Abstract
Chronic liver disease is a major cause of morbidity and mortality worldwide and usually develops over many years, as a result of chronic inflammation and scarring, resulting in end-stage liver disease and its complications. The progression of disease is characterised by ongoing inflammation and consequent fibrosis, although hepatic steatosis is increasingly being recognised as an important pathological feature of disease, rather than being simply an innocent bystander. However, the current gold standard method of quantifying and staging liver disease, histological analysis by liver biopsy, has several limitations and can have associated morbidity and even mortality. Therefore, there is a clear need for safe and non-invasive assessment modalities to determine hepatic steatosis, inflammation and fibrosis. This review covers key mechanisms and the importance of fibrosis and steatosis in the progression of liver disease. We address non-invasive imaging and blood biomarker assessments that can be used as an alternative to information gained on liver biopsy.
Keywords: Hepatic steatosis, Fibrosis, Non-invasive assessment, Blood biomarker, Ultrasound
Core tip: Ongoing hepatic fibrosis and steatosis are well recognised features of chronic liver disease. Liver biopsy is currently the gold standard for assessing the disease although this has an associated but low morbidity and mortality risk. Therefore, alternative methods of non-invasive assessment of liver disease are of relevance and importance. We outline the mechanisms of hepatic fibrosis and steatosis and review uses of non-invasive imaging and blood biomarkers as an alternative to liver biopsy.
CLINICAL PROBLEM
Chronic liver disease is a major cause of morbidity and mortality worldwide. The presence of chronic inflammation and consequent fibrosis leads to the development of cirrhosis and its complications. Whilst the exact prevalence of chronic liver disease is unknown, cirrhosis of the liver was attributed for more than one million deaths worldwide in 2010, although these figures probably reflect heavy under-reporting[1]. The total worldwide prevalence of cirrhosis has been estimated at around 1% with significant regional variation owing to the presence of viral hepatitis, the metabolic syndrome and alcohol consumption[2].
Chronic liver disease has a varied aetiology, including viruses, such as hepatitis B (HBV) and hepatitis C (HCV). Worldwide, over half a billion people may be chronically infected with either of these viruses[3,4]. Metabolic causes include the increasing prevalence of non-alcoholic fatty liver disease (NAFLD). Toxic causes, such as excess alcohol consumption, aflatoxin exposure[5,6] and autoimmune disorders, such as primary biliary cirrhosis and autoimmune hepatitis, contribute to the disease burden. Over half of the deaths attributable to cirrhosis and nearly 80% of those attributable to primary liver cancers occur in those who have chronic HBV and HCV infection[7], while in many developed nations excess alcohol consumption is the commonest cause. In the developing world, aflatoxin exposure further complicates the picture, leading to high rates of hepatocellular carcinoma (HCC).
Chronic liver disease usually takes many years to progress from inflammation, associated with hepatocyte injury, to fibrosis and mostly requires long-term exposure to the causative agent. Progressive scarring or fibrosis develops during the period of time between initiation and end-stage disease. The resulting pre-cirrhotic fibrosis is a target for therapies aimed at reducing the rate of progression to cirrhosis, or even reversal of fibrosis[8].
Effective antiviral therapies and the advent of antifibrotic drugs have led to increasing demand for non-invasive, accurate and reliable biomarkers of hepatic disease severity. It is well recognised that the current “gold standard”, histological analysis of liver biopsy, has limitations and engenders risk to the patient. Sampling variability and the subjective interpretation of scoring systems means that the consistency and representation of the true disease state is questionable. The procedure frequently causes discomfort and if the patient has a malignancy, there is a risk of tumour seeding[9]. Furthermore, there is frequent associated morbidity and a small, but significant, mortality rate[10] in all but a few cases. Liver biopsy is rarely performed in lower income countries, often due to a lack of expertise to interpret results[11]. On the other hand, in the United States and the developed world, magnetic resonance techniques allow a quantitative assessment of different aspects of disease from metabolic markers of inflammation, through to assessment of fibrotic load, markers of portal hypertension and prognostic indicators of the complications of cirrhosis[12]. There is a clear need for reliable and effective non-invasive markers of hepatic inflammation and fibrosis, both for the management of individual patients and for the development of new anti-fibrotic therapies. Novel imaging modalities and non-invasive biomarkers have the potential to fulfil this role and offer significant benefit in treatment monitoring and have the potential also to be of use in resource-constrained settings.
NATURAL HISTORY OF CHRONIC LIVER DISEASE
Chronic liver injury leads to initiation and perpetuation of inflammatory processes, which, by a cascade of inter-related processes and pathways, leads to deposition of fibrous tissue (Figure 1). By convention, fibrosis has been considered potentially reversible, while the end-stage of the pathological process, cirrhosis, has been considered irreversible. However, with elimination of the cause of liver injury, a number of studies have demonstrated regression of all stages of fibrosis in animal models and in humans[13-17]. Elucidation of the process of fibrogenesis enables markers of disease severity and potential targets for therapeutic intervention to be developed.
Figure 1.
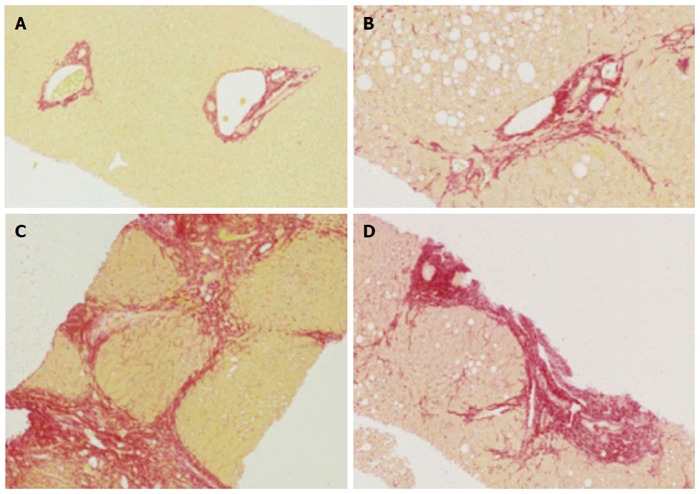
Histology of normal liver, fibrosis and cirrhosis. A: Representative histological images (using Sirius red staining), normal liver; B: Mild to moderate fibrosis with portal tract expansion (METAVIR F = 2, Ishak stage 3); C: Moderate “bridging” fibrosis (METAVIR F = 3, Ishak stage 4); D: Cirrhosis (METAVIR F = 4, Ishak 5 or 6).
KEY MECHANISMS OF FIBROGENESIS
Fibrosis is a dynamic process of hepatic homeostasis mediated by several cellular mediators in response to an inflammatory process. In particular, hepatic stellate cells (HSC) have a central role in the pathogenesis of liver fibrosis[8,18]. These cells comprise 15% of liver cell mass[19]. HSCs are activated following liver injury from a relatively quiescent lipid and vitamin A-storing phenotype to a myofibroblastic phenotype, capable of proliferation, contraction and fibrogenesis. However, other myofibroblastic cell populations have also been shown to be involved in fibrogenesis, including portal fibroblasts[20,21] .
HSC activation occurs in two stages: initiation and perpetuation and each involves characteristic pathways, as described by Friedman[18]. Initiating events may consist of any chronic perturbation of hepatic homeostasis, which is often, but not always associated with the presence of inflammatory cells on liver biopsy. Such perturbations of hepatic homeostasis may lead to the net over-production of unstable reactive oxygen species (ROS) derived from hepatocytes, macrophages, stellate cells and inflammatory cells. ROS are potent mediators of the initiation and perpetuation of liver injury and cause lipid peroxidation of cellular membranes. Perpetuation of HSC activation is comprised of a number of cellular responses, which lead to increased expression and responsiveness to growth factors. Perpetuation is a dynamic process, occurring along a number of pathways associated with different HSC responses. These responses include release of largely proinflammatory cytokines, proliferation and increased contractility of HSCs, chemotaxis of inflammatory cells and the net deposition of pathological ECM[18].
Resolution of fibrosis
For resolution of fibrosis to occur, removal of the injurious agent is a prerequisite. Activation of HSCs has been shown to be crucial for the development of fibrosis, while in recovery and resolution of fibrosis, the number of activated HSCs is reduced. The latter may occur as a result of reversion to the quiescent form or by apoptosis. A return to the quiescent form has been observed in vitro[22], but not in vivo. Apoptosis has been postulated as the predominant mechanism for removal of HSC activity. It has been shown that spontaneous apoptosis of HSCs occurs in vitro. Conversely, it has also been shown that MMP-2 levels correlate with apoptosis and may be stimulated by apoptosis[23] and that TIMP-1, by inhibition of MMP, leads to inhibition of activated HSC apoptosis[24].
HEPATIC STEATOSIS
Liver fibrosis is the key pathological feature of progressive liver disease. However, the accumulation of excessive hepatic triglyceride, hepatic steatosis, is increasingly recognised as an important factor in the pathogenesis of a number of chronic liver diseases, not simply an “innocent bystander”.
Definitions
Hepatic steatosis is a pathological lesion, defined as the presence of large and small vesicles of fat, predominantly triglycerides, accumulating within hepatocytes[25]. Hepatic steatosis is frequently associated with obesity, insulin resistance and dyslipidaemia in those who do not consume excessive quantities of alcoholic drinks, where it is termed non-alcoholic fatty liver disease[26] (NAFLD). Hepatic steatosis may also be a result of secondary causes, which include alcoholism, HCV, severe weight loss, total parenteral nutrition and drugs (such as amiodarone, diltiazem, tamoxifen, steroids and highly active antiretroviral therapy)[27]. NAFLD may thus be considered a syndrome of various aetiologies, excluding alcohol and, by convention, HCV.
Epidemiology
In a systematic review on the epidemiology of NAFLD, Vernon and colleagues reported the prevalence of NAFLD in the United States ranges from 10%-35% in published studies, depending on the investigative assessment modality used and the study population[1]. Worldwide, NAFLD prevalence ranges from 6%-35% with a median of 20%[1].
Key issues in pathophysiology
The development of hepatic steatosis occurs when the rate of synthesis or import of fatty acids by hepatocytes exceeds the rate of export or catabolism. Such an imbalance can occur in a number of ways and is summarised in Figure 2 with increased uptake of fatty acids by hepatocytes in obesity, increased hepatic fatty acid triglyceride synthesis, impaired fatty acid mitochondrial β-oxidation and reduced VLDL and triglyceride synthesis all being components to consider to a greater or lesser extent[28]. Importantly, with respect to hepatic triglyceride export, HCV core protein has been shown to inhibit this process, providing a direct route to hepatic steatosis in HCV infection[29,30].
Figure 2.
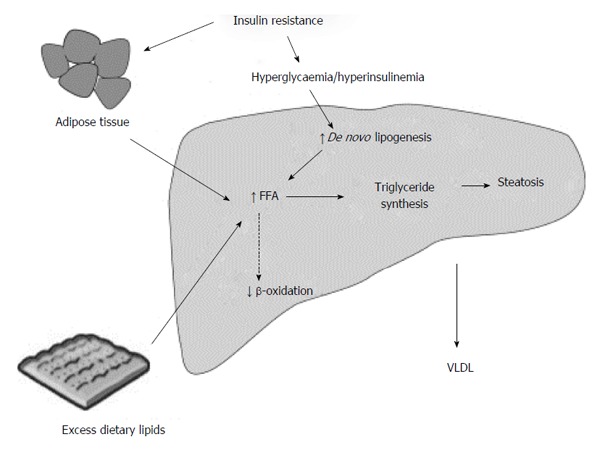
Summary of metabolic mechanisms leading to hepatic steatosis. Reproduced from Dowman et al[155] with permission from Oxford University Press.
CURRENT APPROACHES TO THE ASSESSMENT OF CHRONIC LIVER DISEASE
The evaluation of patients with chronic liver disease is largely based on assessment of the clinical history, physical examination and measurement of non-specific liver enzymes. Most pre-cirrhotic chronic liver disease is asymptomatic, where clinical signs are subtle and non-specific. Clinical chemistry, so-called “liver function tests” (aminotransferases, alkaline phosphatase, bilirubin and gamma-glutamyl transferase) and haematological indices (cell-counts and the prothrombin time) may be abnormal in cirrhotic and pre-cirrhotic disease, but alone, their sensitivity and specificity for the diagnosis and staging of chronic liver disease is limited[2].
Liver biopsy - the “gold standard” for disease assessment
Histological assessment of liver biopsy is the mainstay for the diagnosis and staging of chronic liver disease. Epidemiological and pathophysiological data support fibrosis being the hallmark of chronic liver disease and a predictor of outcome[31]. Most liver biopsies for the assessment of chronic liver disease are performed percutaneously, by passing the biopsy needle between the ribs into the right lobe of the liver. Guidelines for safe and effective liver biopsy have been published by the British Society of Gastroenterology[32].
Validity of histological scoring systems
Fibrosis and inflammation: The interpretation of the histology of liver tissue in chronic liver diseases is based on categorisation or scoring of inflammatory features (grade) and fibrosis and architectural disruption (stage)[33]. The first major scoring system was developed in 1981, and based on the identification of periportal necrosis, intralobular necrosis, portal inflammation and fibrosis, assessed separately and assigned a score[34], although it was later modified to include additional components[35]. The first three components of the score contribute to the necroinflammatory grade, while the fibrosis score, from 0-6, is based on architectural changes in the pattern and expansion of fibrous bands. From a practical perspective, cirrhosis is represented by both stage 5 (incomplete cirrhosis) and 6 (probable or definite cirrhosis).
The METAVIR scoring system was developed to look specifically at HCV-related liver disease. The histological activity is based on piecemeal and lobular necrosis, while the fibrosis stage is scored from 0-4, with 4 representing cirrhosis[36]. All scoring systems are designed to place a numeric value to architectural features. They consist of ordered categorical data representing qualitative and semi-quantitative descriptions, so are not quantitative measures of fibrosis. Studies of liver tissue using digital image analysis have shown that the area of fibrous tissue is not linearly related to the fibrosis score. Moreover, inflammation may cause expansion of fibrous tracts[33]. Nevertheless, histological fibrosis assessment has been validated by successive clinical studies, making the features clinically relevant[31].
Steatosis: Scoring systems quantify fat on the basis of visible hepatic lipid droplets within hepatocytes. The grade of steatosis is based on the proportion of hepatocytes containing visible lipid and is expressed semi-quantitatively on a scale of 0-3 (0, < 5%; 1, 5%-33%; 2, > 33%-66%; 3, > 66%)[37,38]. Grading is considered in the context of other histopathological lesions, such as inflammatory infiltration and ballooning of hepatocytes. However, there are considerable limitations to liver biopsy for the quantification of hepatic lipid. Quantification of lipid is based only on visible lipid droplets, so invisible (for example, membrane) lipid is not included. The assessment is only semi-quantitative, being a two-dimensional estimation of the proportion of hepatocytes including lipid, and not considering the volume of droplets. Finally, the composition of the fatty acid components is not assessed, although, using immunohistochemical techniques, the presence of lipid peroxidation products may be determined[39].
Limitations of liver biopsy
Morbidity and mortality: Percutaneous liver biopsy has a small, but quantifiable, risk of mortality, quoted as between 1 in 1000 and 1 in 10000 patients[10,40,41]. Minor complications, such as post-procedural pain or localised haematoma, occur in between 3% and 30% of cases. More severe complications, including intraperitoneal haemorrhage (requiring transfusion) or perforation of a viscus (including pneumothorax) may occur in 0.3% to 0.6% of cases[32,42]. Tumour seeding following biopsy of suspected carcinomas in cirrhosis may also occur in 2.7% of cases[43]. Good management of clotting disorders and the appropriate use of the transjugular approach for liver biopsy may improve outcome[32].
Sampling variability: Percutaneous liver biopsy typically samples less than 1/50000th of the liver, so any heterogeneity of pathological features may lead to sampling variability[44]. Autopsy studies have demonstrated that cirrhosis may be missed on a single pass liver biopsy in between 10% and 30% of cases[45,46], while a study using laparoscopic biopsy of both left and right lobes of the liver found a difference of at least one fibrosis stage between lobes in over 30% of patients[47]. The size of the liver biopsy specimen influences sampling variability. Smaller biopsy sizes (in length and breadth) were shown to lead to a lower probability of observing characteristics of more severe diseases and, consequently, led to underestimation of disease severity[48].
Subjectivity and inter-observer variation: Histological scoring systems are designed to be objective and reproducible, but interpretation is still a source of error. Inter-observer variability is low for the assessment of fibrosis, but higher for the assessment of activity or inflammation[49]. In a study of intra- and inter-observer variability, agreement was better for fibrosis than inflammation and also amongst experienced pathologists than more junior pathologists[50]. These authors concluded that the experience of the pathologist had more influence on agreement than the characteristics of the biopsy itself. Histology of liver biopsy specimens is still the mainstay for the definitive diagnosis of liver diseases and for investigation of co-existing pathology. Nevertheless, there are risks inherent in the technique and scoring systems should be interpreted in the knowledge of the limitations.
NON-INVASIVE TECHNIQUES
Non-invasive assessment techniques are suited to longitudinal studies as the risk to patients is more limited than liver biopsy. However, the measured parameters may be more susceptible to influence by confounding factors and so specificity is important to consider. Non-invasive tests of chronic liver disease may be broadly divided into serum (or blood) markers and imaging-based technologies.
Serum markers
Serum markers have been studied in detail to detect early fibrotic changes as blood tests are quick and acceptable to patients. While the results are objective, there is the possibility of the presence of confounding factors from extrahepatic disease. These have been reviewed extensively[51-53]. Serum markers may broadly be divided into “indirect” and “direct” markers, single tests and panels. Indirect markers are markers of liver function, which reflect liver fibrosis, while direct markers include serum extracellular matrix components and intermediates of fibrogenesis[54]. The strengths and limitations of the most widely used models are summarized below.
APRI
APRI (AST to Platelet Ratio Index) was proposed as an alternative to biopsy in patients with chronic HCV infection[55] and it is calculated as (AST/upper limit of normal range)/platelet count (109/L) × 100. A recent meta-analysis by Lin and colleagues showed APRI had AUROC scores for the diagnosis of significant fibrosis, severe fibrosis, and cirrhosis of 0.77, 0.80, and 0.83 respectively, demonstrating a potential use for identifying HCV-related fibrosis[56]. However, APRI fails to identify a significant proportion of people in the earlier stages of fibrosis and therefore is limited in its ability to identify only significant and untreated chronic HCV-related fibrosis[55,57].
Enhanced liver fibrosis score
The enhanced liver fibrosis score (ELF®) score (Siemens, Munich, Germany) combines three direct markers of fibrosis including hyaluronic acid (a component of the extracellular matrix), TIMP-1 (an inhibitor of matrix metalloproteinases, which break down collagen) and PIINP (a marker of collagen synthesis at disease site). Therefore, the premise of this scoring system is that a higher score will indicate a higher rate of fibrogenesis. ELF® has been shown to have good performance for the detection of significant fibrosis in chronic HCV (93% sensitivity and 83% specificity)[58] but also in NAFLD (sensitivity 89% and specificity 96%) and ALD (100% sensitivity and 16.7% specificity)[59], although results for the latter two have been less rigorously evaluated. Results also need to be adjusted appropriately as scores can be influenced by gender, age, and sex[60].
FibroTest®
FibroTest® (BioPredictive, Paris, France) uses five different serum markers in its model and has been validated in meta-analysis in multiple aetiologies including NAFLD (AUROC 0.84; 95%CI: 0.76-0.92), alcohol-related liver disease (AUROC 0.86; 95%CI: 0.80-0.92) and both chronic HBV infection (AUROC 0.80; 95%CI: 0.77-0.84) and HCV infection (AUROC 0.85; 95%CI: 0.82-0.87)[61]. However, results are limited by false-positive results, attributed to increases in bilirubin or decreases in haptoglobin; in particular HCV patients on ribavirin. Results can also be affected by acute inflammation, Gilbert’s syndrome and cholestasis[52].
FIB-4 index: The FIB-4 index combines several markers of liver function into the following formula: age (years) × AST [U/L]/(platelets [109/L] × (ALT [U/L]). The FIB-4 index was specifically developed as an alternative to biopsy in patients with chronic HCV infection, although it has shown use in other causes of liver disease. In a study of 529 HCV-infected patients, the FIB-4 index enabled the correct identification of patients with severe fibrosis (F3-F4) and cirrhosis with an Area under Receiver Operated Curve (AUROC) of 0.85 (95%CI: 0.82-0.89) and 0.91 (95%CI: 0.86-0.93), respectively[62]. However, a lack of universal agreement amongst studies for positive and negative cut-off values has proved problematic.
Forns index: The Forns index is another formula that assesses liver function by combining age, cholesterol, gamma-glutamyltranspeptidase and platelet count. Forns and colleagues showed using a best cut-off score of < 4.2, the presence of significant fibrosis (F2-F4) could be excluded with high accuracy (negative predictive value of 96%) in 125 (36%) of 351 patients with chronic HCV infection[63]. The Forns index has also been shown to be more accurate than other serum markers including FIB-4 and aspartate APRI (AUROC 0.795, 0.764 and 0.774 respectively) in the prediction of significant fibrosis in patients with chronic HCV[64].
IMAGING-BASED MODALITIES AS ALTERNATIVES TO BIOPSY
Various imaging modalities have been proposed as alternatives to liver biopsy. Given that there is strong evidence that liver stiffness measurements (LSM) increases with the degree of fibrosis[65,66], the most successful imaging modalities have used techniques to measure liver stiffness and correlate this with the degree of fibrosis, most notably ultrasound-based transient elastography (TE) and acoustic radiation force impulse imaging (ARFI®, Siemens, Munich, Germany). Their potential use in the measurement of hepatic steatosis and differentiation of hepatic steatosis grade has also been investigated. The use of other modalities, including MR techniques, to assess morphological and biochemical changes in chronic liver disease (CLD) will also be discussed.
TE
TE was first developed as Fibroscan® (Echosens, Paris, France), where a vibrator generates low frequency shear waves through the liver which are then transmitted to an ultrasound receiver. The velocity of the waves is dependent on the tissue elasticity and therefore, the rate of propagation through the liver can be used as a measure of liver stiffness and converted into a numerical value (kPa). Meta-analyses have shown TE has a very high sensitivity and specificity for detecting cirrhosis, but its accuracy was much reduced in the earlier stages of fibrosis[67,68]. This is partly due to the lack of validation for stiffness cut-off values in earlier stages of disease, but also possibly due to the multiple processes that contribute to liver stiffness other than fibrosis[67,69]. Fibroscan® machines now have been calibrated to measure hepatic steatosis levels by using a novel Controlled Attenuation Parameter (CAP®; Echosens, Paris, France), with results showing a sensitivity and specificity between 78% and 100% and an excellent correlation between different steatosis grades, determined by the percentage of hepatocytes with fatty infiltration (Spearman Rank ρ = 0.81, P < 10-16)[70]. Its use has been validated in patients with chronic hepatitis C infection (AUROC 0.80; 95%CI: (0.75-0.84) for S ≥ 1, 0.86; 95%CI: 0.81-0.92 for S ≥ 2 and 0.88; 95%CI: 0.73-1.00 for S = 3) respectively and has shown correlation in patients withNAFLD (0.49, P = 0.00069) although this accuracy reduces with increasing steatosis grade in patients with NAFLD[71,72].
Acoustic radiation force impulse imaging®
Acoustic radiation force impulse imaging (ARFI)® (Siemens, Munich, Germany) is another form of ultrasound elastography that measures soft tissue displacement following the exposure of high-energy acoustic pulses. The measurement of displacement can then be quantified and interpreted as a measurement of liver stiffness. Meta-analysis by Friedrich-Rust and colleagues used AUROC to show ARFI® is effective with scores of 0.87 for discriminating significant fibrosis, 0.91 for severe fibrosis and 0.93 for cirrhosis[73]. Additionally, ARFI® has also shown comparable results with TE for detection of significant fibrosis and cirrhosis and was significantly more likely to obtain reliable measurements[74]. However, its uses for detecting earlier stages of fibrosis though remain limited.
ElastPQ®
ElastPQ® (Philips, Best, Netherlands) is a newer ultrasound method of non-invasive assessment of liver stiffness and uses two-dimensional shear wave elastography to generate an absolute measurement of liver stiffness. ElastPQ® showed comparable accuracy to ARFI® when differentiating 176 patients with and without chronic liver disease (83.7% vs 83.1%), although measurements of liver stiffness for ElastPQ® were significantly lower, compared to ARFI® and thus required different cut-off values[75]. Additionally, in a study of 291 patients with chronic HBV, who underwent biopsy or partial hepatectomy, ElastPQ® values showed good correlation between the stage of liver fibrosis and grade of necroinflammatory activity while being unaffected by levels of steatosis[76]. However, whilst ElastPQ® remains an exciting prospect, further comparisons with other non-invasive imaging modalities and liver biopsy are needed.
Standard ultrasound
Ultrasound is widely used clinically to detect hepatic steatosis, but it can also detect the vascular changes of chronic liver disease with contrast enhancement[77]. Ultrasound is also able to detects hepatic steatosis, based on the premise that steatosis causes increased echogenicity of the hepatic parenchyma, leading to a brighter image when compared to the cortex of the ipsilateral kidney[78]. Other conditions, such as fibrosis may also lead to increased echogenicity, resulting in a potential for confusion. A review of the non-invasive measurement of fat content found the sensitivity of ultrasound for the detection of steatosis to range from 60% to 94%, while the specificity ranged from 84% to 95%[79]. However, histologically-assessed mild steatosis resulted in a sensitivity of just 55%[80]. In addition, obesity reduces the accuracy of ultrasound due to technical considerations and increased attenuation of signal caused by subcutaneous fat. Ultrasound performs more poorly for the quantification of hepatic lipid, although subjective grading systems, categorizing steatosis into mild, moderate and severe groups have been proposed[81]. Dynamic microbubble contrast-enhanced studies are thought to exploit the intra- and extrahepatic vascular changes that generate shortening of hepatic vein transit times with increasing disease severity[82,83] (Figure 3).
Figure 3.
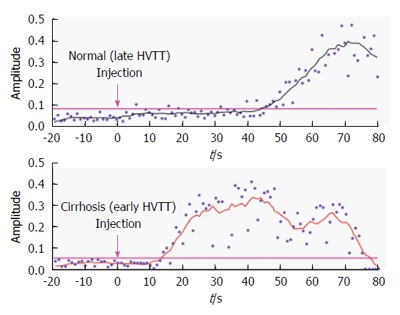
Hepatic vascular transit times in normal patients and patients with cirrhosis. Time intensity curves from the hepatic vein show earlier arrival of contrast in the cirrhotic liver[83].
Computed tomography
CT enables the assessment of hepatic steatosis on the basis of radiographic density[84], although the ability to quantify hepatic lipid is not clear[85]. Ionising radiation associated with CT-scanning confers a small excess risk to subjects, making it less suited for repeated measurements.
Magnetic resonance imaging
T1 and T2 mapping are MR techniques that can be used for in vivo tissue characterisation either individually or in combination. T1 relaxation times correlate with increased levels of extracellular fluid associated with inflammation and fibrosis, whereas T2 mapping primarily reflects the amount of iron deposition[86]. For example, T1 mapping of the liver has used a modified Look-Locker inversion recovery sequence with motion recovery[87] and T2 on multi-gradient-echo acquisition[88]. Since elevated iron levels interfere with T1 measurements, correction algorithms are applied to provide more accurate readings[86]. T1 mapping has shown good correlation with the histological degree of fibrosis in a cohort of 79 patients with chronic liver disease of multiple aetiologies with a ROC of 0.94 for any degree of fibrosis[88].
Additionally, Pavlides and colleagues have used T1 mapping to show a correlation with the degree of liver disease and the risk for developing clinical events in 112 patients. Optimal T1 cut-off points were created by comparing multiparametric MR data with histological staging of fibrosis from previous studies. This was used to create a “liver inflammation and fibrosis” (LIF) staging score. Pavlides subsequently found that patients with severe liver disease (LIF > 3) were at higher risk for developing clinical events, compared to patients with LIF < 1 (P = 0.02) and LIF 1-1.99 (P = 0.03)[86]. T1 mapping has also been shown to differentiate Child-Pugh A patients from Child-Pugh B/C patients effectively (P < 0.00001)[89]. T1 mapping is a promising diagnostic tool that has shown to be effective in differentiating different stages of fibrosis and also has shown potential for predicting clinical events. However, further research is needed to validate effective scoring systems and the influences of other compounding factors for it to become a valid alternative to liver biopsy in clinical practice.
Magnetic resonance elastography
While ultrasound-based transient elastography techniques such as Fibroscan® measure liver stiffness in a defined region (about 5 cm3, right lobe of the liver), Magnetic Resonance Elastography (MRE) can be used to predict fibrosis stage effectively in patients with chronic liver disease, while producing a wider and more representative map of liver stiffness in both 2D and 3D planes. This is performed using similar principles to TE, whereby propagating shear waves are generated and imaged using phase contrast MRI, which includes oscillating motion sensitising gradients (MSGs). The subsequent cyclic spin displacement of protons, which in the presence of synchronised MSGs, are encoded as phase shifts within the MRI signal[90].
MRE has been validated in multiple studies and meta-analyses[91-94]. One meta-analysis by Singh and colleagues using 12 retrospective studies, comprising 697 patients with CLD of varying aetiology, showed MRE had high diagnostic capability for detecting significant fibrosis (F ≥ 2), advanced fibrosis (F ≥ 3), as well as cirrhosis with ROC values of 0.88 (0.84-0.91), 0.93 (0.90-0.95), and 0.92 (0.90-0.94) respectively, as determined by liver biopsy[91]. Another meta-analysis by Su and colleagues, comprising 13 studies and 989 patients, also demonstrated good sensitivity and specificity of MRE for the staging of fibrosis. The pooled sensitivity and specificity for F ≥ 1, F ≥ 2, F ≥ 3 and F = 4 were 0.87 (95%CI: 0.84-0.89) and 0.92 (95%CI: 0.87-0.96), 0.87 (95%CI: 0.84-0.90) and 0.92 (95%CI: 0.89-0.95), 0.88 (95%CI: 0.85-0.91) and 0.91 (95%CI: 0.88-0.93), 0.91 (95%CI: 0.87-0.94) and 0.92 (95%CI: 0.89-0.94), respectively. The pooled ROC values for F ≥ 1, F ≥ 2, F ≥ 3 and F = 4 were 0.9502, 0.9663, 0.9644, and 0.9768, respectively[92]. MRE can also be used to stratify risk of cirrhosis progression in patients with chronic HCV infection[95] and may have greater potential than TE for assessment of NAFLD in patients with high risk of NASH or cirrhosis, due to the multiparametric nature of MRI which allows for a comprehensive assessment of the liver[96].
Recent studies have shown that three-dimensional spin-echo echoplanar imaging (3D-SE-EPI) could have better diagnostic accuracy than conventional two-dimensional gradient-recalled echo (2D-GRE). In a study of 179 patients with either chronic HBV or HCV infection, Shi and colleagues showed AUCs for the characterisation of F ≥ 1, F ≥ 2, F ≥ 3, and F = 4 were 0.957 (95%CI: 0.913-0.983), 0.971 (0.932-0.991), 0.991 (0.961-0.999), and 0.979 (0.942-0.995) for 3D-SE-EPI compared with the AUCs for 2D-GRE at each fibrosis stage which were 0.948 (0.901-0.977), 0.959 (0.915-0.981), 0.979 (0.943-0.995), and 0.976 (0.938-0.994) respectively[97]. A higher diagnostic accuracy for 3D-SE-EPI compared with 2D-GRE has also been shown for patients with NAFLD advanced fibrosis[98].
Proton magnetic resonance spectroscopy
Since a Magnetic Resonance Spectroscopy (1H MRS) spectrum of the liver is dominated by lipid and water resonances, 1H MRS has been used for the assessment of hepatic fat. The percentage liver fat has been estimated from the number of protons in the lipid and water resonances by calibration with hepatic lipid extracts[99]. Such measures have been used to assess racial differences in the prevalence of hepatic steatosis and the technique has also been applied in a population of over 2000 participants[100,101]. Simpler lipid-to-water resonance ratios have been used to compare hepatic steatosis between obese and lean individuals and have demonstrated a change in intrahepatic lipid in response to dietary intervention[102-104]. 1H MRS also has also been applied to the assessment of steatosis in living-donor liver transplantation[105] and for quantification of steatosis in HIV mono-infected individuals who are at greater risk of liver fibrosis and cirrhosis[106].
Proton density fat fraction
MRI techniques can also be used to calculate the proton density fat fraction (PDFF), a marker of hepatic steatosis, using MRS as a reference. PDFF is defined as the ratio of density of mobile protons from triglycerides and the total density of protons from mobile triglycerides and mobile water which then reflects the concentration of fat within that tissue in the absence of confounding factors[107]. PDFF has shown at least equivalence in accuracy for quantifying hepatic steatosis with both 1H MRS and with histological grade, across several studies with various aetiologies of chronic liver disease[108-112]. Additionally, in a retrospective study of data from 506 adults, PDFF estimation accuracy was not affected by age, sex and BMI with the authors concluding these confounders have a clinically negligible effect[113]. Further validation of these techniques would be of benefit as 1H MRS techniques are not widely available.
Phosphorus MRS
Phosphorus (31P) MRS currently remains a research tool and allows observation of metabolites associated with energy metabolism as well as membrane phospholipid turnover. The latter include phosphomonoesters (PME), thought broadly to represent membrane precursors including phosphoethanolamine, phosphocholine and phosphodiesters (PDE), which are thought to represent membrane degradation products including glycerophosphocholine and glycerophosphoethanolamine. Studies in chronic liver disease both in vitro[114,115] and in vivo[116,117] have demonstrated a correlation between the PME/PDE and disease state or severity (Figure 4). A study in patients with acute hepatitis A demonstrated an acute rise in PME/PDE, which decreased with resolution of disease[118]. Moreover, in patients with hepatitis C, the PME/PDE decreased significantly in those responding to antiviral treatment but did not change in non-responders[119].
Figure 4.
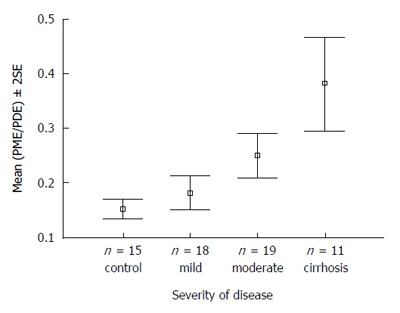
31P magnetic resonance spectroscopy of patients with increasing severity of liver disease vs controls. PME/PDE ratios obtained from in vivo hepatic 31P MRS correlating with severity of liver disease in patients with hepatitis C[116]. MRS: Magnetic resonance spectroscopy; PME: Phosphomonoester; PDE: Phosphodiester.
Combination techniques
Combinations of non-invasive techniques can be used to improve accuracy. Serum biomarker models can be combined to create more accurate algorithms, such as the Fibropaca algorithm, Leroy algorithm and SAFE biopsy, with some studies suggesting their use could reduce the number of liver biopsies by 79%[120]. However, the use of serum biomarkers in combination with imaging techniques has proved highly useful. One study of 183 patients with chronic HCV by Castera and colleagues demonstrated that a combination of serum FibroTest® and ultrasound-based Fibroscan® (TE) (the Bordeaux algorithm) showed an increased AUROC score for F2 (0.88 vs 0.83) and F3 (0.95 vs 0.90), compared to Fibroscan® alone, thus avoiding the need for biopsy in a large proportion of patients[121]. The Anger’s algorithm combines the serum biomarker model, Fibrometer® (Echosens, Paris, France) and ultrasound-based Fibroscan®. TE has also demonstrated good diagnostic accuracy and required significantly fewer biopsies than the Bordeaux algorithm for significant fibrosis (20.2% vs 28.6%, P = 0.02) and cirrhosis (9.3% vs 25.3%, P = 0.001)[122]. Most recently, the role of combination techniques as a cost-effective screening tool has been validated by Harman and colleagues in a community setting in Nottingham, United Kingdom (practice population 10479). High-risk patients were identified using risk factors for chronic liver disease and subsequently investigated them using a serial biomarker algorithm and liver stiffness measurement (Figure 5). Of the 504 identified as being high risk, 62 patients (12.3%) had normal biomarkers and were not further investigated. 378 patients then agreed to undergo TE which found 98 patients (26.8% of valid scans) had clinically significant fibrosis (defined as LSM < 8kPa). Most interestingly, 71/98 patients (72.4%) of these patients had normal liver enzymes and would have been otherwise missed by conventional algorithm models. This techniques also managed to identify 140% more patients with definite cirrhosis (n = 11)[123].
Figure 5.
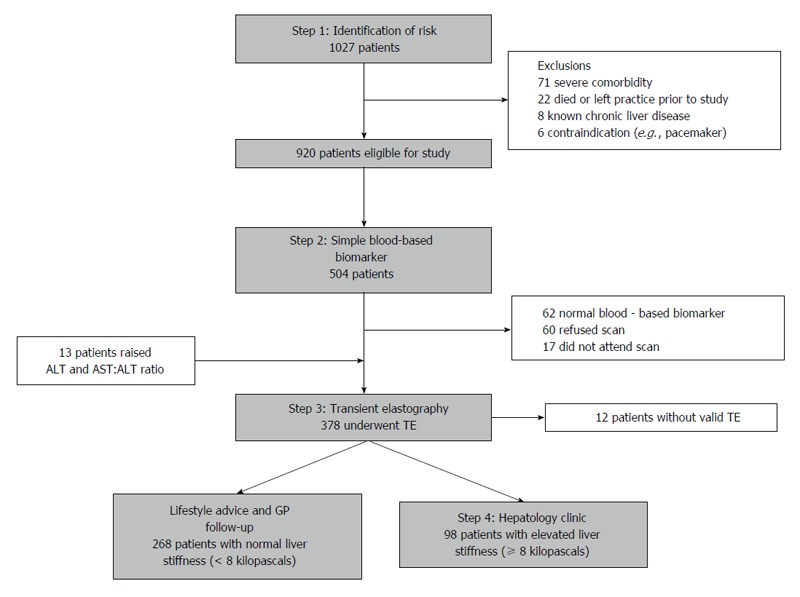
Diagnostic algorithm and patient flow chart of the non-invasive biomarker and transient elastography pathway[123]. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; GP: General practitioner; TE: Transient elastography.
IMAGING MODALITIES TO DETECT FEATURES OF CLD
Imaging modalities are can be used to detect a number of signs or physical parameters, which are of relevance to chronic liver disease.
Morphological changes
The later stages of chronic liver disease are characterised by a number of intra- and extrahepatic structural changes. A number of these structural changes are manifestations or consequences of other pathological processes. The cirrhotic liver is typically small with an uneven border or perimeter. These features are caused by the contraction of thick bands of fibrous tissue interspersed by regenerative nodules. Ultrasound-visible features, such as liver surface nodularity, caudate lobe hypertrophy and the presence of detectable hepatic venous blood flow have been shown to identify those with severe fibrosis or cirrhosis from a cohort of patients with chronic liver disease, although it is doubtful that the severe fibrosis group alone would be identifiable by such a technique[124]. Routine MRI, computed tomography (CT) and ultrasound indices can detect gross morphological changes associated with cirrhosis, but none is sensitive or specific. Moreover, pre-cirrhotic disease is not adequately discriminated[125-127]. However, dynamic superparamagnetic iron oxide-enhanced and gadolinium-enhanced MRI demonstrates reticular-nodular patterns, thought to represent septal hepatic fibrosis, the presence of which, taken with an overall (subjective) qualitative assessment, allows the discrimination of moderate and severe from mild fibrosis[128] (Figure 6). Digital image processing of unenhanced CT scans has enabled assessment of the distribution of high-attenuation patterning, again presumed to represent fibrosis and distinction between moderate and severe fibrosis[129]. However, these techniques do not provide a quantitative measure of disease severity, and a number of the techniques remain unvalidated by other centres. Water molecules are tightly bound in the fibrotic extracellular matrix, providing the rationale behind the application of diffusion-weighted MR imaging to chronic liver disease. An apparent diffusion coefficient (ADC) is derived, representing proton, and hence water, mobility. A reduced ADC is observed in cirrhosis and with increasing fibrosis stage, and has been interpreted as being due to restriction of water diffusion in fibrotic tissue[130,131], or possibly by reduced capillary perfusion[132]. However, the precise relationship between the ADC and fibrosis is currently unclear.
Figure 6.
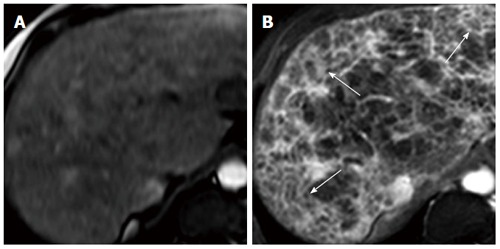
Transverse MR images of cirrhotic liver in vivo[128]. A: SPIO-enhanced two-dimensional spoiled gradient echo (SPGR) image with echotime of 2.65 ms; B: Double-enhanced SPGR image at the same level, showing hyperintense reticulations and hypointense nodules (arrows), thought to represent fibrous septal bands surrounding regenerative nodules.
Portal hypertension
Portal hypertension is the cause of much morbidity and mortality associated with chronic liver disease, through development of varices of porto-systemic anastomoses and through activation of vasodilatory pathways and development of ascites and the hepatorenal syndrome. Increased portal pressure is caused by increased intrahepatic resistance to flow which results from both vascular factors and fibrosis[133]. The structural results of portal hypertension, such as splenomegaly, ascites and the presence of venous collaterals are also readily assessed by conventional imaging techniques, but these features tend to be associated with decompensated cirrhosis and not pre-cirrhotic disease stages. A number of ultrasound-based studies have aimed to assess portal pressure indirectly as a surrogate for disease severity[134]. However, ultrasound Doppler indices of portal flow were found not to correlate reliably with increasing severity of disease[135,136]. A number of studies have pointed to a relationship between portal hypertension and liver stiffness measurement (LSM) measured by Fibroscan®. Foucher and colleagues demonstrated a correlation between liver stiffness measurement and splenomegaly, the presence of oesophageal varices and a history of bleeding varices[137]. A relationship between LSM, measured by Fibroscan® and the presence of varices has also been described, although evidence for the relationship between LSM and size of varices is mixed[138,139]. Vizzutti and colleagues went on to demonstrate correlation between LSM and the hepatic venous pressure gradient (HVPG), particularly at lower HVPG values (< 10-12 mmHg). This represents a complex relationship, which was less apparent at higher HVPG values[139]. It has been stated that the “progressive rise in portal pressure...[is]…due mainly to an increase in intrahepatic vascular resistance from the accumulation of fibrillar extracellular matrix”[140]. However, increased arterial and portal inflow may contribute directly to the liver stiffness, while haemodynamic changes characteristic of advanced portal hypertension, including extrahepatic haemodynamic changes, may not be detected by changes in liver stiffness.
Intrahepatic vascular changes
Vascular remodeling is increasingly seen as a pathological feature of chronic liver disease. In the development of fibrosis, obliteration of the small hepatic and portal veins may lead to a congestive hepatopathy, which is exacerbated by a co-existent hyperdynamic circulation[141]. This results in inflammation and oxidative stress, both triggers for fibrogenesis. Intrahepatic vascular remodeling within the fibrotic liver is performed by the contractile HSCs, mediated by changes in levels of nitric oxide (NO), consequent to derangement of endothelial NO synthase. This contributes to high resistance and constricted sinusoidal vessels[142]. Such mechanisms may also contribute to the development of intrahepatic vascular shunts. Imaging techniques assess changes in physical properties consequent to vascular alteration. While vascular changes occur with increasing fibrosis, imaging techniques do not assess fibrosis directly, so may be considered surrogate markers in this context.
Inflammation and cell turnover
Hepatic inflammation is associated with cellular inflammatory infiltrate, tissue oedema and hepatocyte swelling. Each of these is likely to affect the physical properties of liver tissue and, as such, can be measured by imaging modalities. These properties include: nuclear relaxation (T2), assessed by MR techniques; water perfusion and diffusion, as assessed by DWI; liver stiffness; changes in attenuation, assessed by CT and echogenicity, assessed by B-mode ultrasound.
Liver stiffness
The association between liver stiffness on Fibroscan® and disease activity, or necroinflammatory score on histology has been shown by a step-wise increase of liver stiffness measurements (LSM) with necroinflammatory activity in a cohort of patients with disease of varied aetiology[143]. The relationship between LSM and biochemical activity in patients with chronic viral hepatitis has also been studied using Fibroscan®. The LSM was lower, stage-for-stage, in those with biochemical remission (assessed by ALT) than those with a higher ALT[144]. Studies have specifically addressed the effect of hepatic inflammation on LSM. In one, 18 patients without a past history of liver disease, but with acute viral hepatitis, were studied. The LSM on Fibroscan® at the peak aminotransferase level exceeded 12kPa (the cut-off for prediction of cirrhosis) and furthermore, in all but one subject, the LSM returned to within normal range (below 7kPa). In addition, the LSM correlated with the aminotransferases at onset and with the AST at follow-up[145]. In another Fibroscan® paper, 20 patients with acute hepatitis of varying aetiology were studied. In those followed up longitudinally, the aminotransferases returned to a level commensurate with the fibrosis stage at biopsy[146]. While it is known that acute hepatitis is associated with an inflammatory infiltrate, tissue oedema and hepatocyte swelling, (all of which are likely to affect LSM), there was no histological confirmation of these features in these studies, as liver biopsy was not clinically indicated[147].
The development of ultrasound-based TE has enabled the rapid acquisition of objective liver stiffness measurements in vivo[65]. Multiple regression analysis in early studies demonstrated a relationship between elasticity measurements and fibrosis stage, but not to the histologically-measured disease activity, necroinflammatory score or the degree of steatosis[65,66]. A number of studies have confirmed the correlation between liver stiffness and hepatic fibrosis in chronic HCV infection[66,148], and other chronic hepatic conditions[149-151] with meta-analyses and systematic reviews also being recently published[68,152]. However, studies have also indicated the presence of co-existing factors that may contribute to liver stiffness, including inflammation, portal hypertension and possibly steatosis[137,147,150,151]. It has also been stressed that “biological tissue is a composite material and it is difficult to separate the influence of each component of the tissue on the total in modulus estimates”[153]. Substantial differences in cut-off values for cirrhosis have been observed on Fibroscan® between those with chronic hepatitis and those with alcohol-related chronic liver disease and NAFLD, perhaps representing modification of liver stiffness by coexisting fat[150,151,154]. Thus, there is circumstantial evidence that steatosis affects liver stiffness although the magnitude of the effect is likely to be smaller than that of other contributing factors, such as fibrosis, inflammation and portal hypertension.
Summary
The development of chronic liver disease is a major cause of morbidity and mortality worldwide and usually occurs over many years from progressive fibrosis and associated hepatocellular injury, including steatosis. Non-invasive assessments using imaging modalities and serum markers have now been shown to be effective at detecting significant fibrosis and cirrhosis to at least some degree. However, recent evidence suggests that various combinations of these techniques may be helpful both as an alternative to liver biopsy, which has significant associated morbidity and mortality, and as a cost-effective tool to identify sub-clinical disease.
SUMMARY OF KEY POINTS
Chronic liver disease develops over many years following ongoing injury and is characterised by progressive scarring caused by fibrosis. Hepatic steatosis is increasingly being recognised as an important factor in a number of chronic liver diseases and occurs when the rate of synthesis or import of fatty acids by hepatocytes exceeds the rate of export or catabolism. Serum markers measure both direct and indirect markers of liver fibrosis although these have had limited success as individual markers of fibrosis. Imaging modalities to assess liver disease can be used to quantify morphological changes, portal hypertension, vascular remodelling and liver stiffness associated with increasing fibrosis. The most widely used and successful of these are transient elastography and acoustic radiation force impulse imaging although MRI techniques are very promising. Combination techniques involving transient elastography and various serum markers can provide good diagnostic accuracy and a reduced need for liver biopsy in patients with significant fibrosis. This has also proved successful as a screening tool in for patients in a community setting.
ACKNOWLEDGMENTS
The authors thank the United Kingdom National Institute for Health Research (NIHR) Biomedical Research Facility at Imperial College London for infrastructure support. SDT-R holds grants from the British Medical Research Council and the Wellcome Trust (London, United Kingdom). MMEC is supported by a Fellowship from the Sir Halley Stewart Trust (Cambridge, United Kingdom). SDT-R, MMEC, HKSF and RN have been participant workers in the PROLIFICA project in West Africa, funded by the European Union Framework 7. The authors also thank Dr Jeremy Cobbold for helpful contributions to this review and for ongoing discussions.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare no relevant or potential conflicts of interest.
Peer-review started: September 3, 2016
First decision: September 28, 2016
Article in press: November 16, 2016
P- Reviewer: Im SS, Tziomalos K S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, Murray CJ, Naghavi M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation. Hepatitis C factsheet, 2000. Available from: http://www.who.int/mediacentre/factsheets/fs164/en/index.html.
- 4.World Health Organisation. Hepatitis B factsheet, 2008. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/index.html.
- 5.Campbell TC. Correspondence re: G-S. Qian, et al., A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol., Biomarkers & amp; Prev., 3: 3-10, 1994, and C.C. Harris, Solving the viral-chemical puzzle of human liver carcinogenesis. Cancer Epidemiol., Biomarkers & amp; Prev., 3: 1-2, 1994. Cancer Epidemiol Biomarkers Prev. 1994;3:519–521. [PubMed] [Google Scholar]
- 6.Autrup H, Seremet T, Wakhisi J, Wasunna A. Aflatoxin exposure measured by urinary excretion of aflatoxin B1-guanine adduct and hepatitis B virus infection in areas with different liver cancer incidence in Kenya. Cancer Res. 1987;47:3430–3433. [PubMed] [Google Scholar]
- 7.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 9.Shyamala K, Girish HC, Murgod S. Risk of tumor cell seeding through biopsy and aspiration cytology. J Int Soc Prev Community Dent. 2014;4:5–11. doi: 10.4103/2231-0762.129446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut. 1995;36:437–441. doi: 10.1136/gut.36.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thursz M, Cooke GS, Hall AJ. Hepatitis B treatment in resource poor settings: time for action. Trop Med Int Health. 2010;15:2–4. doi: 10.1111/j.1365-3156.2009.02410.x. [DOI] [PubMed] [Google Scholar]
- 12.Cobbold J, Lim A, Wylezinska M, Cunningham C, Crossey M, Thomas H, Patel N, Cox J, Taylor-Robinson S. Magnetic resonance and ultrasound techniques for the evaluation of hepatic fibrosis. Hepatology. 2006;43:1401–1402; author reply 1402. doi: 10.1002/hep.21217. [DOI] [PubMed] [Google Scholar]
- 13.Hammel P, Couvelard A, O’Toole D, Ratouis A, Sauvanet A, Fléjou JF, Degott C, Belghiti J, Bernades P, Valla D, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344:418–423. doi: 10.1056/NEJM200102083440604. [DOI] [PubMed] [Google Scholar]
- 14.Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427–436. doi: 10.1055/s-2001-17557. [DOI] [PubMed] [Google Scholar]
- 15.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kweon YO, Goodman ZD, Dienstag JL, Schiff ER, Brown NA, Burchardt E, Schoonhoven R, Brenner DA, Fried MW. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol. 2001;35:749–755. doi: 10.1016/s0168-8278(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 17.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 18.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niki T, Pekny M, Hellemans K, Bleser PD, Berg KV, Vaeyens F, Quartier E, Schuit F, Geerts A. Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology. 1999;29:520–527. doi: 10.1002/hep.510290232. [DOI] [PubMed] [Google Scholar]
- 20.Beaussier M, Wendum D, Schiffer E, Dumont S, Rey C, Lienhart A, Housset C. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab Invest. 2007;87:292–303. doi: 10.1038/labinvest.3700513. [DOI] [PubMed] [Google Scholar]
- 21.Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–963. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Sohara N, Znoyko I, Levy MT, Trojanowska M, Reuben A. Reversal of activation of human myofibroblast-like cells by culture on a basement membrane-like substrate. J Hepatol. 2002;37:214–221. doi: 10.1016/s0168-8278(02)00103-4. [DOI] [PubMed] [Google Scholar]
- 23.Preaux AM, D’ortho MP, Bralet MP, Laperche Y, Mavier P. Apoptosis of human hepatic myofibroblasts promotes activation of matrix metalloproteinase-2. Hepatology. 2002;36:615–622. doi: 10.1053/jhep.2002.35279. [DOI] [PubMed] [Google Scholar]
- 24.Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069–11076. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- 25.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 26.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 27.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 28.Nassir F, Rector RS, Hammoud GM, Ibdah JA. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol Hepatol (N Y) 2015;11:167–175. [PMC free article] [PubMed] [Google Scholar]
- 29.Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185–194. doi: 10.1096/fj.01-0396com. [DOI] [PubMed] [Google Scholar]
- 30.Patel JH, Cobbold JF, Thomas HC, Taylor-Robinson SD. Hepatitis C and hepatic steatosis. QJM. 2010;103:293–303. doi: 10.1093/qjmed/hcp192. [DOI] [PubMed] [Google Scholar]
- 31.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 32.Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology. Gut. 1999;45 Suppl 4:IV1–IV11. doi: 10.1136/gut.45.2008.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Standish RA, Cholongitas E, Dhillon A, Burroughs AK, Dhillon AP. An appraisal of the histopathological assessment of liver fibrosis. Gut. 2006;55:569–578. doi: 10.1136/gut.2005.084475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 35.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 36.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 37.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 38.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 39.Paradis V, Kollinger M, Fabre M, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation by-products in chronic liver diseases. Hepatology. 1997;26:135–142. doi: 10.1053/jhep.1997.v26.pm0009214462. [DOI] [PubMed] [Google Scholar]
- 40.Lindor KD, Bru C, Jorgensen RA, Rakela J, Bordas JM, Gross JB, Rodes J, McGill DB, Reading CC, James EM, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology. 1996;23:1079–1083. doi: 10.1002/hep.510230522. [DOI] [PubMed] [Google Scholar]
- 41.Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology. 1978;74:103–106. [PubMed] [Google Scholar]
- 42.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 43.Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–1596. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 44.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 45.Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–525. doi: 10.1016/s0140-6736(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 46.Poniachik J, Bernstein DE, Reddy KR, Jeffers LJ, Coelho-Little ME, Civantos F, Schiff ER. The role of laparoscopy in the diagnosis of cirrhosis. Gastrointest Endosc. 1996;43:568–571. doi: 10.1016/s0016-5107(96)70192-x. [DOI] [PubMed] [Google Scholar]
- 47.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 48.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239–244. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 49.Goldin RD, Goldin JG, Burt AD, Dhillon PA, Hubscher S, Wyatt J, Patel N. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol. 1996;25:649–654. doi: 10.1016/s0168-8278(96)80234-0. [DOI] [PubMed] [Google Scholar]
- 50.Rousselet MC, Michalak S, Dupré F, Croué A, Bedossa P, Saint-André JP, Calès P. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology. 2005;41:257–264. doi: 10.1002/hep.20535. [DOI] [PubMed] [Google Scholar]
- 51.Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol. 2006;44:462–474. doi: 10.1016/j.jhep.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 52.Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology. 2006;43:S113–S120. doi: 10.1002/hep.21046. [DOI] [PubMed] [Google Scholar]
- 53.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912–921. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 54.Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134:1670–1681. doi: 10.1053/j.gastro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 56.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 57.Borsoi Viana MS, Takei K, Collarile Yamaguti DC, Guz B, Strauss E. Use of AST platelet ratio index (APRI Score) as an alternative to liver biopsy for treatment indication in chronic hepatitis C. Ann Hepatol. 2009;8:26–31. [PubMed] [Google Scholar]
- 58.Catanzaro R, Milazzo M, Arona S, Sapienza C, Vasta D, Arcoria D, Marotta F. Diagnostic accuracy of enhanced liver fibrosis test to assess liver fibrosis in patients with chronic hepatitis C. Hepatobiliary Pancreat Dis Int. 2013;12:500–507. doi: 10.1016/s1499-3872(13)60079-x. [DOI] [PubMed] [Google Scholar]
- 59.Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 60.Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59:236–242. doi: 10.1016/j.jhep.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Poynard T, Morra R, Halfon P, Castera L, Ratziu V, Imbert-Bismut F, Naveau S, Thabut D, Lebrec D, Zoulim F, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol. 2007;7:40. doi: 10.1186/1471-230X-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 63.Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 64.Güzelbulut F, Çetınkaya ZA, Sezıklı M, Yaşar B, Ozkara S, Övünç AO. AST-platelet ratio index, Forns index and FIB-4 in the prediction of significant fibrosis and cirrhosis in patients with chronic hepatitis C. Turk J Gastroenterol. 2011;22:279–285. doi: 10.4318/tjg.2011.0213. [DOI] [PubMed] [Google Scholar]
- 65.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 67.Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650–659. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 68.Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 69.Trovato FM, Tognarelli JM, Crossey MM, Catalano D, Taylor-Robinson SD, Trovato GM. Challenges of liver cancer: Future emerging tools in imaging and urinary biomarkers. World J Hepatol. 2015;7:2664–2675. doi: 10.4254/wjh.v7.i26.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13–20. doi: 10.1016/j.clinre.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 71.Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, Poupon R, Cardoso AC, Marcellin P, Douvin C, et al. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan(®): validation in chronic hepatitis C. J Viral Hepat. 2012;19:244–253. doi: 10.1111/j.1365-2893.2011.01534.x. [DOI] [PubMed] [Google Scholar]
- 72.Friedrich-Rust M, Romen D, Vermehren J, Kriener S, Sadet D, Herrmann E, Zeuzem S, Bojunga J. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012;81:e325–e331. doi: 10.1016/j.ejrad.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 73.Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S, et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212–e219. doi: 10.1111/j.1365-2893.2011.01537.x. [DOI] [PubMed] [Google Scholar]
- 74.Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138–1147. doi: 10.1111/liv.12240. [DOI] [PubMed] [Google Scholar]
- 75.Sporea I, Bota S, Grădinaru-Taşcău O, Şirli R, Popescu A. Comparative study between two point Shear Wave Elastographic techniques: Acoustic Radiation Force Impulse (ARFI) elastography and ElastPQ. Med Ultrason. 2014;16:309–314. doi: 10.11152/mu.201.3.2066.164.isp1. [DOI] [PubMed] [Google Scholar]
- 76.Ma JJ, Ding H, Mao F, Sun HC, Xu C, Wang WP. Assessment of liver fibrosis with elastography point quantification technique in chronic hepatitis B virus patients: a comparison with liver pathological results. J Gastroenterol Hepatol. 2014;29:814–819. doi: 10.1111/jgh.12479. [DOI] [PubMed] [Google Scholar]
- 77.Grier S, Lim AK, Patel N, Cobbold JF, Thomas HC, Cox IJ, Taylor-Robinson SD. Role of microbubble ultrasound contrast agents in the non-invasive assessment of chronic hepatitis C-related liver disease. World J Gastroenterol. 2006;12:3461–3465. doi: 10.3748/wjg.v12.i22.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Webb M, Yeshua H, Zelber-Sagi S, Santo E, Brazowski E, Halpern Z, Oren R. Diagnostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. AJR Am J Roentgenol. 2009;192:909–914. doi: 10.2214/AJR.07.4016. [DOI] [PubMed] [Google Scholar]
- 79.Mehta SR, Thomas EL, Bell JD, Johnston DG, Taylor-Robinson SD. Non-invasive means of measuring hepatic fat content. World J Gastroenterol. 2008;14:3476–3483. doi: 10.3748/wjg.14.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114–1122. doi: 10.1053/jlts.2002.36740. [DOI] [PubMed] [Google Scholar]
- 81.Fishbein M, Castro F, Cheruku S, Jain S, Webb B, Gleason T, Stevens WR. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol. 2005;39:619–625. doi: 10.1097/00004836-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 82.Albrecht T, Blomley MJ, Cosgrove DO, Taylor-Robinson SD, Jayaram V, Eckersley R, Urbank A, Butler-Barnes J, Patel N. Non-invasive diagnosis of hepatic cirrhosis by transit-time analysis of an ultrasound contrast agent. Lancet. 1999;353:1579–1583. doi: 10.1016/S0140-6736(98)06373-9. [DOI] [PubMed] [Google Scholar]
- 83.Lim AK, Taylor-Robinson SD, Patel N, Eckersley RJ, Goldin RD, Hamilton G, Foster GR, Thomas HC, Cosgrove DO, Blomley MJ. Hepatic vein transit times using a microbubble agent can predict disease severity non-invasively in patients with hepatitis C. Gut. 2005;54:128–133. doi: 10.1136/gut.2003.030965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bydder GM, Kreel L, Chapman RW, Harry D, Sherlock S, Bassan L. Accuracy of computed tomography in diagnosis of fatty liver. Br Med J. 1980;281:1042. doi: 10.1136/bmj.281.6247.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, Ha HK, Lee MG, Hwang S, Lee SG, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 86.Pavlides M, Banerjee R, Sellwood J, Kelly CJ, Robson MD, Booth JC, Collier J, Neubauer S, Barnes E. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol. 2016;64:308–315. doi: 10.1016/j.jhep.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Messroghli DR, Greiser A, Fröhlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26:1081–1086. doi: 10.1002/jmri.21119. [DOI] [PubMed] [Google Scholar]
- 88.Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, Collier JD, Booth JC, Schneider JE, Wang LM, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60:69–77. doi: 10.1016/j.jhep.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cassinotto C, Feldis M, Vergniol J, Mouries A, Cochet H, Lapuyade B, Hocquelet A, Juanola E, Foucher J, Laurent F, et al. MR relaxometry in chronic liver diseases: Comparison of T1 mapping, T2 mapping, and diffusion-weighted imaging for assessing cirrhosis diagnosis and severity. Eur J Radiol. 2015;84:1459–1465. doi: 10.1016/j.ejrad.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 90.Low G, Kruse SA, Lomas DJ. General review of magnetic resonance elastography. World J Radiol. 2016;8:59–72. doi: 10.4329/wjr.v8.i1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, Hassanein T, Asbach P, Godfrey EM, Yin M, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13:440–451.e6. doi: 10.1016/j.cgh.2014.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su LN, Guo SL, Li BX, Yang P. Diagnostic value of magnetic resonance elastography for detecting and staging of hepatic fibrosis: a meta-analysis. Clin Radiol. 2014;69:e545–e552. doi: 10.1016/j.crad.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Lee VS, Miller FH, Omary RA, Wang Y, Ganger DR, Wang E, Rao S, Levitsky J. Magnetic resonance elastography and biomarkers to assess fibrosis from recurrent hepatitis C in liver transplant recipients. Transplantation. 2011;92:581–586. doi: 10.1097/TP.0b013e31822805fa. [DOI] [PubMed] [Google Scholar]
- 94.Bohte AE, de Niet A, Jansen L, Bipat S, Nederveen AJ, Verheij J, Terpstra V, Sinkus R, van Nieuwkerk KM, de Knegt RJ, et al. Non-invasive evaluation of liver fibrosis: a comparison of ultrasound-based transient elastography and MR elastography in patients with viral hepatitis B and C. Eur Radiol. 2014;24:638–648. doi: 10.1007/s00330-013-3046-0. [DOI] [PubMed] [Google Scholar]
- 95.Takamura T, Motosugi U, Ichikawa S, Sano K, Morisaka H, Ichikawa T, Enomoto N, Onishi H. Usefulness of MR elastography for detecting clinical progression of cirrhosis from child-pugh class A to B in patients with type C viral hepatitis. J Magn Reson Imaging. 2016;44:715–722. doi: 10.1002/jmri.25182. [DOI] [PubMed] [Google Scholar]
- 96.Taouli B, Serfaty L. Magnetic Resonance Imaging/Elastography Is Superior to Transient Elastography for Detection of Liver Fibrosis and Fat in Nonalcoholic Fatty Liver Disease. Gastroenterology. 2016;150:553–556. doi: 10.1053/j.gastro.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 97.Shi Y, Xia F, Li QJ, Li JH, Yu B, Li Y, An H, Glaser KJ, Tao S, Ehman RL, et al. Magnetic Resonance Elastography for the Evaluation of Liver Fibrosis in Chronic Hepatitis B and C by Using Both Gradient-Recalled Echo and Spin-Echo Echo Planar Imaging: A Prospective Study. Am J Gastroenterol. 2016;111:823–833. doi: 10.1038/ajg.2016.56. [DOI] [PubMed] [Google Scholar]
- 98.Loomba R, Cui J, Wolfson T, Haufe W, Hooker J, Szeverenyi N, Ang B, Bhatt A, Wang K, Aryafar H, et al. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. Am J Gastroenterol. 2016;111:986–994. doi: 10.1038/ajg.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Longo R, Pollesello P, Ricci C, Masutti F, Kvam BJ, Bercich L, Crocè LS, Grigolato P, Paoletti S, de Bernard B. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995;5:281–285. doi: 10.1002/jmri.1880050311. [DOI] [PubMed] [Google Scholar]
- 100.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 101.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 102.Thomas EL, Hamilton G, Patel N, O’Dwyer R, Doré CJ, Goldin RD, Bell JD, Taylor-Robinson SD. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122–127. doi: 10.1136/gut.2003.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomas EL, Brynes AE, Hamilton G, Patel N, Spong A, Goldin RD, Frost G, Bell JD, Taylor-Robinson SD. Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:5813–5819. doi: 10.3748/wjg.v12.i36.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Otten J, Mellberg C, Ryberg M, Sandberg S, Kullberg J, Lindahl B, Larsson C, Hauksson J, Olsson T. Strong and persistent effect on liver fat with a Paleolithic diet during a two-year intervention. Int J Obes (Lond) 2016;40:747–753. doi: 10.1038/ijo.2016.4. [DOI] [PubMed] [Google Scholar]
- 105.Chiang HJ, Lin LH, Li CW, Lin CC, Chiang HW, Huang TL, Chen CL, Cheng YF. Magnetic resonance fat quantification in living donor liver transplantation. Transplant Proc. 2014;46:666–668. doi: 10.1016/j.transproceed.2013.11.050. [DOI] [PubMed] [Google Scholar]
- 106.Lui G, Wong VW, Wong GL, Chu WC, Wong CK, Yung IM, Wong RY, Yeung SL, Yeung DK, Cheung CS, et al. Liver fibrosis and fatty liver in Asian HIV-infected patients. Aliment Pharmacol Ther. 2016;44:411–421. doi: 10.1111/apt.13702. [DOI] [PubMed] [Google Scholar]
- 107.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36:1011–1014. doi: 10.1002/jmri.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Idilman IS, Keskin O, Celik A, Savas B, Halil Elhan A, Idilman R, Karcaaltincaba M. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol. 2016;57:271–278. doi: 10.1177/0284185115580488. [DOI] [PubMed] [Google Scholar]
- 109.Bashir MR, Zhong X, Nickel MD, Fananapazir G, Kannengiesser SA, Kiefer B, Dale BM. Quantification of hepatic steatosis with a multistep adaptive fitting MRI approach: prospective validation against MR spectroscopy. AJR Am J Roentgenol. 2015;204:297–306. doi: 10.2214/AJR.14.12457. [DOI] [PubMed] [Google Scholar]
- 110.Tang A, Desai A, Hamilton G, Wolfson T, Gamst A, Lam J, Clark L, Hooker J, Chavez T, Ang BD, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274:416–425. doi: 10.1148/radiol.14140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Horng DE, Hernando D, Reeder SB. Quantification of liver fat in the presence of iron overload. J Magn Reson Imaging. 2016 doi: 10.1002/jmri.25382. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paparo F, Cenderello G, Revelli M, Bacigalupo L, Rutigliani M, Zefiro D, Cevasco L, Amico M, Bandelloni R, Cassola G, et al. Diagnostic value of MRI proton density fat fraction for assessing liver steatosis in chronic viral C hepatitis. Biomed Res Int. 2015;2015:758164. doi: 10.1155/2015/758164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heba ER, Desai A, Zand KA, Hamilton G, Wolfson T, Schlein AN, Gamst A, Loomba R, Sirlin CB, Middleton MS. Accuracy and the effect of possible subject-based confounders of magnitude-based MRI for estimating hepatic proton density fat fraction in adults, using MR spectroscopy as reference. J Magn Reson Imaging. 2016;43:398–406. doi: 10.1002/jmri.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bell JD, Cox IJ, Sargentoni J, Peden CJ, Menon DK, Foster CS, Watanapa P, Iles RA, Urenjak J. A 31P and 1H-NMR investigation in vitro of normal and abnormal human liver. Biochim Biophys Acta. 1993;1225:71–77. doi: 10.1016/0925-4439(93)90124-j. [DOI] [PubMed] [Google Scholar]
- 115.Taylor-Robinson SD, Thomas EL, Sargentoni J, Marcus CD, Davidson BR, Bell JD. Cirrhosis of the human liver: an in vitro 31P nuclear magnetic resonance study. Biochim Biophys Acta. 1995;1272:113–118. doi: 10.1016/0925-4439(95)00074-e. [DOI] [PubMed] [Google Scholar]
- 116.Lim AK, Patel N, Hamilton G, Hajnal JV, Goldin RD, Taylor-Robinson SD. The relationship of in vivo 31P MR spectroscopy to histology in chronic hepatitis C. Hepatology. 2003;37:788–794. doi: 10.1053/jhep.2003.50149. [DOI] [PubMed] [Google Scholar]
- 117.Menon DK, Sargentoni J, Taylor-Robinson SD, Bell JD, Cox IJ, Bryant DJ, Coutts GA, Rolles K, Burroughs AK, Morgan MY. Effect of functional grade and etiology on in vivo hepatic phosphorus-31 magnetic resonance spectroscopy in cirrhosis: biochemical basis of spectral appearances. Hepatology. 1995;21:417–427. [PubMed] [Google Scholar]
- 118.Yamane Y, Umeda M, O’uchi T, Mitsushima T, Nakata K, Nagataki S. Phosphorus-31 nuclear magnetic resonance in vivo spectroscopy of human liver during hepatitis A virus infection. Dig Dis Sci. 1994;39:33–38. doi: 10.1007/BF02090057. [DOI] [PubMed] [Google Scholar]
- 119.Lim AK, Patel N, Hamilton G, Mylvahan K, Kuo YT, Goldin RD, Taylor-Robinson SD. 31P MR spectroscopy in assessment of response to antiviral therapy for hepatitis C virus-related liver disease. AJR Am J Roentgenol. 2007;189:819–823. doi: 10.2214/AJR.07.2418. [DOI] [PubMed] [Google Scholar]
- 120.Sebastiani G, Halfon P, Castera L, Mangia A, Di Marco V, Pirisi M, Voiculescu M, Bourliere M, Alberti A. Comparison of three algorithms of non-invasive markers of fibrosis in chronic hepatitis C. Aliment Pharmacol Ther. 2012;35:92–104. doi: 10.1111/j.1365-2036.2011.04897.x. [DOI] [PubMed] [Google Scholar]
- 121.Castéra L, Sebastiani G, Le Bail B, de Lédinghen V, Couzigou P, Alberti A. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J Hepatol. 2010;52:191–198. doi: 10.1016/j.jhep.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 122.Boursier J, Vergniol J, Sawadogo A, Dakka T, Michalak S, Gallois Y, Le Tallec V, Oberti F, Fouchard-Hubert I, Dib N, et al. The combination of a blood test and Fibroscan improves the non-invasive diagnosis of liver fibrosis. Liver Int. 2009;29:1507–1515. doi: 10.1111/j.1478-3231.2009.02101.x. [DOI] [PubMed] [Google Scholar]
- 123.Harman DJ, Ryder SD, James MW, Jelpke M, Ottey DS, Wilkes EA, Card TR, Aithal GP, Guha IN. Direct targeting of risk factors significantly increases the detection of liver cirrhosis in primary care: a cross-sectional diagnostic study utilising transient elastography. BMJ Open. 2015;5:e007516. doi: 10.1136/bmjopen-2014-007516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89–94. doi: 10.1148/radiol.2272020193. [DOI] [PubMed] [Google Scholar]
- 125.Aubé C, Oberti F, Korali N, Namour MA, Loisel D, Tanguy JY, Valsesia E, Pilette C, Rousselet MC, Bedossa P, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol. 1999;30:472–478. doi: 10.1016/s0168-8278(99)80107-x. [DOI] [PubMed] [Google Scholar]
- 126.Awaya H, Mitchell DG, Kamishima T, Holland G, Ito K, Matsumoto T. Cirrhosis: modified caudate-right lobe ratio. Radiology. 2002;224:769–774. doi: 10.1148/radiol.2243011495. [DOI] [PubMed] [Google Scholar]
- 127.Ito K, Mitchell DG, Hann HW, Kim Y, Fujita T, Okazaki H, Honjo K, Matsunaga N. Viral-induced cirrhosis: grading of severity using MR imaging. AJR Am J Roentgenol. 1999;173:591–596. doi: 10.2214/ajr.173.3.10470885. [DOI] [PubMed] [Google Scholar]
- 128.Aguirre DA, Behling CA, Alpert E, Hassanein TI, Sirlin CB. Liver fibrosis: noninvasive diagnosis with double contrast material-enhanced MR imaging. Radiology. 2006;239:425–437. doi: 10.1148/radiol.2392050505. [DOI] [PubMed] [Google Scholar]
- 129.Romero-Gómez M, Gómez-González E, Madrazo A, Vera-Valencia M, Rodrigo L, Pérez-Alvarez R, Pérez-López R, Castellano-Megias VM, Nevado-Santos M, Alcón JC, et al. Optical analysis of computed tomography images of the liver predicts fibrosis stage and distribution in chronic hepatitis C. Hepatology. 2008;47:810–816. doi: 10.1002/hep.22112. [DOI] [PubMed] [Google Scholar]
- 130.Aubé C, Racineux PX, Lebigot J, Oberti F, Croquet V, Argaud C, Calès P, Caron C. [Diagnosis and quantification of hepatic fibrosis with diffusion weighted MR imaging: preliminary results] J Radiol. 2004;85:301–306. doi: 10.1016/s0221-0363(04)97582-8. [DOI] [PubMed] [Google Scholar]
- 131.Lewin M, Poujol-Robert A, Boëlle PY, Wendum D, Lasnier E, Viallon M, Guéchot J, Hoeffel C, Arrivé L, Tubiana JM, et al. Diffusion-weighted magnetic resonance imaging for the assessment of fibrosis in chronic hepatitis C. Hepatology. 2007;46:658–665. doi: 10.1002/hep.21747. [DOI] [PubMed] [Google Scholar]
- 132.Taouli B, Tolia AJ, Losada M, Babb JS, Chan ES, Bannan MA, Tobias H. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. AJR Am J Roentgenol. 2007;189:799–806. doi: 10.2214/AJR.07.2086. [DOI] [PubMed] [Google Scholar]
- 133.Langer DA, Shah VH. Nitric oxide and portal hypertension: interface of vasoreactivity and angiogenesis. J Hepatol. 2006;44:209–216. doi: 10.1016/j.jhep.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 134.Vilgrain V. Ultrasound of diffuse liver disease and portal hypertension. Eur Radiol. 2001;11:1563–1577. doi: 10.1007/s003300101050. [DOI] [PubMed] [Google Scholar]
- 135.Bernatik T, Strobel D, Hahn EG, Becker D. Doppler measurements: a surrogate marker of liver fibrosis? Eur J Gastroenterol Hepatol. 2002;14:383–387. doi: 10.1097/00042737-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 136.Lim AK, Patel N, Eckersley RJ, Kuo YT, Goldin RD, Thomas HC, Cosgrove DO, Taylor-Robinson SD, Blomley MJ. Can Doppler sonography grade the severity of hepatitis C-related liver disease? AJR Am J Roentgenol. 2005;184:1848–1853. doi: 10.2214/ajr.184.6.01841848. [DOI] [PubMed] [Google Scholar]
- 137.Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kazemi F, Kettaneh A, N’kontchou G, Pinto E, Ganne-Carrie N, Trinchet JC, Beaugrand M. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230–235. doi: 10.1016/j.jhep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 139.Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290–1297. doi: 10.1002/hep.21665. [DOI] [PubMed] [Google Scholar]
- 140.Lim JK, Groszmann RJ. Transient elastography for diagnosis of portal hypertension in liver cirrhosis: is there still a role for hepatic venous pressure gradient measurement? Hepatology. 2007;45:1087–1090. doi: 10.1002/hep.21731. [DOI] [PubMed] [Google Scholar]
- 141.Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238–1247. [PubMed] [Google Scholar]
- 142.Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45:817–825. doi: 10.1002/hep.21564. [DOI] [PubMed] [Google Scholar]
- 143.Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–973. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360–369. doi: 10.1111/j.1365-2893.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 145.Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380–384. doi: 10.1002/hep.22007. [DOI] [PubMed] [Google Scholar]
- 146.Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592–595. doi: 10.1002/hep.22056. [DOI] [PubMed] [Google Scholar]
- 147.Cobbold JF, Taylor-Robinson SD. Transient elastography in acute hepatitis: All that’s stiff is not fibrosis. Hepatology. 2008;47:370–372. doi: 10.1002/hep.22200. [DOI] [PubMed] [Google Scholar]
- 148.Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 149.Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouillères O, de Lédinghen V, Dhumeaux D, Marcellin P, Beaugrand M, et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118–1124. doi: 10.1002/hep.21151. [DOI] [PubMed] [Google Scholar]
- 150.Ganne-Carrié N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, Dhumeaux D, Trinchet JC, Beaugrand M. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511–1517. doi: 10.1002/hep.21420. [DOI] [PubMed] [Google Scholar]
- 151.Yoneda M, Yoneda M, Fujita K, Inamori M, Tamano M, Hiriishi H, Nakajima A. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD) Gut. 2007;56:1330–1331. doi: 10.1136/gut.2007.126417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 153.Yeh WC, Li PC, Jeng YM, Hsu HC, Kuo PL, Li ML, Yang PM, Lee PH. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med Biol. 2002;28:467–474. doi: 10.1016/s0301-5629(02)00489-1. [DOI] [PubMed] [Google Scholar]
- 154.Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48:606–613. doi: 10.1016/j.jhep.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 155.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71–83. doi: 10.1093/qjmed/hcp158. [DOI] [PMC free article] [PubMed] [Google Scholar]


