Abstract
AIM
To analyse the clinical features of patients with the serrated lesions in the upper gastrointestinal tract (UPGI) tract.
METHODS
Patients who underwent routine esophagogastroduodenoscopy (EGD) at the Digestive Endoscopy Centre of General Hospital, Tianjin Medical University between January 2011 and December 2015 were consecutively recruited. Patients with UPGI serrated lesions were consecutively identified. The patients’ demographics and histopathology were recorded. The colorectal findings for patients who underwent colonoscopy simultaneously or within six months were also extracted from the colonoscopy database. In addition, we analysed differences in colorectal neoplasia detection between the study patients and randomly selected patients matched for age and gender who did not exhibit serrated lesions and who also underwent colonoscopy in the same period.
RESULTS
A total of 21 patients out of 98746 patients (0.02%) who underwent EGD were confirmed to have serrated lesions with predominantly crenated, sawtooth-like configurations. The mean age of the 21 patients was (55.3 ± 17.2) years, and 11 patients were male (52.4%). In terms of the locations of the serrated lesions, 17 were found in the stomach (including 3 in the cardia, 9 in the corpus and 5 in the antrum), 3 were found in the duodenum, and 1 was found in the esophagus. Serrated lesions were found in different mucosal lesions, with 14 lesions were detected in polyps (8 hyperplastic polyps and 6 serrated adenomas with low grade dysplasia), 3 detected in Ménétrier gastropathy, 3 detected in an area of inflammation or ulcer, and 1 detected in the intramucosal carcinoma of the duodenum. In addition, colonoscopy data were available for 18 patients, and a significantly higher colorectal adenoma detection rate was observed in the UPGI serrated lesions group than in the randomly selected age- and gender-matched group without serrated lesions who also underwent colonoscopy in the same period (38.9% vs 11.1%, OR = 5.091, 95%CI: 1.534-16.890, P = 0.010). The detection rate of advanced adenoma was also higher in the UPGI serrated lesions group (22.2% vs 4.2%, OR = 6.571, 95%CI: 1.322-32.660, P = 0.028).
CONCLUSION
Serrated lesions in the UPGI were detected in various mucosal lesions with different pathological morphologies. Moreover colonoscopy is recommended for the detection of concurrent colorectal adenoma for these patients.
Keywords: Clinical features, Upper gastrointestinal tract, Serrated lesions, Colorectal adenoma, Colorectal cancer
Core tip: In this retrospective study, the clinical features of the serrated lesions in the upper gastrointestinal tract (UPGI) were analysed. We found that serrated lesions in the UPGI occurred can be found in different mucosal lesions. Furthermore, a significantly higher colorectal adenoma detection rate was observed in the UPGI serrated lesions group than in the randomly selected age- and gender-matched group from our colonoscopy database, and the detection rate of advanced adenoma was also higher in the UPGI serrated group. Therefore, colonoscopy is recommended for the detection of concurrent colorectal adenoma in patients with UPGI serrated lesions.
INTRODUCTION
A new “alternative” pathway by which adenocarcinomas develop from serrated lesions was recently described by Jass and Smith, and it may account for 10% to 30% of all cases of colorectal cancer (CRC)[1-5]. According to the 2010 WHO classification, three subgroups including hyperplastic polyps (HP), traditional serrated adenoma (TSA), and sessile serrated adenoma/polyp have been divided, forming a heterogeneous group of colorectal lesions. However, detailed information about patients with serrated lesions in the upper gastrointestinal tract (UPGI) is very limited.
UPGI nonconventional adenomatous and nonadenomatous types of dysplasia, such as serrated adenoma and dysplasia, have recently been identified. In 1992, Stolte et al[6] revealed a characteristic “hypertrophy” of the parietal cells that was induced by omeprazole, and produced a serrated internal gland profile. In addition, in 2001, Rubio[7] reported the first case of serrated adenoma of the stomach. Since then, serrated dysplasia has been reported in patients with Ménétrier-like lymphocytic gastritis[8], reactive gastropathy[9] and even Barrett’s esophagus[10], and it is epitomised by hypereosinophilic cytoplasm, small oval-shaped nuclei and prominent luminal serration. Serrated adenomas characterized by branched villi exhibiting lateral saw-tooth indentations lined with dysplastic cells or “Christmas-tree-like” serrated configurations have also been detected in the esophagus[11], the stomach[12-20], the duodenum[19,21-27], the pancreas[28] and the gallbladder[29]. Although serrated adenomas are rare, recent reports indicate that 53.4% (39/73) of traditional serrated adenomas in the UPGI are invasive carcinomas[30]. Therefore, analysing the comprehensive clinical features of UPGI serrated lesions is still worthwhile.
Moreover, a meta-analysis was recently performed to assess patients at risk of developing colorectal polyps with upper digestive polyps, and the finding showed that the incidence of colorectal neoplasia was markedly higher in patients with UPGI polyps than in those without UPGI polyps[31]. However the relationship between UPGI serrated lesions and colorectal neoplasia is not clear. Hence, in the present study, we analysed the clinical and pathological features of serrated lesions in the UPGI and also evaluated the colonoscopy findings in the study group.
MATERIALS AND METHODS
Design and patients
Patients who underwent a routine esophagogastroduodenoscopy (EGD) at the Digestive Endoscopy Centre of the General Hospital, Tianjin Medical University between January 2011 and December 2015 were consecutively recruited. Patients who underwent other types examinations, such as therapeutic endoscopy and emergent endoscopy, were not included. The patients’ features, including their age, gender, body mass index (BMI), endoscopy indications, family history of cancer and the size and location of the lesions were extracted from the endoscopy reports and patient questionaires.
In patients with histologically confirmed UPGI serrated lesions, colonoscopy was required simultaneously or within six months. Each patient was compared to 4 randomly selected age- and gender-matched controls without serrated lesions who also underwent a colonoscopy within the same time period. We also analysed the differences in colorectal neoplasia detection in each study patient and in the control group. Patients with a history of colonoscopy polypectomy or surgical resection of the colon or rectum and patients with a family history of CRC, polyposis syndromes or inflammatory bowel disease were excluded. Informed consents for EGD and colonoscopy were obtained from all of the participants before the procedure, and the researchers had access to the patients’ identifying information. The study was approved by the Ethics Committee of the General Hospital, Tianjin Medical University.
Endoscopic procedure
Before undergoing the EGD, all of the patients were asked to undergo a fasting period of at least 12 h and water deprivation for 8 h. Polyethylene glycol lavage was prescribed for bowel preparation and watery diarrhea excretion prior to the procedure indicated adequate intestinal preparation for the colonoscopy. Experienced endoscopists carefully performed the colonoscopies while the patients were under anaesthesia. The cardiopulmonary function was monitored by an anaesthetist, and the patients were maintained under general anaesthesia with intravenous injections of propofol. Electronic gastroscopy (GIF-Q260, Olympus, Tokyo, Japan) and colonoscopy (Olympus CF-Q260, Olympus, Tokyo, Japan) equipment were used for all procedures.
Pathological evaluation
All of the biopsy specimens or resected lesions that collected during the EGD were fixed in 10% formalin within 1 h of removal and then fixed for a minimum of 4 h. All haematoxylin and eosin-stained sections used for the pathological assessment and classification were evaluated by experienced pathologists. Serrated lesions in the UPGI exhibit clinically and molecularly diverse changes with common features, such as crypt luminal morphology characterized by glandular serration. In addition, advanced colorectal adenomas (AA) were defined as tubular adenomas > 10 mm in size, adenomas with villous histology, or high grade dysplasia. Multiple polyps were categorized according to the most advanced lesion.
Statistical analysis
All of the statistical analyses were performed with SPSS 17.0 (Chicago, IL, United States) for Windows. The means and standard deviations (SDs) were calculated for continuous variables. Categorical or constitute data were expressed as percentage. Risks of colorectal neoplasia between patients with serrated lesions in the UPGI and the control group in our database were compared via the χ2 test or Fisher’s exact test. The level of statistically significance was set at two-tailed P < 0.05.
RESULTS
General information on the study group
During the study period, 98746 routine EGDs were performed. A total of 21 patients with serrated lesions that exhibited predominantly crenated, sawtooth-like configurations were diagnosed. The mean age of these 21 patients with serrated lesions was (55.3 ± 17.2) years, and the proportion of males was 52.4% (11/21). The mean BMI of the patients was (24.9 ± 5.8) kg/m2 and 13 patients (61.9%) had a BMI within the normal range. The proportions of patients with a history of smoking, alcohol use, and a family history of gastric cancer were 33.3% (7/21), 47.6% (10/21) and 4.8% (1/21), respectively. The indications for EGD included upper abdominal pain (23.8%, 5/21), nausea, vomiting and reflux (19.0%, 4/21), anemia and edema (19.0%, 4/21), positive fecal occult blood test (14.3%,3/21), a history of gastric polyps (14.3%, 3/21) and dyspepsia (9.5%, 2/21) (Table 1).
Table 1.
General information on patients with serrated lesions in upper gastrointestinal tract
| n (%) | |
| Total | 21 |
| Mean age (yr), mean ± SD | 55.3 ± 17.2 |
| Gender, male | 11 (52.4) |
| Body mass index (kg/m2), mean ± SD | 24.9 ± 5.8 |
| 18.5-23.9 | 13 (61.9) |
| ≥ 24.0 | 8 (38.1) |
| History of smoking | 7 (33.3) |
| Alcohol consumption | 10 (47.6) |
| Family history of gastric cancer | 1 (4.8) |
| Indications for endoscopy | |
| Upper abdominal pain | 5 (23.8) |
| Nausea, vomit and reflux | 4 (19.0) |
| Anemia and edema | 4 (19.0) |
| Positive fecal occult blood test | 3 (14.3) |
| A history of gastric polyps | 3 (14.3) |
| Dyspepsia | 2 (9.5) |
Distribution of serrated lesions detected in UPGI
Table 2 presents the distribution of serrated lesions: 17 lesions were detected in the stomach (including 3 in the cardia, 9 in the corpus and 5 in the antrum), 3 were detected in the duodenum (2 in the duodenal bulb and 1 in the descending part) and 1 was detected in the lower esophagus.
Table 2.
Clinical features of serrated lesions in upper gastrointestinal tract
| n (%) | |
| Size (mm), mean ± SD | 11.7 ± 10.3 |
| ≤ 5 | 8 (38.1) |
| 5-10 | 4 (19.0) |
| 10-20 | 6 (28.6) |
| 20-30 | 1 (4.8) |
| ≥ 30 | 2 (9.5) |
| Distribution | |
| Esophagus | 1 (4.8) |
| Cardia | 3 (14.3) |
| Corpus | 9 (42.9) |
| Antrum | 5 (23.8) |
| Duodenum | 3 (14.3) |
| Morphology | |
| Serrated hyperplasia | 6 (28.6) |
| Hyperplastic polyps | 8 (38.1) |
| Adenoma | 6 (28.6) |
| Adenocarcinoma | 1 (4.8) |
| Situation of serrated lesions in mucosal lesions | |
| Inflammation or ulcer | 3 (14.3) |
| Serrated polyps | 14 (66.7) |
| Ménétrier gastropathy | 3 (14.3) |
| Duodenal cancer | 1 (4.8) |
| Colonoscopy findings | 18 |
| No-polyp | 4 (22.2) |
| Hyperplastic polyps | 7 (38.9) |
| Non-advanced adenomas | 3 (16.7) |
| Advanced adenomas | 4 (22.2) |
| Tubular adenoma with high grade dysplasia | 1 (5.6) |
| Tubulovillous adenoma | 2 (11.1) |
| Adenoma > 10 mm | 1 (5.6) |
Morphology of UPGI serrated lesions
The mean size of the UPGI serrated lesions was (11.7 ± 10.3) mm. The diameter was less than 20 mm in 18 patients, and more than 30 mm in 2 patients. The histopathological features of different serrated lesions were divided into four morphologies: (1) serrated hyperplasia (6/21) which was detected in areas of inflammation or ulcer lesions (3/21) and Ménétrier gastropathy (3/21); (2) HPs (8/21); (3) serrated adenoma with low grade dysplasia (6/21); and (4) Serrated lesion (1/21), which was found in the intramucosal carcinoma of the duodenum. The typical pathological images and clinical features are shown in Figure 1 and Table 2.
Figure 1.
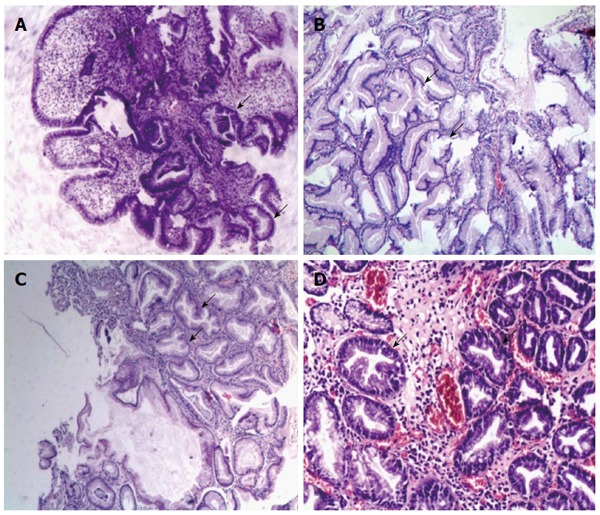
Typical pathological images of serrated lesions in upper gastrointestinal tract. Serrated lesions characterized by epithelial cells with luminal infolding and a serrated growth pattern were shown. A: Serrated hyperplasia in esophagitis (× 40); B: Serrated hyperplasia in the Ménétrier gastropathy: marked foveolar hyperplasia and glandular cysts with serrated lesions in the stomach (× 100); C: Hyperplastic polyp in the stomach: a serrated polyp without overt cytological atypia showed narrowed crypt bases that were predominantly lined with immature cells (× 100); D: Serrated adenoma with low grade dysplasia in the duodenum: a serrated polyp with enlarged nuclei, a pencil-shaped, hyperchromaticity and nuclear stratification (× 100).
Prevalence of colorectal neoplasia in patients with UPGI serrated lesions
A total of 18 patients with UPGI serrated lesions (81.7%, 18/21) underwent a colonoscopy simultaneously or within six months, and 3 patients refused to undergo a colonoscopy. We then evaluated the colonoscopy findings of these patients. Three non-advanced colorectal adenomas (NAA) and 4 AAs (1 tubular adenoma with high grade dysplasia, 2 tubulovillous adenomas and 1 adenoma > 10 mm in size) were found, and all of these colorectal adenomas were detected only in patients with UPGI serrated adenomas or polyps. The remaining colonoscopy reports included 7 colorectal HPs and 4 patients without polyps. CRC was not detected found in 18 patients. The colonoscopy findings in the patients with UPGI serrated lesions are illustrated in Table 3. We also compared the detection rate of colorectal adenoma in the UPGI serrated lesions group with that in the control group in our colonoscopy database (Table 4). A total of 72 age- and gender-matched patients without serrated lesions who had presented to our centre for EGD and colonoscopy were randomly selected as the control group. A significantly higher colorectal adenoma detection rate was observed in the UPGI serrated lesions group than in the control group (38.9% vs 11.1%, OR = 5.091; 95%CI: 1.534-16.890; P = 0.010), and a higher detection rate of advanced adenoma was observed in the UPGI serrated lesions group (22.2% vs 4.2%, OR = 6.571; 95%CI: 1.322-32.660; P = 0.028).
Table 3.
Colonoscopy findings in the patients with serrated lesions in upper gastrointestinal tract
| Serrated lesions in upper gastrointestinal tract mucosal lesions (n) | Colonoscopy findings (n) |
| Inflammation or ulcer (3) | HPs (1) |
| HPs (8) | AA (2), NAA (1), HPs (2) |
| Serrated adenoma (6) | AA (2), NAA (2), HPs (2) |
| Ménétrier gastropathy (3) | HPs (2) |
| Duodenal cancer (1) | Absent |
HPs: Hyperplastic polyps; AA: Advanced adenoma; NAA: Non-advanced adenoma.
Table 4.
Prevalence of colorectal adenoma in patients with serrated lesions in upper gastrointestinal tract and the control group n (%)
| Patients with serrated lesions in UPGI (n = 18) | Average group (n = 72) | OR (95%CI) | P value | |
| Colorectal adenoma | 7 (38.9) | 8 (11.1) | 5.091 (1.534-16.890) | 0.010 |
| Non-advanced adenoma | 3 (16.7) | 5 (6.9) | 2.680 (0.576-12.463) | 0.195 |
| Advanced adenoma | 4 (22.2) | 3 (4.2) | 6.571 (1.322-32.660) | 0.028 |
DISCUSSION
Since 1990, serrated polyps have been commonly found during colonoscopy and recognized as an important process in the development of CRC[1,32,33]. Limited reports focused on the clinical features of UPGI serrated lesions because of the low prevalence of these lesions, and few articles have described serrated adenomas in the UPGI[7-25]. Thus, serrated polyps have not been previously listed in the classifications of the upper digestive tract[34-36]. The present study provides current information on serrated lesions in different UPGI diseases, including inflammation or ulcer, Ménétrier gastropathy, HPs, serrated adenomas, and adenocarcinoma, as well as the serrated profile found in cases of reactive gastropathy[8,9]. We also found that nearly half of the UPGI serrated lesions were located in the gastric corpus, and 2/3 of the lesions were detected in polyps in the current study. In addition, we evaluated the colonoscopy findings of patients with UPGI serrated lesions, and found a significantly higher colorectal adenoma detection rate in the serrated lesions group than in the control group, thus colonoscopy may be recommended to exclude the presence of concurrent colorectal adenomas in these patients.
This study provides the first description the detection and distribution of serrated lesions in the UPGI, and analysed the colonoscopy results of these patients compared with the control group. Simple and readily accepted methods (EGD and colonoscopy) were used, and the possibility of clinical heterogeneity was minimized because of the study setting, which was within a tertiary endoscopic centre. However, several limitations should be mentioned. First, relatively small sample size was used in the present study because of the rarity of serrated lesions, and a statistical analysis of the age, gender, BMI and family history of patients with different mucosal lesions could not be conducted. Second, this study was conducted in a tertiary endoscopic centre, therefore selection bias likely occurred. In addition, more rigorous studies with larger sample sizes from multiple clinical centres are necessary to determine whether patients with UPGI serrated lesions have a higher rate of colorectal adenomas and to ascertain whether these findings similar to those in previous reports[31,37].
Activating mutations of the RAS-RAF-MAPK pathway have been reported to initiate and sustain lesions in the serrated pathway, and the presence of a positive CpG island methylation phenotype and DNA repair genes might play a major role in colorectal neoplastic progression[5,38,39]. Compared with serrated polyps of the colon, extracolonic serrated polyps are virtually undescribed and their genetic alterations are largely unknown. The pathological findings and analysis of the molecular alterations of 13 serrated neoplasms of the small intestine indicated that almost half of the neoplasms demonstrated high-grade dysplasia or were associated with an adenocarcinoma. However, the absence of the BRAFV600E mutation does not support a role for the serrated neoplasia pathway in the development of these lesions, as it does in colorectal serrated polyps[26]. Another report confirmed that oncogenic KRAS mutation was the most common abnormality in extracolonic serrated polyps, whereas a microsatellite instability and a CpG island methylator phenotype were less commonly[19]. Rubio[30] presented a TSA pathway of carcinogenesis in the UPGI, and 53.4% (39/73) of the UPGI TSAs reported in the literature are associated with invasive carcinomas, however, we only detected one case associated with duodenal cancer. The younger average age of the patients with serrated adenoma in our study (62.2 ± 11.4) than that in the past reports (66.4 ± 11.7) compared with that in previous may provide a suitable explanation for this phenomenon. Hence, the mechanism that causes these lesions to evolve into invasive carcinomas remains elusive.
In conclusion, serrated lesions in the UPGI, which represents a rarely described histological phenotype, were observed in various mucosal lesions with different pathological morphologies. Moreover, colonoscopy is recommended to exclude the presence of concurrent colorectal carcinomas in these patients. However, further studies are needed to clarify the clinical significance of these lesions.
ACKNOWLEDGMENTS
We thank all of the doctors, nurses and pathologists who helped to manage the patients who underwent endoscopies in our centre.
COMMENTS
Background
Recently, a new “alternative” pathway by which adenocarcinomas develop from serrated lesions was first described by Jass and Smith, and this pathway may account for 10% to 30% of all cases of colorectal cancer. However, information on upper gastrointestinal (UPGI) serrated lesions is limited. UPGI nonconventional adenomatous and nonadenomatous types of dysplasia, such as serrated adenoma and dysplasia, have been recently identified. Although serrated adenomas are rare, recent reports have indicated that 53.4% (39/73) of traditional serrated adenomas in the UPGI are invasive carcinomas. Therefore, analysing the comprehensive clinical features of the UPGI serrated lesions is still worthwhile.
Research frontiers
Colorectal serrated polyps are recognized as important contributors to colorectal cancer. However, detailed information on upper gastrointestinal serrated lesions is limited. The results of this study contribute to the analysis of the clinical features of serrated lesions in the UPGI, and the findings recommend colonoscopy for the detection of to find concurrent colorectal adenomas in these patients.
Innovations and breakthroughs
In this article, the authors found that serrated lesions in the UPGI occur in different mucosal lesions, such as areas of inflammation and ulcers, hyperplastic polyps, serrated adenomas and Ménétrier gastropathy. Furthermore, a significantly higher colorectal adenoma detection rate was observed in the UPGI serrated lesions group than in the randomly selected age- and gender-matched group from our colonoscopy database, and the detection rate of advanced adenoma was also higher in the UPGI serrated lesions group. Therefore, colonoscopy is recommended for the detection of concurrent colorectal adenomas in patients with UPGI serrated lesions.
Applications
This study shows that serrated lesions in the UPGI occur in different mucosal lesions. Furthermore, patients diagnosed with serrated lesions in the UPGI, should undergo a colonoscopy to detect any concurrent colorectal adenomas.
Terminology
UPGI: Endoscopic examination that includes esophagus, stomach, ampulla and the descending part of the duodenum.
Peer-review
Although the serrated lesions in UPGI are rare in the population, it is very important to understand its clinical and pathological features as such lesions maybe related to invasive carcinoma in UPGI exhibited. Furthermore, authors found in this study that the serrated lesions in UPGI are associated with higher colorectal adenoma detection rate.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Ethics Committee of General Hospital, Tianjin Medical University.
Informed consent statement: Informed consent for esophagogastroduodenoscopy and colonoscopy was obtained from all of the participants before the procedure.
Conflict-of-interest statement: The authors have no conflicts of interest.
Data sharing statement: No additional data are available.
Peer-review started: June 24, 2016
First decision: September 20, 2016
Article in press: November 16, 2016
P- Reviewer: Ahmad Z, Biondi A, Fialova A, Zhao JB S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
References
- 1.Jass JR, Smith M. Sialic acid and epithelial differentiation in colorectal polyps and cancer--a morphological, mucin and lectin histochemical study. Pathology. 1992;24:233–242. doi: 10.3109/00313029209068874. [DOI] [PubMed] [Google Scholar]
- 2.Bordaçahar B, Barret M, Terris B, Dhooge M, Dreanic J, Prat F, Coriat R, Chaussade S. Sessile serrated adenoma: from identification to resection. Dig Liver Dis. 2015;47:95–102. doi: 10.1016/j.dld.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Burgess NG, Tutticci NJ, Pellise M, Bourke MJ. Sessile serrated adenomas/polyps with cytologic dysplasia: a triple threat for interval cancer. Gastrointest Endosc. 2014;80:307–310. doi: 10.1016/j.gie.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Huang CS, Farraye FA, Yang S, O’Brien MJ. The clinical significance of serrated polyps. Am J Gastroenterol. 2011;106:229–240; quiz 241. doi: 10.1038/ajg.2010.429. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien MJ, Zhao Q, Yang S. Colorectal serrated pathway cancers and precursors. Histopathology. 2015;66:49–65. doi: 10.1111/his.12564. [DOI] [PubMed] [Google Scholar]
- 6.Stolte M, Bethke B, Rühl G, Ritter M. Omeprazole-induced pseudohypertrophy of gastric parietal cells. Z Gastroenterol. 1992;30:134–138. [PubMed] [Google Scholar]
- 7.Rubio CA. Serrated neoplasia of the stomach: a new entity. J Clin Pathol. 2001;54:849–853. doi: 10.1136/jcp.54.11.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubio CA, Ost A, Kato Y, Yanagisawa A, Rivera F, Hirota T. Hyperplastic foveolar gastropathies and hyperplastic foveolar gastritis. APMIS. 1997;105:784–792. doi: 10.1111/j.1699-0463.1997.tb05084.x. [DOI] [PubMed] [Google Scholar]
- 9.Mino-Kenudson M, Tomita S, Lauwers GY. Mucin expression in reactive gastropathy: an immunohistochemical analysis. Arch Pathol Lab Med. 2007;131:86–90. doi: 10.5858/2007-131-86-MEIRGA. [DOI] [PubMed] [Google Scholar]
- 10.Odze RD. What the gastroenterologist needs to know about the histology of Barrett’s esophagus. Curr Opin Gastroenterol. 2011;27:389–396. doi: 10.1097/MOG.0b013e328346f551. [DOI] [PubMed] [Google Scholar]
- 11.Rubio CA, Tanaka K, Befrits R. Traditional serrated adenoma in a patient with Barrett’s esophagus. Anticancer Res. 2013;33:1743–1745. [PubMed] [Google Scholar]
- 12.Rubio CA, Lagergren J. Serrated adenomas of the cardia. Anticancer Res. 2004;24:2113–2116. [PubMed] [Google Scholar]
- 13.M’sakni I, Rommani SR, Ben Kahla S, Najjar T, Ben Jilani S, Zermani R. Another case of serrated adenoma of the stomach. J Clin Pathol. 2007;60:580–581. doi: 10.1136/jcp.2006.037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubio CA, Petersson F, Höög A, Jónasson JG, Nesi G, Chandanos E, Lindblad M. Further studies on serrated neoplasias of the cardia: a review and case report. Anticancer Res. 2007;27:4431–4434. [PubMed] [Google Scholar]
- 15.Hasuo T, Semba S, Satake S, Shirasaka D, Aoyama N, Yokozaki H. Superficially elevated-type serrated hyperplastic lesion of the stomach with minute adenocarcinoma. Dig Endosc. 2009;21:101–105. doi: 10.1111/j.1443-1661.2009.00831.x. [DOI] [PubMed] [Google Scholar]
- 16.Köklü S, Başar O, Akbal E, Ibiş M. Gastric serrated adenoma polyp treated with endoscopic band ligation (with video) Surg Laparosc Endosc Percutan Tech. 2010;20:e204–e205. doi: 10.1097/SLE.0b013e3181fd27ab. [DOI] [PubMed] [Google Scholar]
- 17.Rubio CA, Björk J. Serrated adenoma of the stomach: Case report and literature review. World J Gastrointest Endosc. 2013;5:261–264. doi: 10.4253/wjge.v5.i5.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon MJ, Min BH, Lee SM, Park HY, Kang SY, Ha SY, Lee JH, Kim JJ, Park CK, Kim KM. Serrated adenoma of the stomach: a clinicopathologic, immunohistochemical, and molecular study of nine cases. Histol Histopathol. 2013;28:453–462. doi: 10.14670/HH-28.453. [DOI] [PubMed] [Google Scholar]
- 19.Taggart MW, Rashid A, Estrella J, Abraham SC. Serrated Polyps of the Extracolonic Gastrointestinal Tract: Histologic Findings and Genetic Alterations. Laboratory Investigation. 2012;92:182a–182a. [Google Scholar]
- 20.Rubio CA, Schmidt PT. An additional case of gastric serrated adenoma. Anticancer Res. 2014;34:3007–3010. [PubMed] [Google Scholar]
- 21.Rubio CA. Serrated adenoma of the duodenum. J Clin Pathol. 2004;57:1219–1221. doi: 10.1136/jcp.2004.016360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roche HJ, Carr NJ, Laing H, Bateman AC. Hyperplastic polyps of the duodenum: an unusual histological finding. J Clin Pathol. 2006;59:1305–1306. doi: 10.1136/jcp.2005.035022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner PL, Chen YT, Yantiss RK. Immunohistochemical and molecular features of sporadic and FAP-associated duodenal adenomas of the ampullary and nonampullary mucosa. Am J Surg Pathol. 2008;32:1388–1395. doi: 10.1097/PAS.0b013e3181723679. [DOI] [PubMed] [Google Scholar]
- 24.Rosty C, Buchanan DD, Walters RJ, Carr NJ, Bothman JW, Young JP, Brown IS. Hyperplastic polyp of the duodenum: a report of 9 cases with immunohistochemical and molecular findings. Hum Pathol. 2011;42:1953–1959. doi: 10.1016/j.humpath.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Sarbia M, Jüttner S, Bettstetter M, Berndt R. Serrated polyps of the duodenum. Three cases with immunohistological and molecular pathological findings. Pathologe. 2013;34:347–351. doi: 10.1007/s00292-013-1754-5. [DOI] [PubMed] [Google Scholar]
- 26.Rosty C, Campbell C, Clendenning M, Bettington M, Buchanan DD, Brown IS. Do serrated neoplasms of the small intestine represent a distinct entity? Pathological findings and molecular alterations in a series of 13 cases. Histopathology. 2015;66:333–342. doi: 10.1111/his.12469. [DOI] [PubMed] [Google Scholar]
- 27.Iwamuro M, Hori K, Tanaka T, Okada H. Serrated polyp of the duodenum. Gastrointest Endosc. 2015;82:966–97; discussion 967. doi: 10.1016/j.gie.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Rubio CA, Grimelius L, Von Sivers K, Höög A. Intraductal serrated adenoma of the pancreas. A case report. Anticancer Res. 2005;25:3099–3102. [PubMed] [Google Scholar]
- 29.Rubio CA. Serrated adenoma of the gallbladder: a case report. Anticancer Res. 2015;35:3485–3487. [PubMed] [Google Scholar]
- 30.Rubio CA. Traditional serrated adenomas of the upper digestive tract. J Clin Pathol. 2016;69:1–5. doi: 10.1136/jclinpath-2015-203258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu ZJ, Lin Y, Xiao J, Wu LC, Liu JG. Clinical significance of colonoscopy in patients with upper gastrointestinal polyps and neoplasms: a meta-analysis. PLoS One. 2014;9:e91810. doi: 10.1371/journal.pone.0091810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang S, Farraye FA, Mack C, Posnik O, O’Brien MJ. BRAF and KRAS Mutations in hyperplastic polyps and serrated adenomas of the colorectum: relationship to histology and CpG island methylation status. Am J Surg Pathol. 2004;28:1452–1459. doi: 10.1097/01.pas.0000141404.56839.6a. [DOI] [PubMed] [Google Scholar]
- 33.Szylberg Ł, Janiczek M, Popiel A, Marszałek A. Serrated polyps and their alternative pathway to the colorectal cancer: a systematic review. Gastroenterol Res Pract. 2015;2015:573814. doi: 10.1155/2015/573814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao H, Wang B, Zhang Z, Zhang H, Qu R. Distribution trends of gastric polyps: an endoscopy database analysis of 24 121 northern Chinese patients. J Gastroenterol Hepatol. 2012;27:1175–1180. doi: 10.1111/j.1440-1746.2012.07116.x. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein NS, Lewin KJ. Gastric epithelial dysplasia and adenoma: historical review and histological criteria for grading. Hum Pathol. 1997;28:127–133. doi: 10.1016/s0046-8177(97)90095-2. [DOI] [PubMed] [Google Scholar]
- 36.Appelman HD. Pathology of the esophagus, stomach, and duodenum. Contemporary issues in surgical pathology. New York: Churchill Livingstone. IX; 1984. p. 296. [Google Scholar]
- 37.Cao H, He N, Song S, Xu M, Piao M, Yan F, Wang B. Is surveillance colonoscopy necessary for patients with sporadic gastric hyperplastic polyps? PLoS One. 2015;10:e0122996. doi: 10.1371/journal.pone.0122996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoue A, Okamoto K, Fujino Y, Nakagawa T, Muguruma N, Sannomiya K, Mitsui Y, Takaoka T, Kitamura S, Miyamoto H, et al. B-RAF mutation and accumulated gene methylation in aberrant crypt foci (ACF), sessile serrated adenoma/polyp (SSA/P) and cancer in SSA/P. Br J Cancer. 2015;112:403–412. doi: 10.1038/bjc.2014.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson JC. Pathogenesis and management of serrated polyps: current status and future directions. Gut Liver. 2014;8:582–589. doi: 10.5009/gnl14248. [DOI] [PMC free article] [PubMed] [Google Scholar]


