB, Papageorgiou C, Tsetsekou E, Soldatos C, Stefanadis C (2006). Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA study. Atherosclerosis 185: 320–326. [DOI] [PubMed] [Google Scholar]
- Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J et al (2013). Association between posttraumatic stress disorder and inflammation: a twin study. Brain Behav Immun 30: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB (2010). A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev 30: 635–641. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW (1998). Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun 12: 212–229. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF et al (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport MH (1998). Circulating lymphocyte phenotypic surface markers in anxiety disorder patients and normal volunteers. Biol Psychiatry 43: 458–463. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Stein MB (1994). Serum cytokine and soluble interleukin-2 receptors in patients with panic disorder. Anxiety 1: 22–25. [DOI] [PubMed] [Google Scholar]
- Regier DA, Rae DS, Narrow WE, Kaelber CT, Schatzberg AF (1998). Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders. Br J Psychiatry Suppl 34: 24–28. [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA (2005). Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 353: 1711–1723. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C (2004). Hypocortisolism and increased glucocorticoid sensitivity of pro-Inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry 55: 745–751. [DOI] [PubMed] [Google Scholar]
- Rooks C, Veledar E, Goldberg J, Bremner JD, Vaccarino V (2012). Early trauma and inflammation: role of familial factors in a study of twins. Psychosom Med 74: 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Weiner MR, Crowley DJ, Silveri MM, Rauch SL, Jensen JE (2014). Insula and anterior cingulate GABA levels in posttraumatic stress disorder: preliminary findings using magnetic resonance spectroscopy. Depress Anxiety 31: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiecki JA, Byrne C, Galdzicki Z, Srikantan V, Chen L, Poulin M et al (2013). PTSD and DNA methylation in select immune function gene promoter regions: a repeated measures case-control study of U.S. military service members. Front Psychiatry 4: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Fukuda K, Sato T, Kawaguchi H, Suematsu M, Matsuda S et al (2001). ERK and p38 MAPK, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res 89: 661–669. [DOI] [PubMed] [Google Scholar]
- Sarapas C, Cai G, Bierer LM, Golier JA, Galea S, Ising M et al (2011). Genetic markers for PTSD risk and resilience among survivors of the World Trade Center attacks. Dis Markers 30: 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia C, Schiffrin EL (2007). Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci 112: 375–384. [DOI] [PubMed] [Google Scholar]
- Schwarcz R (2004). The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol 4: 12–17. [DOI] [PubMed] [Google Scholar]
- Scott KM, McGee MA, Wells JE, Oakley Browne MA (2008). Obesity and mental disorders in the adult general population. J Psychosom Res 64: 97–105. [DOI] [PubMed] [Google Scholar]
- Segman RH, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, Shalev AY (2005). Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol Psychiatry 10: 500–513 425. [DOI] [PubMed] [Google Scholar]
- Shang J, Fu Y, Ren Z, Zhang T, Du M, Gong Q et al (2014). The common traits of the ACC and PFC in anxiety disorders in the DSM-5: meta-analysis of voxel-based morphometry studies. PloS One 9: e93432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea A, Walsh C, Macmillan H, Steiner M (2005). Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology 30: 162–178. [DOI] [PubMed] [Google Scholar]
- Shin LM, Bush G, Milad MR, Lasko NB, Brohawn KH, Hughes KC et al (2011). Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am J Psychiatry 168: 979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Bush G, Whalen PJ, Handwerger K, Cannistraro PA, Wright CI et al (2007). Dorsal anterior cingulate function in posttraumatic stress disorder. J Trauma Stress 20: 701–712. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB et al (2001). An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry 50: 932–942. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Yamada R, Tabei Y, Otani R, Itoi C, Saito S et al (2011). Damage to the right dorsal anterior cingulate cortex induces panic disorder. J Affect Disord 133: 569–572. [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB (2006). Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry 60: 402–409. [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo IA, Matthews SC, Paulus MP, Stein MB (2009). Initial evidence of a failure to activate right anterior insula during affective set shifting in posttraumatic stress disorder. Psychosom Med 71: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ (2015). Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther 149: 150–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B et al (2011). Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet 156B: 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg EM et al (2009). Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behav Immun 23: 1117–1124. [DOI] [PubMed] [Google Scholar]
- Sondergaard HP, Hansson LO, Theorell T (2004). The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. Clin Chim Acta 342: 93–98. [DOI] [PubMed] [Google Scholar]
- Song C, Li X, Kang Z, Kadotomi Y (2007. a). Omega-3 fatty acid ethyl-eicosapentaenoate attenuates IL-1beta-induced changes in dopamine and metabolites in the shell of the nucleus accumbens: involved with PLA2 activity and corticosterone secretion. Neuropsychopharmacology 32: 736–744. [DOI] [PubMed] [Google Scholar]
- Song Y, Zhou D, Guan Z, Wang X (2007. b). Disturbance of serum interleukin-2 and interleukin-8 levels in posttraumatic and non-posttraumatic stress disorder earthquake survivors in northern China. Neuroimmunomodulation 14: 248–254. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA 3rd, Arnsten A, Charney DS (1999). Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry 46: 1192–1204. [DOI] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Volzke H, Wallaschofski H, John U, Freyberger HJ et al (2010). Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J Psychiatr Res 44: 15–21. [DOI] [PubMed] [Google Scholar]
- Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A et al (1997). Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biol Psychiatry 42: 345–348. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF (2013). Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology 38: 1220–1235. [DOI] [PubMed] [Google Scholar]
- Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM (2007). Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal 19: 251–260. [DOI] [PubMed] [Google Scholar]
- Tavares RG, Tasca CI, Santos CE, Alves LB, Porciuncula LO, Emanuelli T et al (2002). Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem Int 40: 621–627. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE (2006). Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry 60: 819–824. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD (1996). Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry 39: 255–266. [DOI] [PubMed] [Google Scholar]
- Tietjen GE, Khubchandani J, Herial NA, Shah K (2012). Adverse childhood experiences are associated with migraine and vascular biomarkers. Headache 52: 920–929. [DOI] [PubMed] [Google Scholar]
- Tracey KJ (2009). Reflex control of immunity. Nat Rev Immunol 9: 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P, Ruwe WD, Masters B, Parker DE, Hossain A, Trautman RP et al (2004). Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biol Psychiatry 56: 121–128. [DOI] [PubMed] [Google Scholar]
- Tukel R, Arslan BA, Ertekin BA, Ertekin E, Oflaz S, Ergen A et al (2012). Decreased IFN-gamma and IL-12 levels in panic disorder. J Psychosom Res 73: 63–67. [DOI] [PubMed] [Google Scholar]
- Tursich M, Neufeld RW, Frewen PA, Harricharan S, Kibler JL, Rhind SG et al (2014). Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Transl Psychiatry 4: e413 A meta-analysis of 36 independent samples describing incresaed inflammation in those exposure to trauma, regardless of diagnostic criteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylee DS, Chandler SD, Nievergelt CM, Liu X, Pazol J, Woelk CH et al (2015). Blood-based gene-expression biomarkers of post-traumatic stress disorder among deployed marines: a pilot study. Psychoneuroendocrinology 51: 472–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R et al (2010). Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci USA 107: 9470–9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Galea S, Chang SC, Aiello AE, Wildman DE, de los Santos R et al (2011). Gene expression and methylation signatures of MAN2C1 are associated with PTSD. Dis Markers 30: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J et al (2014). An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry 171: 1278–1286. [DOI] [PubMed] [Google Scholar]
- van Veen JF, van Vliet IM, Derijk RH, van Pelt J, Mertens B, Zitman FG (2008). Elevated alpha-amylase but not cortisol in generalized social anxiety disorder. Psychoneuroendocrinology 33: 1313–1321. [DOI] [PubMed] [Google Scholar]
- Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M (2003). Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. J Clin Endocrinol Metab 88: 277–284. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A et al (1999). Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab 84: 2603–2607. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A et al (2004). Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 89: 2119–2126. [DOI] [PubMed] [Google Scholar]
- Vidovic A, Gotovac K, Vilibic M, Sabioncello A, Jovanovic T, Rabatic S et al (2011). Repeated assessments of endocrine- and immune-related changes in posttraumatic stress disorder. Neuroimmunomodulation 18: 199–211. [DOI] [PubMed] [Google Scholar]
- Vieira MM, Ferreira TB, Pacheco PA, Barros PO, Almeida CR, Araujo-Lima CF et al (2010). Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol 229: 212–218. [DOI] [PubMed] [Google Scholar]
- Villablanca AC, Warford C, Wheeler K (2015). Inflammation and cardiometabolic risk in African American Women is reduced by a pilot community-based educational intervention. J Womens Health 25: 188–199. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, de Jonge P, Penninx BW (2013). Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry 3: e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L et al (2007). Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res 41: 744–752. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Zitman FG, van Pelt J, Derijk RH, Verhagen JC, van Dyck R et al (2010). Salivary cortisol levels in persons with and without different anxiety disorders. Psychosom Med 72: 340–347. [DOI] [PubMed] [Google Scholar]
- Wagner EY, Wagner JT, Glaus J, Vandeleur CL, Castelao E, Strippoli MP et al (2015). Evidence for chronic low-grade systemic inflammation in individuals with agoraphobia from a population-based prospective study. PloS One 10: e0123757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JC, Oakley RH, Jewell CM, Cidlowski JA (2001). Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci USA 98: 6865–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T, Skelton K, Phifer J, Jovanovic T, Gillespie CF, Smith A et al (2011). Posttraumatic stress disorder is a risk factor for metabolic syndrome in an impoverished urban population. Gen Hosp Psychiatry 33: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welty FK, Alfaddagh A, Elajami TK (2015). Targeting inflammation in metabolic syndrome. Transl Res 167: 257–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo AP, Gibson G (2015). Blood gene expression profiles suggest altered immune function associated with symptoms of generalized anxiety disorder. Brain Behav Immun 43: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW (2010). Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 34: 1181–1188. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Lowy MT, Giller EL Jr. (1995). Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry 52: 583–593. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M et al (2009). Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry 66: 708–711. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Kaufman S (2005). Circadian rhythm of salivary cortisol in Holocaust survivors with and without PTSD. Am J Psychiatry 162: 998–1000. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J (2007). Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56: 19–32. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I (2011). Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25: 181–213. [DOI] [PubMed] [Google Scholar]
- Zhao J, Liu J, Pang X, Wang S, Wu D, Zhang X et al (2013). Angiotensin II induces C-reactive protein expression via AT1-ROS-MAPK-NF-kappaB signal pathway in hepatocytes. Cell Physiol Biochem 32: 569–580. [DOI] [PubMed] [Google Scholar]
- Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J et al (2014). Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PloS One 9: e94075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieker J, Zieker D, Jatzko A, Dietzsch J, Nieselt K, Schmitt A et al (2007). Differential gene expression in peripheral blood of patients suffering from post-traumatic stress disorder. Mol Psychiatry 12: 116–118. [DOI] [PubMed] [Google Scholar]