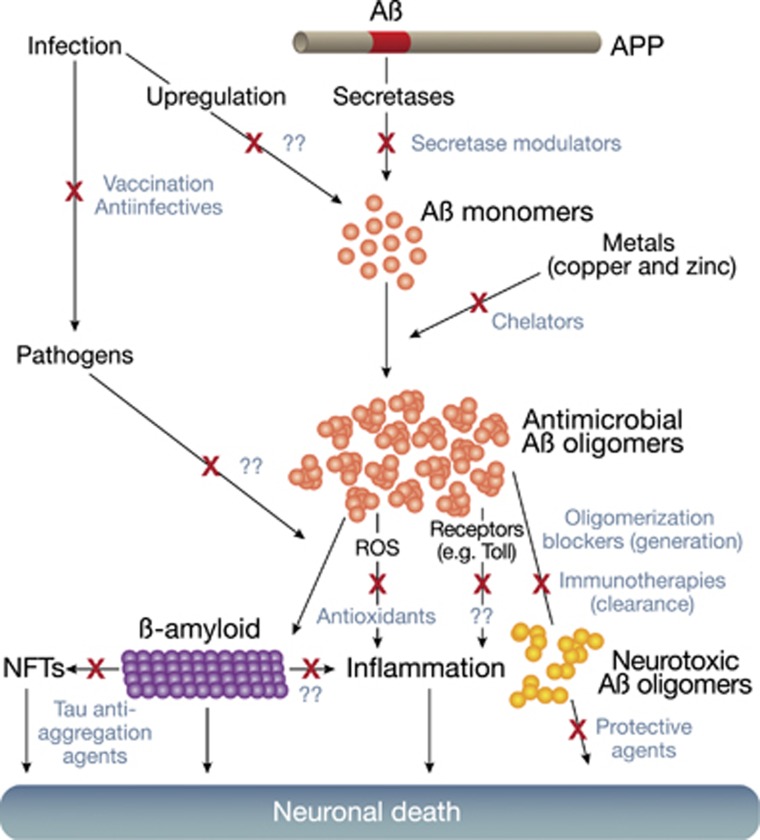
The amyloid cascade hypothesis is the dominant model for Alzheimer's disease (AD) pathology and has guided AD drug development strategies for over two decades. The hypothesis states that excessive cerebral accumulation of β-amyloid plaques from oligomerization of amyloid-β protein (Aβ) drives a neurodegenerative cascade, including generation of neurofibrillary tangles (NFTs) from tau protein. NFTs are a hallmark pathology for AD. Overwhelming genetic and biochemical data support the primacy of Aβ fibrillization pathways in AD pathology. A landmark study by Choi et al (2014) also recently confirmed that β-amyloid generation leads to NFTs in novel 3D human neuronal cell culture models that recapitulate AD pathologies (Choi et al, 2014). However, the roles played by Aβ and β-amyloid in the amyloid cascade model has shifted markedly during the last two decades. Initially, insoluble β-amyloid plaques were viewed as driving neurodegeneration. The key species mediating AD pathology are now thought to be soluble oligomeric intermediates generated during Aβ fibrillization. The near future may see further substantial adjustments to the amyloid cascade model if recent findings of an antimicrobial function for Aβ, and links between AD and innate immune genes (Bertram and Tanzi, 2012) are confirmed. Importantly, new innate immune models of AD amyloidosis are likely to be of considerable interest for ongoing and future AD therapeutic efforts (Figure 1).
Figure 1.
Aβ oligomer pathways and potential targets for therapeutic intervention. Recent studies of ‘resilient' brains with high senile plaque loads have shown β-amyloid alone is insufficient for AD neurodegeneration. Neurofibrillary Tangles (NFTs) and neuroinflammation are also required. Shown are oligomer-mediated pathways contributing to neurodegeneration in AD and potential points for intervention (X). Possible approaches emerging from the antimicrobial peptide role of Aβ are indicated (??) as well as established therapeutic strategies, including secretase modulators that reduce the 42 amino acid Aβ isoforms (eg, GSM, NS-GSI, BSI), copper chelators (eg, PBT2), Aβ oligomerization blockers (eg, PBT2, ELND005, NRM8499), immunotherapies aimed at antibody-mediated clearance of Aβ (eg, Bapi, Sola), antioxidants that attenuate ROS-mediated inflammation (eg, vitamin E, epigallo-catechine gallate, resveratrol), agents purported to be neuroprotective (eg, dimebon), and drugs that inhibit tau aggregation and NTF generation (eg, rhodanine-based and Phenylthiazolyl-hydrazide inhibitors).
In prevailing models of AD amyloidogenesis, Aβ's propensity to oligomerize and generate β-amyloid is viewed as an intrinsically abnormal and exclusively pathological activity. However, recent findings suggest Aβ oligomerization may help protect the brain from infection. Aβ shows potent in vitro microbicidal activities against human pathogens, consistent with identity as an antimicrobial peptide (Soscia et al, 2010). Aβ expression also significantly increases survival of transgenic mice in acute encephalitis models (Kumar et al, 2016). Moreover, fibrillization appears to mediate the protective activities of Aβ in brain and leads to the concentration and entrapment of pathogens within β-amyloid deposits (Kumar et al., 2016). Confirmation of a physiological role for β-amyloid generation would shift the modality of Aβ's pathophysiology from abnormal stochastic behavior toward dysregulated innate immune response. This suggests a response to perceived immunochallenge may mediate AD amyloidosis. This may involve sterile inflammatory stimuli, as with aseptic inflammation diseases. Or alternatively, AD amyloidosis may be driven by subclinical chronic cerebral infection. It remains unclear if infection precedes the establishment of the amyloid cascade in AD brain. However, a large body of data has accrued linking brain pathogens to enhanced β-amyloid deposition (Miklossy, 2011). If an AD infection etiology is confirmed, patients could be given anti-infectives to slow or halt the diseases progression. Vaccination against AD-linked pathogens may also provide long-term protection. In any case, independent of the involvement of infection in AD etiology, the identification of an antimicrobial function for Aβ and emerging roles for innate immune pathways in β-amyloid generation seem poised to take the amyloid cascade hypothesis in new directions. Most importantly, these new findings seem likely to reveal much needed new therapeutic approaches for what has proven a highly intractable disease.
FUNDING AND DISCLOSURE
Our work is supported by grants from NIH (5R01AI081990-02), the Cure Alzheimer's Fund, and The Helmsley Charitable Trust. RDM. is a coinventor on a patent for the use of amyloidogenic peptides such as Aβ as possible clinical antibiotics. The remaining authors declare no conflict of interest.
References
- Bertram L, Tanzi RE (2012). The genetics of Alzheimer's disease. Prog Mol Biol Transl Sci 107: 79–100. [DOI] [PubMed] [Google Scholar]
- Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D'Avanzo C et al (2014). A three-dimensional human neural cell culture model of Alzheimer's disease. Nature 515: 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J et al (2016). Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med 8: 340ra–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J (2011). Emerging roles of pathogens in Alzheimer disease. Expert Rev Mol Med 13: e30. [DOI] [PubMed] [Google Scholar]
- Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B et al (2010). The Alzheimer‘s disease-associated amyloid β-protein is an antimicrobial peptide. PLoS One 5: e9505. [DOI] [PMC free article] [PubMed] [Google Scholar]