Abstract
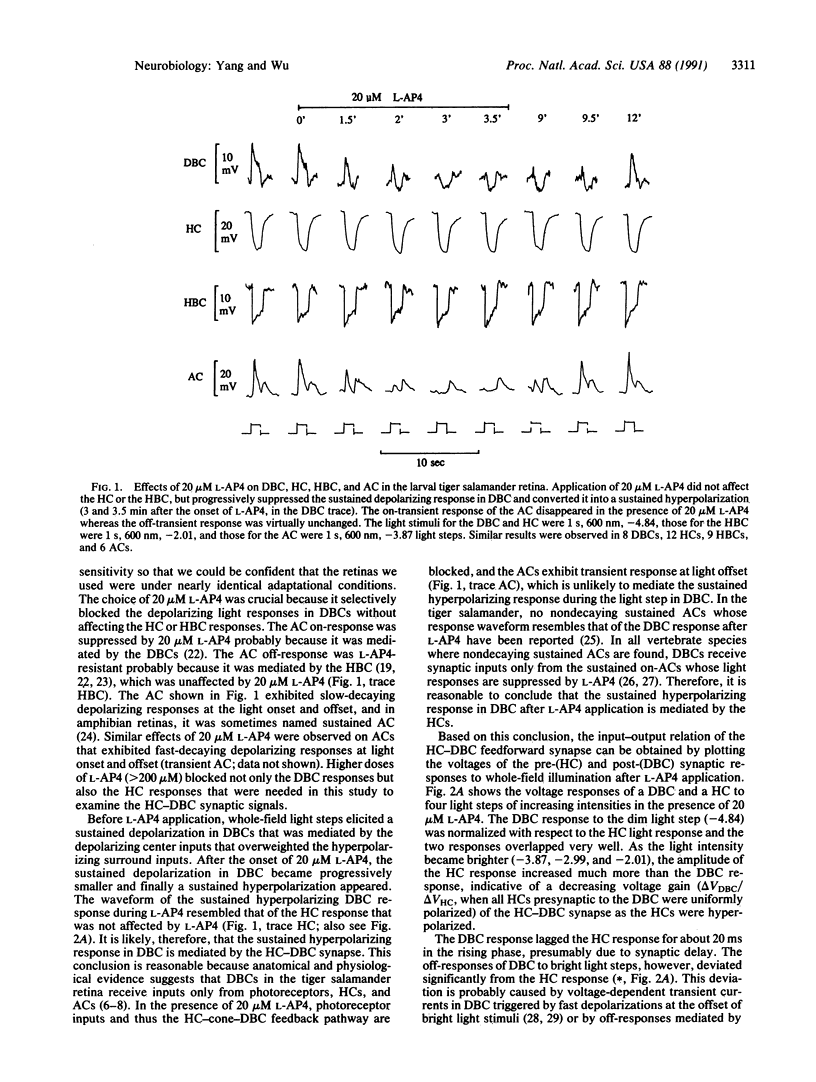
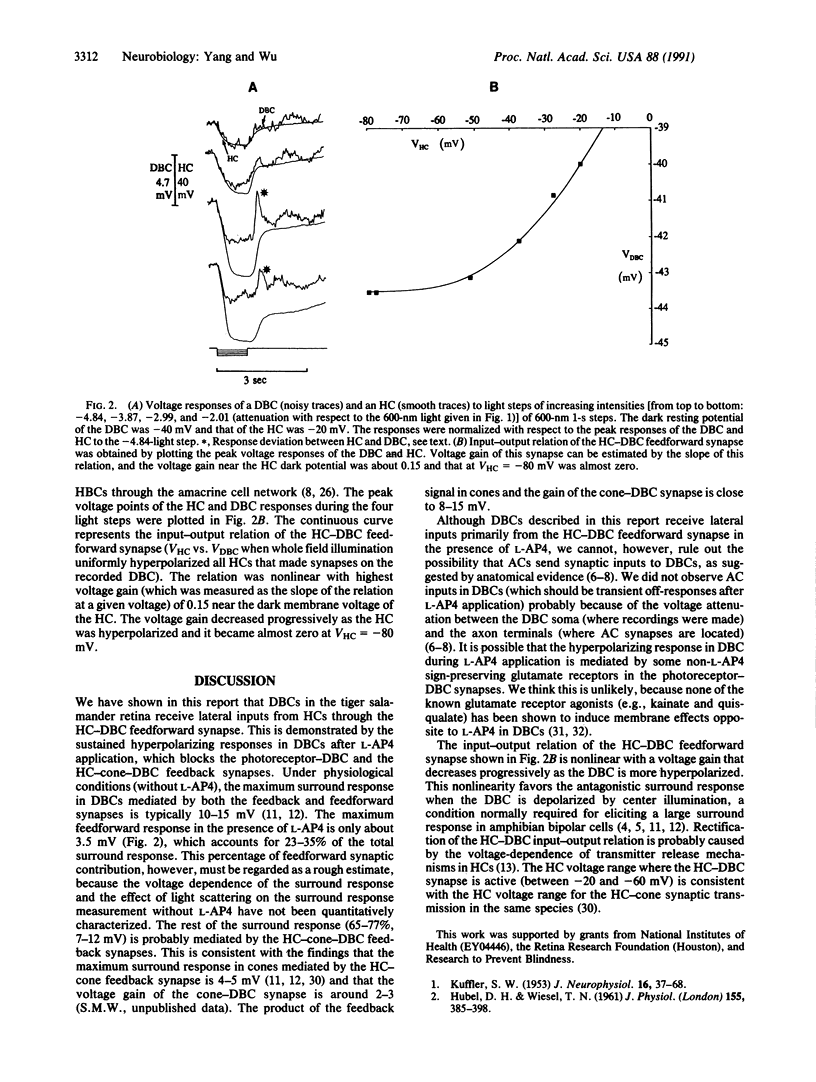
Lateral inhibition is the ubiquitous strategy used by visual neurons for spatial resolution throughout the animal kingdom. It has been a puzzle whether lateral inputs in retinal bipolar cells are mediated by the horizontal cell (HC)-cone feedback synapse, by the HC-bipolar cell feedforward synapse, or by both. By blocking the central inputs of the depolarizing bipolar cells (DBCs) with L-2-amino-4-phosphonobutyrate, we were able to eliminate the contribution of the feedback synapse and to demonstrate the postsynaptic light response in DBCs mediated by the HC-DBC feedforward synapse. The HC-DBC feedforward synapse contributes roughly one-third of the surround response whereas the HC-cone-DBC feedback synapse probably contributes the rest.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkin M. S., Miller R. F. Subtle actions of 2-amino-4-phosphonobutyrate (APB) on the Off pathway in the mudpuppy retina. Brain Res. 1987 Nov 17;426(1):142–148. doi: 10.1016/0006-8993(87)90433-1. [DOI] [PubMed] [Google Scholar]
- Ayoub G. S., Lam D. M. The release of gamma-aminobutyric acid from horizontal cells of the goldfish (Carassius auratus) retina. J Physiol. 1984 Oct;355:191–214. doi: 10.1113/jphysiol.1984.sp015414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Werblin F. S. Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. J Neurophysiol. 1969 May;32(3):315–338. doi: 10.1152/jn.1969.32.3.315. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated from Carassius auratus. J Physiol. 1985 Jan;358:131–152. doi: 10.1113/jphysiol.1985.sp015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philos Trans R Soc Lond B Biol Sci. 1973;265(872):471–489. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Lasansky A., Vallerga S. Horizontal cell responses in the retina of the larval tiger salamander. J Physiol. 1975 Sep;251(1):145–165. doi: 10.1113/jphysiol.1975.sp011085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiafava P. L. Horizontal cells influence membrane potential of bipolar cells in the retina of the turtle. Nature. 1978 Sep 14;275(5676):141–142. doi: 10.1038/275141a0. [DOI] [PubMed] [Google Scholar]
- Naka K. I. The horizontal cells. Vision Res. 1972 Apr;12(4):573–588. doi: 10.1016/0042-6989(72)90153-8. [DOI] [PubMed] [Google Scholar]
- Skrzypek J., Werblin F. Lateral interactions in absence of feedback to cones. J Neurophysiol. 1983 Apr;49(4):1007–1016. doi: 10.1152/jn.1983.49.4.1007. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981 Jan 9;211(4478):182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neurons. Science. 1983 Mar 11;219(4589):1230–1232. doi: 10.1126/science.6131536. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. Characterization of an extended glutamate receptor of the on bipolar neuron in the vertebrate retina. J Neurosci. 1985 Jan;5(1):224–233. doi: 10.1523/JNEUROSCI.05-01-00224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M., Attwell D., Mobbs P., Wilson M. Membrane currents in retinal bipolar cells of the axolotl. J Gen Physiol. 1988 Jan;91(1):49–72. doi: 10.1085/jgp.91.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibos L. N., Werblin F. S. The response properties of the steady antagonistic surround in the mudpuppy retina. J Physiol. 1978 May;278:79–99. doi: 10.1113/jphysiol.1978.sp012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J. I., Tonosaki K. Effect of polarisation of horizontal cells on the on-centre bipolar cell of carp retina. Nature. 1978 Nov 23;276(5686):399–400. doi: 10.1038/276399a0. [DOI] [PubMed] [Google Scholar]
- Vallerga S. Physiological and morphological identification of amacrine cells in the retina of the larval tiger salamander. Vision Res. 1981;21(8):1307–1317. doi: 10.1016/0042-6989(81)90236-4. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Skrzypek J. Formation of receptive fields and synaptic inputs to horizontal cells. Prog Clin Biol Res. 1982;113:181–192. [PubMed] [Google Scholar]
- Werblin F. S. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978 Jul;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. T. Synaptic orgnization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol. 1974 Mar;3(1):1–33. doi: 10.1007/BF01111929. [DOI] [PubMed] [Google Scholar]
- Yang X. L., Wu S. M. Effects of CNQX, APB, PDA, and kynurenate on horizontal cells of the tiger salamander retina. Vis Neurosci. 1989 Sep;3(3):207–212. doi: 10.1017/s0952523800009962. [DOI] [PubMed] [Google Scholar]



