Fifty years ago it was discovered that the dentate gyrus (DG) subfield of the hippocampus is one of the few areas of the brain where new neurons are generated throughout adulthood (Altman, 1963). In more recent years, advances in labeling techniques have allowed for a deeper understanding of how these adult-born granule cells (abGCs) mature and functionally integrate into the adult DG circuit. In humans, it was found that the majority of the DG GCs are subject to exchange, with about ~700 new neurons added to the hippocampus per day (Spalding et al, 2013). These nascent neurons have been implicated in a number of cognitive and mood-related functions, including some behavioral responses to stress and antidepressant treatments, as well as in pattern separation, the segregation of similar inputs into distinct output streams to facilitate memory encoding and discrimination (Kheirbek et al, 2012). However, these roles for abGCs in behavior and local circuit function have been primarily inferred from in vitro slice preparations, which lack the full complement of active inputs, or in vivo experiments where the intrinsic properties or total numbers of abGCs have been manipulated. This has left many questions unanswered, namely, how abGCs function and develop in vivo, and whether they encode information differently from mature GCs (mGCs).
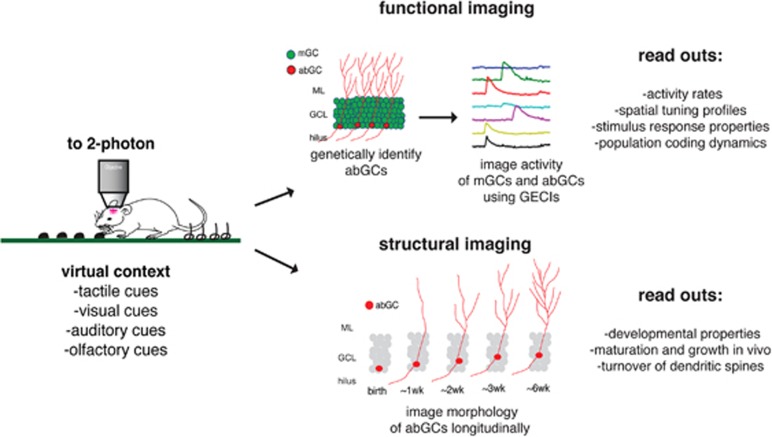
To address these questions, recent studies have applied high-resolution imaging techniques allowing for the first direct observation of abGCs and mGCs in anesthetized and behaving animals. Danielson et al, (2016) used 2-photon calcium imaging in head-fixed behaving mice to simultaneously measure activity patterns in mGCs and abGCs as mice explored a virtual environment (Figure 1). A tamoxifen-inducible Nestin-CreERT2/tdTomato mouse line was injected with a virus, inducing expression of GCaMP6f in all GCs, and pulsed with TMX 6 weeks before imaging to selectively label abGCs 6 weeks and younger with tdTomato. This revealed that abGCs were more active and less spatially tuned than their mature counterparts. In addition, unlike in mGCs, the most active abGCs tended to be the least spatially tuned, consistent with a model in which young abGCs are initially more active, and less spatially tuned, but within 6 weeks become more similar to the less active and more spatially tuned mature cells. Further elucidation of the developmental trajectory of abGCs in vivo has been provided by Goncalves et al, 2016, where abGCs were identified via delivery of a GFP-expressing retrovirus. Longitudinal visualization of dendritic structure by 2-photon microscopy revealed that abGC dendrites underwent overgrowth, followed by pruning as they integrated into the DG circuit, a process that was accelerated by enrichment and exercise. Future studies using these kinds of approaches will begin to elucidate how the unique functional and developmental properties of abGCs facilitate local circuit function and influence hippocampal output to impact behavior in both the mood and cognitive domains.
Figure 1.
Observing abCG dynamics in vivo. In head-fixed mice, virtual contexts are generated via distinct combinations of diverse sensory stimuli. Genetically encoded calcium indicators (GECIs) allow for 2-photon imaging of in vivo activity dynamics of mGCs and abGCs, while retroviral labeling allows for longitudinal analysis of abGC developmental dynamics. Such strategies will accelerate the understanding of how this form of plasticity contributes to hippocampal function and behavior.
The development of novel approaches to visualize and manipulate discrete neuronal elements in vivo is paving the way to a more sophisticated, cell-type-based understanding of circuits that are disrupted in neuropsychiatric illness (Steinberg et al, 2015). As preclinical models indicate adult neurogenesis may have a role in emotional behavior, understanding the mechanisms by which abGCs function in vivo, and how their properties are impacted by environmental manipulations will clarify not only the normal encoding properties of these neurons but provide insight into how they may be targeted for therapeutic interventions.
Funding and disclosure
The authors declare no conflict of interest.
Acknowledgments
MAK is supported by grants from NIMH (R01MH108623 and K01MH099371).
References
- Altman J (1963). Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec 145: 573–591. [DOI] [PubMed] [Google Scholar]
- Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J, Denny CA et al (2016). Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron 90: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves JT, Bloyd CW, Shtrahman M, Johnston ST, Schafer ST, Parylak SL et al (2016). In vivo imaging of dendritic pruning in dentate granule cells. Nat Neurosci 19: 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R (2012). Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci 15: 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB et al (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell 153: 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Christoffel DJ, Deisseroth K, Malenka RC (2015). Illuminating circuitry relevant to psychiatric disorders with optogenetics. Curr Opin Neurobiol 30: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]