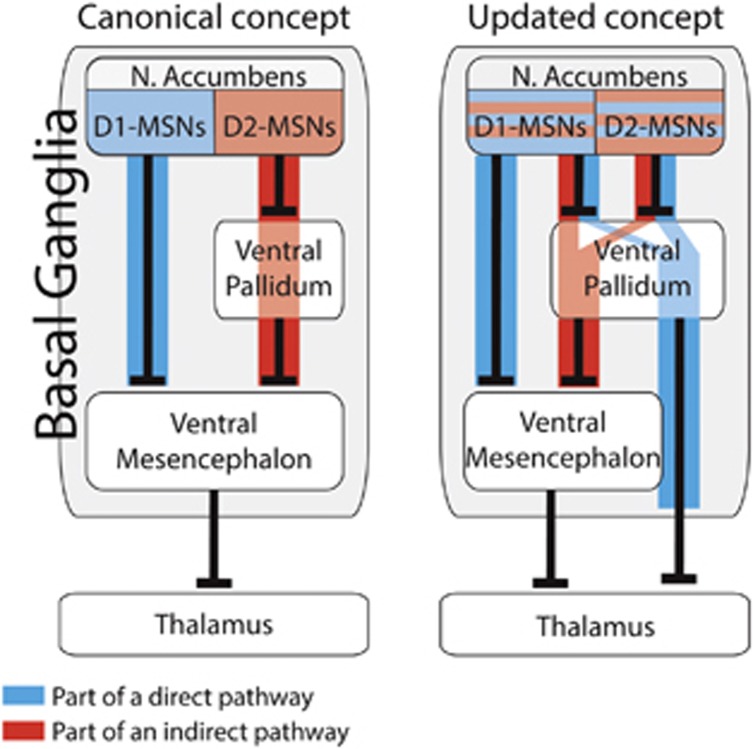
According to current concepts, motivated and addictive behaviors across species depend on the activity of the nucleus accumbens (NAc), and specifically on two groups of projection medium spiny neurons (MSNs). Those that directly inhibit the dopaminergic ventral mesencephalon (VM) (the ‘direct pathway') express the D1 dopamine receptor (D1-MSNs), disinhibit the thalamus and promote motivated behavior. The others, considered to be solely D2-MSNs, inhibit the ventral pallidum (VP), which inhibits the VM (the ‘indirect pathway'), this results in the inhibition of the thalamus and motivated behavior (Figure 1). Importantly, these pathways were originally described for the dorsal striatum in times of relatively limited technology (Albin et al, 1989), and were assumed to be true also for the NAc.
Figure 1.
Past and novel concepts of the direct and indirect pathways of the nucleus accumbens. Left—canonical concept. D1-MSNs and D2-MSNs are completely segregated to direct and indirect pathways. Right—updated concept. Both D1-MSNs and D2-MSNs participate in direct and indirect pathways. Note that MSNs projecting to the VP may be part of a direct or indirect pathway, depending on the target of their downstream VP neuron.
Recent developments in cell-type-specific tools to investigate neural circuits have allowed researchers to evaluate the role of D1-MSNs and D2-MSNs in the expression of motivated and addictive behavior. As expected from the canonical circuitry, NAc D1-MSN activation promotes drug-seeking behavior while D2-MSNs had an inhibitory effect. However, other studies have shown that it is the input to the VP, rather than to the VM, which promotes reinstatement of drug-seeking behavior after withdrawal and that the VP itself, unlike its dorsal counterpart the globus pallidus, directly inhibits the thalamus (Root et al, 2015). Thus, the classic circuitry of the reward system was questioned.
In a recent study (Kupchik et al, 2015), we provided the first definitive evidence that the direct and indirect pathways of the NAc are not coded by MSN cell types (Figure 1). Using optogenetics in transgenic mice combined with neural tracing tools we showed that D1-MSNs comprise a significant portion of the classical indirect pathway by synapsing on VP neurons that project to the VM. Conversely, we showed that NAc D2-MSNs target VP neurons that innervate the thalamus directly. Thus, these D2-MSNs make a direct pathway through the VP that disinhibits the thalamus. These findings are not restricted to the ventral striatum as other emerging studies have observed similar results in the dorsal striatum (Cazorla et al, 2014; Saunders et al, 2015).
These novel findings in the ventral basal ganglia highlight the VP as a central node of the reward system. Other than the classical D1-MSN-to-VM pathway, whether a neuron is part of a direct or indirect pathway is determined in the VP. Curiously, some 15% of VP cells project to both the VM and the thalamus (Tripathi et al, 2013), converging the direct and indirect pathway on the same neuron. Thus, our perspective of the reward system needs to be more nuanced than simply through the prism of the direct and indirect pathways. Understanding this complexity requires deeper analysis of the reward system, and particularly of the understudied VP.
In conclusion, the traditional view of the reward system as being comprised of two distinct cell-type-specific pathways is an oversimplification. Emerging data requires developing a new perspective of the circuitry of the reward system that will guide future treatment strategies for addiction and other related mental health disorders.
Funding and disclosure
The authors declare no conflict of interest.
Acknowledgments
This research was supported by US Public Health Service grants DA03906, DA12513 and DA015369, the Israel Science Foundation grant 1381/15, and a fellowship from the Neuroscience Institute, Medical University of South Carolina.
References
- Albin RL, Young AB, Penney JB (1989). The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375. [DOI] [PubMed] [Google Scholar]
- Cazorla M, de Carvalho FD, Chohan M, Shegda M, Chuhma N, Rayport S et al (2014). Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron 81: 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW (2015). Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci 18: 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Melendez RI, Zaborszky L, Napier TC (2015). The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol 130: 29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Oldenburg IA, Berezovskii VK, Johnson CA, Kingery ND, Elliott HL et al (2015). A direct GABAergic output from the basal ganglia to frontal cortex. Nature 521: 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Prensa L, Mengual E (2013). Axonal branching patterns of ventral pallidal neurons in the rat. Brain Struct Funct 218: 1133–1157. [DOI] [PubMed] [Google Scholar]