Abstract
Carbamylation (or carbamoylation) of lysine residues and protein N-termini is a ubiquitous, non-enzymatic post-translational modification. Carbamylation at sites of inflammation is due to cyanate formation during the neutrophil oxidative burst and may target lysine residues within the antimicrobial peptide LL-37, which is secreted by activated neutrophils. The bactericidal and immunomodulatory properties of LL-37 depend on its structure and cationic nature, which are conferred by arginine and lysine residues. Therefore, carbamylation may affect the biological functions of LL-37. This may be of great importance in the context of using LL-37 as a target for drug development. The present study examined the kinetics and pattern of LL-37 carbamylation to investigate how this modification affects the bactericidal, cytotoxic, and immunomodulatory function of the peptide. The results indicated that LL-37 undergoes rapid modification in the presence of physiological concentrations of cyanate, yielding a spectrum of diverse carbamylated peptides. Mass spectrometry analyses revealed that the N-terminal amino group of Leu-1 was highly reactive and was modified almost instantly by cyanate to generate the predominant form of the modified peptide, named LL37C1. This was followed by the sequential carbamylation of Lys-8, Lys-12, and Lys-15, to yield LL37C8, and LL37C12,15, respectively. Carbamylation had profound and diverse effects on the structure and biological properties of LL-37. In some cases, anti-inflammatory LL-37 was rapidly converted to pro-inflammatory LL-37. Thus, caution should be exercised when treating patients with severe inflammatory conditions, such as sepsis, with pro-inflammatory LL-37.
Keywords: carbamylation, LL-37, immunomodulation
Introduction
Post-translational modifications (PTMs) are pivotal steps in protein maturation. PTMs increase the functional diversity of the proteome and play a key role in multiple cellular processes, including protein-protein interactions, cell signaling, differentiation, and regulation of gene expression [1]. Carbamylation is a ubiquitous, non-enzymatic PTM, in which cyanate (OCN−) reacts with primary amino groups (NH3+) at the N-termini of proteins, and with lysine residues in the polypeptide chain, to generate α-carbamyl amino acids and ε-carbamyl-lysine (homocitrulline), respectively [2,3]. Since urea (a by-product of protein metabolism) and cyanate comprise an equilibrium pair, the level of protein carbamylation is markedly increased in diseases associated with chronic uremia. A recent study identified a novel pathway linking inflammation, myeloperoxidase (MPO), and carbamylation. MPO, a heme peroxidase released by activated neutrophils, catalyzes the formation of cyanate from hydrogen peroxide and thiocyanate, ultimately leading to homocitrullination of proteins [3]. The chemical conversion of positively charged Lys residues to neutral homocitrulline residues affects the charge distribution within a polypeptide chain in a manner that often results in impairment or even loss of function. For example, loss of function upon carbamylation has been reported for matrix metalloproteinase-2, inhibitor of metalloproteinase-2, and insulin [4,5].
The abundance of MPO at inflammatory foci has sparked significant interest in the role of carbamylation in the context of chronic inflammatory diseases and atherogenesis [3,6,7]. Apart from directly affecting protein function and turnover, homocitrulline residues act as neo-epitopes that can trigger primary immune responses, thereby inducing chemotaxis and proliferation of CD4+ T cells and the subsequent production of interferon-γ, interleukin (IL)-10, and IL-17. In addition, antibodies against carbamylated residues have clinical value in that they predict a more erosive progression of rheumatoid arthritis [8,9].
Cationic antimicrobial peptides (CAMPs) are essential components of human innate immunity and are produced by a variety of cells, including epithelial cells [10], keratinocytes [11], and neutrophils [12]. In humans, CAMPs are represented by α- and β-defensins and the cathelicidin-derived LL-37 peptide. The active form of LL-37 is characterized by a high abundance of arginine and lysine residues, which generate a net positive charge of +6 at a natural pH. Apart from killing a wide spectrum of pathogenic bacteria [13,14], LL-37 neutralizes lipopolysaccharide (LPS), functions as a chemoattractant for immune cells (including T cells, monocytes, neutrophils, and mast cells)[15,16], profoundly affects the course of dendritic cell maturation [17], stimulates production of cytokines, chemokines, and their receptors [18,19], and triggers mast cell degranulation [16]. These effects are mediated, at least in part, by activation of at least four different receptors: formyl peptide receptor-like 1 (FPRL1), epidermal growth factor receptor (EGFR), P2X7, and CXCR2. Finally, recent studies show that LL-37 has a direct effect on the cells and might be hemolytic [20] or cytotoxic to peripheral blood mononuclear cells (PBMCs) at high concentrations [21]. At low concentrations (<5 μM), LL-37 induces rapid secondary necrosis of apoptotic human neutrophils [22]. To prevent collateral tissue damage due to exacerbation of inflammation, the activity of LL-37 is strictly controlled by serum proteins [23], most likely apolipoprotein-A1 (apoA-1) [24]. Taken together, all available data indicate that the antibacterial activity of LL-37 is secondary to its immunomodulatory functions. In a twist of the paradigm, it is now generally accepted that the major role of CAMPs is not direct killing of invading microbes, but rather acting as signaling molecules for innate and acquired immunity.
Within the inflammatory milieu, the concomitant release of LL-37 and MPO by activated neutrophils creates the perfect conditions for LL-37 carbamylation. Additionally, lysine residues within CAMPs are crucial for peptide structure and activity, and conversion of these positively charged residues to neutral homocitrulline residues would be expected to abrogate the biological activity of LL-37.
Here, we performed mass spectrometry based sequence analysis and found that LL-37 undergoes rapid carbamylation in the presence of cyanate in a time- and concentration-dependent manner. Unexpectedly, we found that the free amino group of the N-terminal leucine was most susceptible to carbamylation under conditions that reflected the cyanate concentration in the inflammatory milieu. Prolonged incubation resulted in the generation of a mixture of variably carbamylated LL-37 molecules with impaired antimicrobial activity against both Gram-negative and Gram-positive species.
Taken together, these results suggest that carbamylation of LL-37 within an inflammatory environment might actually exacerbate inflammation and be detrimental to the host.
Materials and methods
In vitro carbamylation of LL-37 and mass spectrometry analysis
LL-37 (ProImmune, Oxford, U.K) was carbamylated by incubation with 10, 50 and 100 mM KCNO (Sigma-Aldrich, Oslo, Norway) in 100 mM HEPES (pH 7.8) for 3 hours at 37°C. The reaction was quenched by addition of formic acid to a final concentration of 5% and samples were immediately purified using StageTips (ThermoScientific). The samples were lyophilized and either analyzed directly by LC-MS/MS or subjected to proteolytic digestion with S. aureus protease V8 (1:25 w/w) at 37°C for 16 hours prior to LC-MS/MS analysis. NanoESI-MS/MS analyses were performed on an EASY-nLC II system (ThermoScientific) connected to a TripleTOF 5600 mass spectrometer (AB Sciex) equipped with a NanoSpray III source (AB Sciex) operated under Analyst TF 1.5.1 control. The samples were suspended in 0.1% formic acid, injected, trapped and desalted on a Biosphere C18 column (5 μo, 2 cm x 100 μ I.D; Nano Separations) after which the peptides were eluted from the trap column and separated on a 15-cm analytical column (75 μI i.d.) packed in-house in a pulled emitter with RP ReproSil-Pur C18-AQ 3 μC resin (Dr. Marisch GmbH, Ammerbuch-Entringen, Germany) and connected in-line to the mass spectrometer. The peptides were eluted using a 20 min gradient from either 5%–35% phase B or 5–90% phase B (0.1% formic acid and 90% acetonitrile). The collected MS files were converted to Mascot generic format (MGF) using the AB SCIEX MS Data Converter beta 1.1 (AB SCIEX). The peptide sequence was identified using in-house Mascot search engine (matrix science). Search parameters were allowing two missed cleaving sites and carbamylation as a variable modification. Peptide tolerance and MS/MS tolerance were set to 10 ppm and 0.1 Da respectively.
Peptide synthesis
Native and carbamylated LL-37 were synthesized by ProImmune by using Fmoc solid-phase peptide synthesis, diluted in 0.01% v/v acetic acid and stored at −70°C until use. The peptides were evaluated by mass spectrometry and the average purity was found to be 95.83%.
Circular dichroism (CD) spectroscopy
The secondary structure of the LL-37 analogues was investigated by Circular dichroism (CD) spectroscopy. The experiments were performed using a Jasco J-810 spectropolarimeter. Far UV-spectra were acquired at 37°C in the 195–260 nm range at a scan rate of 50 nm/min and a band width of 1 nm. Three scans were accumulated for each sample and appropriate blanks were subtracted from each spectrum by using the software provided by the instrument manufacturers. The peptides (10 μM) were analyzed upon dilution in a 10 mM sodium phosphate buffer containing 50% v/v trifluorethanol (TFE) or a physiological salt solution resembling plasma (113 mM NaCl, 24 mM NaHCO3, 0.6 mM MgCl2, 1.3 mM CaCl2, 3.9mM KCl) in 1.0-mm quartz cuvettes (Hellma-Analytics, Oslo, Norway). The mean ellipticity was calculated using the formula [θ] = θ/(10·c·l), where θ is the ellipticity (mdeg), 10 is a scaling factor, c is the protein concentration (M) and l is the path length of the cuvette (cm). The helical content (percentage of helix) was estimated by using the CDNN program from the molar ellipticity θ [deg.cm2 dmol-1].
Broth microdilution assay
Frozen samples of S. aureus LS-1, E. coli ATCC 25922 and B. subtilis ATCC 3366 were cultured on horse blood-agar plates at 37°C over night. Few colonies were selected and pre-cultured in 50 ml LB-broth in a shaking incubator (220 rpm, 37°C) over night. The bacteria were diluted 1:100 times in fresh LB-broth and cultured to its mid-log-phase. The bacteria were washed four times at 4000 x g for 5 min (E. coli and S. aureus) or at 6000 x g for 8 min (B. subtilis) at 4°C and thereafter suspended to 1 x 106 CFU/ml in PBS without calcium and magnesium. Native and carbamylated LL-37 described above were diluted to different concentrations in 0.01% v/v acetic acid containing 0.2% w/v bovine serum albumin (Sigma-Aldrich). Thereafter, one part of the peptide solutions were mixed with nine parts bacteria solution to get a final peptide concentration of 1 μg/ml (i.e., 0.2 μM). In addition, one positive control sample containing bacterial solution without additives and one negative control without bacteria were prepared. All samples were incubated for 2 hours at 37°C. Samples containing B. subtilis were incubated on a shaking plate at 220 rpm. 100 μl aliquots of the bacterial mixture were spread on blood agar plates in duplicate or triplicate after being 10-fold diluted in 4 steps (undiluted, 1:10, 1:100, 1:1000, 1:10000). The plates were incubated at 37°C over night. Colony-forming units were counted and the total number was determined from the dilution factor. The experiments were performed under sterile conditions.
Culture of human monocyte-derived macrophages (hMDMs)
Human blood samples were collected from healthy volunteers by using heparin-coated tubes (BD) and diluted 1:1 in PBS without calcium and magnesium. PBMCs were isolated by density gradient separation on Lymphoprep (Axis-Shield Poc AS) and diluted in macrophage medium (RPMI 1640 containing UltraGlutamin supplemented with 10% v/v autologous human serum and penicillin streptavidin; Sigma-Aldrich). Subsequently, 3 x 106 cells/well were plated in 24-well plates (Sarstedt, Nümbrecht, Germany) and incubated for 24 hours at 37°C in 5% CO2. Non-adherent PBMCs were removed by washing with PBS. Adherent cells were cultured in regularly changed macrophage medium for a minimum of 10 days, at which time approximately 10% of the cells had differentiated into human monocyte-derived macrophages (hMDMs).
Cytokine production of hMDMs
Following washing (3x) with PBS mature hMDMs were incubated with RPMI medium containing 0.5 μM native or carbamylated LL-37 together with 100 ng/ml LPS (Sigma-Aldrich) in triplicated wells for 20 hours at 37°C in 5% CO2. RPMI without any additives was used as a negative control and RPMI with LPS was used as a positive control. The supernatants were removed from the wells, centrifuged at 300 x g for 5 min and frozen at -70°C until cytokine analysis. The concentrations of GM-CSF, IFN-γ, IL1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10 and TNF-α were measured using a cytokine human 10-plex panel (Life technologies, Oslo, Norway).
Hemolysis of erythrocytes
Peripheral blood was collected in heparin coated tubes (BD) from healthy volunteers and centrifuged at 800 x g for 10 min at 10°C. The pellet was gently suspended in PBS, twice the original volume, and washed (2x) by centrifugation. The pellet was once again suspended in PBS to the initial blood volume. Thereafter, 4% v/v erythrocyte suspension was mixed with 1–20 μM native and carbamylated LL-37 diluted in PBS, added to a V-bottomed 96-well plate and incubated for 1–6 hours at 37°C. The plate was mixed every 15 min by shaking at 600 rpm. After incubation, the plates were centrifuged at 500 x g for 5 min. The supernatant was collected and the released of hemoglobin was measured at 405 nm.
Chemotaxis of neutrophils
The Insall chamber was used to visualise chemotaxis [25]. For each sample, isolated neutrophils (400 μl in RPMI, final density 1 × 106/ml) were added to acid washed (0.2 M HCl), dried and blocked (7.5%, BSA 400 μl, Sigma) coverslips (22 mm, VWR International), which were then incubated at room temperature (approximately 23°C) for 30 min. to allow the cells to adhere. The coverslip was then inverted and placed at the top of the chemotaxis chamber ensuring that the chemoattractant loading bays were exposed. The desired chemoattractant (80 μl), fMLP (10 nM) or LL-37 and its modified forms (used at 20 μM after assessing a range of concentrations) or control (RPMI media) was injected into the chemoattractant channels. The cell movement was analyzed using a Zeiss Primovert microscope (Carl Zeiss Imaging, Thornwood, NY, USA) and Images captured every 30 s for up to 40 frames per condition using a Q Imaging Retiga 2000R camera (Qimaging, Surry, Canada).
Binding of LL-37 and apo-A1 by surface plasmon resonance
The SPR experiments were performed using a BIACORE 3000 instrument (GE Healthcare). The N-HisTag containing ApoA1 (NH-ApoA1) was immobilized on the surface of a NTA sensor chip (GE Healthcare ) according to manufacturer’s protocol. Shortly, for direct capture of NH-ApoA1, the surface of NTA chip was prepared by 1 min injection of 0.5 mM NiCl2, then NH-ApoA1 at concentration of 2,5 μg/ml diluted in running buffer (10 mM HEPES, 150 mM NaCl, and 0.005% surfactant (v/v), pH 7.4) was injected with a flow rate of 5 μl/min to achieve capture level between 800–1000 resonance units (RU). The surface stabilized ApoA1was primed with running buffer before subsequent assay steps. The binding of all LL-37 analogs were each tested in triplicate in concentration range up to 1000 nM in running buffer. All samples were injected at a flow rate of 5 μl/min. Between experiments, the surfaces were strictly regenerated with two pulses (30 s) of 1 M NaCl at a flow rate of 20 μl/min, followed by an extensive wash procedure with running buffer. All measurements were performed at a constant temperature of 25 °C. Sensorgrams corrected for the reference were aligned, and a blank run was subtracted.
Results
LL-37 undergoes rapid carbamylation, which affects the peptide structure
Multiple studies based on circular dichroism [23], Fourier Transform Infrared [26] and NMR spectroscopy [27] reveal that LL-37 exists as a linear cationic, amphipathic α-helical structure within vesicles, both within the lipid bilayer and in solutions with an ionic composition resembling that of intracellular fluid or plasma. We used mass spectrometry to show that LL-37 undergoes rapid modification in the presence of cyanate, with the number of carbamylated residues increasing in a concentration- and time-dependent manner (Fig. 1A–D). In the presence of 10 mM KCNO (equivalent to cyanate levels found in the inflammatory milieu), multiple forms of carbamylated LL-37 were detected in the reaction mixture after only 10 minutes. Closer analysis of the elution pattern of modified peptides from a RP-HPLC column revealed two distinct forms of LL-37 bearing a single homocitrulline, which were the most commonly detected modifications. Although almost 70% of the modifications were represented by a single site substitution, a variety of peptides carrying multiple (two, three or four) carbamylated residues (Fig. 1B) were also detected. Increasing either the cyanate concentration (to 50 mM and 100 mM) or incubation time shifted the modification pattern. In 50 mM KCNO, previously predominant peptides bearing a single modification were replaced by peptides bearing multiple carbamylations on different Lys residues, resulting in a highly heterogeneous mixture. Peptides of the same molecular mass bearing one or more carbamylated amino groups resolved into several peaks (same color in Fig. 1C), which depended on the particular combination of the amino groups modified. This suggests that all six lysine side chains in LL-37 were equally susceptible to carbamylation. This was confirmed by analysis of LL-37 in the presence of 100 mM cyanate, which showed the presence a of a hetrogenous population of LL-37 with both 5 and 6 carbamylations (Several peaks with same colour Fig. 1C).
Figure 1. The quantity of carbamylated amino acid residues increases with elevated KCNO concentration.
LL-37 was incubated for 3 hours with increasing amounts of KCNO. A) 0 mM KCNO, B) 10 mM KCNO, C) 50 mM KCNO. The samples were analysed by liquid chromatography (LC)-MS/MS and the m/z values corresponding to LL37 with 0 to 7 carbamylated amino acid residues were extracted (colour code according to legend). The KCNO mediated carbamylation results in a heterogeneous population of carbamylated LL-37 as seen by the appearance of multiple peaks. D) The N-terminal leucine (L1) and lysine at position 8 on LL-37 are highly accessible to carbamylation. LL-37 were incubated with 10 mM or 50mM KCNO for 1 hour at 37°C. The position of modification was determined using NanoESI-MS/MS after V8 digestion. Spectral count were calculated by taking the sum of the number of spectra matching the identified peptides.
To address the functional significance of LL-37 carbamylation, we focused our attention on modifications identified after incubating the peptide in an environment mimicking that of the inflammatory milieu. To this end, we first identified the most common carbamylated variants of LL-37 under these conditions. KCNO-treated samples were digested with S. aureus protease V8 and analyzed by LC-MS/MS. By calculating the number of spectra matching the identified peptides (spectral count), we showed that LL-37 bearing a carbamylated N-terminus (α-carbamyl-Leu-1 LL-37; LL37C1) was the predominant peptide form present after 1 h of exposure to 10 mM KCNO. The second most common single-modified peptide was LL-37 bearing homocitrulline residue at position 8 (LL37C8) (Fig. 1D).
Prolonged incubation with KCNO generated a mixture of LL-37 peptide derivatives containing homocitrulline at random positions. Among them, LL-37 bearing a double modification (on Lys-12 and Lys-15; LL-37C12,15) was frequently detected (data not shown). Based on these results, LL37C1, LL-37C8, and LL-37C12,15 were synthesized for further analysis (Table 1).
Table 1.
LL-37 peptides synthesized and examined in this study.
| Peptide | Amino acid sequence |
|---|---|
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES |
| LL-37C1 |
|
| LL-37C8 |
|
| LL-37C12,15 |
|
α-carb-L, leucine carbamylated on the α-carbon; ε-carb-K, lysine carbamylated on the ε-carbon.
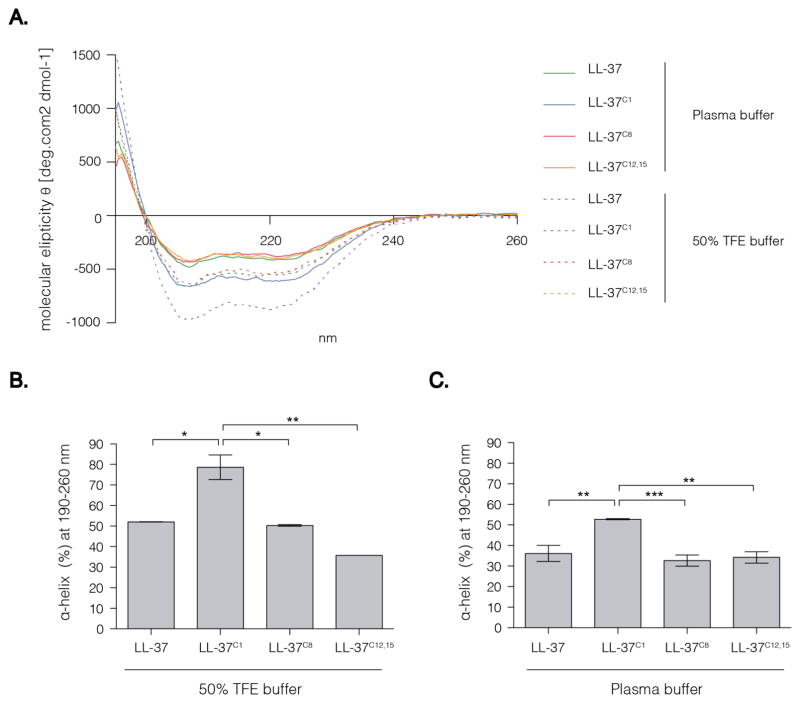
In aqueous solution, LL-37 forms a random coil structure, but adopts an α-helical conformation under physiological conditions; the α-helical structure is also adopted in TFE or lipid bilayer vesicles [26]. In the latter case, NMR studies show that LL-37 comprises three basic parts: an N-terminal α-helix, a C-terminal α-helix, and a C-terminal tail [27]. The hydrophobic surface of LL-37 is bordered by positively charged residues, which enable interaction with negatively charged molecules or structures such as LPS [28], genetic material, and bacterial cell wall components [13,14]. We hypothesized that carbamylation-induced changes in charge and hydrophobicity would have a significant impact on the secondary structure of the peptide and, consequently, its biological activity (which is strictly related to the physiochemical properties of the peptide). We used CD spectroscopy to examine the impact of carbamylation on the capacity of LL-37 to form a helical structure. The far UV CD spectrum of LL-37 in a physiological salt solution resembling blood plasma showed two minima (at 208 and 222 nm) (Fig. 2A). These are characteristic for an α-helical secondary structure. Interestingly, neither single carbamylation of Lys-8 (LL-37C8) nor double carbamylation of Lys-12 and Lys-15 (LL-37C12,15) had any impact on the α-helical structure of LL-37. Conversely, carbamylation of the N-terminal amino group (LL37C1) led to a significant increase in the propensity of the peptide to adopt an α-helical structure (Fig. 2A).
Figure 2. CD spectra of native and carbamylated LL-37.
The peptide concentration were 10 μM. 50% TFE buffer contained 50% TFE diluted in 10mM Sodium Phosphate buffer. The plasma buffer contained 113 mM NaCl, 24 mM NaHCO3, 0.6 mM MgCl2, 1.3 mM CaCl2, 3.9mM KCl. (A) Spectra are the mean of two (50% TFE buffer) or three (plasma buffer) independent experiments. (B) Difference of predicted α-helical content between the peptide analogues in 50% TFE buffer at 190–260 nm. (C) Difference of predicted α-helical content between the peptide analogues in Plasma buffer at 190–260 nm. (B–C) Data are expressed by the mean ± SD. Statistical significance was evaluated by one-way ANOVA followed by Tukey’s multiple comparisons test. p<0.05, **p<0.01; ***p<0.001. TFE, trifluorethanol.*
As expected, incubation with TFE increased the ellipticity and the helical content of the peptides beyond that observed in plasma buffer (Fig. 2B). Under these conditions, CD analysis of the far UV spectra predicted an α-helical content of approximately 80% for LL37C1, but approximately 50% for the native peptide (Fig. 2B). However, the difference between the peptides in TFE was similar to that observed in plasma buffer, i.e., the α-helical composition of LL37C1 was about 30% greater than that of the other peptides (Fig. 2C).
Carbamylation abrogate the bactericidal capacity of LL-37
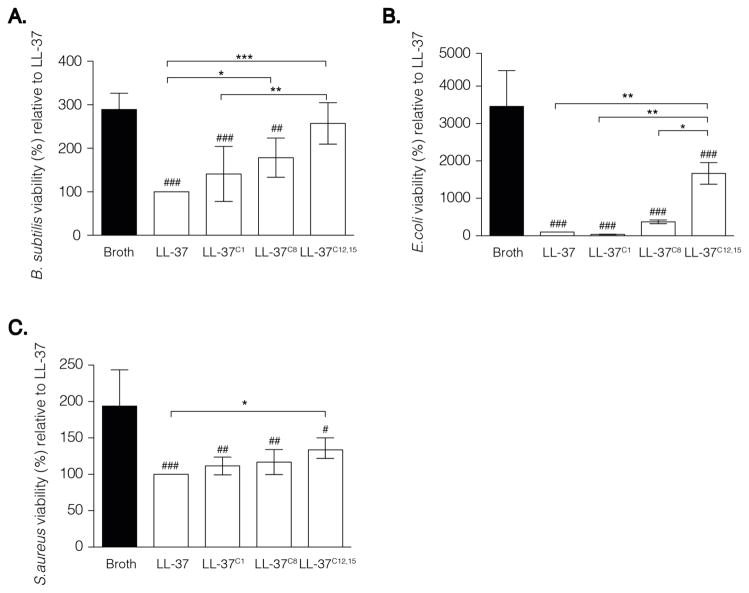
LL-37 interacts with bacteria via electrostatic and hydrophobic interactions resulting in membrane permeabilisation and disruption. A broth micro-dilution assay revealed that the bactericidal activity of LL-37 was profoundly affected by carbamylation. At a peptide concentration of 1 μg/ml (i.e., 0.2 μM), LL37C8 and LL37C12,15 demonstrated impaired ability to inhibit bacterial growth of B. subtilis when compared with the native peptide (p < 0.05 and p < 0.001, respectively; Figure 3A). Additionally, the potential of LL37C12,15 to inhibit the growth of E. coli (p < 0.001; Fig. 3B) and S. aureus (p < 0.05; Fig. 3C) was significantly decreased as compared to native LL-37. Confirming previous observations, that reducing the number of residues on the N terminus of LL-37 had only minor impact on its bactericidal properties, carbamylation of the N-terminal amino group did not affect the antimicrobial capacity of LL37C1 as compared to that of the native peptide (Fig. 3A – C). In addition to its direct microbicidal role, LL-37 is a potent regulator of innate immunity, controlling the response to pathogen-associated molecular patterns including endotoxin (LPS). Electrostatic interaction of the cationic LL-37 with the strongly anionic Lipid A domain of LPS prevents it from binding to TLR receptors expressed by monocytes and macrophages, a keystone event in inflammatory response. Therefore, we investigated whether carbamylation, and thus conversion of cationic Lys residues into neutral homocitrulline will diminish the binding capacity of LL-37 to LPS. hMDMs were exposed to LPS (100 ng/ml) in the presence of the either native LL-37 or carbamylated peptides. Thereafter, the cytokine profile of the supernatants was examined by multiplex analysis. Surprisingly, in contrast to antimicrobial activity, carbamylation did not limit the ability of the peptide to bind LPS. LL-37C1 and LL-37C8 attenuated the pro-inflammatory activity of LPS and blocked secretion of TNF- α and IL-6 from the cells as effectively as the native peptide. Interestingly, we observed statistically significant decrease of TNF- α and IL-6 in supernatants from LPS stimulated macrophages in the presence of LL-37C12,15 as compared to the native peptide (Fig. 4A, B). This might indicate that, as compared to the native peptide, LL-37C12,15 exhibits higher affinity for endotoxin thereof limiting it’s binding to the TLR4 receptors.
Figure 3. Carbamylation abrogate the antimicrobial activity of LL-37.
(A–C) The number of colony forming units formed after incubation with (A) B. subtilis, (B) E. coli and (C) S. aureus. All bacterial species were incubated with carbamylated or native LL-37. Bacterial growth in the presence of native LL-37 was set at 100%. Data are expressed as the mean ± SD. Statistical significance was evaluated by one-way ANOVA followed by Tukey’s multiple comparisons post-test. *p<0.05, **p<0.01; ***p<0.001; whereas #p <0.05; ##p< 0.01; ###p<0.001 as compared to (A–C) no peptide or(D–E) LPS.
Figure 4. Carbamylation does not impair LPS binding capacity of the LL-37.
Human monocyte derived macrophages were incubated with 100 ng/ml LPS in the absence or presence of variously modified peptides. The levels of (A) TNF-α and (B) IL-6 in the supernatants were then measured and LL-37+LPS was set to 100%. Data are expressed as the mean ±SD. Statistical significance was evaluated by one-way ANOVA followed by Tukey’s multiple comparisons post-test. *p<0.05, **p<0.01; ***p<0.001; whereas #p <0.05; ##p< 0.01; ###p<0.001 as compared to LPS.
Carbamylation of LL-37 affects its affinity for Apo-A1
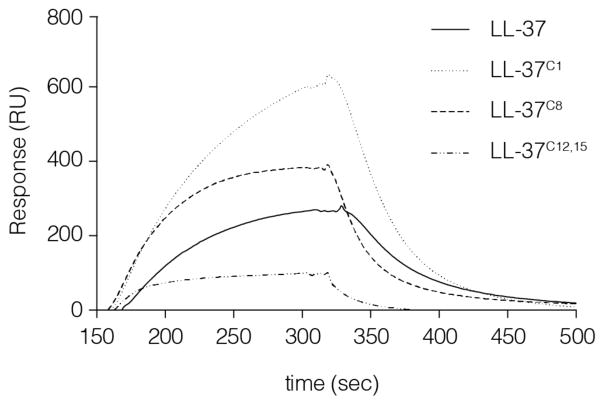
In vivo, LL-37 circulates in a complex with Apo-A1. This carrier protein might function as a scavenger to inhibit the cytotoxic effects of the peptide secondary to its release at inflammatory sites. Therefore, we used surface plasmon resonance to examine whether carbamylation alters the affinity of LL-37 for Apo-A1, which would effectively modulate the concentration of the bioavailable peptide within the inflammatory milieu. We found that Lys-12 and Lys-15 are critical for the interaction between the peptide and Apo-A1. LL-37C12,15 showed a 3-fold lower affinity for Apo-A1 than the native peptide. By contrast, both peptides carrying a single modification, LL-37C8 and LL-37C1, showed significantly stronger binding (1.5 and 3 times higher, respectively) than native LL-37. Taken together, these data indicate that any interference with the charged side chains of the amino acids within the LL-37 polypeptide chain has a significant impact on the peptide’s ability to associate with its carrier protein. This may either reduce or amplify the observed in vivo effects by modulating the amount of accessible LL-37 in the environment (Fig. 5).
Figure 5. Carbamylation of LL-37 reduce the binding capacity to apolipoprotein A1.
Surface plasmon resonance characteristics for the interaction between ApoA1 and carbamylated and native LL-37 at a peptide concentration of 1μM. RU, resonance units
Carbamylation affects the chemotactic capacity of LL-37
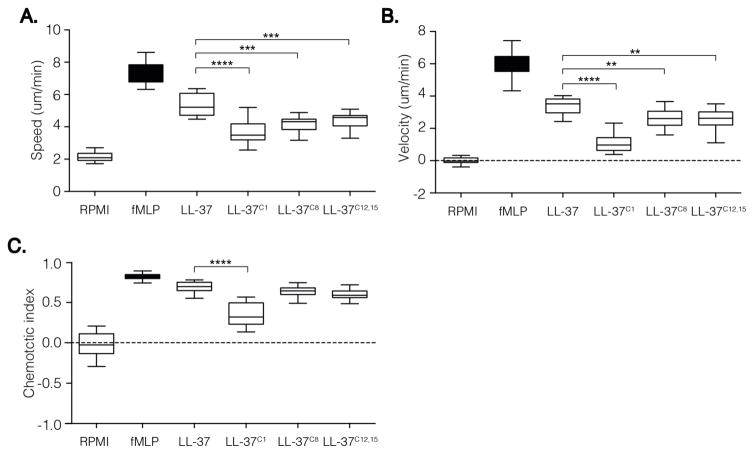
The ability of neutrophils to efficiently reach sites of inflammation is pivotal for efficient elimination of pathogens and restriction of potential tissue damage. LL-37 is a strong chemoattractant for neutrophils, monocytes, and T cells via the FPRL1 receptor [15]. To investigate how carbamylation impacts the chemotactic activity of LL-37, we used a “state of the art” approach that allowed us to observe cell migration in real time. The Insall chambers used in the study provide a gradient for the cells to migrate against, rather than simply exposing cells to the chemoattractant alone. This in turn allows observation of cell migration in more detail as information about speed (average speed of the cell over time in any direction), velocity (average speed of the cell in the direction of the gradient over time), and the directional accuracy of chemotaxis (expressed as the chemotactic index) can be obtained. We found that a peptide concentration of 20 μM was optimal in our assay (tested concentrations, 10 μM–40 μM; data not shown). Both native LL-37 and the carbamylated forms induced neutrophil migration without any apparent toxicity (Fig. 6A, B, C). However, neutrophils were significantly less responsive to the carbamylated versions of LL-37. Both the speed and velocity of migrating neutrophils were significantly lower when LL37C1, LL37C8, and LL37C12,15 were used as chemoattractants. Interestingly, LL37C1 was not only a significantly weaker chemoattractant than the other carbamylated peptides, but it also showed lower directional accuracy. Taken together, these observations suggest that, although all of the homocitrullinated peptides were able to trigger cell migration, carbamylation of the N-terminal amino group had a strong negative effect on directional accuracy.
Figure 6. Carbamylation decrease chemotactic potential of LL-37 peptide.
Analysis of chemotaxis using 20 μM peptide concentration. Neutrophils from 4 healthy volunteers were treated with either native or modified peptide. Extracted values for each individual’s speed, velocity, chemotactic index and resultant vector length were analysed for statistical difference. The midline of each box represents median. The statistical significance was evaluated by one-way ANOVA followed by Tukey’s multiple comparisons post-test: *p < 0.05; **p < 0.01; ***p < 0.001; whereas #p < 0.05; ##p < 0.01; ###p < 0.001 as compared to native peptide.
Carbamylation affects the cytotoxicity of LL-37
LL-37 can be cytotoxic to host cells. Therefore, we used a highly sensitive hemolytic assay to examine the impact of carbamylation on the cell-permeabilizing effects of LL-37. Human red blood cells (hRBC) were incubated with the peptide for 2 h at 37°C. The results showed that LL37C1 exerted a very strong and concentration-dependent lytic effect. The peptide caused a significant increase in hemoglobin release when used at a concentration of 2 μM (# p < 0.05; Fig. 7) as compared to PBS. The half-maximal effective concentration for LL37C1 was 17.9 μM. Conversely, LL37C8 and LL37C12,15 had significantly less capacity to induce membrane permeabilization (Fig. 7); indeed, hRBC were resistant to permeabilization by LL37C12,15 at concentrations of up to 20 μM. Given that the concentration of LL-37 can easily reach 20 μM at sites of inflammation (with levels up to 250 μM reported in psoriatic lesions) [29], permeabilization of membranes might facilitate the extracellular release of various potentially deleterious substances from lysed cells, which may then activate neutrophils and eventually lead to further increases in carbamylation.
Figure 7. Carbamylation significantly affect the hemolytic capacity of LL-37.
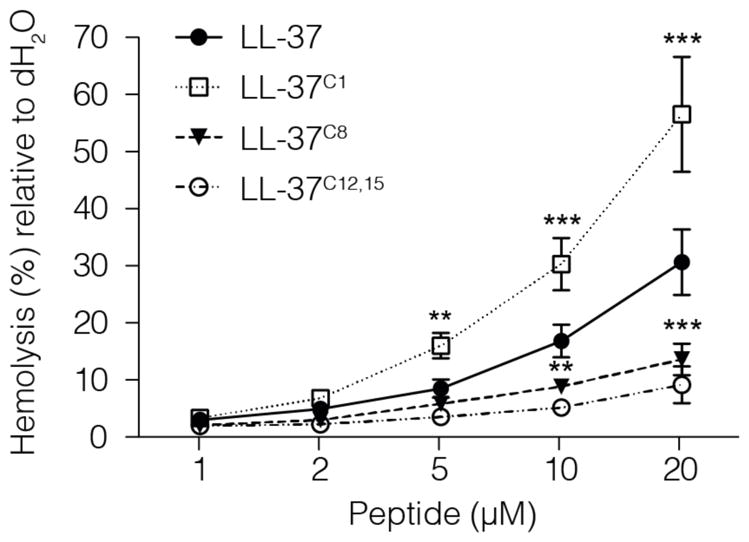
Erythrocytes were incubated for 2 hours with LL-37 analogues at a peptide concentration ranging from 1–20 μM. The hemolytic activity of the peptides was evaluated by recording the release of hemoglobin at 405 nm from human erythrocytes upon incubation. Data represent 4 individual experiments and are expressed as means ±SD. The statistical significance was evaluated by one-way ANOVA followed by Dunnett’s multiple comparisons post-test: *p < 0.05; **p < 0.01; ***p < 0.001 when compared with LL-37 at a peptide concentration of 2 μM.
Discussion
Host antimicrobial peptides, in particular LL-37, are potential novel therapeutics, mainly due to their wound healing, antiseptic, and antimicrobial properties[14,30,31]. In the era of rapidly increasing drug resistance among pathogens, novel treatments based on LL-37-derived peptides are very much needed. In addition, the immunostimulatory and cytotoxic properties of LL-37 may be useful tools for cancer treatment [32], or even as a vaginal contraceptive [33]. It is, however, important to keep in mind that the effector functions of LL-37 are most often executed at local sites of inflammation, and mainly in the context of neutrophil activation and subsequent release of MPO and H2O2 [34]. Thus, LL-37 is mainly present in microenvironments that foster carbamylation. Therefore, it is important to examine the impact of carbamylation on the immunomodulatory and cytotoxic functions of LL-37 prior to the clinical administration of cathelicidin-derived peptides. Here, we explored the effects of carbamylation on the biological functions of LL-37.
We found that in the presence of cyanate, LL-37 undergoes rapid modification to generate a pool of peptides, each with a diverse carbamylation pattern. Mass spectrometry analyses revealed that the N-terminal amino group of leucine residues is highly reactive and is modified almost instantaneously in the presence of 10 mM KCNO. Thus, LL37C1 is most likely the predominant form of carbamylated LL-37 in vivo. Even though carbamylation at this site did not affect the bactericidal properties of the peptide, LL-37C1 lost the ability to function as a chemoattractant for neutrophils. Concurrently, LL-37C1 gained significant RBC-lysing capacity; indeed, it was almost 3-fold more cytotoxic than native LL-37 in a hemolytic assay. Because tissue injury/necrosis results in increased receptor-dependent immune cell migration in response to released intracellular components[35,36], we cannot rule out the possibility that LL37C1 indirectly triggers cell migration in vivo in response to damage to surrounding tissue. By stark contrast, although the chemoattractive capacity of LL-37C8 and LL-37C12,15 was diminished, neutrophils retained directional accuracy in response to the stimuli. This suggests that, despite the reduced affinity of Lys-8 and Lys-12/Lys-15 carbamylated peptides for FPRL-1, they were still able to bind the receptor. At the same time, LL-37C8 and LL-37C12,15 were significantly less toxic to hBRCs, with the latter exhibiting no hemolytic effects at a concentration of 20 μM. This observation confirms those of previous studies showing that truncation or blocking [26] of the N-terminus reduces the cytotoxicity of LL-37 while at the same time leaving bactericidal activity unchanged [37]. Indeed, only LL-37C12,15 showed lower bactericidal activity than the native peptide against B. subtilis, E. coli, and S. aureus. In addition, Xhindoli and colleagues showed that oligomerization of LL-37 results in increased formation of α-helices, leading to an increased capacity to permeabilize erythrocytes and monocytes; this suggests that a parallel arrangement of the peptides favors aggregation via interaction between the N-termini [26]. In agreement with these results, we showed that LL37C1 was about 50% more α-helical than the native peptide, both in a secondary structure-inducing environment (50% TFE buffer) and in a plasma-mimicking buffer. Since loss of the N-terminal charge due to carbamylation increases the hydrophobicity of this region [38], it is possible that LL37C1 is more prone to aggregation due to reduced electrostatic repulsion between the molecules. This would increase its propensity to form α-helices and explain the increased cytotoxicity. Intriguingly, the ability of LL-37C8 and LL-37C12,15 to lyse neutrophils and erythrocytes was different. Neutrophil membranes are zwitterionic and interact with LL-37 independently of the overall charge of the peptide [22,39]. By contrast, the erythrocyte membrane contains sialic acid, which results in a negatively charged cell surface; therefore, it is more susceptible to lysis by cationic peptides. Thus, it is not surprising that the reduced cationicity conferred by carbamylation results in impaired hemolysis but does not affect the lysis of neutrophils. Interestingly, this difference was not evident in the case of LL37C1. However, the uncompromising capacity of LL37C1 to lyse both erythrocytes and neutrophils (data not shown) emphasizes the involvement of a hydrophobic N-terminus in this process.
The antimicrobial and cytotoxic activities of LL-37 are effectively inhibited in human plasma. This latency is due to the interaction between LL-37 and its carrier protein, apoA-1 [24]; this interaction is dependent on the hydrophobicity of the N-terminus of LL-37 [40] as well as on its α-helical content [41]. In line with these results, we found that LL37C1 showed significantly higher affinity for apo-A1 (300%) than the native peptide, indicating that the cytotoxic effects of LL37C1 may be limited by an Apo-A1-dependent protective mechanism. However, LL37C1 may be detrimental to the host if generated at inflammatory foci that are poorly infiltrated by blood plasma. By contrast, LL37C12,15 had lower affinity for apo-A1. Since Apo-A1 has a net negative charge [42], it is likely that the significant loss of electrostatic interactions upon carbamylation of two or more Lys residues impairs L-37 binding to apo-A1.
Upon carbamylation, the bactericidal activity of LL-37 is compromised. Again, this is most likely related to the loss of two positive charges when Lys residues are converted to homocitrulline. It is likely that the reduced electrostatic attraction between LL-37C12,15 and bacterial membranes results in impaired antimicrobial activity against B. subtilis, E. coli, and S. aureus. Interestingly, even though the bactericidal domain of LL-37 has been mapped to the C-terminal region (amino acids 17–29) [43], LL-37C8 showed impaired bactericidal activity against B. subtilis. Thus, the lysine at position 8 is important for efficient killing of this Gram-positive bacterium.
Interestingly, LL-37C12,15 was the most effective LPS-detoxifying agent among all tested forms of LL-37, i.e., this carbamylated peptide efficiently attenuated the LPS-induced production of TNF-α, an important mediator of endotoxic shock [44]. In addition to directly binding LPS via electrostatic and hydrophobic interactions, LL-37 also interacts with the LPS receptor, CD14, to block LPS-induced macrophage activation [45]. We hypothesize that carbamylation of Lys-12 and Lys-15 increases the binding of LL-37 to this receptor. From a clinical point of view, it is tempting to speculate that LL37C12,15 provides therapeutic protection against septic shock without being cytotoxic. Nevertheless, it must be kept in mind that further carbamylation may alter the biological activity of the peptide.
In summary, carbamylation has a profound impact on the bactericidal, cytotoxic, and pro-inflammatory activity of LL-37, which may have detrimental consequences for the host. The pattern of carbamylation is dependent on the OCN− concentration and time of exposure, suggesting that the subsequent effects of this modification will be difficult to foresee and, therefore, control in vivo. Thus, we suggest that caution should be exercised when administering cathelicidin-derived peptides to patients with diseases manifested by inflammation, such as severe infections or sepsis.
References
- 1.Deribe YL, Pawson T, Dikic I. Post-translational modifications in signal integration. Nat Struct Mol Biol. 2010 Jun;17:666–672. doi: 10.1038/nsmb.1842. [DOI] [PubMed] [Google Scholar]
- 2.Stark GR, Smyth DG. The use of cyanate for the determination of NH2-terminal residues in proteins. J Biol Chem. 1963 Jan;238:214–226. [PubMed] [Google Scholar]
- 3.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Hörkkö S, Barnard J, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007 Oct;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 4.Kraus LM, Kraus AP. Carbamoylation of amino acids and proteins in uremia. Kidney Int Suppl. 2001 Feb;78:S102–107. doi: 10.1046/j.1523-1755.2001.59780102.x. [DOI] [PubMed] [Google Scholar]
- 5.Oimomi M, Hatanaka H, Yoshimura Y, Yokono K, Baba S, Taketomi Y. Carbamylation of insulin and its biological activity. Nephron. 1987;46:63–66. doi: 10.1159/000184303. [DOI] [PubMed] [Google Scholar]
- 6.Holzer M, Zangger K, El-Gamal D, Binder V, Curcic S, Konya V, et al. Myeloperoxidase-derived chlorinating species induce protein carbamylation through decomposition of thiocyanate and urea: novel pathways generating dysfunctional high-density lipoprotein. Antioxid Redox Signal. 2012 Oct 15;17:1043–1052. doi: 10.1089/ars.2011.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asci G, Basci A, Shah SV, Basnakian A, Toz H, Ozkahya M, et al. Carbamylated low-density lipoprotein induces proliferation and increases adhesion molecule expression of human coronary artery smooth muscle cells. Nephrol Carlton Vic. 2008 Dec;13:480–486. doi: 10.1111/j.1440-1797.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 8.Mydel P, Wang Z, Brisslert M, Hellvard A, Dahlberg LE, Hazen SL, et al. Carbamylation-dependent activation of T cells: a novel mechanism in the pathogenesis of autoimmune arthritis. J Immunol Baltim Md 1950. 2010 Jun 15;184:6882–6890. doi: 10.4049/jimmunol.1000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi J, Knevel R, Suwannalai P, van der Linden MP, Janssen GMC, van Veelen PA, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A. 2011 Oct 18;108:17372–17377. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A. 1998 Aug 4;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frohm M, Agerberth B, Ahangari G, Stâhle-Bäckdahl M, Lidén S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997 Jun 13;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 12.Sørensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001 Jun 15;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 13.Dürr UHN, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006 Sep;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Duplantier AJ, van Hoek ML. The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds. Front Immunol. 2013;4:143. doi: 10.3389/fimmu.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Yang, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000 Oct 2;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002 May;106:20–26. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson DJ, Currie AJ, Reid GSD, Bowdish DME, MacDonald KL, Ma RC, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol Baltim Md 1950. 2004 Jan 15;172:1146–1156. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 18.Pistolic J, Cosseau C, Li Y, Yu JJ, Filewod NCJ, Gellatly S, et al. Host defence peptide LL-37 induces IL-6 expression in human bronchial epithelial cells by activation of the NF-kappaB signaling pathway. J Innate Immun. 2009;1:254–267. doi: 10.1159/000171533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montreekachon P, Chotjumlong P, Bolscher JGM, Nazmi K, Reutrakul V, Krisanaprakornkit S. Involvement of P2X(7) purinergic receptor and MEK1/2 in interleukin-8 up-regulation by LL-37 in human gingival fibroblasts. J Periodontal Res. 2011 Jun;46:327–337. doi: 10.1111/j.1600-0765.2011.01346.x. [DOI] [PubMed] [Google Scholar]
- 20.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J. 1999 Aug 1;341( Pt 3):501–513. [PMC free article] [PubMed] [Google Scholar]
- 21.Bowdish DME, Davidson DJ, Hancock REW. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci. 2005 Feb;6:35–51. doi: 10.2174/1389203053027494. [DOI] [PubMed] [Google Scholar]
- 22.Li H-N, Barlow PG, Bylund J, Mackellar A, Björstad A, Conlon J, et al. Secondary necrosis of apoptotic neutrophils induced by the human cathelicidin LL-37 is not proinflammatory to phagocytosing macrophages. J Leukoc Biol. 2009 Oct;86:891–902. doi: 10.1189/jlb.0209050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson J, Gudmundsson GH, Rottenberg ME, Berndt KD, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998 Feb 6;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Agerberth B, Löthgren A, Almstedt A, Johansson J. Apolipoprotein AI binds and inhibits the human antibacterial/cytotoxic peptide LL-37. J Biol Chem. 1998 Dec 11;273:33115–33118. doi: 10.1074/jbc.273.50.33115. [DOI] [PubMed] [Google Scholar]
- 25.Hazeldine J, Harris P, Chapple IL, Grant M, Greenwood H, Livesey A, et al. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell. 2014 Aug;13:690–698. doi: 10.1111/acel.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xhindoli D, Pacor S, Guida F, Antcheva N, Tossi A. Native oligomerization determines the mode of action and biological activities of human cathelicidin LL-37. Biochem J. 2014 Jan 15;457:263–275. doi: 10.1042/BJ20131048. [DOI] [PubMed] [Google Scholar]
- 27.Porcelli F, Verardi R, Shi L, Henzler-Wildman KA, Ramamoorthy A, Veglia G. NMR structure of the cathelicidin-derived human antimicrobial peptide LL-37 in dodecylphosphocholine micelles. Biochemistry (Mosc) 2008 May 20;47:5565–5572. doi: 10.1021/bi702036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995 Apr;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002 Oct 10;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 30.Ciornei CD, Sigurdardóttir T, Schmidtchen A, Bodelsson M. Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob Agents Chemother. 2005 Jul;49:2845–2850. doi: 10.1128/AAC.49.7.2845-2850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, et al. Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin Diagn Lab Immunol. 2002 Sep;9:972–982. doi: 10.1128/CDLI.9.5.972-982.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chuang C-M, Monie A, Wu A, Mao C-P, Hung C-F. Treatment with LL-37 peptide enhances antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer. Hum Gene Ther. 2009 Apr;20:303–313. doi: 10.1089/hum.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srakaew N, Young CD, Sae-wu A, Xu H, Quesnel KL, di Brisco R, et al. Antimicrobial host defence peptide, LL-37, as a potential vaginal contraceptive. Hum Reprod Oxf Engl. 2014 Apr;29:683–696. doi: 10.1093/humrep/deu018. [DOI] [PubMed] [Google Scholar]
- 34.Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol Off J Can Soc Allergy Clin Immunol. 2006 Sep 15;2:98–108. doi: 10.1186/1710-1492-2-3-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng LG, Qin JS, Roediger B, Wang Y, Jain R, Cavanagh LL, et al. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J Invest Dermatol. 2011 Oct;131:2058–2068. doi: 10.1038/jid.2011.179. [DOI] [PubMed] [Google Scholar]
- 36.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CCM, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010 Oct 15;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 37.Wong JH, Ng TB, Legowska A, Rolka K, Hui M, Cho CH. Antifungal action of human cathelicidin fragment (LL13-37) on Candida albicans. Peptides. 2011 Oct;32:1996–2002. doi: 10.1016/j.peptides.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Kanaori K, Nosaka AY. Characterization of human calcitonin fibrillation in aqueous urea solution by 1H NMR spectroscopy. Biochemistry (Mosc) 1996 Oct 1;35:12671–12676. doi: 10.1021/bi961013l. [DOI] [PubMed] [Google Scholar]
- 39.Ding B, Soblosky L, Nguyen K, Geng J, Yu X, Ramamoorthy A, et al. Physiologically-relevant modes of membrane interactions by the human antimicrobial peptide, LL-37, revealed by SFG experiments. Sci Rep. 2013;3:1854. doi: 10.1038/srep01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sørensen O, Bratt T, Johnsen AH, Madsen MT, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is bound to lipoproteins in plasma. J Biol Chem. 1999 Aug 6;274:22445–22451. doi: 10.1074/jbc.274.32.22445. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Johansson J, Agerberth B, Jörnvall H, Griffiths WJ. The antimicrobial peptide LL-37 binds to the human plasma protein apolipoprotein A-I. Rapid Commun Mass Spectrom RCM. 2004;18:588–589. doi: 10.1002/rcm.1361. [DOI] [PubMed] [Google Scholar]
- 42.Sparks DL, Lund-Katz S, Phillips MC. The charge and structural stability of apolipoprotein A-I in discoidal and spherical recombinant high density lipoprotein particles. J Biol Chem. 1992 Dec 25;267:25839–25847. [PubMed] [Google Scholar]
- 43.Li X, Li Y, Han H, Miller DW, Wang G. Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J Am Chem Soc. 2006 May 3;128:5776–5785. doi: 10.1021/ja0584875. [DOI] [PubMed] [Google Scholar]
- 44.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]; Classical article. J Immunol Baltim Md 1950. 2008 Jul 1;181:7–9. [Google Scholar]
- 45.Rosenfeld Y, Papo N, Shai Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible modes of action. J Biol Chem. 2006 Jan 20;281:1636–1643. doi: 10.1074/jbc.M504327200. [DOI] [PubMed] [Google Scholar]