Abstract
AIM
To identify predictors for synchronous liver metastasis from resectable pancreatic ductal adenocarcinoma (PDAC) and assess unresectability of synchronous liver metastasis.
METHODS
Retrospective records of PDAC patients with synchronous liver metastasis who underwent simultaneous resections of primary PDAC and synchronous liver metastasis, or palliative surgical bypass, were collected from 2007 to 2015. A series of pre-operative clinical parameters, including tumor markers and inflammation-based indices, were analyzed by logistic regression to figure out predictive factors and assess unresectability of synchronous liver metastasis. Cox regression was used to identify prognostic factors in liver-metastasized PDAC patients after surgery, with intention to validate their conformance to the indications of simultaneous resections and palliative surgical bypass. Survival of patients from different groups were analyzed by the Kaplan-Meier method. Intra- and post-operative courses were compared, including complications. PDAC patients with no distant metastases who underwent curative resection served as the control group.
RESULTS
CA125 > 38 U/mL (OR = 12.397, 95%CI: 5.468-28.105, P < 0.001) and diabetes mellitus (OR = 3.343, 95%CI: 1.539-7.262, P = 0.002) independently predicted synchronous liver metastasis from resectable PDAC. CA125 > 62 U/mL (OR = 5.181, 95%CI: 1.612-16.665, P = 0.006) and age > 62 years (OR = 3.921, 95%CI: 1.217-12.632, P = 0.022) correlated with unresectability of synchronous liver metastasis, both of which also indicated a worse long-term outcome of liver-metastasized PDAC patients after surgery. After the simultaneous resections, patients with post-operatively elevated serum CA125 levels had shorter survival than those with post-operatively reduced serum CA125 levels (7.7 mo vs 16.3 mo, P = 0.013). The survival of liver-metastasized PDAC patients who underwent the simultaneous resections was similar to that of non-metastasized PDAC patients who underwent curative pancreatectomy alone (7.0 mo vs 16.9 mo, P < 0.001), with no higher rates of either pancreatic fistula (P = 0.072) or other complications (P = 0.230) and no greater impacts on length of hospital stay (P = 0.602) or post-operative diabetic control (P = 0.479).
CONCLUSION
The criterion set up by CA125 levels could facilitate careful diagnosis of synchronous liver metastases from PDAC, and prudent selection of appropriate patients for the simultaneous resections.
Keywords: CA125, Pancreatic ductal adenocarcinoma, Liver metastasis, Unresectability, Prognosis
Core tip: The presence of liver metastasis from pancreatic ductal adenocarcinoma (PDAC) usually deprives patients of opportunities for resection of PDAC. We utilized a series of clinical parameters for pre-operative evaluation of PDAC with synchronous liver metastasis, including diagnosis and assessment of unresectability. The criterion set up by serum CA125 levels could facilitate the careful judgement of the occurrence of synchronous liver metastases from PDAC, and the prudent selection of appropriate patients for simultaneous resections for primary PDAC and synchronous liver metastasis, for the sake of prolonged survival and substantial reduction in morbidity and mortality.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive and progressive malignancy with increasing incidence and death rates[1,2]. Despite the steady improvement in survival for most cancers, progress has been limited for PDAC, for which the 5-year relative survival rate for all stages combined is 8%[2]. The rate of resection for primary PDAC is only 10%-20%, and approximately 50% of new PDAC cases are discovered to have distant metastases[3]. Some distant micro-metastases are undetectable at diagnosis through a thorough pre-operative imaging tests including positron emission tomography/computed tomography (PET/CT), and may only be confirmed by exploration during planned curative resection. Even those patients undergoing curative pancreatectomy are still at a 25%-50% risk of developing distant metastases[4-6]. The dismal prognosis of PDAC with distant metastasis has been acknowledged by its 5-year relative survival rate of 1%[7].
PDAC shows a remarkable preference for the liver to metastasize due to its portal venous blood draining and lymphatic spread. Weh et al[8] summarized that the incidence of liver metastasis from PDAC ranged from 25% to 75%. About 12% of unsuspected liver metastases are not discovered until surgery, and liver metastasis reduces the survival of patients with PDAC to 5 mo[9,10]. Currently, chemotherapy remains the mainstay of treatment for liver metastasis from PDAC, with two combination chemotherapy regimens-FOLFIRINOX (bolus plus infusional fluorouracil, leucovorin, irinotecan, and oxaliplatin regimen) and gemcitabine plus nanoparticle albumin-bound paclitaxel-emerging as new standards[11-13].
The doctrine that the presence of liver metastasis from resectable PDAC contradicts a curative resection and indicates a palliative surgical bypass, deprives patients of an incremental benefit from simultaneous curative resections for primary and metastatic PDAC, even at a R1 status. An unconventional surgical option to curatively resect primary PDAC and synchronous liver metastasis may be merely justified by prolonged survival, a longer recurrence-free interval and, at least, no more surgical-related morbidity and mortality. Pancreaticoduodenectomy (PD) combined with additional organ resection has been indicated for locally advanced PDAC with the same safety as PD alone[14]. Even if palliative PD can be performed instead, patients can benefit from significantly longer survival and low morbidity rate[15,16]. Thus, simultaneous curative resections for primary PDAC and synchronous liver metastasis can also be advocated on highly individual basis. However, the threshold comprised of conventional clinical indexes has not been first established to pre-operatively distinguish the occurrence of liver metastasis among patients with resectable PDAC. And, the criterion also needs to be set for selection for patients whom the simultaneous resections favor in a proper sense.
As the predictive accuracy of serum CA125 levels has been reported in a two-center clinical study where we were involved[17,18], here we highlighted the relationships between serum CA125 levels and both synchronous liver metastasis from PDAC and unresectability of liver-metastasized PDAC, and focused on the long-term outcome of liver-metastasized PDAC patients after individualized surgeries indicated by serum CA125 levels.
MATERIALS AND METHODS
Patients
Sixty-nine patients with resectable primary PDAC and synchronous liver metastasis who underwent surgery at the Huashan Hospital between March 2007 and December 2015 were identified in a prospective database. Of these, 30 patients underwent simultaneous curative resections for primary PDAC and synchronous liver metastases, and 39 patients underwent palliative surgical bypass prior to gemcitabine-based chemotherapy due to unresectable liver metastases. All data collected was consented by these patients and approved by the Ethical Committee and Institutional Review Board. Only patients with histologically confirmed PDAC and liver metastasis who underwent surgery were included in the current study. Patients with unresectable primary PDAC, neuroendocrine tumor, cystadenocarcinoma, ampullary cancer, distal bile duct, and duodenal carcinoma as well as extrahepatic metastatic disease such as serosal implants or peritoneal metastases were not considered in the study. To investigate the predictors for synchronous liver metastasis, 138 patients with no evidence of distant metastases who underwent curative resection for primary PDAC alone were selected at the same period mentioned above for matching with the control group in a 1:2 fashion. These patients were matched as closely as possible to the baseline characteristics of the liver metastasis cohort.
Pre-operative evaluation
Routine pre-operative diagnostics consisted of a baseline history, physical examination and clinical laboratory tests and imaging tests. The tumor markers CA19-9, CA125 and CEA were used as serum diagnostic tools. Ultrasonography, computed tomography scanning and PET were performed in all instances. Pre-operative biliary drainage, endoscopic retrograde biliary drainage or percutaneous transhepatic cholangial drainage was indicated for jaundice.
Surgical procedures
Depending on the location of primary PDAC, curative resection was performed as pancreatoduodenectomy, or total pancreatectomy, or distal splenopancreatectomy, accompanied by lymphadenectomy. Selected patients underwent portal/superior mesenteric vein resection and artificial blood vessel replacement. The number and distribution of metastatic diseases, which were assessed by intra-operative ultrasonographic measurement once more to detect liver micro-metastases under suspicious conditions, determined the extent of liver resection. During the laparotomy, the abdomen was completely staged. Given that no acknowledged guidelines of surgery for liver metastasis from PDAC offered the use of reference to surgeons, the decision for resection was made by the intention to reach a R0 status in both the pancreas and the liver and a good performance status (American Society of Anesthesiologists ASA classification ≤ III). The palliative Roux-en Y bypass was constituted by retrocolic end-to-side hepaticojejunal anastomosis and antecolic gastroenterostomy.
Data collection
The following data were assessed prospectively for each patient: demographics, pre-operative symptoms and previous history, histology of primary PDAC and synchronous liver metastasis, pre-operative treatments, blood parameters, operative details, post-operative course. Among them, plasma fibrinogen and platelets have been shown to play a possible role of both predictive and prognostic factors of distant metastasis[19,20]. Blood neutrophil-lymphocyte ratio (NLR), lymphocyte-monocyte ratio (LMR), platelet-lymphocyte ratio (PLR) and prognostic nutritional index (PNI, albumin [g/L] + 5×total lymphocyte count [× 109/L]) have acted as inflammation-based indices to predict the clinical outcome of primary or metastasized cancers after surgery or chemotherapy[21-25] as well as the association with metastasized cancer burden[26-27]. Body-mass-index (BMI), NLR, LMR, PLR and PNI were obtained by calculation during the initial evaluation. All pathologic specimens were reviewed through intra-operative frozen section analysis or routine paraffin section analysis by two independent pathologists to unanimously confirm the diagnosis of primary PDAC and synchronous liver metastasis. Post-operative course included post-operative morbidity such as pancreatic fistula, and mortality defined as any death during hospitalization and within 30 d of surgery. Follow-up information was obtained through review of the medical records and direct contact with the patients. When the date of death was inaccessible, patients were censored at the last contact or record from hospitalization or oncological outpatient clinics.
Statistical analysis
Summary statistics were reported using mean or median values where appropriate. Student’s t-test or analysis of variance was used for mean comparison of continuous variables distributed normally, whereas Mann-Whitney U test or Kruskal-Wallis H test was used to compare skewed continuous variables. Fisher’s exact test or Pearson’s χ test was used to compare frequencies of categorical variables among groups. The cutoff value of fibrinogen, NLR, LMR, PLR, PNI and platelet was determined by widely accepted thresholds[19,20,23,28], allowing comparison with the available literature. Serum CA19-9 level of 400 U/mL used for indicating distant metastasis of PDAC[29,30] was adopted as a cutoff for logistic regression analysis and Cox regression analysis. According to receiver operating characteristic (ROC) curve, an optimal cutoff serum CA125 level of 38 U/mL was identified for analysis of predictors for synchronous liver metastasis, and 62 U/mL for assessment of unresectability for synchronous liver metastasis and overall survival for PDAC patients with synchronous liver metastasis. Predictors for synchronous liver metastasis from PDAC and unresectability for synchronous liver metastasis were estimated by logistic regression analyses. Prognostic factors for overall survival were estimated by Cox proportional hazards models. The Kaplan-Meier method was used to analyze the overall survival from the date of surgery. Differences in survival were examined using the log-rank test. A two-sided P value of < 0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed by utilizing SPSS statistics 20 (IBM corporation, Armonk, NY, United States).
RESULTS
Patient characteristics
Table 1 shows the clinicopathologic characteristics of 69 PDAC patients (group A and B) with liver metastasis, who were the focus of the study, and 138 PDAC patients (group C) with no distant metastases, who were enrolled as a matched group. In the case group, the majority of 69 patients were male (n = 47, 68.1%) with an overall mean age of 62.6 years. According to ASA grading system, 21 (30.4%) patients were evaluated as grade I, 46 (66.7%) as grade II, and 2 (2.9%) as grade III. The primary PDAC site was largely head or neck (n = 53, 76.8%). Primary PDAC displayed venous invasion in 22 (31.9%) patients and lymph node invasion in 14 (46.7%) patients.
Table 1.
Clinicopathologic characteristics of 207 pancreatic ductal adenocarcinoma patients undergoing surgery
| Parameter |
No. of patients |
|||
| Simultaneous resections (Group A) | Palliative surgical bypass (Group B) | Total (Group A + Group B) | Pancreatectomy alone (Group C) | |
| n = 30 | n = 39 | n = 69 | n = 138 | |
| Mean age ± SD, yr | 62.2 ± 10.0 | 63.0 ± 10.4 | 62.6 ± 10.1 | 58.8 ± 10.6 |
| Sex (Female) | 10 | 12 | 22 | 45 |
| ASA | ||||
| I | 11 | 10 | 21 | 37 |
| II | 19 | 27 | 46 | 97 |
| III | 0 | 2 | 2 | 4 |
| IV | 0 | 0 | 0 | 0 |
| Primary tumor location | ||||
| Head/neck | 15 | 38 | 53 | 106 |
| Body/tail | 15 | 1 | 16 | 32 |
| Median primary tumor size [IQR], cm | 4.0 (2.5-5.0) | - | - | 3.0 (2.0-3.5) |
| Pathology (PDAC) | 30 | 39 | 69 | 138 |
| TNM stage | ||||
| I | 0 | 0 | 0 | 22 |
| IIA | 0 | 0 | 0 | 22 |
| IIB | 0 | 0 | 0 | 94 |
| III | 0 | 0 | 0 | 0 |
| IV | 30 | 39 | 69 | 0 |
| Primary tumor differentiation | ||||
| Well/moderate | 13 | - | - | 71 |
| Poor | 17 | - | - | 67 |
| Ki67 [IQR], % | 20 (8-30) | - | - | 30 (15-50) |
| Venous invasion | 3 | 19 | 22 | 37 |
| Lymph node invasion | 14 | - | - | 82 |
| Hepatic metastasis | 30 | 39 | 69 | 0 |
| Surgery for primary tumor | ||||
| Total pancreatectomy | 1 | 0 | 1 | 5 |
| Pancreaticoduodenectomy | 11 | 0 | 11 | 95 |
| Distal pancreatectomy | 18 | 0 | 18 | 38 |
| Palliative bypass | 0 | 39 | 39 | 0 |
Among these 69 patients, 30 patients (group A) underwent simultaneous curative resections for primary PDAC as well as synchronous liver metastasis, and 39 patients (group B) underwent palliative surgical bypass. The curative resections for primary PDAC included PD (n = 11, 36.7%), distal pancreatectomy (n = 18, 60.0%) and total pancreatectomy (n = 1, 3.3%), with portal/superior mesenteric vein resection and artificial blood vessel replacement (n = 3, 2.2%). The mean age at the time of surgery was 62.2 years in group A, and 63.0 years in group B. Half of group A and 38 of group B suffered from adenocarcinoma of the head/neck of the pancreas. Fifteen of group A and 20 of group B were found to have unexpected liver metastases by direct-view or intra-operative ultrasonographic measurement during surgeries. The proportion of the successful simultaneous resections was triennially rising across the study period (Figure 1).
Figure 1.
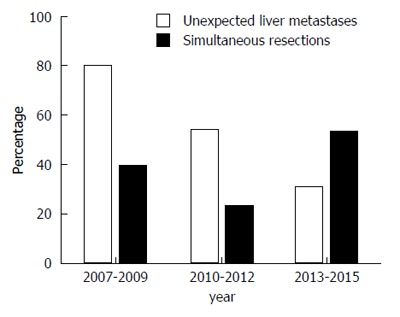
Trends in the occurrence of unexpected liver metastases identified during surgeries and implementation of the simultaneous resections among all cases across the study period.
Most patients of the matched cohort (group C) were male (n = 93, 68.1%) with an overall mean age of 58.8 years. Thirty-seven (26.8%) patients were evaluated as ASA grade I, 97 (70.3%) as grade II, and 4 (2.9%) as grade III. As adenocarcinoma of the head/neck of the pancreas (n = 106) accounted for 76.8% of all resectable pancreatic adenocarcinoma, the majority of surgical options were PD (n = 95, 68.9%), and the rest were distal pancreatectomy (n = 38, 27.5%) and total pancreatectomy (n = 5, 3.6%). Thirty-seven (26.8%) patients underwent portal/superior mesenteric vein resection and artificial blood vessel replacement (n = 3, 2.2%) due to venous invasion. After lymphadenectomy, 82 of group C were found to have lymph node invasion.
Predictors for synchronous liver metastasis from PDAC
To determine which pre-operative factors are independent predictors for synchronous liver metastasis from PDAC, a univariate analysis was performed for preliminary screening of clinical parameters followed by a stepwise logistic regression analysis of the occurrence of synchronous liver metastasis from PDAC. In univariate analysis, there was a trend toward a higher incidence of no synchronous liver metastases in patients with CA19-9 > 400 U/mL (P < 0.001), CA125 > 38 U/mL (P < 0.001), CEA > 5 U/mL (P = 0.002), NLR > 5 (P = 0.026), and diabetes mellitus (P = 0.017) (Table 2). In multivariate analysis, both CA125 > 38 U/mL (OR = 12.397, 95%CI: 5.468-28.105, P < 0.001) and diabetes mellitus (OR = 3.343, 95%CI: 1.539-7.262; P = 0.002) were determined to independently predict synchronous liver metastasis from PDAC (Table 3). The area under the ROC curve (AUC) of serum CA125 level was 0.821 (95%CI: 0.752-0.891), with sensitivity of 71.01% and specificity of 87.61% at the threshold of 38 U/mL.
Table 2.
Predictors of synchronous liver metastasis from resectable pancreatic ductal adenocarcinoma
| Parameter | Total (Group A + Group B) | Pancreatectomy alone (Group C) | P value (univariate) |
| n = 69 | n = 138 | ||
| Age, yr | |||
| ≤ 62 | 32 | 80 | |
| > 62 | 37 | 58 | 0.116 |
| Sex | |||
| Male | 47 | 93 | |
| Female | 22 | 45 | 0.916 |
| BMI | |||
| < 18 kg/m2 | 6 | 11 | 0.196 |
| 18-25 kg/m2 | 54 | 101 | |
| > 25 kg/m2 | 9 | 26 | 0.965 |
| Smoke | |||
| No | 50 | 97 | |
| Yes | 19 | 41 | 0.745 |
| ASA | |||
| I | 21 | 37 | |
| II-III | 48 | 101 | 0.584 |
| CA19-9 | |||
| ≤ 400 U/mL | 40 | 116 | |
| > 400 U/mL | 29 | 22 | < 0.001 |
| CA125 | |||
| ≤ 38 U/mL | 20 | 121 | |
| > 38 U/mL | 49 | 26 | < 0.001 |
| CEA | |||
| ≤ 5 U/mL | 42 | 112 | |
| > 5 U/mL | 27 | 26 | 0.002 |
| Fibrinogen | |||
| ≤ 4.0 g/L | 50 | 101 | |
| > 4.0 g/L | 19 | 37 | 0.912 |
| NLR | |||
| ≤ 5 | 59 | 131 | |
| > 5 | 10 | 7 | 0.026 |
| PLR | |||
| ≤ 150 | 36 | 78 | |
| > 150 | 33 | 60 | 0.553 |
| PNI | |||
| > 45 | 42 | 101 | |
| ≤ 45 | 27 | 37 | 0.072 |
| Platelet | |||
| ≤ 250 × 109/L | 53 | 108 | |
| > 250 × 109/L | 16 | 30 | 0.813 |
| Jaundice | |||
| No | 34 | 78 | |
| Yes | 35 | 60 | 0.431 |
| Albumin | |||
| > 35 g/L | 42 | 82 | |
| ≤ 35 g/L | 27 | 56 | 0.841 |
| Diabetes mellitus | |||
| No | 39 | 101 | |
| Yes | 30 | 37 | 0.017 |
| Pancreatitis | |||
| No | 42 | 93 | |
| Yes | 27 | 45 | 0.354 |
Table 3.
Multivariate analysis of predictors of synchronous liver metastasis
| Parameter | Odds ratio | 95%CI | P value |
| CA19-9 | |||
| ≤ 400 U/mL | |||
| > 400 U/mL | 2.398 | 0.909-6.327 | 0.077 |
| CA125 | |||
| ≤ 38 U/mL | |||
| > 38 U/mL | 12.397 | 5.468-28.105 | < 0.001 |
| CEA | |||
| ≤ 5 U/mL | |||
| > 5 U/mL | 0.672 | 0.249-1.817 | 0.434 |
| NLR | |||
| ≤ 5 | |||
| > 5 | 0.934 | 0.283-3.083 | 0.911 |
| Diabetes mellitus | |||
| No | |||
| Yes | 3.343 | 1.539-7.262 | 0.002 |
Risk factors for unresectability of synchronous liver metastasis
Table 4 compares the clinical parameters between the curative group (group A) and the palliative group (group B), all of whom had primary PDAC and synchronous liver metastasis. In univariate analysis, the probability of unresectability was significantly increased when patients presented with age > 62 (P = 0.004), CA19-9 > 400 U/mL (P = 0.026), CA125 > 62 U/mL (P = 0.001) and albumin ≤ 35 g/L (P = 0.021). In multivariate analysis, one clinical index and one tumor marker, age > 62 (OR = 3.921, 95%CI: 1.217-12.632, P = 0.022) and CA125 > 62 U/mL (OR = 5.181, 95%CI: 1.612-16.665, P = 0.006), were found to correlate with increased unresectability when a 62-U/mL threshold of CA125 was used (Table 5). The AUC of serum CA125 level was 0.701 (95%CI: 0.576-0.826), with sensitivity of 71.79% and specificity of 70.00% at the threshold of 62 U/mL.
Table 4.
Risk factors for unresectability of synchronous liver metastasis from pancreatic ductal adenocarcinoma
| Parameter | Simultaneous resections (Group A) | Palliative surgical bypass (Group B) | P value (univariate) |
| n = 30 | n = 39 | ||
| Age, yr | |||
| ≤ 62 | 20 | 12 | |
| > 62 | 10 | 27 | 0.004 |
| Sex | |||
| Male | 20 | 27 | |
| Female | 10 | 12 | 0.821 |
| BMI | |||
| < 18 kg/m2 | 2 | 4 | 0.664 |
| 18-25 kg/m2 | 23 | 31 | |
| > 25 kg/m2 | 5 | 4 | 0.472 |
| Smoke | |||
| No | 23 | 27 | |
| Yes | 7 | 12 | 0.494 |
| ASA | |||
| I | 11 | 10 | |
| II-III | 19 | 29 | 0.326 |
| CA19-9 | |||
| ≤ 400 U/mL | 22 | 18 | |
| > 400 U/mL | 8 | 21 | 0.026 |
| CA125 | |||
| ≤ 38 U/mL | 11 | 9 | |
| > 38 U/mL | 19 | 30 | 0.221 |
| CA125 | |||
| ≤ 62 U/mL | 21 | 11 | |
| > 62 U/mL | 9 | 28 | 0.001 |
| CEA | |||
| ≤ 5 U/mL | 22 | 20 | |
| > 5 U/mL | 8 | 19 | 0.066 |
| Fibrinogen | |||
| ≤ 4.0 g/L | 21 | 29 | |
| > 4.0 g/L | 9 | 10 | 0.688 |
| NLR | |||
| ≤ 5 | 26 | 33 | |
| > 5 | 4 | 6 | 0.811 |
| PLR | |||
| ≤ 150 | 17 | 19 | |
| > 150 | 13 | 20 | 0.513 |
| PNI | |||
| > 45 | 22 | 20 | |
| ≤ 45 | 8 | 19 | 0.066 |
| Platelet | |||
| ≤ 250 × 109/L | 26 | 27 | |
| > 250 × 109/L | 4 | 12 | 0.097 |
| Albumin | |||
| > 35 g/L | 23 | 19 | |
| ≤ 35 g/L | 7 | 20 | 0.021 |
| Diabetes mellitus | |||
| No | 17 | 22 | |
| Yes | 13 | 17 | 0.983 |
| Pancreatitis | |||
| No | 22 | 20 | |
| Yes | 8 | 19 | 0.066 |
Table 5.
Multivariate analysis of risk factors for unresectability of synchronous liver metastasis from pancreatic ductal adenocarcinoma
| Parameter | Odds ratio | 95%CI | P value |
| Age, yr | |||
| ≤ 62 | |||
| > 62 | 3.921 | 1.217-12.632 | 0.022 |
| CA19-9, U/mL | |||
| ≤ 400 L | |||
| > 400 | 1.760 | 0.517-5992 | 0.366 |
| CA125, U/mL | |||
| ≤ 62 | |||
| > 62 | 5.181 | 1.612-16.665 | 0.006 |
| Albumin | |||
| > 35 g/L | |||
| ≤ 35 g/L | 1.796 | 0.516-6.253 | 0.357 |
Prognostic factors for PDAC patients with synchronous liver metastasis
Following their respective surgeries, PDAC patients with synchronous liver metastasis had a decreased median survival compared to those with PDAC and no distant metastasis (7.0 mo vs 16.9 mo, P < 0.001) (Figure 2). However, the median survival of patients who underwent simultaneous curative resections for primary PDAC and liver metastasis was not significantly different from that of those who underwent curative resection for primary PDAC alone (15.7 mo vs 16.9 mo, P = 0.085). Unfortunately, patients who underwent palliative bypass merely had a 4.4-mo survival because of unresectable liver metastases.
Figure 2.
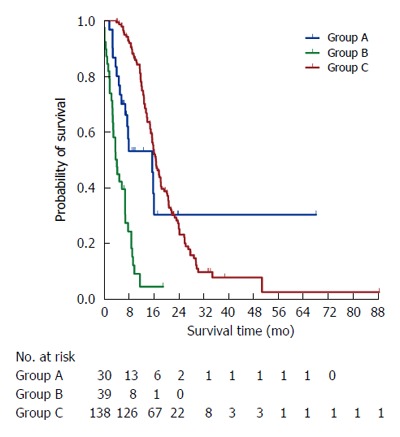
Kaplan-Meier survival curves of PDAC patients with synchronous liver metastasis who underwent simultaneous resections for primary PDAC and synchronous liver metastasis (group A) and palliative surgical bypass (group B) and PDAC patients with no distant metastases who underwent curative resection for primary PDAC alone (group C).
Prognostic factors for overall survival of PDAC patients with synchronous liver metastasis were illustrated using Cox regression analysis (Table 6). Univariate analysis revealed that patients with age > 62 (P = 0.006), CA19-9 > 400 U/mL (P = 0.042) and CA125 > 62 U/mL (P = 0.003) tended to have a diminished survival. Multivariate analysis demonstrated that age > 62 (HR = 2.191, 95%CI: 1.182-4.060, P = 0.013) and CA125 > 62 U/mL (HR = 2.601, 95%CI: 1.403-4.832, P = 0.002) were still retained as significant and independent prognostic factors for long-term survival. Moreover, after the simultaneous resections, patients with post-operatively elevated serum CA125 levels had shorter survival than those with post-operatively reduced serum CA125 levels (7.7 mo vs 16.3 mo, P = 0.013) (Figure 3).
Table 6.
Cox regression analysis of prognostic factors in pancreatic ductal adenocarcinoma patients with synchronous liver metastasis undergoing surgery
| Parameter | n | Median OS (95%CI) (mo) | Univariate analysis |
Multivariate analysis |
||
| P value | Hazard ratio | 95%CI | P value | |||
| Age, yr | ||||||
| ≤ 62 | 32 | 9.988 (5.215-14.760) | ||||
| > 62 | 37 | 4.534 (3.294-5.774) | 0.006 | 2.191 | 1.182-4.060 | 0.013 |
| Sex | ||||||
| Male | 47 | 5.388 (2.454-8.322) | ||||
| Female | 22 | 7.129 (5.820-8.439) | 0.428 | |||
| BMI, kg/m2 | ||||||
| < 18 | 6 | 4.008 (0.000-8.701) | 0.939 | |||
| 18-25 | 54 | 6.998 (5.147-8.849) | ||||
| > 25 | 9 | 7.721 (1.548-13.894) | 0.548 | |||
| Smoke | ||||||
| No | 50 | 7.031 (4.606-9.455) | ||||
| Yes | 19 | 5.979 (0.607-11.352) | 0.317 | |||
| ASA | ||||||
| I | 21 | 9.988 (3.524-16.452) | ||||
| II-III | 48 | 6.998 (3.803-10.193) | 0.273 | |||
| Primary tumor location | ||||||
| Head/neck | 53 | 6.998 (4.910-9.086) | ||||
| Body/tail | 16 | 7.129 (3.438-10.820) | 0.762 | |||
| CA19-9, U/mL | ||||||
| ≤ 400 | 40 | 7.984 (6.454-9.513) | ||||
| > 400 | 29 | 4.008 (2.832-5.185) | 0.042 | 1.398 | 0.773-2.527 | 0.267 |
| CA125, U/mL | ||||||
| ≤ 62 | 32 | 9.035 (7.052-11.017) | ||||
| > 62 | 37 | 4.008 (2.801-5.215) | 0.003 | 2.601 | 1.403-4.823 | 0.002 |
| CEA, U/mL | ||||||
| ≤ 5 | 42 | 7.721 (6.340-9.102) | ||||
| > 5 | 27 | 5.191 (1.847-8.535) | 0.320 | |||
| Fibrinogen, g/L | ||||||
| ≤ 4.0 | 50 | 7.129 (5.373-8.886) | ||||
| > 4.0 | 19 | 5.191 (2.575-7.807) | 0.533 | |||
| NLR | ||||||
| ≤ 5 | 59 | 7.031 (5.123-8.939) | ||||
| > 5 | 10 | 4.008 (0.000-9.812) | 0.495 | |||
| PLR | ||||||
| ≤ 150 | 36 | 7.031 (4.545-9.517) | ||||
| > 150 | 33 | 5.979 (3.287-8.672) | 0.851 | |||
| PNI | ||||||
| > 45 | 42 | 7.129 (6.237-8.022) | ||||
| ≤ 45 | 27 | 5.848 (3.396-8.300) | 0.890 | |||
| Platelet | ||||||
| ≤ 250 × 109/L | 53 | 6.998 (5.018-8.978) | ||||
| > 250 × 109/L | 16 | 7.097 (0.957-13.236) | 0.993 | |||
| Jaundice | ||||||
| No | 34 | 7.721 (4.540-10.901) | ||||
| Yes | 35 | 6.998 (3.593-10.403) | 0.446 | |||
| Biliary drainage | ||||||
| No | 45 | 7.129 (5.245-9.014) | ||||
| Yes | 24 | 5.027 (1.872-8.181) | 0.878 | |||
| Bilirubin, μmol/L | ||||||
| ≤ 50 | 58 | 6.998 (4.918-9.078) | ||||
| > 50 | 11 | 7.031 (1.408-12.651) | 0.448 | |||
| Albumin, g/L | ||||||
| > 35 | 42 | 7.097 (4.893-9.300) | ||||
| ≤ 35 | 27 | 5.027 (2.171-7.882) | 0.799 | |||
| Diabetes mellitus | ||||||
| No | 39 | 6.998 (4.177-9.819) | ||||
| Yes | 30 | 6.998 (4.424-9.572) | 0.300 | |||
| Pancreatitis | ||||||
| No | 42 | 5.979 (4.876-9.186) | ||||
| Yes | 27 | 6.998 (2.516-11.480) | 0.789 | |||
Figure 3.
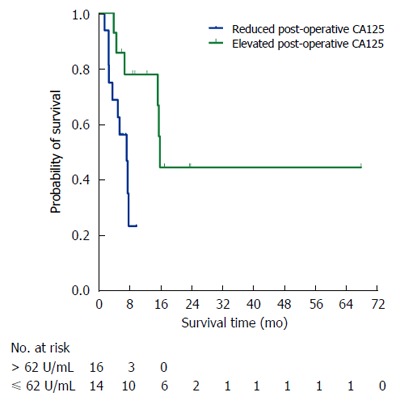
Kaplan-Meier survival curves according to post-operative CA125 levels of PDAC patients with synchronous liver metastasis who underwent simultaneous resections for primary PDAC and synchronous liver metastasis (group A).
Comparison of intra- and post-operative courses
During the intra-operative period, mean operative time, median blood loss and frequency of intra-operative RBC transfusion were similar between patients in group A and group C (P = 0.494, P = 0.780, P = 0.691) (Table 7). During the post-operative course, pancreatic fistula was the most frequent complication in both group A (n = 9, 40.9%) and group C (n = 22, 33.3%). Only one patient in group A had two complications, pancreatic fistula and cerebral infarction. Three patients in group C had two complications, pancreatic fistula and delayed gastric emptying, delayed gastric emptying and gastrointestinal hemorrhage, pancreatic fistula and pneumonia respectively. The rates of pancreatic fistula and other complications in group A were not significantly greater than in group C (P = 0.072, P = 0.230). As for alteration of post-operative glycemic status by surgery, 53.8% (n = 7) of patients in group A with diabetes mellitus were cured of diabetes post-operatively, whereas 46.2% (n = 6) had either no change in diabetic status or experienced worsening of glucose control. Among patients without pre-operative diabetes mellitus in group A, 35.3% (n = 6) remained nondiabetic post-operatively and 64.7% (n = 11) developed new-onset. These two surgical options of group A and C did not have significantly different impacts on post-operative diabetic control (P = 0.602). Two in group A and 4 in group C were readmitted because of biliary reflux or delayed gastric emptying. There was no in-hospital mortality in either group. The median length of hospital stay in group A was not longer than in group C due to additional resection for synchronous liver metastases (P = 0.479).
Table 7.
Comparison of perioperative parameters in different cohorts of patients undergoing surgery
| Parameter |
No. of patients |
P value | |
| Simultaneous resections (Group A) n = 30 | Pancreatectomy alone (Group C) n = 138 | ||
| Mean operative time, min | 344.3 | 380.5 | 0.494 |
| Median blood loss, mL | 400 | 400 | 0.780 |
| Intra-operative RBC transfusion | 12 | 61 | 0.691 |
| Complication | |||
| Pancreatic fistula | 9 | 22 | 0.072 |
| Any other | 13 | 44 | 0.230 |
| Biliary fistula | 0 | 0 | |
| Chylous fistula | 1 | 3 | |
| Delayed gastric emptying | 4 | 10 | |
| Intra-abdominal infection | 6 | 28 | |
| Gastrointestinal hemorrhage | 0 | 1 | |
| Cerebral infarction | 1 | 0 | |
| Pneumonia | 1 | 2 | |
| Post-operative diabetes mellitus | 0.602 | ||
| Dissolved | 7 | 15 | |
| New-onset | 6 | 12 | |
| Persistent | 6 | 22 | |
| Re-admission | 2 | 4 | |
| In-hospital mortality | 0 | 0 | |
| Hospital stay, d | 18 | 19 | 0.479 |
DISCUSSION
Although incidental liver metastases from PDAC identified during surgeries are not an unusual finding to surgeons, intra-operative surprises probably make patients lose opportunities to receive a more rational treatment modality which surgeons have weighed the pros and cons of. Accurate detection of liver metastases from PDAC and proper selection of patients who are likely to benefit from simultaneous curative resections for primary PDAC and synchronous liver metastasis are a great challenge for individualized therapy for PDAC. As pancreatectomy is performed with relatively high morbidity and mortality, assessment of unresectability of synchronous liver metastases under the circumstance of resectable primary PDAC, needs an objective standard using a series of pre-operative clinical parameters. In the present study, we identified a pre-operative serum signature of CA125 levels over 38 U/mL as one of the predictors for synchronous liver metastasis from PDAC. Serum CA125 levels over 62 U/mL were found not only to imply unresectability for synchronous liver metastasis, but also to indicate a poor survival for PDAC patients with synchronous liver metastasis. These suggest that PDAC patients with synchronous liver metastasis predicted by serum CA125 levels over 38 U/mL could be appropriate for and, more importantly, benefit from the simultaneous resections if serum CA125 levels range between 38 U/mL and 62 U/mL.
Since CA125 has been extensively used as a biomarker of various types of cancers, its diagnostic and prognostic values are gradually attracting great attention for PDAC. Recently it was reported in a two-center clinical study that elevated serum CA125 levels were more pronounced in patients with the metastasis-associated burden, especially liver metastasis[17]. Elevated serum CA125 levels in patients with gastric adenocarcinoma were also observed with the presence of peritoneal metastases and lymph node metastases[31,32]. On the contrary to the similar elevated serum CA19-9 levels in all stages of PDAC, serum CA125 levels for PDAC with distant metastasis were shown to be higher than that for early or locally advanced PDAC[33]. Our result indicated that patients with PDAC were more likely to have synchronous liver metastasis if serum CA125 level exceeded 38 U/mL instead of serum CA19-9 level higher than 1000 U/mL. Thus, it is inferred that serum CA125 levels are insensitive to primary PDAC.
In addition to differential diagnosis, serum CA125 levels reflected the extent of liver metastases as well. We found that the median serum CA125 levels of unexpected liver metastases was lower than that of detected liver metastases (52 U/mL vs 72 U/mL, P = 0.009). The median serum CA125 levels of liver metastases with fewer than 5 nodules smaller than 3 cm, or more than 3 nodules larger than 3 cm, or intermediate nodules was, respectively, 37 U/mL, 67 U/mL and 486 U/mL[17]. Of note, even though patients received curative resection for primary PDAC and post-operatively displayed a decrease in serum CA19-9 level, an early distant metastasis and poor survival still troubled those who did not experience a decrease in serum CA125 levels[17,18], as we observed. Furthermore, CA125 expression in PDAC was also found to directly correlate with tumor stage, grade and metastasis[34,35], and to increase along with loss of differentiation of PDAC[35], which denotes the tendency for distant metastasis[36,37]. Primary PDAC expressed CA125 under the same intensity as metastatic lesions did, demonstrating the maintenance of PDAC for CA125 expression during the metastatic process[35]. Therefore, we believe that CA125 is an effective pre-operative factor for monitoring synchronous liver metastasis from PDAC.
Given that CA19-9 can be influenced by obstructive jaundice or pancreatitis[38] and cannot be detected due to lack of the Lewis antigen[39], CA125 characterized by secretory stability is considered more suitable for objective judgement. Regarding the unresectability of cancer, CA125 has been widely utilized as the therapeutic strategy[40-44]. Compared with CA19-9, the most common tumor marker evaluated in patients with PDAC, CA125 as a predictor for unresectability of primary PDAC had a superior ROC area of 0.81, with a cutoff level of 19.7U/mL[45]. Moreover, elevated CA125 levels over the selected threshold could distinguish factually unresectable PDAC from equivocally resectable PDAC judged by multidetector CT[45]. In the present study, we analyzed a series of clinical parameters, including tumor markers, and found that serum CA125 levels over 62 U/mL might signify unresectability of synchronous liver metastasis even if primary PDAC could be curatively resected at a R0 status. Considering that serum CA125 levels also implied the extent of liver metastasis[17] and that the location and number of liver metastases determined the feasibility and method of surgery[46], our findings were quite deducible and rational. Taken together with predictability of synchronous liver metastasis by serum CA125 level over 38 U/mL, a narrow range of serum CA125 level from 38 U/mL to 62 U/mL denoted simultaneous resectability of primary PDAC and synchronous liver metastasis.
In spite of uneventful curative resections, prolonged survival does not necessarily belong to all patients. On one hand, it was demonstrated that resected patients with pre-operative serum CA125 levels over 18.4 U/mL survived less than half of the life time as those with lower serum CA125 levels (11.3 mo vs 25.3 mo)[17]. More importantly, unlike CA19-9, no discrepancies of predictability by CA125 were found in PDAC patients with hyperbilirubinemia[47]. It was determined in the two-center clinical study that the combination of CA19-9 over 1000 U/mL and either CA125 or CEA indicated a worse surgical outcome, with a median survival of 7.0 mo vs 18.2 mo for the validation cohort from our hospital[18]. In addition, as a good response to curative surgery, deceasing CA125 levels after pancreatectomy were associated with longer survival time as well (40.8 mo vs 14.6 mo)[17]. Our data also reflected that patients with elevated serum CA125 levels did not display a survival advantage following the simultaneous resections. Associated with the incidence of liver metastasis, co-expression of CA125 and mesothelin could signify unfavorable outcome in PDAC patients (19.0 mo vs 34.8 mo)[48]. In this study, we showed that pretreatment serum CA125 level over 62 U/mL was useful for indicating a worse outcome for PDAC patients with synchronous liver metastasis. These imply that our surgical option for primary PDAC and synchronous liver metastasis determined by serum CA125 levels does have an impact on patient survival and that the simultaneous curative resections do improve clinical outcome. Aggressive therapeutic regimens may be more advantageous in patients with lower serum CA125 levels.
On the other hand, Dünschede et al[49] claimed shorter survival in patients with synchronous liver metastasis undergoing simultaneous resections than in those treated by gemcitabine (8.0 mo vs 11 mo) despite no statistical differences. However, resection for metachronous liver metastases instead of gemcitabine might extend survival in highly selected patients. Meanwhile, Klein et al[50] reported that no similar survival was achieved by pancreatectomy and simultaneous liver resection for PDAC, albeit at a R0 status, compared with pancreatectomy for non-metastasized PDAC (13.0 mo vs 26.5 mo)[50] (Table 8). On the contrary, we showed that the survival of PDAC patients with synchronous liver metastasis who underwent simultaneous curative resections (15.7 mo) was not only longer than that of those who underwent palliative surgical bypass alone (4.4 mo) but also similar to that of patients with non-metastasized PDAC who underwent curative pancreatectomy alone (16.9 mo). Such discrepancy with the previous two studies can be explained by inconspicuous residual lesion after liver resection misjudged by pre-operative or intra-operative assessment. In accordance with our data, De Jong et al[51] demonstrated that overall survival appeared not to be different in the patients who underwent PD and liver-directed therapy compared with those with no evidence of liver metastasis who underwent PD (17.7 mo vs 17.9 mo). Therefore, our result of Cox regression analysis showing that serum CA125 levels less than 62 U/mL were independently associated with a prolonged survival, justified our criterion of serum CA125 level as appropriate for simultaneous resections for primary PDAC and synchronous liver metastasis, and certified its benefit for survival of patients with synchronous liver metastasis from PDAC.
Table 8.
Survival data from published studies with simultaneous resections of primary pancreatic ductal adenocarcinoma and synchronous liver metastasis
|
Simultaneous resections |
Palliative surgical bypass or chemotherapy |
Pancreatectomy alone |
P value | ||||
| Median (mo) | n | Median (mo) | n | Median (mo) | n | ||
| Adam et al[57] (2006) | NA1 | 41 | - | - | - | - | - |
| Yamada et al[58] (2006) | 15.0 | 6 | - | - | - | - | - |
| Gleisner et al[59] (2007) | 5.9 | 17 | - | - | - | - | - |
| Shrikhande et al[60] (2007) | 7.9 | 10 | - | - | - | - | - |
| De Jong et al[51] (2010) | 17.7 | NA | - | - | 17.9 | NA | 0.730 |
| De Jong et al[61] (2010) | 13.02 | 14 | - | - | - | - | - |
| Dünschede et al[49] (2010) | 8.0 | 9 | 11 | 5 | - | - | - |
| Seelig et al[62] (2010) | 11.8 | 4 | - | - | - | - | - |
| Klein et al[50] (2012) | 13.0 | 7 | - | - | 26.5 | 13 | NA |
| Tachezy et al[63] (2016) | 14.5 | 69 | 7.5 | 69 | - | - | < 0.001 |
Five-year survival of 20% was provided;
The median survival of 25 patients with pancreatic ductal adenocarcinoma (PDAC) and cholangiocarcinoma.
Now that pancreatectomy itself is associated with significant morbidity and mortality, a simultaneous liver resection may carry the extraneous risks influencing overall survival, such as bile leak, hemorrhage, or liver abscess[52,53]. The risk of developing a liver abscess reached nearly 40%-50% for liver-directed therapy radiofrequency ablation of liver tumors in patients with a biliary tract procedure such as an enterobiliary anastomosis or biliary stenting[54]. As for liver resection for metastasized PDAC, it is noteworthy that the construction of a biliary-enteric anastomosis during PD may be one of the induction factors of a liver abscess. The development of post-operative complications has been found to be detrimental to survival and to lead to early recurrence in PDAC patients[55,56]. However, our study found no liver-specific complications caused by liver resection and no more severe pancreatic fistula caused by pancreatectomy, suggesting relative safety of simultaneous resections with similar morbidity compared with standard pancreatectomy alone.
The current study had several limitations. Despite a time span of 8 years, only a relatively small sample size of patients was identified as having primary PDAC and synchronous liver metastasis who underwent either simultaneous resections or palliative surgical bypass. As such, this study had limited statistical power. Meanwhile, there may have been selection bias in whether PDAC patients with synchronous liver metastasis were chosen for surgery. For example, if some PDAC patients with resectable tumor of body or tail of the pancreas and unresectable synchronous liver metastases did not present with biliary or upper digestive obstruction, they usually underwent gemcitabine-based chemotherapy instead of palliative surgical bypass first and were excluded from our study. In addition, the focus of our study was the impact of pre-operative factors on diagnosis of liver metastasis and selection of suitable patients for simultaneous resections, which overlooks the influence of intra-or post-operative factors on overall and recurrence-free survival. Furthermore, the role of serum CA125 levels in clinical prediction for metachronous liver metastasis from PDAC was not investigated and hence cannot provide guidance for all patients with liver metastasis from PDAC.
In conclusion, diagnosis and treatment of liver metastasis from PDAC must be individualized in the era of precision medicine because of its highly malignant biological behavior. Serum CA125 level over 38 U/mL predicts synchronous liver metastasis from PDAC, and serum CA125 level over 62 U/mL is associated with unresectability of metastatic disease burden. The criterion set up by serum CA125 levels facilitates the careful diagnosis of synchronous liver metastases from PDAC pre-operatively and the prudent selection of appropriate patients for simultaneous resections for primary PDAC and synchronous liver metastasis for the sake of prolonged survival and substantially reduced morbidity or mortality. Therefore, simultaneous resections for primary PDAC and synchronous liver metastasis are justified by prolonged survival in patients selected by serum CA125. It is foreseeable that the indication for the simultaneous resections for precisely diagnosed liver-metastasized PDAC will be extended with the development of surgical techniques and thus more PDAC patients will have a clear survival benefit.
COMMENTS
Background
Approximately 50% of new pancreatic ductal adenocarcinoma (PDAC) cases are discovered to have distant metastases. The doctrine that the presence of liver metastasis from resectable PDAC contradicts a curative resection and indicates a palliative surgical bypass, deprives patients of an incremental benefit from simultaneous curative resections for primary and metastatic PDAC. Diagnosis of synchronous liver metastasis from PDAC and assessment of unresectability are still challenging to surgeons.
Research frontiers
The two-center clinical study we were involved in reported that elevated serum CA125 levels are more pronounced in patients with the metastasis-associated burden. On the contrary to the similar elevated serum CA19-9 levels in all stages of PDAC, serum CA125 levels for PDAC with distant metastasis were higher than that for early or locally advanced PDAC. Serum CA125 levels also imply the extent of liver metastasis, and the location and number of liver metastases determine the feasibility and method of surgery. Therefore, we hypothesized that CA125 might be an effective pre-operative factor for monitoring synchronous liver metastasis from PDAC, and that CA125 could predict unresectability of synchronous liver metastasis.
Innovations and breakthroughs
PDAC patients with synchronous liver metastasis predicted by serum CA125 levels over 38 U/mL might be appropriate for and, more importantly, benefit from simultaneous resections if serum CA125 levels range between 38 U/mL and 62 U/mL. The survival of PDAC patients with synchronous liver metastasis who underwent simultaneous curative resections was not only longer than that of those who underwent palliative surgical bypass alone but also similar to that of patients with non-metastasized PDAC who underwent curative pancreatectomy alone.
Applications
The criterion set up by serum CA125 levels facilitates the careful judgement of occurrence of synchronous liver metastases from PDAC, and the prudent selection of appropriate patients for simultaneous resections for primary PDAC and synchronous liver metastasis, for the sake of prolonged survival and substantially reduced morbidity or mortality.
Terminology
Liver metastasis is a remarkable preference of PDAC to disseminate due to its portal venous blood draining and lymphatic spread, and drastically reduces the survival of patients with PDAC. Simultaneous curative resection is an unconventional surgical option for synchronous liver metastasis, which requires further justification by prolonged survival, a longer recurrence-free interval and, at least, no more surgical-related morbidity and mortality.
Peer-review
This research is original and very interesting for publication.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Fudan University Huashan Hospital Ethical Committee and Institutional Review Board.
Informed consent statement: All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare no conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: August 5, 2016
First decision: September 5, 2016
Article in press: October 19, 2016
P- Reviewer: Cvetanovic A S- Editor: Qi Y L- Editor: Filipodia E- Editor: Wang CH
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Louvet C, Philip PA. Accomplishments in 2007 in the treatment of metastatic pancreatic cancer. Gastrointest Cancer Res. 2008;2:S37–S41. [PMC free article] [PubMed] [Google Scholar]
- 4.Papavasiliou P, Hoffman JP, Cohen SJ, Meyer JE, Watson JC, Chun YS. Impact of preoperative therapy on patterns of recurrence in pancreatic cancer. HPB (Oxford) 2014;16:34–39. doi: 10.1111/hpb.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michalski CW, Erkan M, Hüser N, Müller MW, Hartel M, Friess H, Kleeff J. Resection of primary pancreatic cancer and liver metastasis: a systematic review. Dig Surg. 2008;25:473–480. doi: 10.1159/000184739. [DOI] [PubMed] [Google Scholar]
- 6.Fujioka S, Misawa T, Okamoto T, Gocho T, Futagawa Y, Yanaga K. Predictors for postoperative liver metastasis in patients with resectable pancreatic cancer. Int Surg. 2008;93:324–330. [PubMed] [Google Scholar]
- 7.Lu F, Poruk KE, Weiss MJ. Surgery for oligometastasis of pancreatic cancer. Chin J Cancer Res. 2015;27:358–367. doi: 10.3978/j.issn.1000-9604.2015.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weh HJ, Steiner P, Crone-Münzebrock W, Hossfeld DK. [Diagnosis and spontaneous course of liver metastases] Chirurg. 1991;62:710–714. [PubMed] [Google Scholar]
- 9.Kneuertz PJ, Cunningham SC, Cameron JL, Torrez S, Tapazoglou N, Herman JM, Makary MA, Eckhauser F, Wang J, Hirose K, et al. Palliative surgical management of patients with unresectable pancreatic adenocarcinoma: trends and lessons learned from a large, single institution experience. J Gastrointest Surg. 2011;15:1917–1927. doi: 10.1007/s11605-011-1665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toomey P, Childs C, Luberice K, Ross S, Rosemurgy A. Nontherapeutic celiotomy incidence is not affected by volume of pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2013;79:781–785. [PubMed] [Google Scholar]
- 11.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 13.Ko AH. Progress in the treatment of metastatic pancreatic cancer and the search for next opportunities. J Clin Oncol. 2015;33:1779–1786. doi: 10.1200/JCO.2014.59.7625. [DOI] [PubMed] [Google Scholar]
- 14.Nikfarjam M, Sehmbey M, Kimchi ET, Gusani NJ, Shereef S, Avella DM, Staveley-O’Carroll KF. Additional organ resection combined with pancreaticoduodenectomy does not increase postoperative morbidity and mortality. J Gastrointest Surg. 2009;13:915–921. doi: 10.1007/s11605-009-0801-2. [DOI] [PubMed] [Google Scholar]
- 15.Lillemoe KD, Cameron JL, Yeo CJ, Sohn TA, Nakeeb A, Sauter PK, Hruban RH, Abrams RA, Pitt HA. Pancreaticoduodenectomy. Does it have a role in the palliation of pancreatic cancer? Ann Surg. 1996;223:718–725; discussion 725-728. doi: 10.1097/00000658-199606000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhlmann K, de Castro S, van Heek T, Busch O, van Gulik T, Obertop H, Gouma D. Microscopically incomplete resection offers acceptable palliation in pancreatic cancer. Surgery. 2006;139:188–196. doi: 10.1016/j.surg.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Xu HX, Wang WQ, Wu CT, Xiang JF, Liu C, Long J, Xu J, Fu de L, Ni QX, et al. Serum CA125 is a novel predictive marker for pancreatic cancer metastasis and correlates with the metastasis-associated burden. Oncotarget. 2016;7:5943–5956. doi: 10.18632/oncotarget.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Xu H, Wang W, Wu C, Chen Y, Yang J, Cen P, Xu J, Liu C, Long J, et al. A preoperative serum signature of CEA+/CA125+/CA19-9 ≥ 1000 U/mL indicates poor outcome to pancreatectomy for pancreatic cancer. Int J Cancer. 2015;136:2216–2227. doi: 10.1002/ijc.29242. [DOI] [PubMed] [Google Scholar]
- 19.Guo Q, Zhang B, Dong X, Xie Q, Guo E, Huang H, Wu Y. Elevated levels of plasma fibrinogen in patients with pancreatic cancer: possible role of a distant metastasis predictor. Pancreas. 2009;38:e75–e79. doi: 10.1097/MPA.0b013e3181987d86. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Gao J, Bai M, Liu R, Li H, Deng T, Zhou L, Han R, Ge S, Huang D, et al. The pretreatment platelet and plasma fibrinogen level correlate with tumor progression and metastasis in patients with pancreatic cancer. Platelets. 2014;25:382–387. doi: 10.3109/09537104.2013.827782. [DOI] [PubMed] [Google Scholar]
- 21.Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long J, Liu L, Liu C, Xu J, Ni Q, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22:670–676. doi: 10.1245/s10434-014-4021-y. [DOI] [PubMed] [Google Scholar]
- 22.Shirai Y, Shiba H, Sakamoto T, Horiuchi T, Haruki K, Fujiwara Y, Futagawa Y, Ohashi T, Yanaga K. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery. 2015;158:360–365. doi: 10.1016/j.surg.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Neal CP, Cairns V, Jones MJ, Masood MM, Nana GR, Mann CD, Garcea G, Dennison AR. Prognostic performance of inflammation-based prognostic indices in patients with resectable colorectal liver metastases. Med Oncol. 2015;32:144. doi: 10.1007/s12032-015-0590-2. [DOI] [PubMed] [Google Scholar]
- 24.Xue P, Kanai M, Mori Y, Nishimura T, Uza N, Kodama Y, Kawaguchi Y, Takaori K, Matsumoto S, Uemoto S, et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med. 2014;3:406–415. doi: 10.1002/cam4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi Q, Geng Y, Sun M, Wang P, Chen Z. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology. 2015;15:145–150. doi: 10.1016/j.pan.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama Y, Gotohda N, Shibasaki H, Nomura S, Kinoshita T, Hayashi R. Usefulness of the neutrophil/lymphocyte ratio measured preoperatively as a predictor of peritoneal metastasis in patients with advanced gastric cancer. Surg Today. 2014;44:2146–2152. doi: 10.1007/s00595-014-0917-1. [DOI] [PubMed] [Google Scholar]
- 27.Cetinkunar S, Guzel H, Emre Gokce I, Erdem H, Gulkan S, Aktimur R, Kucuk B, Imamoglu I, Kargici H. High levels of platelet/lymphocyte ratio are associated with metastatic gastric cancer. J BUON. 2015;20:78–83. [PubMed] [Google Scholar]
- 28.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, Foulis AK, Horgan PG, McMillan DC. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Kim TH, Han SS, Park SJ, Lee WJ, Woo SM, Yoo T, Moon SH, Kim SH, Hong EK, Kim DY, et al. CA 19-9 level as indicator of early distant metastasis and therapeutic selection in resected pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:e743–e748. doi: 10.1016/j.ijrobp.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Yoo T, Lee WJ, Woo SM, Kim TH, Han SS, Park SJ, Moon SH, Shin KH, Kim SS, Hong EK, et al. Pretreatment carbohydrate antigen 19-9 level indicates tumor response, early distant metastasis, overall survival, and therapeutic selection in localized and unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:e623–e630. doi: 10.1016/j.ijrobp.2011.02.063. [DOI] [PubMed] [Google Scholar]
- 31.Streppel MM, Vincent A, Mukherjee R, Campbell NR, Chen SH, Konstantopoulos K, Goggins MG, Van Seuningen I, Maitra A, Montgomery EA. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;43:1755–1763. doi: 10.1016/j.humpath.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujimura T, Kinami S, Ninomiya I, Kitagawa H, Fushida S, Nishimura G, Kayahara M, Shimizu K, Ohta T, Miwa K. Diagnostic laparoscopy, serum CA125, and peritoneal metastasis in gastric cancer. Endoscopy. 2002;34:569–574. doi: 10.1055/s-2002-33228. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto K, Haga Y, Yoshimura R, Egami H, Yokoyama Y, Akagi M. Comparative effectiveness of the tumour diagnostics, CA 19-9, CA 125 and carcinoembryonic antigen in patients with diseases of the digestive system. Gut. 1987;28:323–329. doi: 10.1136/gut.28.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang K, Tan E, Sayegh Z, Centeno B, Malafa M, Coppola D. Cancer Antigen 125 (CA125, MUC16) Protein Expression in the Diagnosis and Progression of Pancreatic Ductal Adenocarcinoma. Appl Immunohistochem Mol Morphol. 2016 doi: 10.1097/PAI.0000000000000368. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Haridas D, Chakraborty S, Ponnusamy MP, Lakshmanan I, Rachagani S, Cruz E, Kumar S, Das S, Lele SM, Anderson JM, et al. Pathobiological implications of MUC16 expression in pancreatic cancer. PLoS One. 2011;6:e26839. doi: 10.1371/journal.pone.0026839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamisawa T, Isawa T, Koike M, Tsuruta K, Okamoto A. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas. 1995;11:345–349. doi: 10.1097/00006676-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413–422. doi: 10.5858/133.3.413. [DOI] [PubMed] [Google Scholar]
- 38.Marrelli D, Caruso S, Pedrazzani C, Neri A, Fernandes E, Marini M, Pinto E, Roviello F. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg. 2009;198:333–339. doi: 10.1016/j.amjsurg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 39.Hamada E, Taniguchi T, Baba S, Maekawa M. Investigation of unexpected serum CA19-9 elevation in Lewis-negative cancer patients. Ann Clin Biochem. 2012;49:266–272. doi: 10.1258/acb.2011.011213. [DOI] [PubMed] [Google Scholar]
- 40.Chi DS, Venkatraman ES, Masson V, Hoskins WJ. The ability of preoperative serum CA-125 to predict optimal primary tumor cytoreduction in stage III epithelial ovarian carcinoma. Gynecol Oncol. 2000;77:227–231. doi: 10.1006/gyno.2000.5749. [DOI] [PubMed] [Google Scholar]
- 41.Byrom J, Widjaja E, Redman CW, Jones PW, Tebby S. Can pre-operative computed tomography predict resectability of ovarian carcinoma at primary laparotomy? BJOG. 2002;109:369–375. doi: 10.1111/j.1471-0528.2002.01216.x. [DOI] [PubMed] [Google Scholar]
- 42.Kouba EJ, Lentz A, Wallen EM, Pruthi RS. Clinical use of serum CA-125 levels in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder. Urol Oncol. 2009;27:486–490. doi: 10.1016/j.urolonc.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 43.Fleming ND, Cass I, Walsh CS, Karlan BY, Li AJ. CA125 surveillance increases optimal resectability at secondary cytoreductive surgery for recurrent epithelial ovarian cancer. Gynecol Oncol. 2011;121:249–252. doi: 10.1016/j.ygyno.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Pelissier A, Bonneau C, Chéreau E, de La Motte Rouge T, Fourchotte V, Daraï E, Rouzier R. CA125 kinetic parameters predict optimal cytoreduction in patients with advanced epithelial ovarian cancer treated with neoadjuvant chemotherapy. Gynecol Oncol. 2014;135:542–546. doi: 10.1016/j.ygyno.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Luo G, Xiao Z, Long J, Liu Z, Liu L, Liu C, Xu J, Ni Q, Yu X. CA125 is superior to CA19-9 in predicting the resectability of pancreatic cancer. J Gastrointest Surg. 2013;17:2092–2098. doi: 10.1007/s11605-013-2389-9. [DOI] [PubMed] [Google Scholar]
- 46.Frankel TL, D’Angelica MI. Hepatic resection for colorectal metastases. J Surg Oncol. 2014;109:2–7. doi: 10.1002/jso.23371. [DOI] [PubMed] [Google Scholar]
- 47.Chen T, Zhang MG, Xu HX, Wang WQ, Liu L, Yu XJ. Preoperative serum CA125 levels predict the prognosis in hyperbilirubinemia patients with resectable pancreatic ductal adenocarcinoma. Medicine (Baltimore) 2015;94:e751. doi: 10.1097/MD.0000000000000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Einama T, Kamachi H, Nishihara H, Homma S, Kanno H, Takahashi K, Sasaki A, Tahara M, Okada K, Muraoka S, et al. Co-expression of mesothelin and CA125 correlates with unfavorable patient outcome in pancreatic ductal adenocarcinoma. Pancreas. 2011;40:1276–1282. doi: 10.1097/MPA.0b013e318221bed8. [DOI] [PubMed] [Google Scholar]
- 49.Dünschede F, Will L, von Langsdorf C, Möhler M, Galle PR, Otto G, Vahl CF, Junginger T. Treatment of metachronous and simultaneous liver metastases of pancreatic cancer. Eur Surg Res. 2010;44:209–213. doi: 10.1159/000313532. [DOI] [PubMed] [Google Scholar]
- 50.Klein F, Puhl G, Guckelberger O, Pelzer U, Pullankavumkal JR, Guel S, Neuhaus P, Bahra M. The impact of simultaneous liver resection for occult liver metastases of pancreatic adenocarcinoma. Gastroenterol Res Pract. 2012;2012:939350. doi: 10.1155/2012/939350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Jong MC, Farnell MB, Sclabas G, Cunningham SC, Cameron JL, Geschwind JF, Wolfgang CL, Herman JM, Edil BH, Choti MA, et al. Liver-directed therapy for hepatic metastases in patients undergoing pancreaticoduodenectomy: a dual-center analysis. Ann Surg. 2010;252:142–148. doi: 10.1097/SLA.0b013e3181dbb7a7. [DOI] [PubMed] [Google Scholar]
- 52.Schlag P, Hohenberger P, Herfarth C. Resection of liver metastases in colorectal cancer--competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol. 1990;16:360–365. [PubMed] [Google Scholar]
- 53.Takemura N, Saiura A, Koga R, Arita J, Yoshioka R, Ono Y, Sano T, Yamamoto J, Kokudo N, Yamaguchi T. Long-term results of hepatic resection for non-colorectal, non-neuroendocrine liver metastasis. Hepatogastroenterology. 2013;60:1705–1712. doi: 10.5754/hge13078. [DOI] [PubMed] [Google Scholar]
- 54.Elias D, Di Pietroantonio D, Gachot B, Menegon P, Hakime A, De Baere T. Liver abscess after radiofrequency ablation of tumors in patients with a biliary tract procedure. Gastroenterol Clin Biol. 2006;30:823–827. doi: 10.1016/s0399-8320(06)73327-9. [DOI] [PubMed] [Google Scholar]
- 55.Aoyama T, Murakawa M, Katayama Y, Yamaoku K, Kanazawa A, Higuchi A, Shiozawa M, Morimoto M, Yoshikawa T, Yamamoto N, et al. Impact of postoperative complications on survival and recurrence in pancreatic cancer. Anticancer Res. 2015;35:2401–2409. [PubMed] [Google Scholar]
- 56.Kamphues C, Bova R, Schricke D, Hippler-Benscheidt M, Klauschen F, Stenzinger A, Seehofer D, Glanemann M, Neuhaus P, Bahra M. Postoperative complications deteriorate long-term outcome in pancreatic cancer patients. Ann Surg Oncol. 2012;19:856–863. doi: 10.1245/s10434-011-2041-4. [DOI] [PubMed] [Google Scholar]
- 57.Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M, Jaeck D, Saric J, Le Treut YP, Belghiti J, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244:524–535. doi: 10.1097/01.sla.0000239036.46827.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamada H, Hirano S, Tanaka E, Shichinohe T, Kondo S. Surgical treatment of liver metastases from pancreatic cancer. HPB (Oxford) 2006;8:85–88. doi: 10.1080/13651820500472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gleisner AL, Assumpcao L, Cameron JL, Wolfgang CL, Choti MA, Herman JM, Schulick RD, Pawlik TM. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer. 2007;110:2484–2492. doi: 10.1002/cncr.23074. [DOI] [PubMed] [Google Scholar]
- 60.Shrikhande SV, Kleeff J, Reiser C, Weitz J, Hinz U, Esposito I, Schmidt J, Friess H, Büchler MW. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2007;14:118–127. doi: 10.1245/s10434-006-9131-8. [DOI] [PubMed] [Google Scholar]
- 61.de Jong MC, Tsai S, Cameron JL, Wolfgang CL, Hirose K, van Vledder MG, Eckhauser F, Herman JM, Edil BH, Choti MA, et al. Safety and efficacy of curative intent surgery for peri-ampullary liver metastasis. J Surg Oncol. 2010;102:256–263. doi: 10.1002/jso.21610. [DOI] [PubMed] [Google Scholar]
- 62.Seelig SK, Burkert B, Chromik AM, Tannapfel A, Uhl W, Seelig MH. Pancreatic resections for advanced M1-pancreatic carcinoma: the value of synchronous metastasectomy. HPB Surg. 2010;2010:579672. doi: 10.1155/2010/579672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tachezy M, Gebauer F, Janot M, Uhl W, Zerbi A, Montorsi M, Perinel J, Adham M, Dervenis C, Agalianos C, et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery. 2016;160:136–144. doi: 10.1016/j.surg.2016.02.019. [DOI] [PubMed] [Google Scholar]


