Abstract
Pericentromeric γ-satellite DNA is organized in constitutive heterochromatin structures. It comprises a 234 bp sequence repeated several thousands times surrounding the centromeric sequence of all murine chromosomes. Potential binding sites for transcription factor Yin Yang 1 (YY1), a repressor or activator of several cellular and viral genes, are present in pericentromeric γ-satellite DNA. Using gel retardation and chromatin immunoprecipitation, we demonstrate in this work that YY1 specifically interacts in vitro and in vivo with γ-satellite DNA. Using immunoFISH and confocal microscopy we show that YY1 specifically co-localizes with pericentromeric γ-satellite DNA clusters organized in constitutive heterochromatin in murine L929 and 3T3 fibroblasts cell lines. Immunoelectron microscopy experiments further confirmed YY1 localization in heterochromatic areas. Overall, our results demonstrate for the first time that a fraction of YY1 is directly associated with constitutive heterochromatin structures. This association appears physiologically relevant since the association of YY1 with pericentromeric γ-satellite DNA observed in cycling 3T3 fibroblasts strongly diminished in quiescent (G0) 3T3 fibroblasts. We discuss the implications of these results in the context of heterochromatin formation as well as with regard to the YY1-induced repression of euchromatic genes.
INTRODUCTION
Yin Yang 1 (YY1) is a ubiquitous, highly conserved transcription factor which activates or represses several different eukaryotic genes among which are c-Myc, c-Fos, p53, α-actin, surf-2, grp78, IgH enhancer, β-casein, Igκ 3′enhancer, ρ-globin, ε-globin, IFN-γ, IFN-β as well as some viral promoters (1,2). The promoter context, intracellular YY1 concentration, YY1 post-translational modifications as well as factors and cofactors interacting with YY1 can determine the activator/repressor nature of YY1. YY1 has been shown to interact with a wide variety of transcription factors such as c-Myc, SP1 and E1A and chromatin remodeling enzymes such as histone deacetylases (HDACs) and histone acetyltransferases (HATs) [reviewed in (1)]. The capacity of YY1 to interact with HATs such as CBP/p300 and PCAF (3–5) could explain its activator function. In contrast, the repressive function of YY1 could be due to YY1 binding to HDACs (6,7). YY1 is known as a nuclear matrix protein (8). Disruption of the YY1 gene is lethal at early stages of development (9). Also, YY1 has been shown to be a Polycomb group (PcG) protein (10). The last two observations point out to a role for YY1 during development.
Formation of heterochromatin, a process that regulates eukaryotic gene silencing [reviewed in (11,12)], requires covalent modifications of DNA (i.e. cytosine methylation) and histones (i.e. methylation of Lys9 of H3 and hypoacetylation of H3 and H4) as well as the association of particular non-histone proteins such as non-histone heterochromatin protein 1 (HP1). Methylation of lysine 9 of histone H3 is important for the binding of HP1, the best characterized proteins found in heterochromatin. Tethering of mammalian HP1 to a reporter gene (13) as well as overexpression of mammalian HP1α and β (14) has been shown to induce gene repression. Also, specific transcriptional repression of some euchromatic genes by Drosophila HP1 has been reported recently (15).
The permanently inactive form of heterochromatin, called constitutive heterochromatin, is composed of repetitive sequences, primarily pericentromeric and telomeric (12). Constitutive heterochromatin is replicated late in the S phase and can be maintained through mitotic and meiotic cell divisions. On electron microphotographs, constitutive heterochromatin appears condensed [reviewed in (16)]. In constitutive heterochromatin, genes are presented in low density. The structure of heterochromatin is characterized by loss of nuclease hypersensitive sites and by regular nucleosomes (17,18). In constitutive heterochromatin, histones are hypoacetylated, histone H3 is trimethylated at Lys 9 and cytosines are hypermethylated (19–21). While methylation of Lys 9 of histone H3 and HP1 binding is critical for heterochromatin formation and gene silencing in pericentromeric heterochromatin, they are less important for heterochromatin formation at the telomeres (22,23).
In this work, we have analyzed the capacity of YY1, an HDAC-interacting transcription factor and PcG protein, to interact with a constitutive heterochromatin component, the pericentromeric γ-satellite DNA. We show that YY1 has the capacity to directly bind in vivo and in vitro to pericentromeric γ-satellite DNA through the specific recognition of YY1 DNA binding sites present in these sequences. Interestingly, the association of YY1 with γ-satellite DNA was disrupted during the transition from cycling to quiescent (G0) cells.
MATERIALS AND METHODS
Cell culture
Murine L929 cell culture was as previously described (24,25). Murine NIH3T3 cells were cultured in DMEM medium with 10% decomplemented fetal calf serum. Cells were plated at a density of 20 000 per square centimeter. For serum starvation (quiescence, G0) of NIH3T3 cells, subconfluent cells were placed in DMEM medium with 0.5% fetal calf serum for 72 h as described in (26,27).
Confocal microscopy
The cells were observed with a Leica-DMRBE microscope with TCS 4D confocal head. The merged images were analyzed by the Scanware (LeicaLasertechnik GmbH) or Image J programs. The pixel fluorograms were used to identify double-labeled pixels as described in (28). Double-labeled pixels were displayed in white on the co-localization overlay images (Figures 2 and 4).
Figure 2.
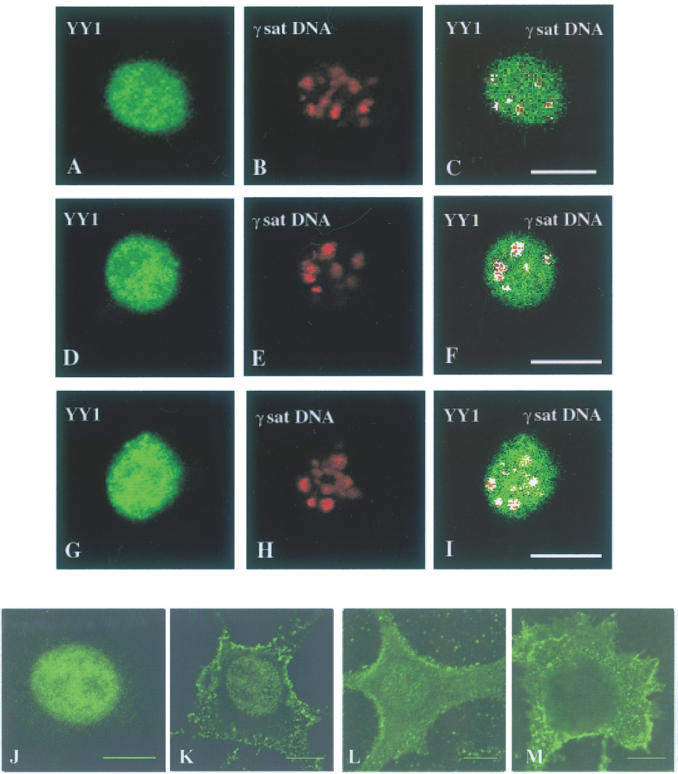
Transcription factor YY1 co-localizes with pericentromeric γ-satellite DNA clusters. Co-localization of endogenous YY1 with pericentromeric γ-satellite DNA was studied by immunoFISH technique in asynchronously growing L929 mouse fibroblasts viewed by confocal microscopy. Each row represents a single optical section of the same nucleus. Left panels (A, D and G) show YY1 subnuclear distribution in three independent L929 cells revealed with anti-YY1 H-10 monoclonal antibody. Middle panels (B, E and H) correspond to FISH analysis of pericentromeric γ-satellite DNA subnuclear localization using γ-satellite plasmid as a probe. Merged images of YY1 with centromeric γ-satellite DNA are shown on right panels for each set (C, F and I) with double-labeled pixels displayed in white. The monoclonal anti-YY1 H-10 antibody was pre-incubated with 0 ng (J), 500 ng (K), 1000 ng (L) or 2000 ng (M) of GST–YY1 before being added to L929 cells as in (A, D and G). Scale bar, 10 μm.
Figure 4.
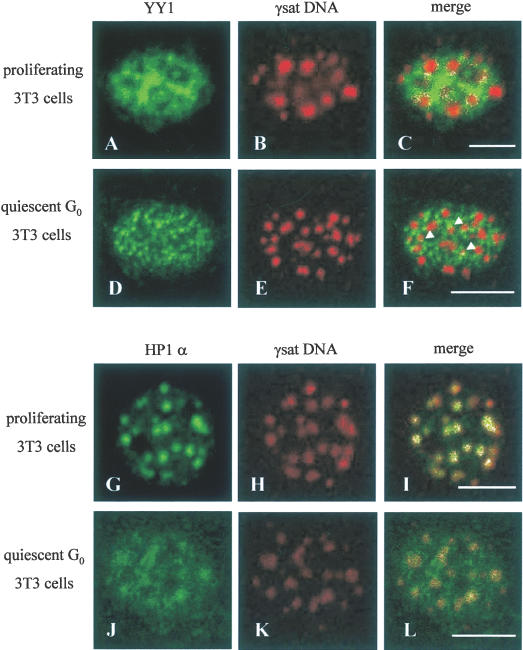
YY1 co-localizes with pericentromeric γ-satellite DNA in cycling but not quiescent (G0) NIH3T3 fibroblasts. Co-localization of endogenous YY1 or HP1α with pericentromeric γ-satellite DNA was studied by immunoFISH technique in either asynchronously growing (cycling) (A–C and G–I) or quiescent (G0) (D–F and J–L) NIH3T3 mouse fibroblasts by confocal microscopy. Each row represents a single optical section of the same nucleus. Panels (A–C) analyze YY1 subnuclear distribution revealed with anti-YY1 H-10 monoclonal antibody compared to FISH labelling of pericentromeric γ-satellite DNA using γ-satellite plasmid as a probe in cycling cells. Panels (D–F) analyze YY1 subnuclear distribution revealed with anti-YY1 H-10 monoclonal antibody compared to FISH labelling of pericentromeric γ-satellite DNA using γ-satellite plasmid as a probe in quiescent cells. In panel (F), arrows indicate small foci of co-localization of YY1 with γ-sat DNA in quiescent 3T3 cells. Panels (G–I) analyze HP1α subnuclear distribution revealed with anti-HP1α 2HP 1H5 monoclonal antibody compared to FISH labelling of pericentromeric γ-satellite DNA using γ-satellite plasmid as a probe in cycling cells. Panels (J–L) analyze HP1α subnuclear distribution revealed with anti-HP1α 2HP 1H5 monoclonal antibody compared to FISH labelling of pericentromeric γ-satellite DNA using γ-satellite plasmid as a probe in quiescent cells. Double-labeled pixels are displayed in white. Scale bar, 10 μm.
Immunoelectron microscopy
Immunogold labeling was essentially as described in (29). Cells were fixed with 1% paraformaldehyde, permeabilized with 0.2% Triton X-100, incubated sequentially with anti-YY1 C-20 rabbit polyclonal antibodies (diluted 1:100) and protein A-Au10 nm. Cells were observed with Jeol 120CX microscope.
Fluorescent in situ hybridization coupled to immunofluorescence (immunoFISH)
ImmunoFISH was carried out essentially as described in (30) with minor modifications. Cells were fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min, then in methanol at −20°C for 10 min, permeabilized with 0.1% Triton in PBS for 5 min and labeled with primary anti-YY1 monoclonal H-10 antibody (Santa Cruz Biotechnology) or anti-HP1α 2HP 1H5 monoclonal antibody (Euromedex) and secondary antibodies [Alexa 488 goat anti-mouse IgG (Molecular Probes)]. Then cells were post-fixed with 4% paraformaldehyde in PBS for 3 min, permeabilized in 0.1% Triton in PBS for 3 min, treated with 0.1 M Tris–HCl, pH 7.0 for 2 min and 2× SSC twice for 2 min. Cells were dehydrated in 70, 80, 90 and 100% ethanol at 4°C for 2 min each and dried. Then cells were treated with 100 μg/ml RNase A for 45 min at 37°C, washed, dehydrated, dried and subjected to in situ hybridization. Cells were hybridized with 10 ng γ-satellite PBS plasmid containing eight copies of 234 bp satellite repeat (31) (a generous gift of Dr Niall Dillon) directly labeled with fluoroRED (Amersham-Pharmacia). The probe was labeled by nick translation using standard protocols. The probe in 75% formamide, 10% dextran sulfate, 2× SSC, 2.5 μg single-stranded DNA from salmon sperm (Boehringer) (final volume 50 μl) was denatured at 95°C for 5 min. Hybridization was performed on slides for 5 min at 80°C (to denature DNA in cells) and then overnight at 37°C. After hybridization, coverslips were washed in 2× SSC for 30 min at 37°C, 1× SSC for 30 min at RT and 0.5× SSC for 30 min at RT and then mounted.
Expression and purification of recombinant GST-YY1 protein
The plasmid encoding glutathione S-transferase (GST)–YY1 was obtained from Dr Martin Montecino (Chile). The GST fusion protein was isolated by transformation of the plasmid into Escherichia coli BL21 strain followed by batch purification using glutathione agarose (Sigma) and the procedure recommended by the manufacturer except that PBS was used as washing buffer instead of the recommended PBS-T. The purity of the protein was evaluated by SDS–PAGE followed by Coomassie Blue staining and western blot analysis using anti-YY1 specific antibodies (Santa Cruz). The protein concentration was determined by Bradford assay.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) with nuclear extracts was carried out as described previously (2). The following wild-type and mutated oligonucleotides annealed with complementary strands were used: γ-sat1 (5′-AGTTTTCTCGCCATATTCCAGG-3′), γ-sat1m (5′-AGTTTTCTCGggATATTCCAGG-3′), γ-sat2 (5′-CCTACAGTGGACATTTCTAAAT-3′), γ-sat2m (5′-CCTACAGTGGggATTTCTAAAT-3′). For the supershift experiments, nuclear extracts were incubated with poly(dI–dC) and 1 or 2 μg of anti-YY1 monoclonal antibody (H-10, Santa Cruz Biotechnology) or 1 or 2 μg of anti-IRF-3 rabbit polyclonal antibodies (FL-425, Santa Cruz Biotechnology) for 1 h at 4°C before adding the corresponding double-stranded 32P-labeled oligonucleotides. For the competition experiments 30-, 60- or 90-fold excess of the corresponding unlabeled oligonucleotides were added at the same time as the 32P-labeled oligonucleotide. In case of EMSA with GST–YY1, 300 and 600 ng of fusion protein were incubated with indicated oligonucleotides.
Chromatin immunoprecipitation (ChIP) assays
ChIP assays on L929 cells were carried out as previously described (2) using 5 μl of polyclonal anti-YY1 antibodies (H-414 Santa Cruz Biotechnology) or 5 μl of polyclonal anti-acetyl-Histone H4(Lys8) antibodies (Upstate Biotechnology) in Figure 1F or 5 μl of anti-YY1 monoclonal antibody H-10 (Santa Cruz Biotechnology) and anti-HP1α 2HP 1H5 monoclonal antibody (Euromedex) in Figure 5. PCR analysis of immunoprecipitated DNA was performed using ‘sense’ oligonucleotide 5′-TAT GGC GAG GAA AAC TGA AA-3′ as 5′ primer and ‘anti-sense’ oligonucleotide 5′-TTC ACG TCC TAA AGT GTG TAT-3′ as 3′ primer to reveal the pericentromeric γ-satellite DNA. PCR conditions were as follows: denaturation at 94°C for 4 min, 13 cycles with denaturation at 94°C for 30 s, annealing at 54°C for 30 s, and elongation at 72°C for 30 s and at 72°C for 10 min.
Figure 1.
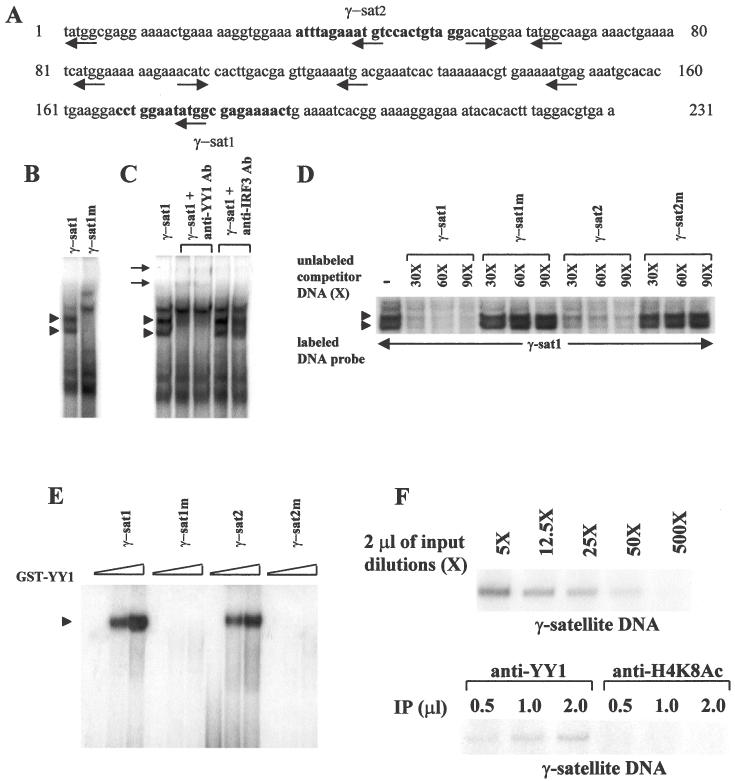
YY1 binds to γ-satellite DNA in vitro and in vivo. (A) The sequence of the centromeric γ-satellite DNA is shown (34) with the potential YY1 binding sites underlined with arrows indicating the 5′ to 3′ orientation. Two 22mer sequences of oligonucleotides γ-sat1 and γ-sat2 are indicated in bold face. (B) Electrophoretic mobility shift assays. L929 nuclear extracts were mixed with 32P-labeled γ-sat1 or mutated γ-sat1m double-stranded oligonucleotide probes and subjected to EMSA. (C) L929 nuclear extracts were incubated with 1 or 2 μg of anti-YY1 antibodies (lanes 2 and 3), or 1 or 2 μg of anti-IRF3 antibodies (lanes 4 and 5) and subjected to EMSA with 32P-labeled γ-sat1. (D) Competition experiments. EMSA with 32P-labeled γ-sat1 incubated with L929 nuclear extracts was carried out in the presence of an excess (30×, 60× and 90×) of corresponding unlabeled oligonucleotide probes γ-sat1, γ-sat1m, γ-sat2 and γ-sat2m. In all EMSA experiments, 1 μg of sonicated unlabeled poly(dI–dC) was used as non-specific competitor DNA. (E) EMSA with GST–YY1. Increasing amounts of GST-YY1 were mixed with 32P-labeled γ-sat1, γ-sat1m, γ-sat2 or γ-sat2m oligonucleotide probes and subjected to EMSA. (F) Chromatin immunoprecipitation (ChIP) assays. Crosslinked chromatin from L929 cells immunoprecipitated with anti-YY1 and anti-H4K8Ac antibodies (IP) was analyzed by PCR using primers corresponding to pericentromeric γ-satellite DNA sequence.
Figure 5.
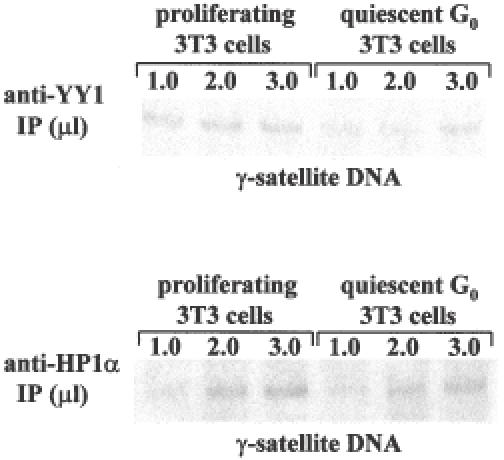
YY1 and HP1α dissociate from γ-satellite DNA in quiescent G0 cells. Chromatin immunoprecipitation (ChIPs) assays. Crosslinked chromatin from proliferating or quiescent G0 3T3 cells immunoprecipitated with anti-YY1 and anti-HP1α antibodies (IP) was analyzed by PCR using primers corresponding to pericentromeric γ-satellite DNA sequence.
RESULTS
YY1 interacts with pericentromeric γ-satellite DNA in vitro and in vivo
DNA regions organized into constitutive heterochromatin are characterized by the presence of highly repetitive non-coding DNA sequences consisting primarily of tandem repetitive satellite sequences found near centromeres. It has been postulated and demonstrated that association/dissociation of genes with pericentromeric heterochromatin represents a mechanism of transcriptional regulation when the association leads to gene transcriptional repression (16,32).
The YY1 protein has been shown to specifically bind a consensus sequence containing a conserved 5′-(C/a/t)CAT-3′ core motif (33). In Figure 1A, we show that the pericentromeric γ-satellite DNA sequence (34) contains multiple potential binding sites for YY1 whose corresponding core motifs are indicated by an arrow with a 5′ to 3′ orientation. In order to test the capacity of YY1 to form protein–DNA complexes with γ-satellite sequences a double-stranded 22mer oligonucleotide probe (γ-sat1) containing one of the potential YY1 binding sites, present in γ-satellite regions, was used (see Materials and Methods) in the electrophoretic mobility shift assays. Murine L929 nuclear extracts were prepared and incubated with oligonucleotide probe γ-sat1 or the corresponding mutated oligonucleotide probe γ-sat1m carrying a mutation in the YY1 core motif. As it can be observed in Figure 1B, several complexes were formed with oligonucleotide probe γ-sat1. Two of these complexes, indicated by arrowheads, disappeared in the presence of the mutated oligonucleotide probe γ-sat1m (Figure 1B, lane 2). In order to confirm the presence of YY1 in these protein–DNA complexes, nuclear extracts were incubated in the presence of 1 or 2 μg of anti-YY1 antibodies before adding γ-sat1 oligonucleotide probe. As it can be observed in Figure 1C, the complexes indicated by arrowheads were supershifted in the presence of antibodies specific for YY1 (compare lane 1 with the lanes 2 and 3, arrows) confirming the presence of YY1 in these complexes. No supershift was observed in the presence of irrelevant anti-IRF3 antibodies (Figure 1C, lanes 4 and 5).
In order to test the capacity of YY1 to bind to other potential YY1 binding sites present in pericentromeric γ-satellite DNA, we carried out competition experiments. In these experiments, 32P-labeled γ-sat1 oligonucleotide probe was incubated with nuclear extracts in the presence of an excess of unlabeled oligonucleotide probes γ-sat1, γ-sat1m, γ-sat2 (corresponding to another potential YY1 binding site present in γ-satellite DNA) (Figure 1A) and γ-sat2m (carrying a mutation in the γ-sat2 YY1 core motif) (see Materials and Methods). As expected, γ-sat1 but not γ-sat1m competed for the γ-sat1-YY1 complexes (Figure 1D, arrowheads). It can also be observed in Figure 1D that sequence γ-sat2 but not γ-sat2m also competed for the γ-sat1–YY1 complexes indicating that YY1 could also specifically bind to oligonucleotide γ-sat2 which contained a YY1 binding site.
In order to confirm the capacity of YY1 to directly bind the YY1 binding sites present in pericentromeric γ-satellite DNA, EMSA was carried out with GST–YY1 in the presence of oligonucleotides γ-sat1, γ-sat1m, γ-sat2 and γ-sat2m. As expected, GST–YY1-bound oligonucleotide probes γ-sat1 and γ-sat2 but not γ-sat1m and γ-sat2m (Figure 1E, arrowhead) indicating that YY1 can directly bind γ-satellite DNA in vitro.
In order to analyze the capacity of YY1 to bind pericentromeric γ-satellite DNA in vivo we performed chromatin immunoprecipitation experiments. Formaldehyde-linked DNA–protein complexes were precipitated with anti-YY1 antibodies and then analyzed by PCR using specific primers corresponding to γ-satellite repeats. As can be observed in Figure 1F, anti-YY1 antibodies precipitated endogenous pericentromeric γ-satellite DNA indicating that YY1 is bound in vivo to this DNA. As a control, an aliquot of the same formaldehyde-linked DNA–protein complexes was also precipitated with antibodies against acetylated K8 of histone H4 which is found in euchromatin, but not in heterochromatin. The DNA precipitated with anti-AcK8H4 antibodies was also analyzed by PCR using primers specific for γ-satellite repeats under the same conditions as those used with anti-YY1 precipitates. As shown in Figure 1F, no pericentromeric γ-satellite DNA was precipitated by anti-AcK8H4 antibodies confirming the specificity of binding of YY1 to pericentromeric γ-satellite DNA.
YY1 co-localizes with pericentromeric heterochromatin γ-satellite DNA
By using the technique of fluorescent in situ hybridization coupled with immunofluorescence (immunoFISH) the capacity of YY1 to co-localize with pericentromeric γ-satellite DNA was analyzed. The results of these experiments carried out with murine fibroblastic L929 cells are illustrated in Figure 2. Left panels (Figure 2A, D and G) show the nuclear distribution of YY1 detected with anti-YY1 monoclonal H-10 antibody in three different L929. Middle panels (Figure 2B, E and H) show the distribution of the γ-satellite DNA detected by FISH using a fluoroRED-labeled DNA probe for the γ-satellite sequence which stained the clusters of heterochromatin. The merged images (Figure 2C, F and I) demonstrate that YY1 is not excluded from pericentromeric heterochromatin and indicates that a fraction of protein YY1 strongly co-localized with γ-satellite DNA clusters. In order to verify that the YY1-signal obtained with the anti-YY1 monoclonal antibody H-10 was specific, the antibody was pre-incubated overnight at 4°C with 0, 500, 1000 or 2000 ng of GST–YY1 (Figure 2J, K, L and M, respectively) before being added to the cells. The YY1-nuclear signal partially disappeared after pre-incubation with 500 and 1000 ng (Figure 2K and L) of GST–YY1 and completely disappeared after pre-incubation with 2000 ng (Figure 2M) of GST–YY1 confirming therefore the specificity of the YY1-signal obtained with the H-10 antibody.
A fraction of YY1 localizes at the border between heterochromatic and euchromatic zones
To confirm the localization of YY1 with respect to heterochromatin clusters, we carried out electron microscopy studies with L929 fibroblastic cells labeled with anti-YY1 antibodies and protein A gold complex. Transcription factor YY1 was detected at the borders between euchromatin (low contrast zones) and heterochromatin (electron dense, highly contrasted zones) (Figure 3, open arrowheads), and in euchromatic areas away from heterochromatin (Figure 3, arrows) but barely inside heterochromatic zones, some of which correspond to perinucleolar heterochromatin (Figure 3, closed arrowheads).
Figure 3.
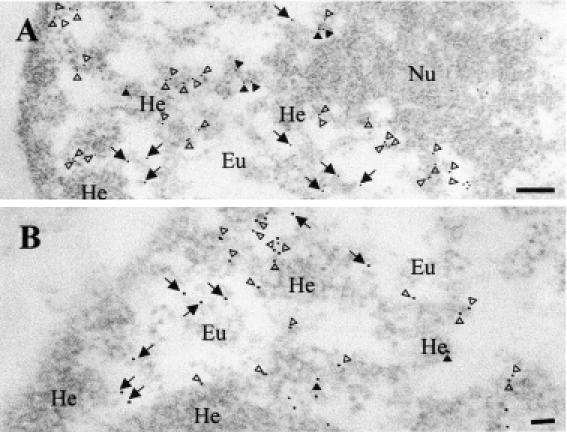
YY1 is localized in heterochromatic and euchromatic zones at the level of electron microscopy. Ultrastructural analysis of YY1 localization in the nuclei of L929 cells was carried out using indirect immunogold electron microscopy with rabbit anti-YY1 C-20 polyclonal antibodies and protein A-Au, 10 nm. Panels (A and B) correspond to two independent L929 cells at different magnifications. Eu, euchromatin; He, heterochromatin; Nu, nucleolus. YY1 is localized in Eu (arrows), at the borders between Eu and He (open arrowheads) and in He (closed arrowheads). Scale bars: (A) 200 nm; (B) 100 nm.
YY1 general nuclear distribution was modified during the transition from proliferation to quiescence
In order to analyze the functional significance of YY1 nuclear distribution in general and YY1-pericentromeric γ-satellite DNA interaction in particular we studied the distribution of YY1 comparatively with this DNA in cycling and quiescent cells. L929 fibroblasts used in our previous experiments cannot easily be driven into a quiescent (G0) state; therefore, we used NIH3T3 fibroblasts which become readily quiescent after incubation in low serum medium (0.5% fetal calf serum for 24–96 h) (26,27). We analyzed the distribution of YY1 comparatively with pericentromeric γ-satellite DNA in NIH3T3 fibroblasts, either in non-synchronized cycling or quiescent (G0) cells (incubated in low serum medium for 72 h). For this purpose, we used immunoFISH technique. In Figure 4A–F we show two nuclei from cells in different culture conditions indicated above. Left panels (Figure 4A and D) present the nuclear distribution of YY1 detected with anti-YY1 monoclonal H-10 antibody. The general distribution of YY1 was clearly modified during the transition from cycling to quiescent cells. In cycling cells, YY1 is diffusely distributed (Figure 4A), while in quiescent (G0) cells YY1 shows granular distribution (Figure 4D). Middle panels (Figure 4B and E) show the distribution of pericentromeric γ-satellite DNA organized in patches which was detected by FISH using a fluoroRED-labeled DNA probe for γ-satellite sequence. The right panels (Figure 4C and F) present the merged images. A fraction of YY1 is co-localized with γ-satellite DNA clusters in cycling NIH3T3 fibroblasts mainly at the periphery of γ-satellite DNA patches. Most of this peripheral co-localization is lost in quiescent (G0) cells (see co-localized pixels on merged images in Figure 4C and F) concomitant with YY1 redistribution. However, a small portion of YY1 still remained co-localized with γ-satellite clusters, visible as small white foci indicated by an arrow in Figure 4F.
HP1α is a non-histone protein known to be one of the major components of pericentromeric heterochromatin. Thus, we analyzed the distribution of HP1α comparatively with pericentromeric γ-satellite DNA using immunoFISH technique as well. In Figure 4G–L we show the analysis of this distribution either in cycling or quiescent (G0) NIH3T3 cells. Left panels (Figure 4G and J) demonstrate the nuclear distribution of HP1α shown with anti-HP1α 2HP 1H5 monoclonal antibody. Middle panels (Figure 4H and K) present the distribution of pericentromeric γ-satellite DNA. The right panels (Figure 4I and L) show the merged images. In cycling NIH3T3 cells, HP1α is organized in clusters which coincide with those of pericentromeric γ-satellite DNA. In quiescent cells, the distribution of HP1α becomes more diffuse and it partially loses its association with clusters of pericentromeric γ-satellite DNA.
We have also compared the capacity of YY1 and HP1α to directly interact with γ-satellite DNA repeats in cycling versus quiescent cells using ChIP assays. Formaldehyde-linked DNA–protein complexes isolated from 3T3 cycling or quiescent G0 cells were precipitated with anti-YY1 and anti-HP1α antibodies and then analyzed by PCR using specific primers corresponding to γ-satellite repeats. The results are shown in Figure 5. In agreement with the results obtained during immunoFISH experiments shown in Figure 4, both antibodies immunoprecipitated less γ-sat DNA from quiescent cells compared to cycling cells. In the case of HP1α, and, to a lesser extent, also in the case of YY1, a small fraction of the protein still remained associated to γ-satellite DNA in quiescent G0 cells.
DISCUSSION
Even though the main trademarks of constitutive pericentromeric heterochromatin are known, much remains to be understood concerning its organization and function, especially, concerning the mechanisms that could govern heterochromatin-dependent gene silencing of euchromatic genes.
Protein YY1 is a transcription factor previously identified as an activator and/or repressor of several euchromatic genes. In this work, we demonstrate that a fraction of the transcription factor YY1 can co-localize with and directly bind to γ-satellite DNA clusters organized in constitutive pericentromeric heterochromatin. We show that YY1 can bind to several potential binding sites present in γ-satellite DNA. This interaction occurs not only in vitro but also in vivo. We show here for the first time, electron microscopy studies of the nuclear localization of YY1 which suggest that a fraction of YY1 localizes at the borders between euchromatic and heterochromatic areas. These results obtained during electron microscopy were in agreement with our results obtained with the immunoFISH technique that showed YY1 co-localization with γ-satellite DNA, mainly at the periphery of pericentromeric clusters.
YY1 co-localization with γ-satellite DNA strongly resembles that of Ikaros with pericentromeric DNA (35). Similar to Ikaros, YY1 displays the capacity to establish protein–protein interactions with transcription regulators and bind to DNA sequence present in promoter regions as well as pericentromeric γ-satellite repeats. Much remains to be understood concerning the macromolecular architecture of constitutive heterochromatin edifices. The capacity of transcription factors such as YY1 and Ikaros to establish protein–protein and protein–DNA interactions with partners present in euchromatin as well as heterochromatin may explain why both these factors localize at the periphery rather than the center of pericentromeric heterochromatin clusters. Unlike Ikaros, which is present only in lymphocytes, YY1 is a ubiquitous transcription factor present in several cell types. The conservation of the YY1 binding sites on satellite sequences from mouse to man (36), regardless of variations normally present in pericentromeric DNA sequences, suggests a possible functionality of these sequences. In Figure 6, we illustrate a model similar to the one proposed for the Ikaros protein, suggesting that YY1 binding to pericentromeric γ-satellite DNA could lead to the targeting of proteins required for heterochromatization, such as HDACs (Figure 6A), or to the targeting of genes via YY1-associated factors (Figure 6B) to the zone of pericentromeric heterochromatin inducing their silencing.
Figure 6.
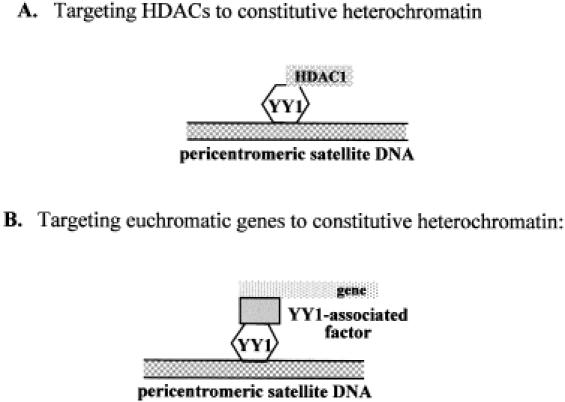
Models of YY1 function in relation with constitutive heterochromatin. (A) YY1 targets HDACs to constitutive heterochromatin. YY1 associated with HDAC1 binds a specific sequence present in pericentromeric γ-satellite DNA repeat. (B) YY1 targets specific euchromatic genes to constitutive heterochromatin. YY1 bound to pericentromeric γ-satellite DNA interacts with YY1 associated factor which in turn binds to a specific euchromatic gene.
YY1 belongs to the Polycomb Group (PcG) family of proteins involved in the repression of homeotic genes. It has been shown that Drosophila DNA binding PcG protein PHO is a homolog of YY1 (10). The observations that YY1, which is essential for development, interacts with heterochromatin structures make a possible link between these two silencing systems. This hypothesis is strengthened by the observation that human PcG complex associates with pericentromeric heterochromatin (37). The data we present in this work open up a wide range of new possibilities concerning YY1-induced silencing of euchromatic genes in relation with constitutive heterochromatin, heterochromatin-like structures and perhaps PcG silencing systems.
Recently, data has been accumulated concerning the role of YY1 in cell cycle and differentiation regulation. The levels of YY1 are regulated in response to insulin-like growth factor 1 (IGF-1) (27). Moreover, ectopic expression of YY1 induces quiescent CASM cells, U2OS and Molt4 lymphocytes to progress to the S phase of the cell cycle. Altogether, these data suggest that up-regulation of YY1 activity is one of the cellular responses to growth factor stimulation inducing therefore DNA synthesis and cell cycle progression in a variety of cell types. YY1 can regulate several genes coding for the proteins involved in DNA synthesis. YY1 binding sites are present in the cell-cycle-regulated genes such as those for E2F (38), dihydrofolate reductase (39), cdc6 (40), histone H3.2 (41) as well as for histone H4 (42). It was shown that YY1 binding to the H3.2 promoter is necessary for its optimal activation during G1/S transition (41). It has been suggested that such a mechanism could be functional also in the regulation of the histone H4 promoter which contain multiple YY1 binding sites. The disruption of YY1 gene in mouse leads to pre-implantation lethality indicating that YY1 can regulate genes essential for rapid proliferation and differentiation of mouse embryos in early development (9).
In this study, we show that the general distribution of YY1 is modified during the transition from cycling to quiescence. Particularly, YY1 co-localization with pericentromeric γ-satellite DNA observed in cycling cells is diminished in quiescent (G0) cells. Results obtained using ChIP assays indicated that not only co-localization but also direct association of YY1 with pericentromeric DNA was almost completely abolished during the transition from proliferation to quiescence. In relation with this, it is interesting to note that in quiescent cells YY1 forms a complex with retinoblastoma protein (Rb) which disappears in serum-stimulated cells (43). YY1 in complex with Rb loses its capacity to bind DNA. We suggest that the formation of a complex between YY1 and Rb could be one of the reasons for the dissociation of YY1 from pericentromeric γ-satellite DNA in quiescent (G0) cells.
We observed in our study that the transition to quiescent (G0) state is accompanied not only by the dissociation of YY1 from pericentromeric heterochromatin but also by partial dissociation from these structures of one of the major non-histone protein of heterochromatin, HP1α. We suggest that the dissociation of HP1α from pericentromeric heterochromatin could induce its redistribution in the nucleus. HP1α could be translocated to other places in the nucleus where it might now repress a number of euchromatic genes which is a characteristic feature of the quiescent (G0) state. According to this hypothesis, pericentromeric heterochromatin could serve as a reservoir of repressive proteins. In relation with this, it is interesting to note that the binding of different HP1 subtypes to centromeres was studied during the cell cycle in human cells, and the domains of HP1 responsible for its localization at interphase and metaphase centromeres have been determined (44). Interestingly, the C-terminal fragment of HP1α including its chromo shadow domain was necessary for its localization to the metaphase centromere, while the N-terminal fragment of HP1β including its chromo domain was necessary for its localization to the interphase centromere.
Recently, it has been shown that HP1 proteins can interact with a pentapeptide present in other proteins as well as in the chromo shadow domain of HP1 itself (45). We found this pentapeptide PRVHV in the C-terminal part of YY1 between zinc fingers 1 and 2. We are presently analyzing the capacity of YY1 to interact with HP1α in cycling as well as in quiescent (G0) cells.
The data we present here indicate that transition from the cycling to the quiescent (G0) state is accompanied by an important redistribution of some of the constituents of pericentromeric heterochromatin. The exact meaning of this phenomenon is not yet understood. The fact that such redistribution affects the association of the transcription factor YY1 with pericentromeric heterochromatin makes us postulate that such reorganization of heterochromatin structures might be related to major changes in the overall level of transcription associated with these opposite states of the cell.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Gérard Géraud and Christophe Chamot for confocal microscopy analysis, Sylvette Reposo for the help with electron microscopy experiments and Dr Niall Dillon for the gift of γ-satellite plasmid. This work was supported by the Centre National de la Recherche Scientifique and by grant from the Association pour la Recherche sur le Cancer (ARC 3236). E.A.S. was a recipient of a fellowship from the Fondation pour la Recherche Médicale (FRM) until April 2002 and presently from Association Française contre les Myopathies (AFM).
REFERENCES
- 1.Thomas M.J. and Seto,E. (1999) Unlocking the mechanisms of transcription factor YY1: are chromatin modifying enzymes the key? Gene, 236, 197–208. [DOI] [PubMed] [Google Scholar]
- 2.Weill L., Shestakova,E. and Bonnefoy,E. (2003), Transcription factor YY1 binds to the murine interferon-beta promoter and regulates its transcriptional capacity with a dual activator/repressor role. J. Virol., 77, 2903–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austen M., Luscher,B. and Luscher-Firzlaff,J.M. (1997) Characterization of the transcriptional regulator YY1. The bipartite transactivation domain is independent of interaction with the TATA box-binding protein, transcription factor IIB, TAFII55, or cAMP-responsive element-binding protein (CBP)-binding protein. J. Biol. Chem., 272, 1709–1717. [DOI] [PubMed] [Google Scholar]
- 4.Galvin K.M. and Shi,Y. (1997) Multiple mechanisms of transcriptional repression by YY1. Mol. Cell. Biol., 17, 3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.S., Galvin,K.M., See,R.H., Eckner,R., Livingston,D., Moran,E. and Shi,Y. (1995) Relief of YY1 transcriptional repression by adenovirus E1A is mediated by E1A-associated protein p300. Genes Dev., 9, 1188–1198. [DOI] [PubMed] [Google Scholar]
- 6.Coull J.J., Romerio,F., Sun, J-M., Volker,J.L., Galvin,K.M., Davie,J.R., Shi,Y., Hansen,U. and Margolis,D.M. (2000) The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J. Virol., 74, 6790–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang W.M., Inouye,C., Zeng,Y., Bearss,D. and Seto,E. (1996) Transcriptional repression by YY1 is mediated by interaction with a mammalian homolog of the yeast global regulator RPD3. Proc. Natl Acad. Sci. USA, 93, 12845–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo B., Odgren,P.R., van Wijnen,A.J., Last,T.J., Nickerson,J., Penman,S., Lian,J.B., Stein,J.L. and Stein G.S. (1995) The nuclear matrix protein NMP-1 is the transcription factor YY1. Proc. Natl Acad. Sci. USA, 92, 10526–10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donohoe M.E., Zhang,X., McGinnis,L., Biggers,J., Li,E. and Shi,Y. (1999) Targeted disruption of mouse Yin Yang 1 transcription factor results in peri-implantation lethality. Mol. Cell. Biol., 19, 7237–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown J.L., Mucci,D., Whiteley,M., Dirksen,M-L. and Kassis,J.A. (1998) The Drosophila Polycomb group gene pleiohomeotic encodes a DNA binding protein with homology to the transcription factor YY1. Mol. Cell, 1, 1057–1064. [DOI] [PubMed] [Google Scholar]
- 11.Eissenberg J.C. and Elgin,S.C.R. (2000) The HP1 protein family: getting a grip on chromatin. Curr. Opin. Genet. Dev., 10, 204–210. [DOI] [PubMed] [Google Scholar]
- 12.Richards E.J. and Elgin,S.C.R. (2002) Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell, 108, 489–500. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen A.L., Ortiz,J.A., You,J., Oulad-Abdelghani,M., Khechumian,R., Gansmuller,A., Chambon,P. and Losson,R. (1999) Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J., 22, 6385–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang K-K. and Worman,H.J. (2002) Gene regulation by human orthologs of Drosophila heterochromatin protein 1. Biochem. Biophys. Res. Com., 293, 1217–1222. [DOI] [PubMed] [Google Scholar]
- 15.Hwang K-K., Eissenberg,J. and Worman,H.J. (2001) Transcriptional repression of euchromatic genes by Drosophila heterochromatin protein 1 and histone modifiers. Proc. Natl Acad. Sci. USA, 98, 11423–11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henikoff S. (2000) Heterochromatin function in complex genomes. Biochim. Bhioph. Acta, 1470, 01–08. [DOI] [PubMed] [Google Scholar]
- 17.Grewal S.I.S. and Elgin,S.C.R. (2002) Heterochromatin: new possibilities for the inheritance of structure. Curr. Opin. Cell Genet. Dev., 12, 178–187. [DOI] [PubMed] [Google Scholar]
- 18.Sun F.L., Cuaycong,M.H. and Elgin,S.C. (2001) Long-range nucleosome ordering is associated with gene silencing in Drosophila melanogaster pericentromeric heterochromatin. Mol. Cell. Biol., 21, 2867–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters A.H.F.M., Kubicek,S., Mechtler,K., O'Sullivan,R.J., Derijck,A.A.H.A., Perez-Burgos,L., Kohlmaier,A., Opravil,S., Tachibana,M., Shinkai,Y., Martens,J.H.A. and Jenuwein,T. (2003) Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol. Cell, 12, 1577–1589. [DOI] [PubMed] [Google Scholar]
- 20.Bachman K.E., Rountree,M.R. and Baylin,S.B. (2001) Dnmt3a and Dnmt3b are transcriptional repressors that exhibit unique localization properties to heterochromatin. J. Biol. Chem., 276, 32282–32287. [DOI] [PubMed] [Google Scholar]
- 21.Okano M., Bell,D.W., Haber,D.A. and Li,E. (1999) DNA methyl-transferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell, 99, 247–257. [DOI] [PubMed] [Google Scholar]
- 22.Cryderman D.E., Morris,E.J., Biessmann,H., Elgin,S.C. and Wallrath,L.L. (1999) Silencing at Drosophila telomeres: nuclear organization and chromatin structure play critical roles. EMBO J., 18, 3724–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekwall K., Cranston,G. and Allshire,R.C. (1999) Fission yeast mutants that alleviate transcriptional silencing in centromeric flanking repeats and disrupt chromosome segregation. Genetics, 153, 1153–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonnefoy E., Bandu,M.-T. and Doly,J. (1999) Specific binding of high-mobility-group I (HMGI) protein and histone H1 to the upstream AT-rich region of the murine beta interferon promoter: HMGI protein acts as a potential antirepressor of the promoter. Mol. Cell. Biol., 19, 2803–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shestakova E., Bandu,M-T., Doly,J. and Bonnefoy,E. (2001) Inhibition of histone deacetylation induces constitutive derepression of the beta interferon promoter and confers antiviral activity. J. Virol., 75, 3444–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw R.J., McClatchey,A.I. and Jacks,T. (1998) Regulation of the neurofibromatosis type 2 tumor suppressor protein, merlin, by adhesion and growth arrest stimuli. J. Biol. Chem., 273, 7757–7764. [DOI] [PubMed] [Google Scholar]
- 27.Flanagan J.R. (1995) Autologous stimulation of YY1 transcription factor expression: role of an insulin-like growth factor. Cell Growth Differ., 6, 185–190. [PubMed] [Google Scholar]
- 28.Demandolx D. and Davoust,J. (1997) Multicolor analysis and local image correlation in confocal microscopy. J. Microsc., 185, 21–36. [Google Scholar]
- 29.Humbel B.M., De Jong,M.D.M., Muller,W.H. and Verkleij,A.J. (1998) Pre-embedding immunolabeling for electron microscopy: an evaluation of permeabilization methods and markers. Microsc. Res. Tech., 42, 43–58. [DOI] [PubMed] [Google Scholar]
- 30.Robert-Fortel I., Junera,H.R., Geraud,G. and Hernandez-Verdun,D. (1993) Three-dimensional organization of the ribosomal genes and Ag-NOR proteins during interphase and mitosis in PtK1 cells studied by confocal microscopy. Chromosoma, 102, 146–157. [DOI] [PubMed] [Google Scholar]
- 31.Lundgren M., Chow,C-M., Sabbattini,P., Georgiou,A., Minaee,S. and Dillon,N. (2000) Transcription factor dosage affects changes in higher order chromatin structure associated with activation of a heterochromatic gene. Cell, 103, 733–743. [DOI] [PubMed] [Google Scholar]
- 32.Brown K.E., Guest,S.S., Smale,S.T., Hahm,K., Merkenschlager,M. and Fisher,A.G. (1997) Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell, 91, 845–854. [DOI] [PubMed] [Google Scholar]
- 33.Hyde-DeRuyscher R.P., Jennings,E. and Shenk,T. (1995) DNA binding sites for the transcriptional activator/repressor YY1. Nucleic Acids Res., 23, 4457–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vissel B. and Choo,K.H. (1989) Mouse major (γ) satellite DNA is highly conserved and organized into extremely long tandem arrays: implications for recombination between nonhomologous chromosomes. Genomics, 5, 407–414. [DOI] [PubMed] [Google Scholar]
- 35.Cobb B.S., Morales-Alcelay,S., Kleiger,G., Brown,K.E., Fisher,A.G. and Smale,S.T. (2000), Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes Dev., 14, 2146–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldini A., Ried,T., Shridhar,V., Ogura,K., D'Aiuto,L., Rocchi,M. and Ward,D.C. (1993) An alphoid DNA sequence conserved in all human and great ape chromosomes: Evidence for ancient centromeric sequences at human chromosomal regions 2q21 and 9q13. Hum. Genet., 90, 577–583. [DOI] [PubMed] [Google Scholar]
- 37.Saurin A.J., Shiels,C., Williamson,J., Satijn,D.P.E., Otte,A.P., Sheer,D. and Freemont,P.S. (1998) The human Polycomb Group Complex associates with pericentromeric heterochromatin to form a novel nuclear domain. J. Cell Biol., 142, 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsiao K.M., McMahon,S.L. and Farnham,P.J. (1994) Multiple DNA elements are required for the growth regulation of the mouse E2F1 promoter. Gene Dev., 8, 1526–1537. [DOI] [PubMed] [Google Scholar]
- 39.Farnham P.J. and Means,A.L. (1990) Sequences downstream of the transcription initiation site modulate the activity of the murine dihydrofolate reductase promoter. Mol. Cell Biol., 10, 1390–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hateboer G., Wobst,A., Petersen,B.O., Le Cam,L., Vigo,E., Sardet,C. and Helin,K. (1998) Cell cycle-regulated expression of mammalian CDC6 is dependent on E2F. Mol. Cell. Biol., 18, 6679–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu F. and Lee,A.S. (2001) YY1 as a regulator of replication-dependent hamster histone H3.2 promoter and an interactive partner of AP-2. J. Biol. Chem., 276, 28–34. [DOI] [PubMed] [Google Scholar]
- 42.Last T.J., van Wijnen,A.J., Birnbaum,M.J., Stein,G.S. and Stein,J.L. (1999) Multiple interactions of the transcription factor YY1 with human histone H4 gene regulatory elements. J. Cell. Biochem., 72, 507–516. [PubMed] [Google Scholar]
- 43.Petkova V., Romanowski,M.J., Sulijoadikusumo, I., Rohne,D., Kang,P., Shenk,T. and Usheva,A. (2001) Interaction between YY1 and the retinoblastoma protein. J. Biol. Chem., 276, 7932–7936. [DOI] [PubMed] [Google Scholar]
- 44.Hayakawa T., Haraguchi,T., Masumoto,H. and Hiraoka,Y. (2003) Cell cycle behavior of human HP1 subtypes: distinct molecular domains of HP1 are required for their centromeric localization during interphase and metaphase. J. Cell Sci., 116, 3327–3338. [DOI] [PubMed] [Google Scholar]
- 45.Smothers J.F. and Henikoff,S. (1999) The HP1 chromo shadow domain binds a consensus peptide pentamer. Curr. Biol., 10, 27–30. [DOI] [PubMed] [Google Scholar]


