Abstract
AIM
Evaluate the association between phase angle and the development of hepatic encephalopathy in the long-term follow-up of cirrhotic patients.
METHODS
This was a prospective cohort study. Clinical, nutritional and biochemical evaluations were performed. Mann-Whitney’s U and χ2 tests were used as appropriate. Kaplan-Meier curves and Cox proportional Hazards analysis were used to evaluate the prediction and incidence of hepatic encephalopathy.
RESULTS
Two hundred and twenty were included; the most frequent etiology of cirrhosis was hepatitis C infection, 52% of the patients developed hepatic encephalopathy (18.6% covert and 33.3% overt); the main precipitating factors were infections and variceal bleeding. Kaplan-Meier curves showed a higher proportion of HE in the group with low phase angle (39%) compared to the normal phase angle group (13%) (P = 0.012). Furthermore, creatinine and phase angle remained independently associated to hepatic encephalopathy in the Cox regression multivariate analysis [hazard ratio = 1.80 (1.07-3.03)].
CONCLUSION
In our cohort of patients low phase angle was associated with an increased incidence of hepatic encephalopathy. Phase angle is a useful nutritional marker that evaluates cachexia and could be used as a part of the integral assessment in patients with cirrhosis.
Keywords: Malnutrition, Hepatic encephalopathy, Phase angle, Cachexia, Prognosis
Core tip: Malnutrition in cirrhosis is a known risk factor for mortality and the development of complications. We used phase angle derived from bioelectrical impedance that reflects cachexia, the type of malnutrition seen in chronic diseases. We found an association between the presence of low phase angle and higher incidence of hepatic encephalopathy in the 48 mo of follow-up compared to the group with normal phase angle, demonstrating the importance of this complication. We proposed a nutritional marker that can predict the development of hepatic encephalopathy with the advantage of being a non-invasive, inexpensive and bedside method.
INTRODUCTION
Hepatic encephalopathy (HE) is defined as a spectrum of neuropsychiatric abnormalities observed in patients with liver dysfunction, in the absence of other explanatory medical conditions; this disorder may significantly impair the daily function, quality of life and survival of patients with cirrhosis[1]. The pathophysiology of HE is complex, and multiple mechanisms have been implicated in its development. Apart from hyperammonemia, other factors such as oxidative stress, inflammation, false neurotransmitters, genetic factors and cerebral hemodynamics are known to trigger HE[2,3].
As complications arise from cirrhosis, the different stages of malnutrition follow including depletion of muscle mass sarcopenia, depletion of both muscle and fat mass and the release of inflammatory mediators cachexia[4], affecting the normal functioning of different organs such as the brain. Some aspects of malnutrition share common mechanisms with those observed in patients with HE, including increased oxidative stress and systemic inflammation[5]. This is especially important in cachexia, where loss of muscle and fat mass together with systemic inflammation contribute to detrimental general status[6,7].
Loss of skeletal muscle is related to poor ammonia clearance by decreasing the amount of glutamine synthetase - a key enzyme related to ammonia scavenging - which catalyzes the conversion of ammonia and glutamate into glutamine[8,9]. Substantial evidence has linked oxidative stress with malnutrition; in fact, the underlying mechanism includes low bioavailability of antioxidants, enhanced production of free radicals and impaired functioning of free radical scavenging enzymes[10]. Finally, cachexia-driven inflammation and oxidative stress might further impair brain function leading to hepatic encephalopathy[5].
Among the current available methods for the assessment of the nutritional status, bioelectrical impedance-derived phase angle (PhA), is regarded as an useful tool indicating the balance between cell hydration and body mass, which is finally translated into tissue homeostasis and nutritional status[11]. Low values of PhA reflect poor nutritional status typically found in clinical settings such as cancer, human immunodeficiency virus and cirrhosis. The clinical application of PhA in patients with cirrhosis and HE includes the evaluation of cachexia[12-14].
Therefore, the aim of this study was to evaluate the association between PhA and the HE development in the long-term follow-up of cirrhotic patients.
MATERIALS AND METHODS
This was a prospective cohort study. Patients attending Gastroenterology and Hepatology clinics at a third-level hospital (Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”, México City, México) between March 2009 and June 2010 were screened for study enrollment. This study was designed and conducted according to the principles of the Declaration of Helsinki and was approved by the local Institutional Ethics Committee (REF 1652). Informed consent was obtained from each participant.
Patients
We included patients with an age of 18 to 65 years, and diagnosed with cirrhosis based on the combination of clinical features, radiological imaging, presence of portal hypertension, compatible biochemical parameters, and/or confirmatory liver biopsy. Inclusion was not restricted to etiology of cirrhosis. Patients with acute or chronic renal failure, an episode of overt HE in the previous 6 mo, with current treatment for HE, major surgery within four weeks before recruitment, pregnancy, active alcoholism, acute disease such as infections, use of neuropsychiatric drugs, hypothyroidism and extremity amputation, were excluded.
Clinical assessment
All patients underwent clinical evaluation to evaluate the presence of ascites, edema, and hepatic encephalopathy. HE was classified according to West Haven criteria. If diagnosis of overt HE was made during follow-up, anti-ammonia treatment (lactulose, antibiotics, L-ornithine-L-aspartate) was prescribed, and resolution/improvement of HE manifestations was confirmed at a follow-up visit.
Biochemical tests including liver function tests, international normalized ratio (INR), creatinine, sodium, and ammonia levels were obtained within the first week of study enrollment. Disease severity was determined according to Child-Pugh (CP) and Model for End-Stage Liver Disease (MELD) scores[15,16].
Bioelectrical impedance analysis and phase angle analysis
The BIA measurement was performed using RJL systems Quantum IV (Clinton Township, MI, United States) applying alternating electric currents of 800 μA at 50 kHz with the aid of Ag/AgCl source and sensor electrodes to obtain R, Xc and phase angle. BIA was performed within the first week of enrollment after an overnight fasting in supine position with arms and legs abducted from the body. Source and sensor electrodes were placed on the dorsum of the hand and foot on the right side of the body, respectively. The BIA-derived PhA was obtained according to standard calculations.
Cachexia can be defined as loss of muscle and fat mass, accompanied by an inflammatory component given by the presence of chronic disease[6], PhA has been shown to be associated to inflammation as well as muscle and fat depletion[11,14]. Cachexia was defined as when the patient presented a PhA value ≤ 4.9° based on the specific cut-off for our population[17] and specifically loss of muscle mass and fat mass that was obtained through a combined evaluation of PhA and vector analysis (RXc graph).
Statistical analysis
Kolmogorov-Smirnov test was performed to test data distribution. To compare groups U of Mann-Whitney and Chi-square test (χ2) were used. Incidence of HE was assessed with Kaplan-Meier curves, using the log rank test to compare the curves, followed by a multivariate Cox regression analysis. To obtain the best regression model, backward elimination model was selected. Statistical analysis was carried out using GraphPad Prism® 5 and SPSS v21 (SPSS Inc., Armonk, New York, United States).
RESULTS
The total population consisted of 220 outpatients (60% females), with a mean follow-up time of 34 ± 9.8 mo. The main etiology of cirrhosis was hepatitis C virus (HCV) (36%), followed by non-alcoholic steatohepatitis (NASH) (18%), primary biliary cirrhosis (17%), alcohol (10%), autoimmune hepatitis (10%), and other causes (9%). A total of 35% of patients were categorized as CP A, 47% CP B, and 18% CP C.
The total population was then divided into two groups, depending on HE development during follow-up; there were no significant differences in age, BMI and mid-arm muscle circumference among groups; however CP, MELD score and its components, as well as phase angle, sodium, hemoglobin and ammonia were worse in the HE group (Table 1).
Table 1.
Clinical and demographic characteristics of the study population according to the presence of hepatic encephalopathy
| HE (n = 115) | Non-HE (n = 105) | P value | |
| Age (yr) | 54.2 ± 10.3 | 51.8 ± 11.7 | 0.109 |
| BMI (kg/m2) | 26.4 (21.3-30.7) | 27.4 (24-30.5) | 0.185 |
| MAMC (cm) | 22.3 (20.3-26.4) | 24.4(21.1-26.98) | 0.230 |
| Phase angle (°) | 4.6 (4.0-5.5) | 5.3 (4.6-6.1) | 0.000 |
| Child-Pugh (points) | 8 (7-10) | 6 (5-8) | 0.000 |
| MELD score | 13 (10-15) | 10 (8-12) | 0.000 |
| Total bilirubin (mg/dL) | 2.1 (1.3-3.4) | 1.5 (1.0-2.6) | 0.001 |
| Albumin (mg/dL) | 3.4 ± 0.6 | 2.9 ± 0.6 | 0.000 |
| INR | 1.3 (1.2-1.4) | 1.2 (1.1-1.3) | 0.005 |
| Creatinine (mg/dL) | 0.84 (0.67-1.09) | 0.72 (0.63-0.83) | 0.000 |
| Sodium (mEq/L) | 136 (133-139) | 139 (136-141) | 0.000 |
| Hemoglobin (g/dL) | 12.2 (10.3-14.3) | 14.0 (12.1-15.3) | 0.000 |
| Ammonia (μ/dL) | 83.9 (50.9-123.3) | 56.3 (37.6-80.3) | 0.000 |
Data presented as mean ± SD, median (P25-P75). HE: Hepatic encephalopathy; BMI: Body mass index; MAMC: Mid-arm muscle circumference; MELD: Model for End-Stage Liver Disease; INR: International normalized ratio.
In total, during the 48 months of follow-up, 52% of the patients developed HE; from this 18.6% was covert HE and 33.3% was overt HE. From the total population 16.4% of the patients had more than one episode of HE during follow-up. Mean PhA was significantly lower in patients that showed persistent HE when compared to the group without persistent HE 4.6 ± 1.17 vs 5.2 ± 1.18 (P = 0.017).
When the analysis of HE development was stratified by nutritional status according to PhA ≤ or > 4.9°, there was a clear difference between groups. The incidence of HE was significantly higher in patients with cachexia, as evidenced by low phase angle, when compared to patients compared to well-nourished patients, 39% vs 13%, respectively (P = 0.012) (Figure 1).
Figure 1.
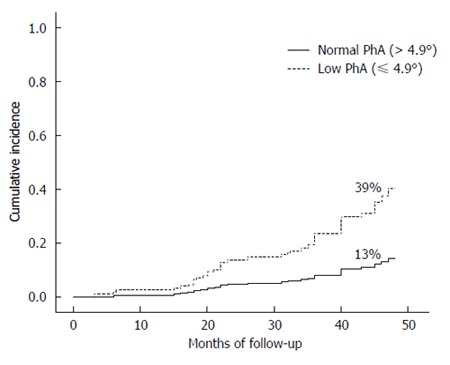
Development of hepatic encephalopathy according to phase angle during the follow-up period of 48 mo (P = 0.012). PhA: Phase angle.
The main precipitating factors of HE were infections different from spontaneous bacterial peritonitis (SBP) 26.7%, this included urinary tract infections and respiratory infections mainly, the second most common factor was variceal bleeding 23.3%, followed by constipation 15.6% and SBP 12.2%. Together, infections, non-SBP and SBP, comprised 38.9% of these factors (Figure 2).
Figure 2.
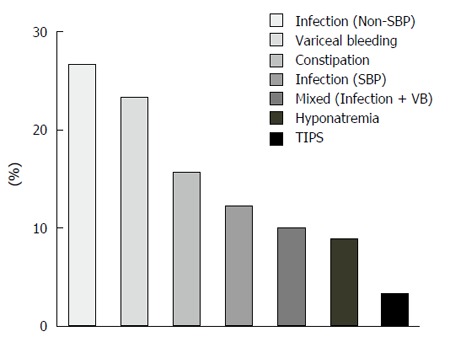
Precipitating factors of hepatic encephalopathy in the cohort. SBP: Spontaneous bacterial peritonitis; VB: Variceal bleeding.
The precipitating factors were then evaluated to search for differences between the group with low PhA and normal PhA; in the group with low PhA infections were higher (26.3% vs 16.3%), and specifically from the group of patients with SBP as precipitating factor 70% had low PhA, although this did not reach statistical significance (P = 0.332 and P = 0.166).
For the multivariate analysis a stepwise Cox regression using Backward elimination method for variables with P values > 0.05 was used; several variables known to be associated to HE were initially included in the univariate analysis their association to the development of HE where PhA, sodium, hemoglobin, ammonia and creatinine were statistically significant; however, after multivariate adjustment, only PhA and creatinine remained independently associated to HE (Table 2).
Table 2.
Characteristics associated with hepatic encephalopathy in the Cox regression model
| Univariate analysis | HR | 95%CI | P value | β |
| Phase angle (≤ 4.9°) | 1.597 | 1.081-2.358 | 0.019 | 0.468 |
| Sodium (mEq/L) | 0.941 | 0.906-0.977 | 0.002 | -0.061 |
| INR | 2.270 | 1.154-4.465 | 0.018 | 0.820 |
| Hemoglobin (g/dL) | 0.897 | 0.836-0.962 | 0.002 | -0.109 |
| Ammonia (μ/dL) | 1.006 | 1.002-1.010 | 0.005 | 0.006 |
| Creatinine (mg/dL) | 4.112 | 1.954-8.654 | 0.000 | 1.414 |
| Total bilirubin (mg/dL) | 1.031 | 0.966-1.100 | 0.362 | 0.030 |
| Multivariate analysis | ||||
| Phase angle (≤ 4.9°) | 1.806 | 1.076-3.031 | 0.025 | 0.591 |
| Sodium (mEq/L) | 0.957 | 0.912-1.003 | 0.065 | -0.044 |
| Creatinine (mg/dL) | 4.116 | 1.573-10.767 | 0.004 | 1.415 |
2-Log-likelihood: Block 0 = 557.59, Block 1 = 540.42, P = 0.000. HR: Hazard ratio; INR: International normalized ratio.
DISCUSSION
The importance of nutritional status in cirrhosis has been widely recognized; it was included in the original description of the Child-Turcotte-Pugh score, and, since then, the influence of nutritional status in cirrhosis and its complications has been studied in different clinical scenarios[18].
Through the years, the need for improved methods for the nutritional assessment in patients with cirrhosis has become evident, mainly due to the bias induced by fluid retention and decreased hepatic synthetic function[19].
In this study, we defined malnutrition, and specifically cachexia, based on phase angle, a direct marker obtained from BIA, previously validated as a marker of nutritional status related to mortality in cirrhosis[17].
The use of this nutritional marker has some advantages over other available methods including reproducibility, ease of use, low cost and accuracy. Low values of PhA have been linked to disrupted cell membrane and cell death, as well as changes in hydration status in different clinical settings[11,12]. Based on these facts, in patients with cirrhosis and HE, a low PhA might reflect low-grade edema and astrocyte swelling[20]. On the other hand, a low PhA translates directly into poor nutritional status, indicating mainly cachexia. The importance of recognizing cachexia in cirrhosis resides in the three components inherent to this condition: loss of muscle and adipose tissue as well as inflammation. Both sarcopenia and inflammation are important pathophysiological mechanisms related to the development and progression of HE[21]. In this cohort of patients, anthropometric measurements such as triceps skinfold thickness and mid-arm muscle circumference showed no difference between the patients with and without hepatic encephalopathy.
The results of our study show an association between the presence of cachexia and the subsequent development of HE. There are no studies showing the long-term effect of cachexia and the risk of developing HE, although malnutrition and HE has been evaluated in some studies. One cross-sectional study found an association between the presence of HE and muscle depletion, using handgrip muscle strength and other nutritional markers[21-23]. Another study found higher proportion of HE in patients with sarcopenia evaluated through computed tomography (CT) compared to patients without sarcopenia (60% vs 49%, P = 0.1), however, no follow-up data was available[24].
Malnutrition could influence the development of HE in different ways. The brain, liver, gut and muscle play an important role in maintaining ammonia metabolism in cirrhosis and might be directly affected by malnutrition[2]. For instance, the muscle function could be damaged since ammonia scavengers through the conversion from ammonia and glutamate into glutamine by the enzyme GS. The disruption of the intestinal barrier in cirrhosis and further increase in intestinal permeability and endotoxin levels, are involved in increased cytokine production, inflammation, and risk of infections[25]. Cachexia has been linked to dysbiosis which can further contribute to increased ammonia production, damage to the intestinal epithelial cells, and increased inflammation, ultimately leading to HE[26].
The main precipitating factors of HE episodes in this cohort were infections, followed by variceal bleeding. It has been established that malnutrition in any condition is related to a higher incidence of infections[27], thus, it can very likely be a possible explanation of the higher incidence of HE in malnourished patients, besides altered ammonia detoxification.
In the univariate analysis, biochemical parameters including sodium, INR, hemoglobin, ammonia and creatinine, as well as PhA were significantly related to the development of HE, and after backward multivariate adjustment, only creatinine and PhA remained significant. Altogether these data support the long-term effect of malnutrition in the development of HE.
The main benefits of this study are the prospective design and the long-term follow-up, which is essential to evaluate the causal effect of cachexia in the development of hepatic encephalopathy, thus it is not only a cross-sectional association, but also a validated and easily reproducible nutritional marker. There were also limitations in this study, individual components of malnutrition (fat mass, muscle mass) cannot be specifically addressed, since only the direct measurements were used, due to the limitations of conventional BIA using prediction equations to calculate indirect markers. Likewise, the cut-off value is specific of our population; therefore it needs to be tested in different populations.
In our cohort of patients low PhA was associated with an increased incidence of HE. PhA is a useful nutritional marker that evaluates cachexia, and could be used as a part of the integral assessment in patients with cirrhosis. Whether improving nutritional status can prevent or delay the development of episodes of HE warrants further investigation.
ACKNOWLEDGMENTS
The authors would like to thank Frau. Dr. Johanna Reissing for her support during the writing of the manuscript. Astrid Ruiz-Margáin and Ricardo Macias-Rodríguez were supported by CONACYT/UNAM. Ricardo Macias Rodriguez was supported by Fundación para la Salud y Educación "Salvador Zubirán". Francisco Javier Cubero is a Ramón y Cajal Researcher (RYC-2014-15242).
COMMENTS
Background
Malnutrition is seen in 40%-90% of the patients with cirrhosis and has been associated to the progression of complications and higher mortality. Some mechanisms such as oxidative stress and systemic inflammation that are involved in the pathophysiology of hepatic encephalopathy (HE) are increased in patients with malnutrition, therefore malnutrition could be associated to HE.
Research frontiers
Some studies have shown the cross-sectional association between malnutrition and HE, however, the long-term effect of malnutrition on the subsequent development of HE has not been fully addressed. Furthermore there is a growing need of a reliable method, capable of easily diagnosing malnutrition that allows multiple measurements over time and that is also related to prognosis.
Innovations and breakthroughs
In this manuscript we propose phase angle as a nutritional marker that reflects malnutrition and specifically cachexia. The authors were able to demonstrate that phase angle has the ability to predict the development of HE. Phase angle is an easily accessible marker that is obtained from a bedside method; therefore, it is useful even in patients with high grades of HE, which is not achieved by the conventional methods of nutritional assessment.
Applications
Phase angle could be easily implemented both in outpatients and hospitalized patients with cirrhosis given that it is derived from a portable, non-invasive method which makes it completely suitable for daily clinical practice; it can be performed safely as often as needed with a very low cost compared to other accurate methods such as computed tomography-scan. And finally this marker could be part of the medical and nutritional follow-up helping to evaluate the effect of any dietary or nutritional interventions.
Terminology
In physical science, phase angle is defined as the arc tangent of the reactance and resistance obtained with bioelectrical impedance. In clinical terms, phase angle is a nutritional prognostic marker that is related to the integrity of cell membranes and tissue quality. Cachexia can be defined as loss of muscle and fat mass, accompanied by a pro-inflammatory component present in chronic diseases.
Peer-review
This manuscript addresses a clearly defined and relevant issue in the field of Gastroenterology, the process of malnutrition in general, and cachexia in particular and its association with hepatic encephalopathy. The focus is on the reliability of phase angle data directly generated through a noninvasive, cost-effective, reproducible method being able to predict successfully the development of HE which is an improvement over the current situation in managing these patients.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study approved by the Institutional review board statement.
Clinical trial registration statement: This study was registerted in ClinicalTrials.gov with reference No. NCT02023177, found in the URL: https://ClinicalTrials.gov/ct2/show/NCT02023177?term=aldo+toore&rank=7.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Data sharing statement: No additional data are available.
Peer-review started: August 9, 2016
First decision: September 21, 2016
Article in press: October 30, 2016
P- Reviewer: He ST, Xavier-Elsas P S- Editor: Qi Y L- Editor: A E- Editor: Liu WX
References
- 1.Córdoba J. New assessment of hepatic encephalopathy. J Hepatol. 2011;54:1030–1040. doi: 10.1016/j.jhep.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437–447. doi: 10.1016/j.jhep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Macías-Rodríguez RU, Duarte-Rojo A, Cantú-Brito C, Sauerbruch T, Ruiz-Margáin A, Trebicka J, Green-Gómez M, Díaz Ramírez JB, Sierra Beltrán M, Uribe-Esquivel M, et al. Cerebral haemodynamics in cirrhotic patients with hepatic encephalopathy. Liver Int. 2015;35:344–352. doi: 10.1111/liv.12557. [DOI] [PubMed] [Google Scholar]
- 4.Rolland Y, Abellan van Kan G, Gillette-Guyonnet S, Vellas B. Cachexia versus sarcopenia. Curr Opin Clin Nutr Metab Care. 2011;14:15–21. doi: 10.1097/MCO.0b013e328340c2c2. [DOI] [PubMed] [Google Scholar]
- 5.Görg B, Qvartskhava N, Bidmon HJ, Palomero-Gallagher N, Kircheis G, Zilles K, Häussinger D. Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology. 2010;52:256–265. doi: 10.1002/hep.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plauth M, Schütz ET. Cachexia in liver cirrhosis. Int J Cardiol. 2002;85:83–87. doi: 10.1016/s0167-5273(02)00236-x. [DOI] [PubMed] [Google Scholar]
- 7.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 8.Duarte-Rojo A, Torres-Vega MA, Villamil-Ramírez H, Estradas J, Domínguez-López A, Sánchez-Muñoz F, Orea-Tejeda A, Castillo-Martínez L, Miliar-García A, Macías-Rodríguez RU, et al. Changes in peripheral blood mononuclear cells glutamine synthetase mRNA after exercise in healthy volunteers: exploring an alternative proposal for non hepatic ammonia metabolism. Rev Invest Clin. 2012;64:164–172. [PubMed] [Google Scholar]
- 9.Adeva MM, Souto G, Blanco N, Donapetry C. Ammonium metabolism in humans. Metabolism. 2012;61:1495–1511. doi: 10.1016/j.metabol.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Dumas JF, Goupille C, Julienne CM, Pinault M, Chevalier S, Bougnoux P, Servais S, Couet C. Efficiency of oxidative phosphorylation in liver mitochondria is decreased in a rat model of peritoneal carcinosis. J Hepatol. 2011;54:320–327. doi: 10.1016/j.jhep.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner RN, Chumlea WC, Roche AF. Bioelectric impedance phase angle and body composition. Am J Clin Nutr. 1988;48:16–23. doi: 10.1093/ajcn/48.1.16. [DOI] [PubMed] [Google Scholar]
- 12.Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;86:509–516. doi: 10.1007/s00421-001-0570-4. [DOI] [PubMed] [Google Scholar]
- 13.Barbosa-Silva MC, Barros AJ. Bioelectrical impedance analysis in clinical practice: a new perspective on its use beyond body composition equations. Curr Opin Clin Nutr Metab Care. 2005;8:311–317. doi: 10.1097/01.mco.0000165011.69943.39. [DOI] [PubMed] [Google Scholar]
- 14.Stobäus N, Pirlich M, Valentini L, Schulzke JD, Norman K. Determinants of bioelectrical phase angle in disease. Br J Nutr. 2012;107:1217–1220. doi: 10.1017/S0007114511004028. [DOI] [PubMed] [Google Scholar]
- 15.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 16.Child CG, Turcotte JG. Surgery and portal hypertension. In: The liver and portal hypertension., editor. Edited by CG Child. Philadelphia: Saunders; 1964. pp. 50–64. [Google Scholar]
- 17.Ruiz-Margáin A, Macías-Rodríguez RU, Duarte-Rojo A, Ríos-Torres SL, Espinosa-Cuevas Á, Torre A. Malnutrition assessed through phase angle and its relation to prognosis in patients with compensated liver cirrhosis: a prospective cohort study. Dig Liver Dis. 2015;47:309–314. doi: 10.1016/j.dld.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 19.Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chavarria L, Alonso J, García-Martínez R, Simón-Talero M, Ventura-Cots M, Ramírez C, Torrens M, Vargas V, Rovira A, Córdoba J. Brain magnetic resonance spectroscopy in episodic hepatic encephalopathy. J Cereb Blood Flow Metab. 2013;33:272–277. doi: 10.1038/jcbfm.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merli M, Giusto M, Lucidi C, Giannelli V, Pentassuglio I, Di Gregorio V, Lattanzi B, Riggio O. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28:281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 22.Sörös P, Böttcher J, Weissenborn K, Selberg O, Müller MJ. Malnutrition and hypermetabolism are not risk factors for the presence of hepatic encephalopathy: a cross-sectional study. J Gastroenterol Hepatol. 2008;23:606–610. doi: 10.1111/j.1440-1746.2007.05222.x. [DOI] [PubMed] [Google Scholar]
- 23.Kalaitzakis E, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Jalan R, Björnsson E. Malnutrition and diabetes mellitus are related to hepatic encephalopathy in patients with liver cirrhosis. Liver Int. 2007;27:1194–1201. doi: 10.1111/j.1478-3231.2007.01562.x. [DOI] [PubMed] [Google Scholar]
- 24.Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, Beaumont C, Tandon P, Esfandiari N, Sawyer MB, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20:640–648. doi: 10.1002/lt.23863. [DOI] [PubMed] [Google Scholar]
- 25.Norman K, Pirlich M, Schulzke JD, Smoliner C, Lochs H, Valentini L, Bühner S. Increased intestinal permeability in malnourished patients with liver cirrhosis. Eur J Clin Nutr. 2012;66:1116–1119. doi: 10.1038/ejcn.2012.104. [DOI] [PubMed] [Google Scholar]
- 26.Pirlich M, Norman K, Lochs H, Bauditz J. Role of intestinal function in cachexia. Curr Opin Clin Nutr Metab Care. 2006;9:603–606. doi: 10.1097/01.mco.0000241671.09676.d8. [DOI] [PubMed] [Google Scholar]
- 27.Chandra RK. Nutrition, immunity and infection: from basic knowledge of dietary manipulation of immune responses to practical application of ameliorating suffering and improving survival. Proc Natl Acad Sci USA. 1996;93:14304–14307. doi: 10.1073/pnas.93.25.14304. [DOI] [PMC free article] [PubMed] [Google Scholar]


